Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule
Abstract
1. Introduction
2. Mechanism of Action of β-Hydroxybutyrate
2.1. Intermediate Metabolite During Energy Metabolism
2.2. Signaling Molecule
| HDAC Class | Members | Cellular Localization | Co-Factor Dependency | Expression | Main Target | Upstream Regulation | References |
|---|---|---|---|---|---|---|---|
| Class I | HDAC1 HDAC2 HDAC3 HDAC8 | Nucleus (HDAC1-2); Nuclear, cytoplasmic, plasma membrane, also mitochondria (HDAC3); Primarily cytoplasmic, also nucleolar (HDAC8) | Zn2+ | - HDAC 1-3: Broad/ubiquitous, high in proliferative tissues - HDAC 8: Restricted to smooth muscle lineage | - HDAC 1-3: Core histones (H3, H4) at gene promoters, Non-histone proteins: p53, Rb, E2F1, NF-κB, MEF2 - HDAC8: Core histones (H3, H4), Non-histone proteins: SMC3, ERRα, ARID1A, p53, BMF, inv(16), fusion protein, α-tubulin | - HDAC1-2: Sin3, NuRD, coREST, - HDAC2: Nitric oxide, - HDAC3: SMRT/NCoR, - HDAC8: cAMP-CREB1 pathway, TGF-β/SMAD3/4 signaling complex | [21,22,23,24,25,26,27] |
| Class IIa | HDAC4 HDAC5 HDAC7 HDAC9 | Cytoplasm and nucleus (HDAC4); Mainly nuclear, can shuttle to cytoplasm (HDAC5); Nucleus and cytoplasm (HDAC7-9) | Zn2+ | Tissue-specific; High in skeletal muscle, heart brain, thymus; Alternative splicing HDAC9 | Core histones (mainly H3), Non-histone proteins: MEF2, Runx, HIFα, Smad7, NF-kB, MyoD | Phosphorylation Calcium/calmodulin-dependent kinases (CaMKs), protein kinase, D1, cAMP pathway Transcriptional regulation: Sp1/Sp3 (for HDAC4) | [28,29,30,31,32] |
| Class IIb | HDAC6 HDAC10 | Cytoplasmic, can shuttle to nucleus under certain conditions (HDAC6); Mainly cytoplasmic (HDAC10) | Zn2+ | Heart, skeletal muscle, and brain | Non-histone proteins: α-tubulin, HSP90, cortactin | Phosphorylation (e.g., PKCζ, PKCα, ERK1/2, CK2, GRK2, GSK3β, Aurora A), dynamic protein–protein interactions and cellular stress signals | [33,34,35] |
| Class III (Sirtuins) | SIRT1-7 | Nucleus (SIRT1, SIRT6); Cytoplasm (SIRT2); Mitohondria (SIRT3-SIRT5); Nucleus (SIRT6); Nucleolar (SIRT7) | NAD+ | Variable: SIRT1/2, broadly expressed in tissues; SIRT3-5, mitochondrial, high in metabolically active tissues; SIRT6, broadly expressed, with notable levels in brain, liver, heart, spleen, adipose tissue, and immune cells; SIRT7, highly expressed in peripheral tissue with high proliferative activity (liver, spleen, testis). Less so in the brain and is found in immune and endothelial cells | Histone deactylation: H1K26, H3K9, H3K14, H3K18, H3K56, H4K6, H4K6, H4K12, H4K16, Non histone: p53, NF-kB RelA/p65 subunit, PGC-1α, FOXO 1/3/4, HSF1, HIF1α, P300, TIP60 | AMPK, oxidative stress (conditions that elevate NAD+) | [36,37,38,39,40] |
| Class IV | HDAC11 | Nucleus and cytoplasm | Brain, kidney, heart, skeletal muscle | Histone and non-histone proteins (d.g., CDT1); Regulates immune responses by controlling IL-10 expression | Non-coding RNAs (ncRNAs), transcriptional regulations. | [41,42] |
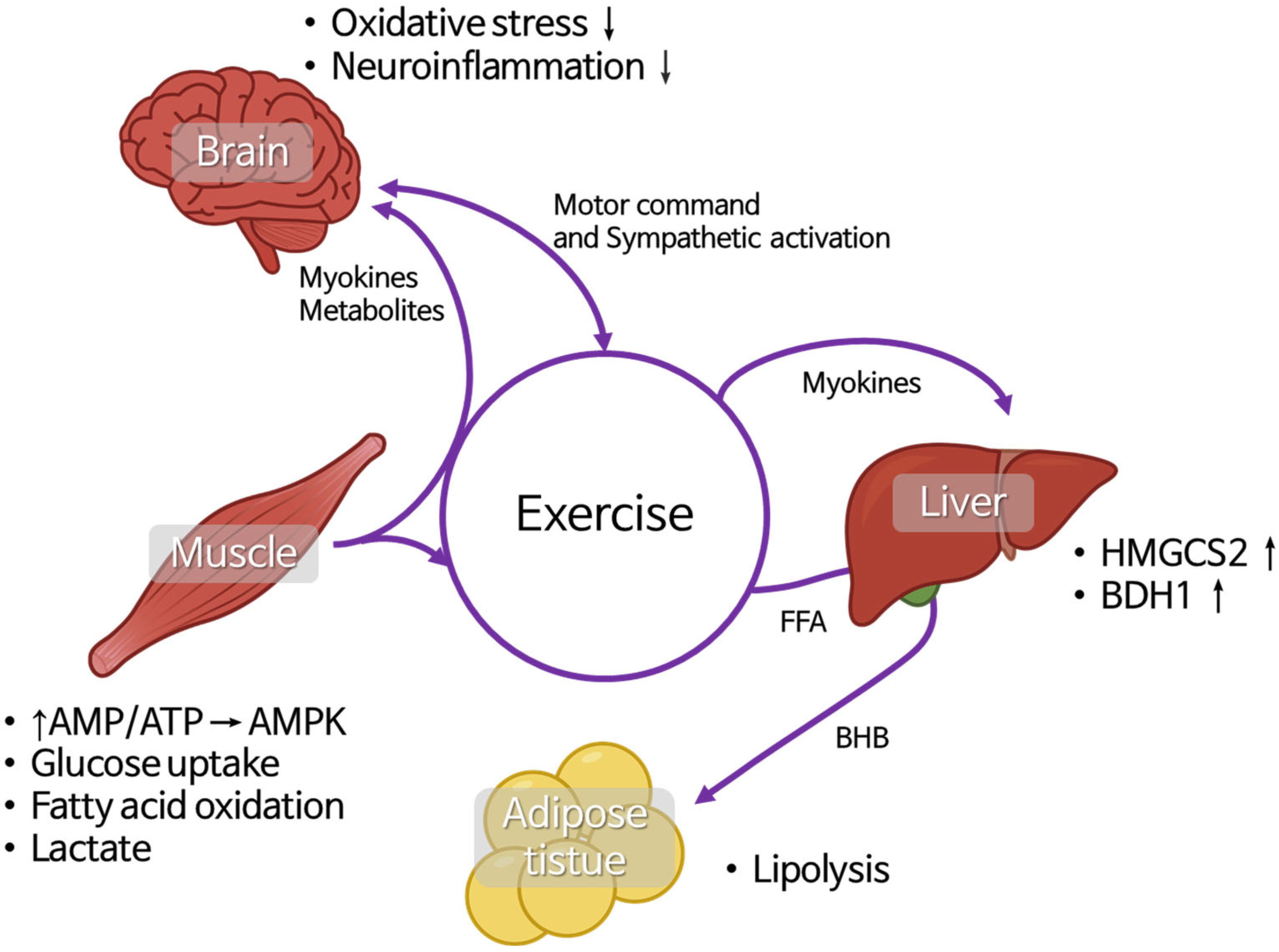
2.3. Muscle–Liver–Adipose-Tissue and Brain Crosstalk During Exercise and Its Relationship with BHB
2.4. Circadian Regulation of BHB Metabolism and Signaling
3. Role of β-Hydroxybutyrate in Diseases
3.1. Sarcopenia
3.2. Cancer and Cachexia
3.3. Diabetes Mellitus
3.4. Cardiovascular Disease
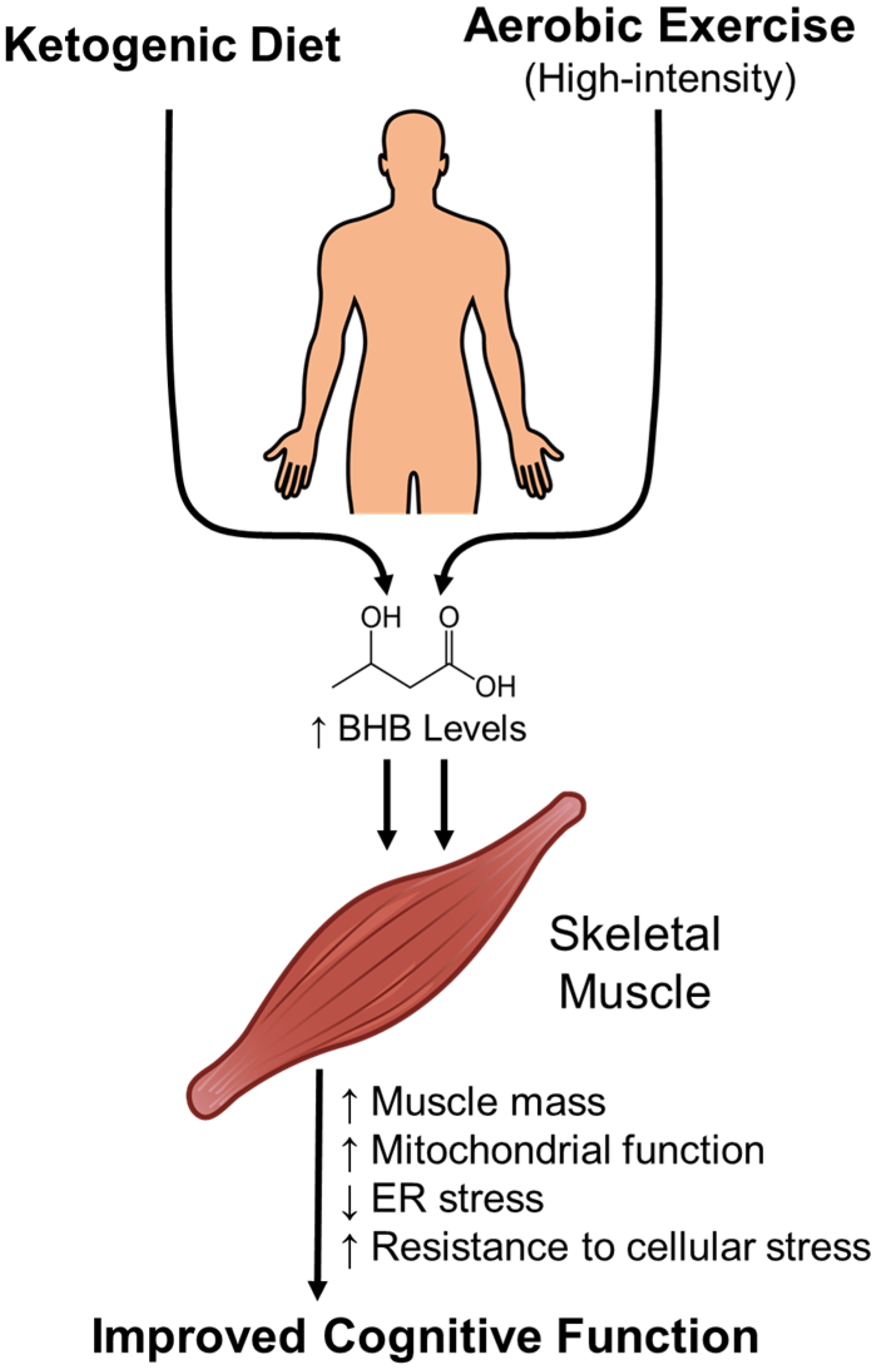
4. Lifestyle Interventions to Enhance β-Hydroxybutyrate Levels
5. Potential Therapeutic Applications of β-Hydroxybutyrate
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ehtiati, S.; Hatami, B.; Khatami, S.H.; Tajernarenj, K.; Abdi, S.; Sirati-Sabet, M.; Hasemi, S.A.H.G.; Ahmadzade, R.; Hamed, N.; Goudarzi, M.; et al. The Multifaceted Influence of Beta-Hydroxybutyrate on Autophagy, Mitochondrial Metabolism, and Epigenetic Regulation. J. Cell. Biochem. 2025, 126, e70050. [Google Scholar] [CrossRef]
- Møller, N. Ketone Body, 3-Hydroxybutyrate: Minor Metabolite—Major Medical Manifestations. J. Clin. Endocrinol. Metab. 2020, 105, 2884–2892. [Google Scholar] [CrossRef]
- Nesterova, V.V.; Babenkova, P.I.; Brezgunova, A.A.; Samoylova, N.A.; Sadovnikova, I.S.; Semenovich, D.S.; Andrianova, N.V.; Gureev, A.P.; Plotnikov, E.Y. Differences in the Effect of Beta-Hydroxybutyrate on the Mitochondrial Biogenesis, Oxidative Stress and Inflammation Markers in Tissues from Young and Old Rats. Biochemistry 2024, 89, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Dakhili, S.A.T.; Ussher, J.R.; Dyck, J.R.B. The therapeutic potential of ketones in cardiometabolic disease: Impact on heart and skeletal muscle. Am. J. Physiol. Cell Physiol. 2024, 326, C551–C566. [Google Scholar] [CrossRef]
- Kim, S.; Park, D.H.; Moon, S.; Gu, B.; Mantik, K.E.K.; Kwak, H.B.; Ryu, J.K.; Kang, J.H. Ketogenic diet with aerobic exercise can induce fat browning: Potential roles of β-hydroxybutyrate. Front. Nutr. 2024, 11, 1443483. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.C.; Seeds, W.A.; Miller, A.C.; Mahajan, V.R.; Curtis, W.M. COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm. Oxidative Med. Cell. Longev. 2020, 2020, 6401341. [Google Scholar] [CrossRef]
- Kyoung Lee, A.; Kim, D.H.; Bang, E.J.; Choi, Y.J.; Chung, H.Y. β-Hydroxybutyrate suppresses lipid accumulation in aged liver through GPR109A-mediated signaling. Aging Dis. 2020, 11, 777–790. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Han, Y.-M.; Bedarida, T.; Ding, Y.; Somba, B.K.; Lu, Q.; Wang, Q.; Song, P.; Zou, M.H. β-Hydroxybutyrate Prevents Vascular Senescence through hnRNP A1-Mediated Upregulation of Oct4. Mol. Cell 2018, 71, 1064–1078.e5. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, X.; Zhou, T.; Li, D.; Peng, J.; Xu, Y.; Huang, W. β-Hydroxybutyrate as an epigenetic modifier: Underlying mechanisms and implications. Heliyon 2023, 9, e21098. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Plourde, G.; Roumes, H.; Suissa, L.; Hirt, L.; Doche, É.; Pellerin, L.; Bouzier-Sore, A.-K.; Quintard, H. Neuroprotective effects of lactate and ketone bodies in acute brain injury. J. Cereb. Blood Flow Metab. 2024, 44, 1078–1088. [Google Scholar] [CrossRef]
- Curcio, A.; Rocca, R.; Alcaro, S.; Artese, A. The Histone Deacetylase Family: Structural Features and Application of Combined Computational Methods. Pharmaceuticals 2024, 17, 620. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P. Inside HDAC with HDAC inhibitors. Eur. J. Med. Chem. 2010, 45, 2095–2116. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Sauders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Li, B.; Yu, Y.; Liu, K.; Zhang, Y.; Geng, Q.; Zhang, F.; Li, Y.; Qi, J. β-Hydroxybutyrate inhibits histone deacetylase 3 to promote claudin-5 generation and attenuate cardiac microvascular hyperpermeability in diabetes. Diabetologia 2021, 64, 226–239. [Google Scholar] [CrossRef]
- Xiao, T.; Fu, Y.; Zhu, W.; Xu, R.; Xu, L.; Zhang, P.; Du, Y.; Chieng, J.; Jiang, H. HDAC8, A Potential Therapeutic Target, Regulates Proliferation and Differentiation of Bone Marrow Stromal Cells in Fibrous Dysplasia. Stem Cells Transl. Med. 2019, 8, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, G.; Su, F.; Cai, Y.; Shi, L.; Meng, Y.; Liu, Z.; Sun, J.; Wang, M.; Qian, M.; et al. HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Res. 2020, 48, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Zwinderman, M.R.H.; De Weerd, S.; Dekker, F.J. Targeting HDAC complexes in asthma and COPD. Epigenomes 2019, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef]
- Weichert, W.; Röske, A.; Gekeler, V.; Beckers, T.; Stephan, C.; Jung, K.; Fritzsche, F.R.; Niespore, S.; Denkert, C.; Dietel, M.; et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer 2008, 98, 604–610. [Google Scholar] [CrossRef]
- Jin, K.L.; Pak, J.H.; Park, J.-Y.; Choi, W.H.; Lee, J.-Y.; Kim, J.-H.; Nam, J.-H. Expression profile of histone deacetylases 1, 2 and 3 in ovarian cancer tissues. J. Gynecol. Oncol. 2008, 19, 185. [Google Scholar] [CrossRef]
- Chiocca, S.; Segré, C.V. Regulating the regulators: The post-translational code of class i HDAC1 and HDAC2. J. Biomed. Biotechnol. 2011, 2011, 690848. [Google Scholar] [CrossRef]
- Molinari, S.; Imbriano, C.; Moresi, V.; Renzini, A.; Belluti, S.; Lozanoska-Ochser, B.; Gigli, G.; Cedola, A. Histone deacetylase functions and therapeutic implications for adult skeletal muscle metabolism. Front. Mol. Biosci. 2023, 10, 1130183. [Google Scholar] [CrossRef]
- Shanmugam, G.; Rakshit, S.; Sarkar, K. HDAC inhibitors: Targets for tumor therapy, immune modulation and lung diseases. Transl. Oncol. 2022, 16, 101312. [Google Scholar] [CrossRef]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br. J. Clin. Pharmacol. 2021, 87, 4577–4597. [Google Scholar] [CrossRef]
- Liu, F.; Pore, N.; Kim, M.; Ranh Voong, K.; Dowling, M.; Maity, A.; Kao, G.D. Regulation of Histone Deacetylase 4 Expression by the SP Family of Transcription Factors. Mol. Biol. Cell 2006, 17, 585–597. [Google Scholar] [CrossRef]
- Han, A.; He, J.; Wu, Y.; Liu, J.O.; Chen, L. Mechanism of recruitment of class II histone deacetylases by myocyte enhancer factor-2. J. Mol. Biol. 2005, 345, 91–102. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, M.; Wang, B.; Zheng, Y.; Jiang, D.; Zhao, L.; Mamun, M.A.A.; Kang, H.; Nie, H.; Zhang, X.; et al. New insights into the non-enzymatic function of HDAC6. Biomed. Pharmacother. 2023, 161, 114438. [Google Scholar] [CrossRef]
- Liu, N.; Xiong, Y.; Li, S.; Ren, Y.; He, Q.; Gao, S.; Zhou, J.; Shui, W. New HDAC6-mediated deacetylation sites of tubulin in the mouse brain identified by quantitative mass spectrometry. Sci. Rep. 2015, 5, 16869. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.M.; Aldana-Masangkay, G.I. The role of HDAC6 in cancer. J. Biomed. Biotechnol. 2011, 2011, 875824. [Google Scholar] [CrossRef]
- Bonomi, R.E.; Riordan, W.; Gelovani, J.G. The Structures, Functions, and Roles of Class III HDACs (Sirtuins) in Neuropsychiatric Diseases. Cells 2024, 13, 1644. [Google Scholar] [CrossRef] [PubMed]
- Budayeva, H.G.; Cristea, I.M. Human sirtuin 2 localization, transient interactions, and impact on the proteome point to its role in intracellular trafficking. Mol. Cell. Proteom. 2016, 15, 3107–3125. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.; Wu, D. Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front. Neurosci. 2021, 15, 614107. [Google Scholar] [CrossRef]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2023, 13, 29. [Google Scholar] [CrossRef]
- Sahakian, E.; Chen, J.; Powers, J.J.; Chen, X.; Maharaj, K.; Deng, S.L.; Lienlaf, M.; Wang, H.W.; Cheng, F.; Sodré, A.L.; et al. Essential role for histone deacetylase 11 (HDAC11) in neutrophil biology. J. Leukoc. Biol. 2017, 102, 475–486. [Google Scholar] [CrossRef]
- Chen, H.; Xie, C.; Chen, Q.; Zhuang, S. HDAC11, an emerging therapeutic target for metabolic disorders. Front. Endocrinol. 2022, 13, 989305. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, D.; Giménez-Cassina, A. Ketone Bodies in the Brain Beyond Fuel Metabolism: From Excitability to Gene Expression and Cell Signaling. Front. Mol. Neurosci. 2021, 14, 732120. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Gan, L.; Fang, J.; Zhang, J.; Yu, X.; Guo, H.; Cai, D.; Cui, H.; Gou, L.; Deng, J.; et al. Beta-Hydroxybutyrate: A Dual Function Molecular and Immunological Barrier Function Regulator. Front. Immunol. 2022, 13, 805881. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H.; Yamahara, K.; Yasuda-Yamahara, M.; Kume, S. Emerging Pathophysiological Roles of Ketone Bodies. Physiology 2024, 39, 167–177. [Google Scholar] [CrossRef]
- Luo, S.; Yang, M.; Han, Y.; Zhao, H.; Jiang, N.; Li, L.; Chen, W.; Li, C.; Yang, J.; Liu, Y.; et al. β-Hydroxybutyrate against Cisplatin-Induced acute kidney injury via inhibiting NLRP3 inflammasome and oxidative stress. Int. Immunopharmacol. 2022, 111, 109101. [Google Scholar] [CrossRef]
- Han, Y.M.; Ramprasath, T.; Zou, M.H. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp. Mol. Med. 2020, 52, 548–555. [Google Scholar] [CrossRef]
- Carbone, A.M.; Borges, J.I.; Suster, M.S.; Sizova, A.; Cora, N.; Desimine, V.L.; Lymperopoulos, A. Regulator of G-Protein Signaling-4 Attenuates Cardiac Adverse Remodeling and Neuronal Norepinephrine Release-Promoting Free Fatty Acid Receptor FFAR3 Signaling. Int. J. Mol. Sci. 2022, 23, 5803. [Google Scholar] [CrossRef]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef]
- Mizuta, K.; Sasaki, H.; Zhang, Y.; Matoba, A.; Emala, C.W. The short-chain free fatty acid receptor FFAR3 is expressed and potentiates contraction in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1248–L1260. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Muscle-to-Brain Signaling Via Myokines and Myometabolites. Brain Plast. 2022, 8, 43–63. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol. Cell. Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Sparke, R.; Davies, S. Starvation, exercise and the stress response. Anaesth. Intensive Care Med. 2023, 24, 624–629. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Starvation Ketosis and the Kidney. Am. J. Nephrol. 2021, 52, 467–478. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Sun, K.; Li, F.; Hu, D.; Chen, J.; Kong, F.; Xie, Y. Liver-Derived Ketogenesis via Overexpressing HMGCS2 Promotes the Recovery of Spinal Cord Injury. Adv. Biol. 2024, 8, 2300481. [Google Scholar] [CrossRef]
- Gómora-García, J.C.; Montiel, T.; Hüttenrauch, M.; Salcido-Gómez, A.; García-Velázquez, L.; Ramiro-Cortés, Y.; Gomora, J.C.; Castro-Obregón, S.; Massieu, L. Effect of the Ketone Body, D-β-Hydroxybutyrate, on Sirtuin2-Mediated Regulation of Mitochondrial Quality Control and the Autophagy–Lysosomal Pathway. Cells 2023, 12, 486. [Google Scholar] [CrossRef]
- Jayashankar, S.S.; Arifin, K.T.; Nasaruddin, M.L. β-Hydroxybutyrate Regulates Activated Microglia to Alleviate Neurodegenerative Processes in Neurological Diseases: A Scoping Review. Nutrients 2023, 15, 524. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, X.; He, Y.; Xie, Y.; Xu, F.; Xu, Y.; Huang, W. Function and mechanism of histone β-hydroxybutyrylation in health and disease. Front. Immunol. 2022, 13, 981285. [Google Scholar] [CrossRef]
- Hu, X.; Peng, J.; Tang, W.; Xia, Y.; Song, P. A circadian rhythm-restricted diet regulates autophagy to improve cognitive function and prolong lifespan. Biosci. Trends 2023, 17, 356–368. [Google Scholar] [CrossRef]
- Laposky, A.D.; Bass, J.; Kohsaka, A.; Turek, F.W. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008, 582, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Dyar, K.A.; Treebak, J.T.; Jepsen, S.L.; Ehrlich, A.M.; Ashcroft, S.P.; Trost, K.; Kunzke, T.; Prade, V.M.; Small, L.; et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 2022, 34, 329–345.e8. [Google Scholar] [CrossRef] [PubMed]
- Mezhnina, V.; Ebeigbe, O.P.; Velingkaar, N.; Poe, A.; Sandlers, Y.; Kondratov, R.V. Circadian clock controls rhythms in ketogenesis by interfering with PPARα transcriptional network. Proc. Natl. Acad. Sci. USA 2022, 119, e2205755119. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feng, D.; Everett, L.J.; Bugge, A.; Lazar, M.A. Circadian epigenomic remodeling and hepatic lipogenesis: Lessons from HDAC3. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 49–55. [Google Scholar] [CrossRef]
- Grimaldi, B.; Nakahata, Y.; Kaluzova, M.; Masubuchi, S.; Sassone-Corsi, P. Chromatin remodeling, metabolism and circadian clocks: The interplay of CLOCK and SIRT1. Int. J. Biochem. Cell Biol. 2009, 41, 81–86. [Google Scholar] [CrossRef]
- Shetty, A.; Hsu, J.W.; Manka, P.P.; Syn, W.K. Role of the Circadian Clock in the Metabolic Syndrome and Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2018, 63, 3187–3206. [Google Scholar] [CrossRef]
- Bolshette, N.; Ibrahim, H.; Reinke, H.; Asher, G. Circadian regulation of liver function: From molecular mechanisms to disease pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 695–707. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, S.; Wei, X.; Liu, K.; Peng, Y.; Yu, M.; Chen, J.; Zhu, J.; Huang, K.; Pan, S. β-Hydroxybutyrate inhibits FOXO3a by histone H3K9 β-Hydroxybutyrylation to ameliorate stroke-related sarcopenia. J. Funct. Foods 2024, 120, 106365. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huan, T.; Wu, X.; Chen, M.; et al. Histone β-hydroxybutyrylation is critical in reversal of sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. β-hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int. J. Mol. Sci. 2018, 19, 2247. [Google Scholar] [CrossRef] [PubMed]
- Tawadrous, M. The Interplay Between Beta-Hydroxybutyrate, Insulin and Glucose on Regulating MCF7 Cell Cycle Status. 2020. Available online: http://hdl.handle.net/10315/38004 (accessed on 21 July 2025).
- Gonzatti, M.B.; Goldberg, E.L. Ketone bodies as chemical signals for the immune system. Am. J. Physiol. Cell Physiol. 2024, 326, C707–C711. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahmod, A.I.; Kamal, A.; Rashid, H.M.; Alashqar, A.M.D.; Khater, S.; Jamal, D.; Waly, M. Ketogenic diet in cancer prevention and therapy: Molecular targets and therapeutic opportunities. Curr. Issues Mol. Biol. 2021, 43, 558–589. [Google Scholar] [CrossRef]
- Fulman-Levy, H.; Cohen-Harazi, R.; Levi, B.; Argaev-Frenkel, L.; Abramovich, I.; Gottlieb, E.; Hoffman, S.; Koman, I.; Nesher, E. Metabolic alterations and cellular responses to β-Hydroxybutyrate treatment in breast cancer cells. Cancer Metab. 2024, 12, 16. [Google Scholar] [CrossRef]
- Badameh, P.; Akhlaghi Tabar, F.; Mohammadipoor, N.; Rezaei, R.; Ranjkesh, R.; Maleki, M.H.; Vakili, O.; Shafiee, S.M. Differential effects of β-hydroxybutyrate and α-ketoglutarate on HCT-116 colorectal cancer cell viability under normoxic and hypoxic low-glucose conditions: Exploring the role of SRC, HIF1α, ACAT1, and SIRT2 genes. Mol. Genet. Genom. 2025, 300, 14. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Wong, A.C.; Lundgren, P.; Golos, A.M.; Descamps, H.C.; Dohnalová, L.; Cramer, Z.; Tian, Y.; Yueh, B.; Eskiocak, O.; et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 2022, 605, 160–165. [Google Scholar] [CrossRef]
- Shirian, F.I.; Karimi, M.; Alipour, M.; Salami, S.; Nourbakhsh, M.; Nekufar, S.; Safari-Alighiarloo, N.; Tavakoli-Yaraki, M. Beta hydroxybutyrate induces lung cancer cell death, mitochondrial impairment and oxidative stress in a long term glucose-restricted condition. Mol. Biol. Rep. 2024, 51, 567. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, N.; Wang, Z.; Wang, Q.; Han, J.; Khan, M.A.; Riaz, A.; Chang, C. β-hydroxybutyrate inhibits cellular proliferation, EMT, stemness, migration, and invasion in glioma cells. Precis. Nutr. 2023, 2, e00038. [Google Scholar] [CrossRef]
- Shakery, A.; Pourvali, K.; Ghorbani, A.; Fereidani, S.S.; Zand, H. Beta-hydroxybutyrate promotes proliferation, migration and stemness in a subpopulation of 5FU treated SW480 Cells: Evidence for metabolic plasticity in colon cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3287–3294. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol. Metab. 2019, 30, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.T.; Ramsamooj, S.; Liang, R.J.; Grover, R.; Hwang, S.K.; Goncalves, M.D.S. Systemic Ketone Replacement Does Not Improve Survival or Cancer Cachexia in Mice with Lung Cancer. Front. Oncol. 2022, 12, 903157. [Google Scholar] [CrossRef] [PubMed]
- Ohiagu, F.O.; Chikezie, P.C.; Chikezie, C.M. Pathophysiology of diabetes mellitus and its Complications: Metabolic events and control. Biomed. Res. Ther. 2021, 8, 4243–4257. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Hosseini, F.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Dietary carbohydrate and the risk of type 2 diabetes: An updated systematic review and dose–response meta-analysis of prospective cohort studies. Sci. Rep. 2022, 12, 2491. [Google Scholar] [CrossRef]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Akkus, G.; Sert, M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J. Diabetes 2022, 13, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Liu, X.; Chen, X.; Zhang, S.; Chen, Y.; Chen, J.; Chen, J.; Wu, J.; Chen, G.-Q. 3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in type 2 diabetic mice. Signal Transduct. Target. Ther. 2023, 8, 190. [Google Scholar] [CrossRef]
- Jung, J.; Park, W.Y.; Kim, Y.J.; Kim, M.; Choe, M.; Jin, K.; Seo, J.H.; Ha, E. 3-Hydroxybutyrate Ameliorates the Progression of Diabetic Nephropathy. Antioxidants 2022, 11, 381. [Google Scholar] [CrossRef]
- Neudorf, H.; Islam, H.; Falkenhain, K.; Oliveira, B.; Jackson, G.S.; Moreno-Cabañas, A.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. Effect of the ketone beta-hydroxybutyrate on markers of inflammation and immune function in adults with type 2 diabetes. Clin. Exp. Immunol. 2024, 216, 89–103. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, C.; Xie, M. Beta-Hydroxybutyrate, Friend or Foe for Stressed Hearts. Front. Aging 2021, 2, 681513. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Xiong, J.; Bai, L.; Tang, H.; Wang, B.; Guo, B.; Qiu, Y.; Li, G.; Gong, M.; et al. β-Hydroxybutyrate Facilitates Homeostasis of Hypoxic Endothelial Cells After Myocardial Infarction via Histone Lysine β-Hydroxybutyrylation of CPT1A. JACC Basic Transl. Sci. 2025, 10, 588–607. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Zhang, X.; Gao, Y.; Wang, H.; Li, Y.; He, D.; Jia, W. Comparative proteomics reveals that fatty acid metabolism is involved in myocardial adaptation to chronic hypoxic injury. PLoS ONE 2024, 19, e0305571. [Google Scholar] [CrossRef]
- Chen, Y.; You, Y.; Wang, X.; Jin, Y.; Zeng, Y.; Pan, Z.; Li, D.; Ling, W. β-Hydroxybutyrate Alleviates Atherosclerotic Calcification by Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis via AMPK/Nrf2 Pathway. Nutrients 2025, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, M.; Proudfoot, D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: A potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008, 103, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Rnlö, J.Ä.; Dluzen, D.F.; Nowak, C. Atherosclerotic Aortic Calcification-Associated Polymorphism in HDAC9 and Associations with Mortality, Cardiovascular Disease, and Kidney Disease. iScience 2020, 23, 101253. [Google Scholar] [CrossRef]
- Lei, M.; Lin, H.; Shi, D.; Hong, P.; Song, H.; Herman, B.; Liao, Z.; Yang, C. Molecular mechanism and therapeutic potential of HDAC9 in intervertebral disc degeneration. Cell. Mol. Biol. Lett. 2023, 28, 104. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Croteau, S.; Hardy, P. Histone deacetylase (HDAC) 9: Versatile biological functions and emerging roles in human cancer. Cell. Oncol. 2021, 44, 997–1017. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Bavi, H.; Baker, J.S.; Moro, T.; Mancin, L.; Paoli, A. Ketogenic diets, physical activity and body composition: A review. Br. J. Nutr. 2022, 127, 1898–1920. [Google Scholar] [CrossRef]
- Dilliraj, L.N.; Schiuma, G.; Lara, D.; Strazzabosco, G.; Clement, J.; Giovannini, P.P.; Trapella, C.; Narducci, M.; Rizzo, R. The Evolution of Ketosis: Potential Impact on Clinical Conditions. Nutrients 2022, 14, 3613. [Google Scholar] [CrossRef]
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and parkinson’s disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef]
- Hartman, A.L.; Vining, E.P.G. Clinical aspects of the ketogenic diet. Epilepsia 2007, 48, 31–42. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Milder, J.B.; Liang, L.P.; Patel, M. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008, 106, 1044–1051. [Google Scholar] [CrossRef]
- Polito, R.; La Torre, M.E.; Moscatelli, F.; Cibelli, G.; Valenzano, A.; Panaro, M.A.; Monda, M.; Messina, A.; Monda, V.; Pisanelli, D. The Ketogenic Diet and Neuroinflammation: The Action of Beta-Hydroxybutyrate in a Microglial Cell Line. Int. J. Mol. Sci. 2023, 24, 3102. [Google Scholar] [CrossRef]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell 2021, 20, e13322. [Google Scholar] [CrossRef]
- Schmidt, M.; Pfetzer, N.; Schwab, M.; Strauss, I.; Kämmerer, U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011, 8, 54. [Google Scholar] [CrossRef]
- Egashira, R.; Matsunaga, M.; Miyake, A.; Hotta, S.; Nagai, N.; Yamaguchi, C.; Takeuchi, M.; Moriguchi, M.; Tonari, S.; Nakano, M.; et al. Long-Term Effects of a Ketogenic Diet for Cancer. Nutrients 2023, 15, 2334. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Ibrahim, N.; Bredefeld, C.; Foo, S.; Lim, V.; Gutman, D.; Huggins, L.-A.; Hegele, R.A. Ketogenic diets, not for everyone. J. Clin. Lipidol. 2021, 15, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Neudorf, H.; Little, J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2024, 47, 100677. [Google Scholar] [CrossRef]
- García-Juárez, M.; García-Rodríguez, A.; Cruz-Carrillo, G.; Flores-Maldonado, O.; Becerril-Garcia, M.; Garza-Ocañas, L.; Torre-Villalvazo, I.; Camacho-Morales, A. Intermittent Fasting Improves Social Interaction and Decreases Inflammatory Markers in Cortex and Hippocampus. Mol. Neurobiol. 2024, 62, 1511–1535. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.; Mosha, M.; Weinstein, D.A.; Riba-Wolman, R. Fasting ketone levels vary by age: Implications for differentiating physiologic from pathologic ketotic hypoglycemia. J. Pediatr. Endocrinol. Metab. 2023, 36, 667–673. [Google Scholar] [CrossRef]
- Berger, B.; Jenetzky, E.; Köblös, D.; Stange, R.; Baumann, A.; Simstich, J.; Michalsen, A.; Schmelzer, K.-M.; Martin, D.D. Seven-day fasting as a multimodal complex intervention for adults with type 1 diabetes: Feasibility, benefit and safety in a controlled pilot study. Nutrition 2021, 86, 111169. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.E.; Bae, J.H.; Lee, J.H.; Shin, H.E.; Zhang, D.D.; Cho, S.C.; Song, W. Effects of exercise-induced beta-hydroxybutyrate on muscle function and cognitive function. Physiol. Rep. 2021, 9, e14497. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Chambers, M.; Kamendulis, L.M.; Temmerman, J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2013, 8, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Kuter, K.Z.; Olech, Ł.; Głowacka, U.; Paleczna, M. Increased beta-hydroxybutyrate level is not sufficient for the neuroprotective effect of long-term ketogenic diet in an animal model of early parkinson’s disease. Exploration of brain and liver energy metabolism markers. Int. J. Mol. Sci. 2021, 22, 7556. [Google Scholar] [CrossRef]
- Wan, S.-R.; Teng, F.-Y.; Fan, W.; Xu, B.-T.; Li, X.-Y.; Tan, X.-Z.; Guo, M.; Gao, C.-L.; Zhang, C.-X.; Jiang, Z.-Z.; et al. BDH1-mediated βOHB metabolism ameliorates diabetic kidney disease by activation of NRF2-mediated antioxidative pathway. Aging 2023, 15, 13384–13410. [Google Scholar] [CrossRef]
- Cuenoud, B.; Hartweg, M.; Godin, J.P.; Croteau, E.; Maltais, M.; Castellano, C.A.; Carpentier, A.C.; Cunnane, S.C. Metabolism of Exogenous D-Beta-Hydroxybutyrate, an Energy Substrate Avidly Consumed by the Heart and Kidney. Front. Nutr. 2020, 7, 503509. [Google Scholar] [CrossRef]
- Pimentel-Suarez, L.I.; Soto-Mota, A. Evaluation of the safety and tolerability of exogenous ketosis induced by orally administered free beta-hydroxybutyrate in healthy adult subjects. BMJ Nutr. Prev. Health 2023, 6, 122–126. [Google Scholar] [CrossRef]
- Gregor, A.N.; Delerive, P.; Cuenoud, B.; Monnard, I.; Redeuil, K.; Harding, C.O.; Gillingham, M.B. D-BHB supplementation before moderate-intensity exercise suppresses lipolysis and selectively blunts exercise-induced long-chain acylcarnitine increase in pilot study of patients with long-chain fatty acid oxidation disorders. Mol. Genet. Metab. 2025, 144, 109070. [Google Scholar] [CrossRef]
- Thomsen, H.H.; Rittig, N.; Johannsen, M.; Møller, A.B.; Jørgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: Anticatabolic impact of ketone bodies. Am. J. Clin. Nutr. 2018, 108, 857–867. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal 2016, 28, 917–923. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Xiao, W. β-hydroxybutyrate as an anti-aging metabolite. Nutrients 2021, 13, 3420. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]

| Cancer type | Model | Key Effects of BHB | Mechanism | Reference |
|---|---|---|---|---|
| Colorectal | In vivo: CAC mice and organoids | ↓ Tumor growth, ↑ Expression of Hopx | HCAR2 | [82] |
| Lung cancer | In vitro: A549 | ↑ Apoptosis, ↑ ROS, ↑ Mitochondrial dysfunction | CD133, CD44, and SOX-9 downregulation | [83] |
| Glioma | In vitro: Glioma U251 cells | ↓ Angiogenesis, Invasion of glioma cells, ↓ EMT process, ↓ Stemness of glioma | GFβ/PI3K/Akt/GSK- 3β and Wnt5a/β-catenin/Snail pathways | [84] |
| Colon cancer | In vitro: 5FU treated SW480 | ↑ Expression of genes associated with stemness and mitochondrial biogenesis, ↓ Expression genes related to glycolytic program and differentiation | 5FU-treated SW480 have the ability to use metabolic energy source of BHB, as well as stemness features also increasing to survive | [85] |
| Study Type | Model/System Used | Intervention or Focus | Main Findings | References |
|---|---|---|---|---|
| Clinical | Adults at risk of T2D (Study 1) | Ex vivo BHB treatment of LPS-stimulated leukocytes | ↓ IL-1β, and TNF-α, IL-6; ↑ IL-10 and IL-1RA | [98] |
| T2D patients (Study 2) | Study single oral dose of ketone monoester (KME) | ↑ plasma IL-10 at 90 min post a single KME dose, coinciding with peak blood BHB in T2D | ||
| T2D patients (Study 3) | 14 days of KME supplementation (3 times a day) + ex vivo BHB on leukocyte | No change in plasma cytokines or immune cells; ex vivo effects still observed in glucose- and time-dependent manner | ||
| Clinical | Obese patients with advanced diabetic nephropathy | 12-week very low-calorie ketogenic diet + exercise | ↓ weight (12%), ↓ albuminuria (reduction 36%, ns), ↓ serum creatinine and cystatin C | [124] |
| In vivo | 6-ODHA-induced Parkinson’s disease in rats | Strict ketogenic diet (7 weeks total) | No neuroprotection despite hyperketonemia: slight behavioral improvement | [125] |
| In vivo and in vitro | HK-2 cells, C57 BKS db/db mice, diabetic kidney tissue from patients | BDH1 overexpression, BHB supplementation, ketogenic diet | BDH1 is downregulated in DKD; its overexpression or BHB/KD reverses oxidative stress, inflammation, fibrosis via NRF2 activation | [126] |
| In vitro and in vivo | HK-2 cells and db/db mice with diabetic nephrophaty | KD, 3-OHB treatment | 3-OHB delayed DN progression; ↑ Autophagy (LC3-II, beclin), ↑ AMPK/NRF2, ↓ ROS; Reduced albuminuria and mesangial expansion cell | [97] |
| Intervention | Disease/Effect | Subject | Status | Clinical Trial No | Phase |
|---|---|---|---|---|---|
| Beta Hydroxybutyra Ester (KetoneaID ke4) | ALS | Patients with ALS | Recruiting | NCT04820478 | N.A. |
| Dietary Supplement: 3-OHB (Oral) | T2D, Ketosis | Patients with T2D | Completed | NCT05263401 | N.A. |
| Dietary Supplement: BHB | Ketosis, Ketonemia | Healthy individuals | Completed | NCT05980858 | N.A. |
| Dietary Supplement: D-BHB | Ketosis | Healthy individuals | Completed | NCT04881526 | N.A. |
| BHB supplementation (Oral) | Chron’s Disease, IBD | Patients with IBS | Recruiting | NCT06351124 | Phase 1 and 2 |
| Dietary Supplement: KME vs. BHB vs. 1,3-Butanediol | Ketosis | Healthy individuals | Completed | NCT05273411 | N.A. |
| Dietary Supplement: 3-OHB salt (NaCl) | Healthy, Incretin Effect, Ketosis | Healthy individuals | Completed | NCT03935841 | N.A. |
| Dietary Supplement: KME vs. Control Supplement: carbohydrate-fat placebo (fructose, corn and canola oil 50:50 ratio) | Preservation of muscle mass and function (i.e., protein synthesis, insulin sensitivity, mitochondrial function) | Healthy individuals | Completed | NCT05679596 | N.A. |
| Dietary Supplement: 3-OHB + Whey vs. Whey | Maintenance of muscle mass in catabolic status (Healthy individuals with LPS injection) | Healthy individuals | Completed | NCT04064268 | N.A. |
| Dietary Supplement: KME vs. Placebo supplement | SCD | Adults with SCD (55 to 75 years old) | Not yet recruiting | NCT06588946 | Phase 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pali, D.V.; Kim, S.; Mantik, K.E.K.; Lee, J.-B.; So, C.-Y.; Moon, S.; Park, D.-H.; Kwak, H.-B.; Kang, J.-H. Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule. Int. J. Mol. Sci. 2025, 26, 7362. https://doi.org/10.3390/ijms26157362
Pali DV, Kim S, Mantik KEK, Lee J-B, So C-Y, Moon S, Park D-H, Kwak H-B, Kang J-H. Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule. International Journal of Molecular Sciences. 2025; 26(15):7362. https://doi.org/10.3390/ijms26157362
Chicago/Turabian StylePali, Dwifrista Vani, Sujin Kim, Keren Esther Kristina Mantik, Ju-Bi Lee, Chan-Young So, Sohee Moon, Dong-Ho Park, Hyo-Bum Kwak, and Ju-Hee Kang. 2025. "Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule" International Journal of Molecular Sciences 26, no. 15: 7362. https://doi.org/10.3390/ijms26157362
APA StylePali, D. V., Kim, S., Mantik, K. E. K., Lee, J.-B., So, C.-Y., Moon, S., Park, D.-H., Kwak, H.-B., & Kang, J.-H. (2025). Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule. International Journal of Molecular Sciences, 26(15), 7362. https://doi.org/10.3390/ijms26157362






