Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons
Abstract
1. Introduction
2. Results
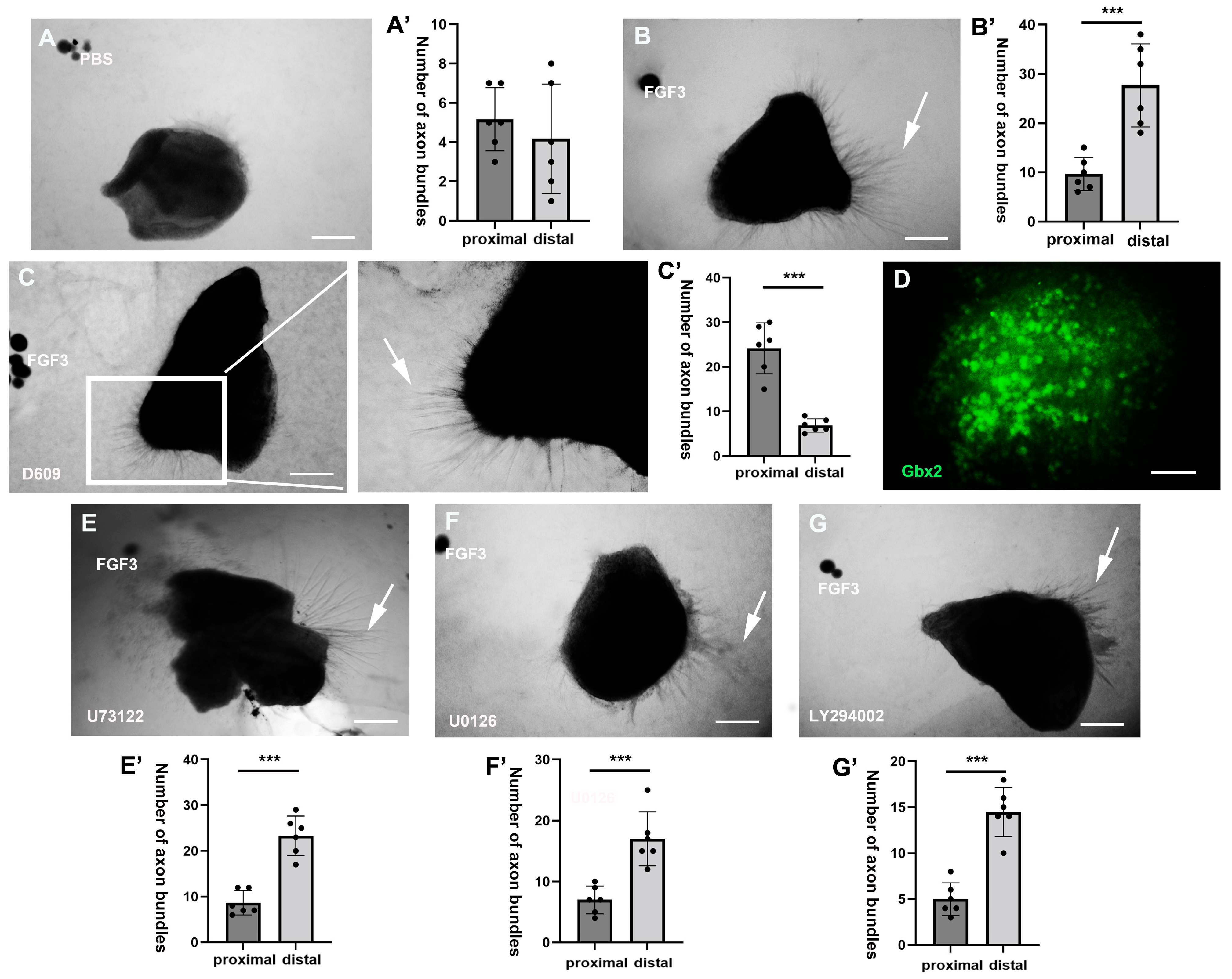
2.1. PC-PLC Pathway Mediates the Direct TCA Chemorepulsion of FGF3
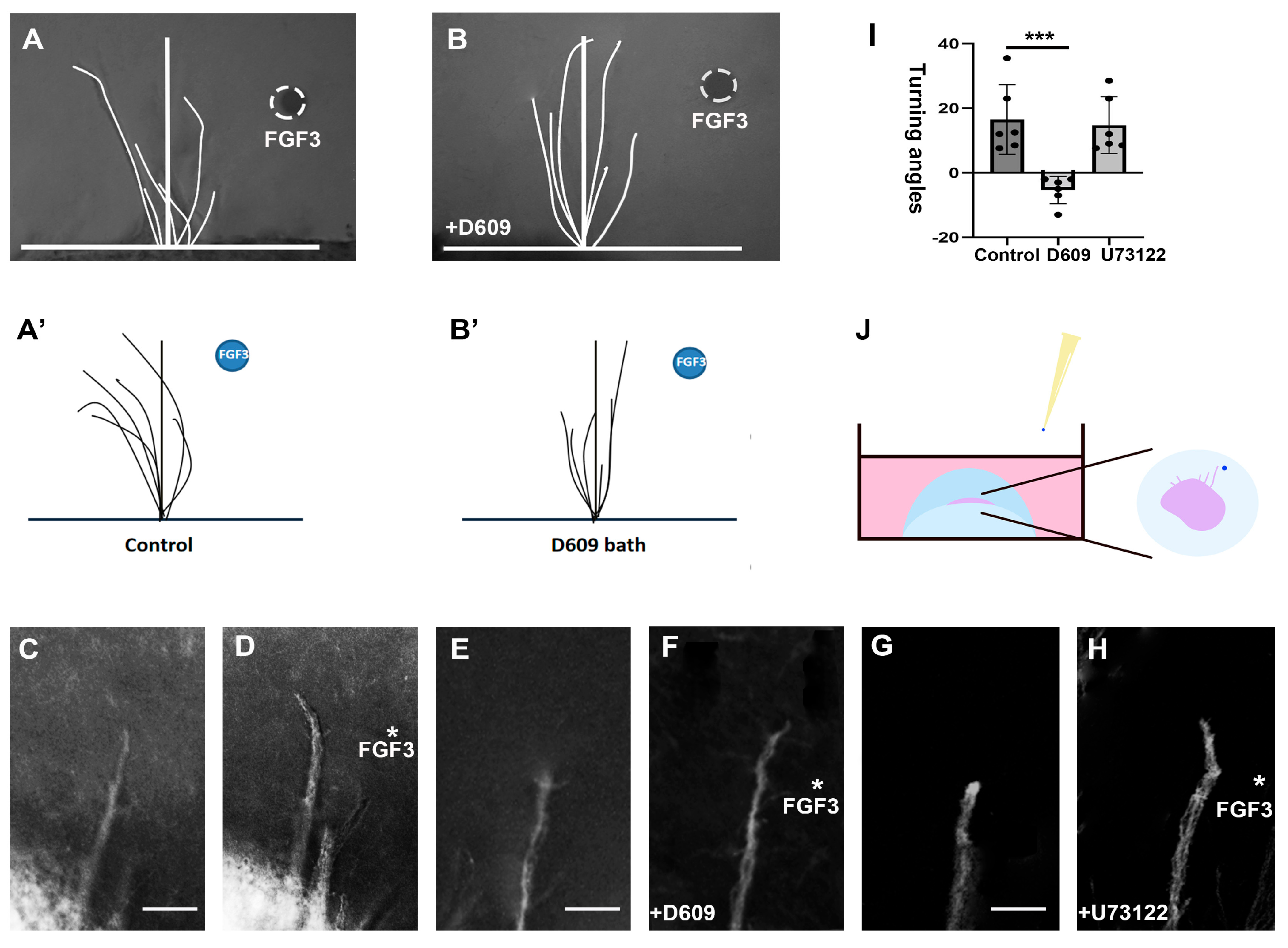
2.2. PC–PLC Pathway Is Necessary for TCA Repulsion In Vivo
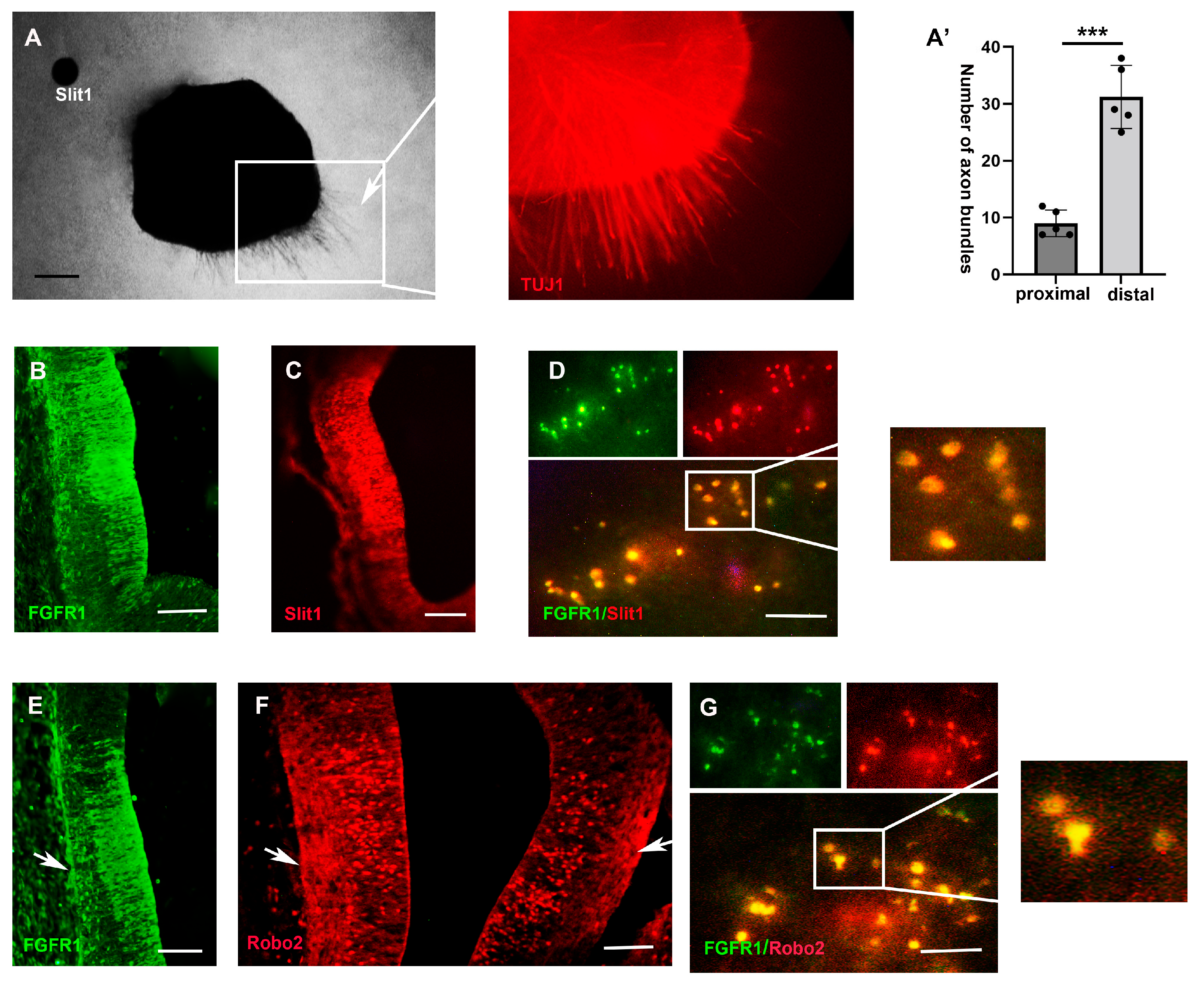
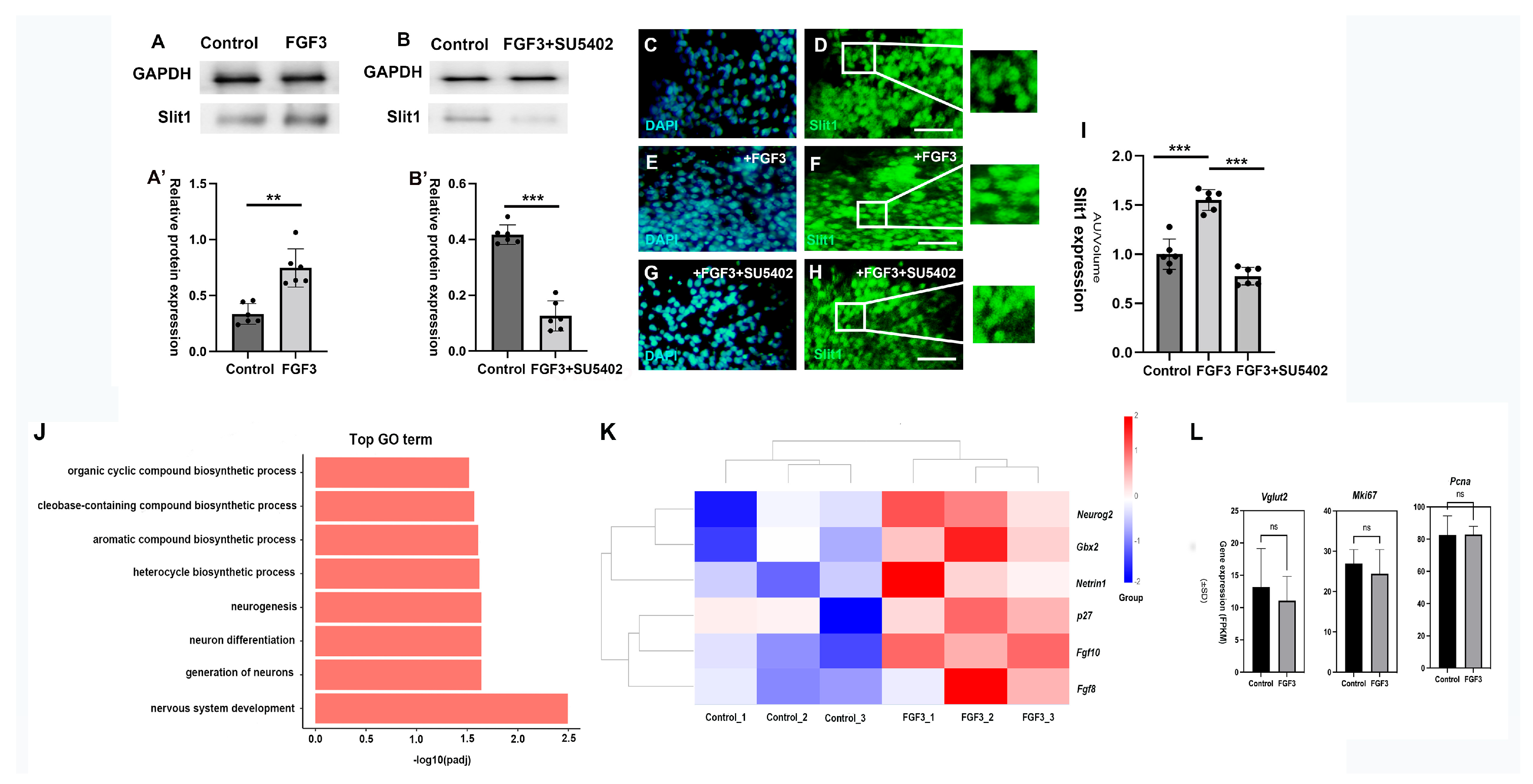
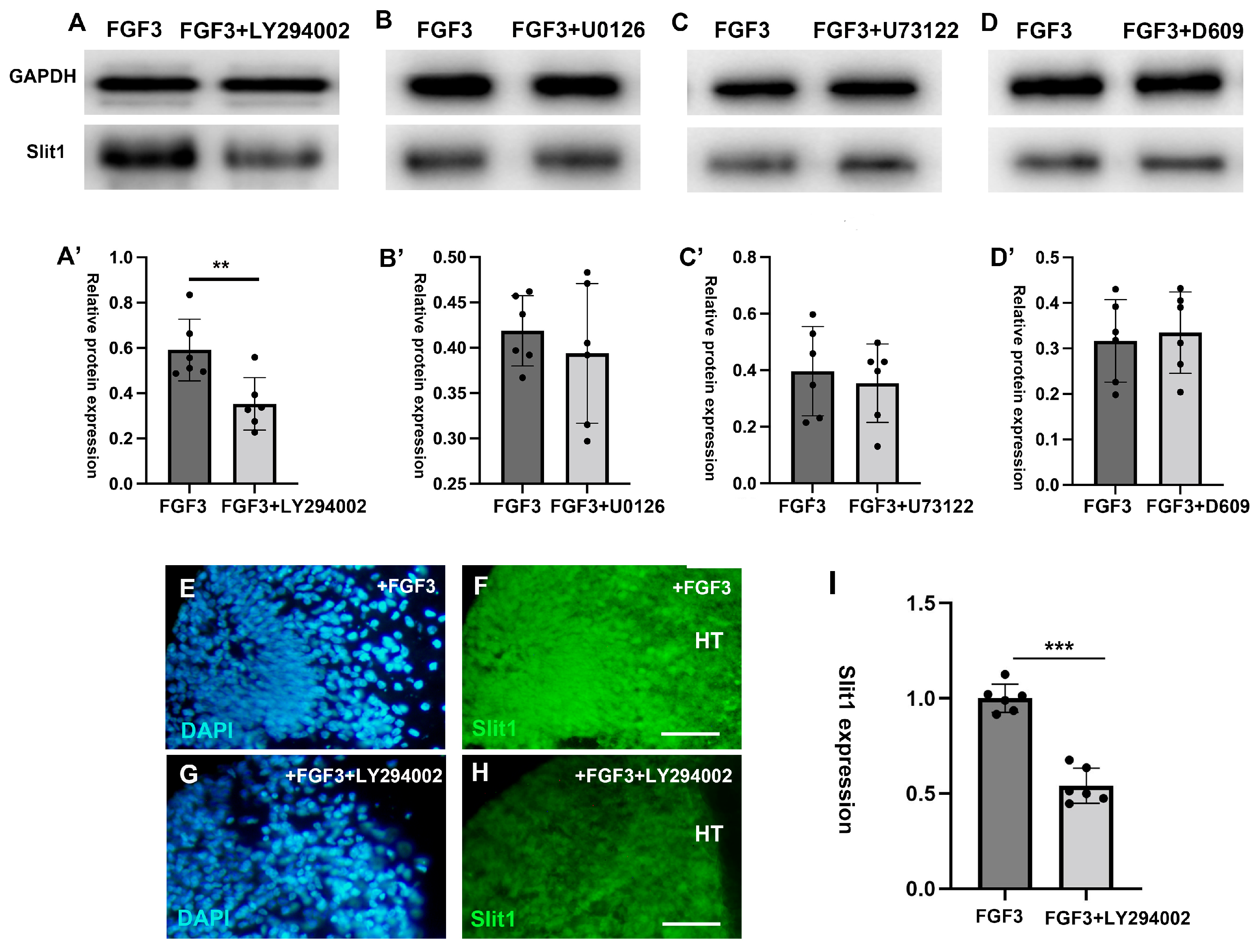
2.3. FGF3 Signaling Regulates the Expression of Slit1
2.4. Indirect Repulsive Effects of FGF3 on TCAs Is Induced by the PI3K Downstream Pathway
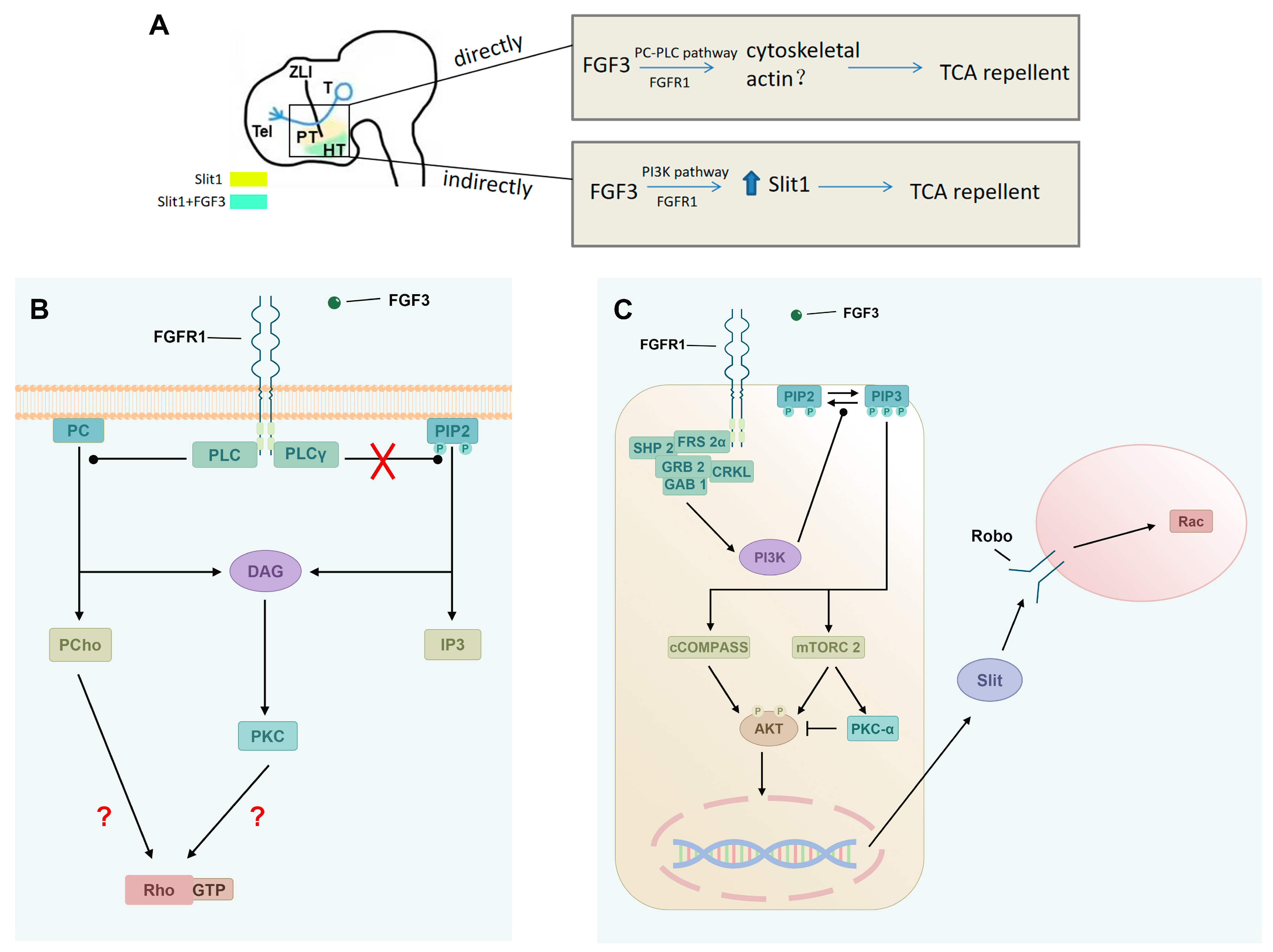
3. Discussion
3.1. Direct Axonal Repellent Effects of FGFs Through the PC-PLC Pathway
3.2. FGF3 Induces Slit1 in the Diencephalon
3.3. Contribution of PI3K Signaling to Indirect TCA Axon Guidance
4. Materials and Methods
4.1. Explant Culture
4.2. Immunofluorescence Analyses
4.3. Bead Implantation
4.4. Western Blot Analysis
4.5. RNA-Seq of Chick Diencephalon
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145, dev151415. [Google Scholar] [CrossRef]
- Desbois, M.; Grill, B. Molecular regulation of axon termination in mechanosensory neurons. Development 2024, 151, dev202945. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, P.; Liu, X.; Cheng, R.; Zhang, S.; Qian, X.; Liu, F. Roles of Fibroblast Growth Factors in the Axon Guidance. Int. J. Mol. Sci. 2023, 24, 10292. [Google Scholar] [CrossRef]
- Tome, D.; Dias, M.S.; Correia, J.; Almeida, R.D. Fibroblast growth factor signaling in axons: From development to disease. Cell Commun. Signal. 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Short, C.A.; Onesto, M.M.; Rempel, S.K.; Catlett, T.S.; Gomez, T.M. Familiar growth factors have diverse roles in neural network assembly. Curr. Opin. Neurobiol. 2021, 66, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.A.; Echevarria, D.; Ahn, C.P.; Sudarov, A.; Joyner, A.L.; Mason, I.J.; Martinez, S.; Martin, G.R. Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 2008, 135, 889–898. [Google Scholar] [CrossRef]
- Molnar, Z.; Kwan, K.Y. Development and Evolution of Thalamocortical Connectivity. Cold Spring Harb. Perspect. Biol. 2024, 16, a041503. [Google Scholar] [CrossRef]
- Gezelius, H.; Lopez-Bendito, G. Thalamic neuronal specification and early circuit formation. Dev. Neurobiol. 2017, 77, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Pogoda, H.M.; Pearson, C.A.; Ohyama, K.; Lohr, H.; Hammerschmidt, M.; Placzek, M. Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development 2013, 140, 1111–1122. [Google Scholar] [CrossRef]
- Liu, K.; Lv, Z.; Huang, H.; Yu, S.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF3 from the Hypothalamus Regulates the Guidance of Thalamocortical Axons. Dev. Neurosci. 2020, 42, 208–216. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. New developments in the biology of fibroblast growth factors. WIREs Mech. Dis. 2022, 14, e1549. [Google Scholar] [CrossRef]
- Webber, C.A.; Chen, Y.Y.; Hehr, C.L.; Johnston, J.; McFarlane, S. Multiple signaling pathways regulate FGF-2-induced retinal ganglion cell neurite extension and growth cone guidance. Mol. Cell. Neurosci. 2005, 30, 37–47. [Google Scholar] [CrossRef]
- Sullivan, K.G.; Bashaw, G.J. Intracellular Trafficking Mechanisms that Regulate Repulsive Axon Guidance. Neuroscience 2023, 508, 123–136. [Google Scholar] [CrossRef]
- Braisted, J.E.; Ringstedt, T.; O’Leary, D.D. Slits are chemorepellents endogenous to hypothalamus and steer thalamocortical axons into ventral telencephalon. Cereb. Cortex 2009, 19 (Suppl. 1), i144–i151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 2017, 7, 6. [Google Scholar] [CrossRef]
- Alexander, J.; Keles, G.; Killingsworth, J.; Bronson, R.; Perez, C.; Sawmiller, D.; Shytle, R.D. Autism, heparan sulfate and potential interventions. Exp. Neurol. 2022, 353, 114050. [Google Scholar] [CrossRef]
- Song, S.; Kang, M.; Lee, J.; Yang, Y.R.; Lee, H.; Kim, J.-I.; Kim, B.; Choi, H.-S.; Hong, E.-B.; Nam, M.-H.; et al. Role of phospholipase Cη1 in lateral habenula astrocytes in depressive-like behavior in mice. Exp. Mol. Med. 2025, 57, 872–887. [Google Scholar] [CrossRef]
- Yang, J.-L.J.; Bertolesi, G.E.; Dueck, S.; Hehr, C.L.; McFarlane, S. The Expression of Key Guidance Genes at a Forebrain Axon Turning Point Is Maintained by Distinct Fgfr Isoforms but a Common Downstream Signal Transduction Mechanism. eNeuro 2019, 6, ENEURO.0086-19. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mizushima, S.; Tamada, A.; Yamamoto, N.; Takashima, S.; Murakami, F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J. Neurosci. 2009, 29, 4044–4055. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Ohkubo, Y.; Maragnoli, M.E.; Rasin, M.-R.; Schwartz, M.L.; Sestan, N.; Vaccarino, F.M. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat. Neurosci. 2006, 9, 787–797. [Google Scholar] [CrossRef]
- Webber, C.A.; Hyakutake, M.T.; McFarlane, S. Fibroblast growth factors redirect retinal axons In Vitro and In Vivo. Dev. Biol. 2003, 263, 24–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, N.; Inui, K.; Matsuyama, Y.; Harada, A.; Hanamura, K.; Murakami, F.; Ruthazer, E.S.; Rutishauser, U.; Seki, T. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J. Neurosci. 2000, 20, 9145–9151. [Google Scholar] [CrossRef] [PubMed]
- Eurtivong, C.; Leung, E.; Sharma, N.; Leung, I.K.H.; Reynisson, J. Phosphatidylcholine-Specific Phospholipase C as a Promising Drug Target. Molecules 2023, 28, 5637. [Google Scholar] [CrossRef]
- Kim, H.Y.; Suh, P.G.; Kim, J.I. The Role of Phospholipase C in GABAergic Inhibition and Its Relevance to Epilepsy. Int. J. Mol. Sci. 2021, 22, 3149. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Q.; Xu, Z.; Liu, F. FGF3 Directs the Pathfinding of Prethalamic GABAergic Axons. Int. J. Mol. Sci. 2023, 24, 14998. [Google Scholar] [CrossRef]
- Dehay, C.; Savatier, P.; Cortay, V.; Kennedy, H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J. Neurosci. 2001, 21, 201–214. [Google Scholar] [CrossRef]
- Liu, K.; Lv, Z.; Huang, H.; Li, M.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF10 regulates thalamocortical axon guidance in the developing thalamus. Neurosci. Lett. 2020, 716, 134685. [Google Scholar] [CrossRef]
- Gilardino, A.; Farcito, S.; Zamburlin, P.; Audisio, C.; Lovisolo, D. Specificity of the second messenger pathways involved in basic fibroblast growth factor-induced survival and neurite growth in chick ciliary ganglion neurons. J. Neurosci. Res. 2009, 87, 2951–2962. [Google Scholar] [CrossRef]
- Boscher, C.; Mège, R.-M. Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell. Signal. 2008, 20, 1061–1072. [Google Scholar] [CrossRef]
- Williams, E.-J.; Walsh, F.S.; Doherty, P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 2003, 160, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Essmann, C.L.; Ryan, K.R.; Elmi, M.; Bryon-Dodd, K.; Porter, A.; Vaughan, A.; McMullan, R.; Nurrish, S. Activation of RHO-1 in cholinergic motor neurons competes with dopamine signalling to control locomotion. PLoS ONE 2018, 13, e0204057. [Google Scholar] [CrossRef]
- Topp, S.; Stigloher, C.; Komisarczuk, A.Z.; Adolf, B.; Becker, T.S.; Bally-Cuif, L. Fgf signaling in the zebrafish adult brain: Association of Fgf activity with ventricular zones but not cell proliferation. J. Comp. Neurol. 2008, 510, 422–439. [Google Scholar] [CrossRef]
- Okumura, A.; Hayashi, T.; Ebisawa, M.; Yoshimura, M.; Sasagawa, Y.; Nikaido, I.; Umesono, Y.; Mochii, M. Cell type-specific transcriptome analysis unveils secreted signaling molecule genes expressed in apical epithelial cap during appendage regeneration. Dev. Growth Differ. 2019, 61, 447–456. [Google Scholar] [CrossRef]
- Niftullayev, S.; Lamarche-Vane, N. Regulators of Rho GTPases in the Nervous System: Molecular Implication in Axon Guidance and Neurological Disorders. Int. J. Mol. Sci. 2019, 20, 1497. [Google Scholar] [CrossRef]
- Yang, S.; Weske, A.; Du, Y.; Valera, J.M.; Johnson, A.N. FGF signaling directs myotube guidance by regulating Rac activity. Development 2020, 147, dev183624. [Google Scholar] [CrossRef] [PubMed]
- Srahna, M.; Leyssen, M.; Choi, C.M.; Fradkin, L.G.; Noordermeer, J.N.; Hassan, B.A. A signaling network for patterning of neuronal connectivity in the Drosophila brain. PLoS Biol. 2006, 4, e348. [Google Scholar] [CrossRef]
- Yang, J.-L.J.; Bertolesi, G.E.; Hehr, C.L.; Johnston, J.; McFarlane, S. Fibroblast growth factor receptor 1 signaling transcriptionally regulates the axon guidance cue slit1. Cell. Mol. Life Sci. 2018, 75, 3649–3661. [Google Scholar] [CrossRef] [PubMed]
- Atkinson-Leadbeater, K.; Bertolesi, G.E.; Hehr, C.L.; Webber, C.A.; Cechmanek, P.B.; McFarlane, S. Dynamic Expression of Axon Guidance Cues Required for Optic Tract Development Is Controlled by Fibroblast Growth Factor Signaling. J. Neurosci. 2010, 30, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Magnani, D.; Morlé, L.; Hasenpusch-Theil, K.; Paschaki, M.; Jacoby, M.; Schurmans, S.; Durand, B.; Theil, T. The ciliogenic transcription factor Rfx3 is required for the formation of the thalamocortical tract by regulating the patterning of prethalamus and ventral telencephalon. Hum. Mol. Genet. 2015, 24, 2578–2593. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef]
- Forthmann, B.; Aletta, J.M.; Lee, Y.W.; Terranova, C.; Birkaya, B.; Stachowiak, E.K.; Stachowiak, M.K.; Claus, P. Coalition of Nuclear Receptors in the Nervous System. J. Cell. Physiol. 2015, 230, 2875–2880. [Google Scholar] [CrossRef]
- Haenzi, B.; Moon, L.D.F. The Function of FGFR1 Signalling in the Spinal Cord: Therapeutic Approaches Using FGFR1 Ligands after Spinal Cord Injury. Neural. Plast. 2017, 2017, 2740768. [Google Scholar] [CrossRef]
- Gestrich, C.; Grieco, K.; Lidov, H.G.; Baird, L.C.; Fehnel, K.P.; Yeo, K.K.; Meredith, D.M.; Alexandrescu, S. H3K27-altered diffuse midline gliomas with MAPK pathway alterations: Prognostic and therapeutic implications. J. Neuropathol. Exp. Neurol. 2023, 83, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, C.N.; Ross, J.; Hennick, K.M.; Wu, S.-R.; Paranjape, N.; Leonard, R.; Wang, J.C.; Keefe, M.G.; Pavlovic, B.J.; et al. Thalamocortical organoids enable In Vitro modeling of 22q11.2 microdeletion associated with neuropsychiatric disorders. Cell Stem Cell 2024, 31, 421–432.e8. [Google Scholar] [CrossRef] [PubMed]
- Gudernova, I.; Vesela, I.; Balek, L.; Buchtova, M.; Dosedelova, H.; Kunova, M.; Pivnicka, J.; Jelinkova, I.; Roubalova, L.; Kozubik, A.; et al. Multikinase activity of fibroblast growth factor receptor (FGFR) inhibitors SU5402, PD173074, AZD1480, AZD4547 and BGJ398 compromises the use of small chemicals targeting FGFR catalytic activity for therapy of short-stature syndromes. Hum. Mol. Genet. 2016, 25, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. The PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2015, 7, a011189. [Google Scholar] [CrossRef]
- Chinnaiya, K.; Placzek, M. A Methodology for the Enzymatic Isolation of Embryonic Hypothalamus Tissue and Its Acute or Post-Culture Analysis by Multiplex Hybridisation Chain Reaction. Bio-Protocol 2023, 13, e4898. [Google Scholar] [CrossRef]
- Dupin, I.; Lokmane, L.; Dahan, M.; Garel, S.; Studer, V. Subrepellent doses of Slit1 promote Netrin-1 chemotactic responses in subsets of axons. Neural Dev. 2015, 10, 5. [Google Scholar] [CrossRef]
- Shukla, S.; Shariat-Madar, Z.; Walker, L.A.; Tekwani, B.L. Mechanism for neurotropic action of vorinostat, a pan histone deacetylase inhibitor. Mol. Cell. Neurosci. 2016, 77, 11–20. [Google Scholar] [CrossRef]
- Lin, M.; Li, P.; Liu, W.; Niu, T.; Huang, L. Germacrone alleviates okadaic acid-induced neurotoxicity in PC12 cells via M1 muscarinic receptor-mediated Galphaq (Gq)/phospholipase C beta (PLCbeta)/ protein kinase C (PKC) signaling. Bioengineered 2022, 13, 4898–4910. [Google Scholar] [CrossRef] [PubMed]
- Frith, T.J.; Granata, I.; Wind, M.; Stout, E.; Thompson, O.; Neumann, K.; Stavish, D.; Heath, P.R.; Ortmann, D.; Hackland, J.O.; et al. Human axial progenitors generate trunk neural crest cells In Vitro. eLife 2018, 7, e35786. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Y.; Huang, D.; Luo, X.; Zhang, L.; Zhu, X.; Zhang, X.; Liu, Z.; Han, J.Y.; Xiong, J.W. Miconazole protects blood vessels from MMP9-dependent rupture and hemorrhage. Dis. Model. Mech. 2017, 10, 337–348. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, J.; Chen, Q.; Dong, Y.; Gao, H.; Liu, F. Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons. Int. J. Mol. Sci. 2025, 26, 7361. https://doi.org/10.3390/ijms26157361
Li K, Li J, Chen Q, Dong Y, Gao H, Liu F. Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons. International Journal of Molecular Sciences. 2025; 26(15):7361. https://doi.org/10.3390/ijms26157361
Chicago/Turabian StyleLi, Kejuan, Jiyuan Li, Qingyi Chen, Yuting Dong, Hanqi Gao, and Fang Liu. 2025. "Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons" International Journal of Molecular Sciences 26, no. 15: 7361. https://doi.org/10.3390/ijms26157361
APA StyleLi, K., Li, J., Chen, Q., Dong, Y., Gao, H., & Liu, F. (2025). Direct and Indirect Downstream Pathways That Regulate Repulsive Guidance Effects of FGF3 on Developing Thalamocortical Axons. International Journal of Molecular Sciences, 26(15), 7361. https://doi.org/10.3390/ijms26157361




