Protective Effect of Camellia japonica Extract on 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis in an SKH-1 Mouse Model
Abstract
1. Introduction
2. Results
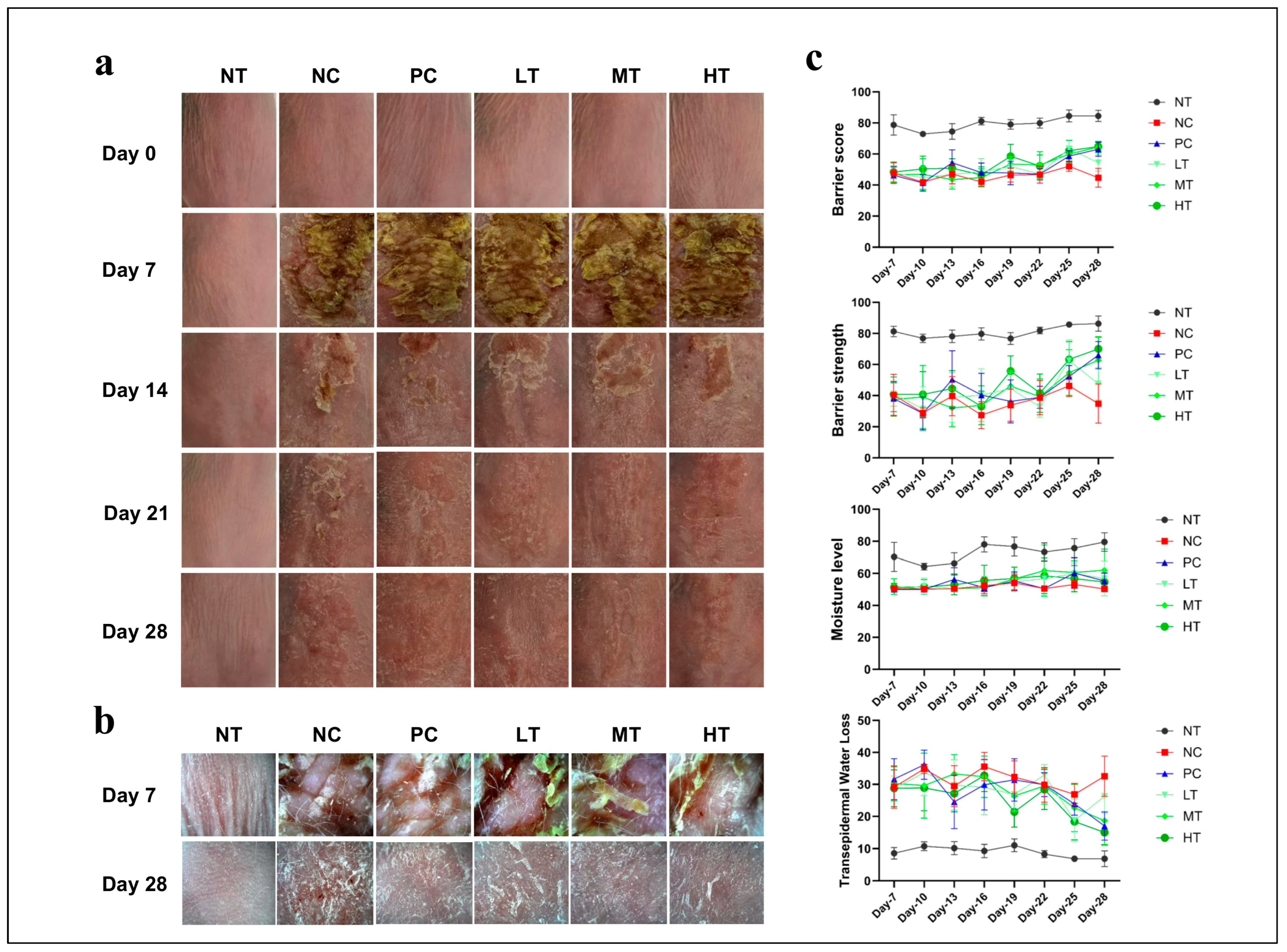
2.1. Effects of Camellia japonica Extract on AD-like Skin Lesions
2.2. Effects of Camellia japonica Extract on AD-like Skin Lesions Investigated Through Histopathological Analysis
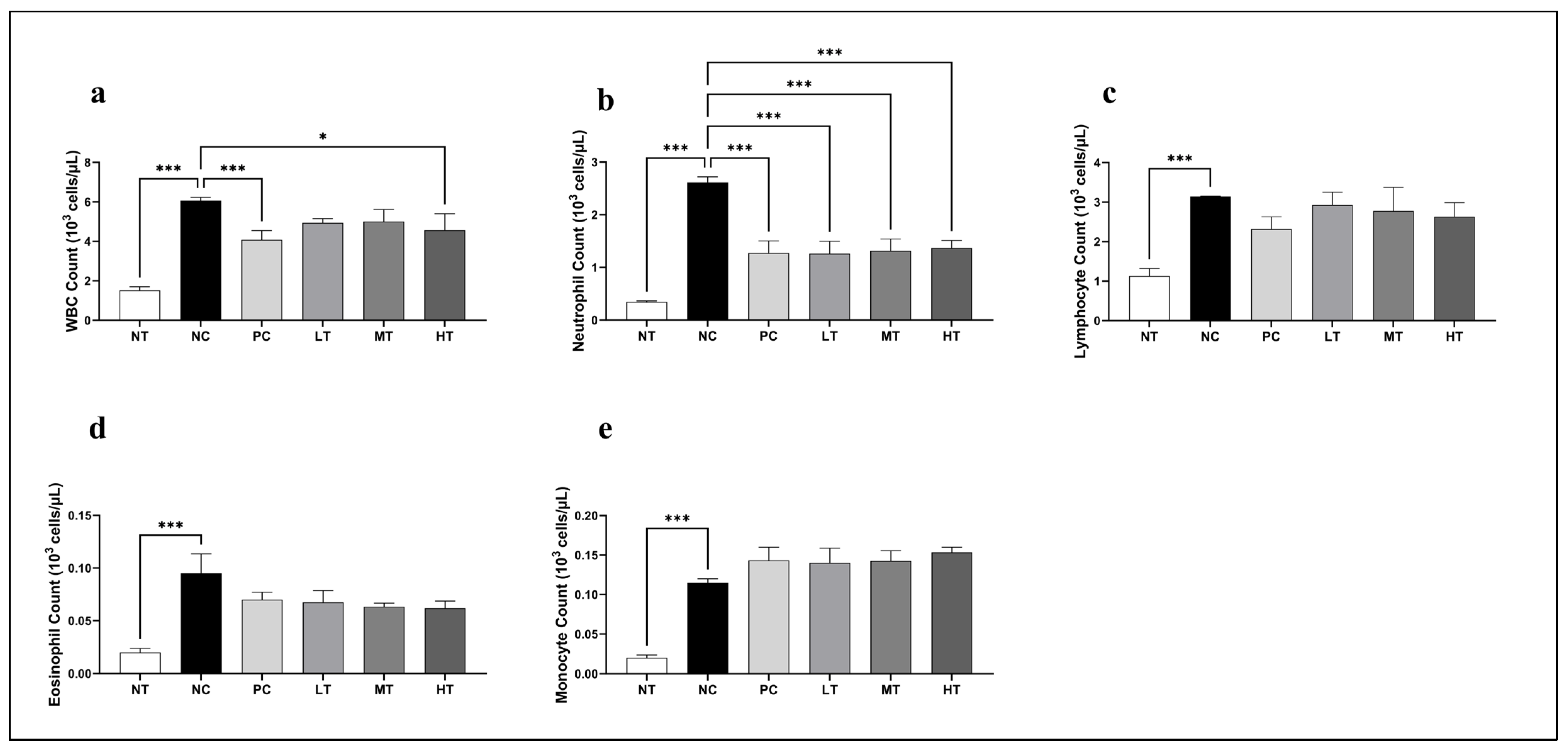
2.3. Effects of Camellia japonica Extract on Systemic Immune Cell
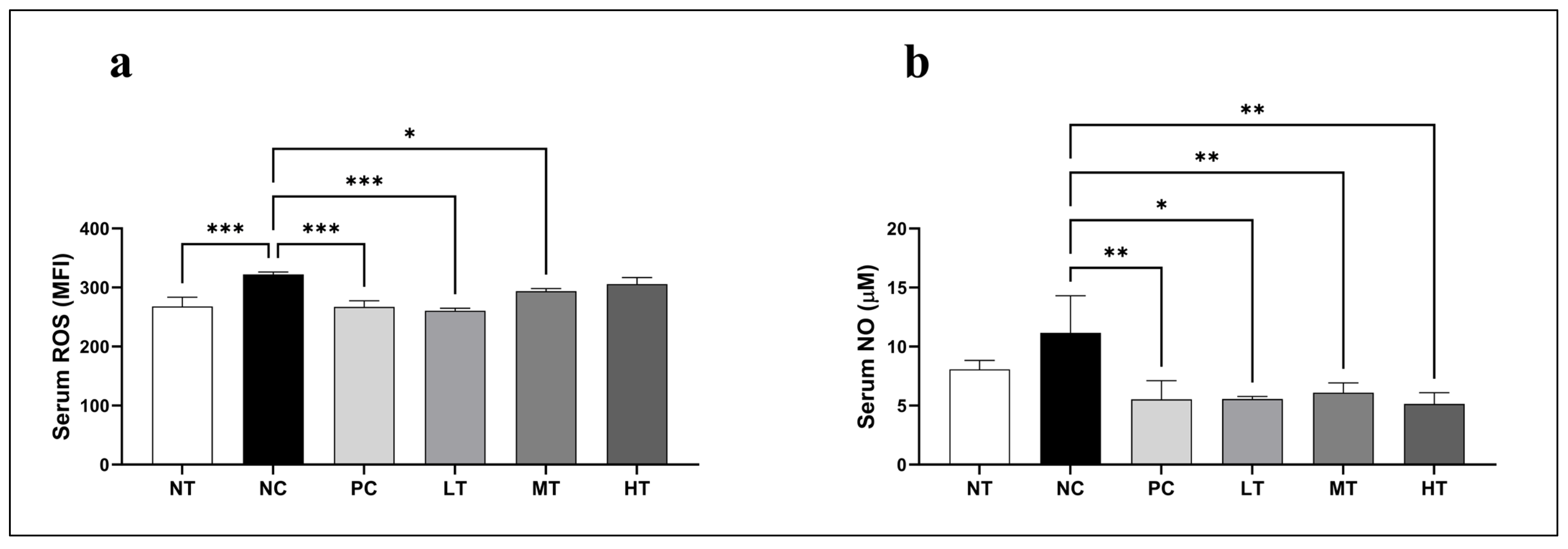
2.4. Effects of Camellia japonica Extract on Oxidative Stress Profiles
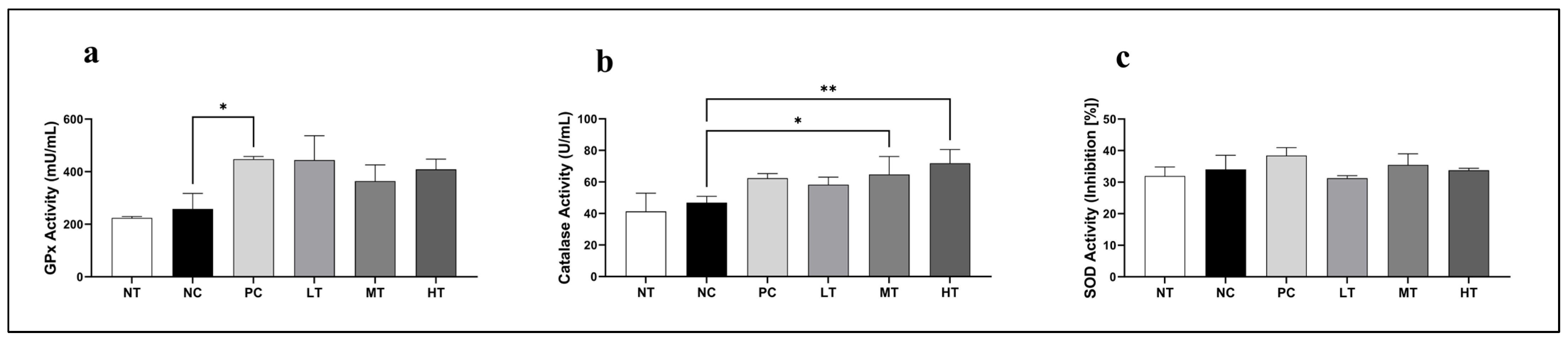
2.5. Effects of Camellia japonica Extract on Antioxidative Enzymatic Activities
2.6. Effects of Camellia japonica Extract on Key Allergy-Associated Biomarkers
2.7. Effects of Camellia japonica Extract on Serum Pro-Inflammatory, Th1 and Th2 Cytokine Profiles
3. Discussion
4. Materials and Methods
4.1. Preparation of Camellia japonica Extract
4.2. Experimental Animals
4.3. Dermatitis Induction
4.4. Evaluation of Skin Lesion Severity
4.5. Serum Sample Preparation
4.6. Histopathologic Examination
4.7. WBC and Differential Count Analysis
4.8. Detection of ROS Level in Serum
4.9. Detection of Serum NO Levels
4.10. Detection of Serum Antioxidant Activities
4.11. Detection of Serum Lactate Dehydrogenase Enzyme Activities
4.12. Detection of Total IgE Level in Serum
4.13. Detection of Total Serum Histamine Level
4.14. Detection of Total Serum TSLP Level
4.15. Detection of Serum Cytokine Levels
4.16. Statistical Analysis Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| CAT | Catalase |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| DNCB | 2,4-Dinitrochlorobenzene |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| H&E | Hematoxylin and eosin |
| IFN-γ | Interferon-gamma |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| LDH | Lactate dehydrogenase |
| NADH | Nicotinamide adenine dinucleotide |
| NIH | National Institutes of Health |
| NO | Nitric oxide |
| NO2− | Nitrite |
| p38 MAPK | p38 mitogen-activated protein kinase |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SOD | Superoxide dismutase |
| TEWL | Transepidermal water loss |
| TNF-α | Tumor necrosis factor-alpha |
| TSLP | Thymic stromal lymphopoietin |
| WBCs | White blood cells |
References
- Guttman-Yassky, E.; Nograles, K.E.; Krueger, J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: Clinical and pathologic concepts. J. Allergy Clin. Immunol. 2011, 127, 1110–1118. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Lee, H.H.; Patel, K.R.; Singam, V.; Rastogi, S.; Silverberg, J.I. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 1526–1532.e7. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y.; Fuxench, Z.C.C. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018, 121, 340–347. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Schaper, K.; Rossbach, K.; Köther, B.; Stark, H.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacol. Res. 2016, 113, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhu, L.; Ruan, T.; Jia, J.; Mo, X.; Yan, F.; Liu, J.; Zhang, Y.; Chen, D. Comparative study of mouse models of atopic dermatitis. Heliyon 2025, 11, e41989. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.E.; Leung, D.Y. Pathophysiology of atopic dermatitis: Clinical implications. In Allergy and Asthma Proceedings; OceanSide Publications, Inc: East Providence, RI, USA, 2019; pp. 84–92. [Google Scholar]
- Luo, Y.; Hu, J.; Zhou, Z.; Zhang, Y.; Wu, Y.; Sun, J. Oxidative stress products and managements in atopic dermatitis. Front. Med. 2025, 12, 1538194. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, P.; Li, Q.; Han, Z. Oxidative stress and gut microbiome in inflammatory skin diseases. Front. Cell Dev. Biol. 2022, 10, 849985. [Google Scholar] [CrossRef] [PubMed]
- Bajgai, J.; Xingyu, J.; Fadriquela, A.; Begum, R.; Kim, D.H.; Kim, C.-S.; Kim, S.-K.; Lee, K.-J. Effects of mineral complex material treatment on 2,4-dinitrochlorobenzene-induced atopic dermatitis like-skin lesions in mice model. BMC Complement. Med. Ther. 2021, 21, 82. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.; Younis, S.M.; Al Tamimi, M.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clinical importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- De Simoni, E.; Candelora, M.; Belleggia, S.; Rizzetto, G.; Molinelli, E.; Capodaglio, I.; Ferretti, G.; Bacchetti, T.; Offidani, A.; Simonetti, O. Role of antioxidants supplementation in the treatment of atopic dermatitis: A critical narrative review. Front. Nutr. 2024, 11, 1393673. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef]
- Vela, P.; Salinero, C.; Sainz, M. Phenological growth stages of Camellia japonica. Ann. Appl. Biol. 2013, 162, 182–190. [Google Scholar] [CrossRef]
- Karadeniz, F.; Oh, J.H.; Kim, H.R.; Ko, J.; Kong, C.-S. Camellioside A, isolated from Camellia japonica flowers, attenuates UVA-induced production of MMP-1 in HaCaT keratinocytes via suppression of MAPK activation. Exp. Ther. Med. 2021, 21, 16. [Google Scholar] [CrossRef]
- Páscoa, R.N.; Teixeira, A.M.; Sousa, C. Antioxidant capacity of Camellia japonica cultivars assessed by near-and mid-infrared spectroscopy. Planta 2019, 249, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Masaki, H. Anti-photoaging capability of antioxidant extract from Camellia japonica leaf. Exp. Dermatol. 2014, 23, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.T.P.; Choi, Y.-S.; Bae, H.-J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Kim, J.H.; Choi, G.N.; Kwak, J.H.; Kim, D.-O.; Heo, H.J. Protective effects of extract with phenolics from camellia (Camellia japonica) leaf against oxidative stress-induced neurotoxicity. Food Sci. Biotechnol. 2010, 19, 1347–1353. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, M.Y. Phytochemical profile, antioxidant, antimicrobial and antipancreatic lipase activities of fermented Camellia japonica L leaf extracts. Trop. J. Pharm. Res. 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Selvakumar, K.; Hwa, K.Y.; Sami, P.; Kumaresan, M. Biogenic fabrication of gold nanoparticles using Camellia japonica L. leaf extract and its biological evaluation. J. Mater. Res. Technol. 2019, 8, 1412–1418. [Google Scholar] [CrossRef]
- Park, J.C.; Hur, J.M.; Park, J.G.; Hatano, T.; Yoshida, T.; Miyashiro, H.; Min, B.S.; Hattori, M. Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 2002, 16, 422–426. [Google Scholar] [CrossRef]
- Kim, S.-B.; Jung, E.-S.; Shin, S.-W.; Kim, M.-H.; Kim, Y.-S.; Lee, J.-S.; Park, D.-H. Anti-inflammatory activity of Camellia japonica oil. BMB Rep. 2012, 45, 177–182. [Google Scholar] [CrossRef]
- Nam, H.H.; Nan, L.; Choo, B.K. Inhibitory effects of Camellia japonica on cell inflammation and acute rat reflux esophagitis. Chin. Med. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, J.Y.; Choi, J.K.; Han, E.; Song, C.-L.; Lee, J.; Cho, Y.S. Effects of the extracts from fruit and stem of Camellia japonica on induced pluripotency and wound healing. J. Clin. Med. 2018, 7, 449. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Woolf, R.; Smith, C.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bae, C.S.; Seo, N.S.; Na, C.S.; Yoo, H.Y.; Oh, D.S.; Bae, M.S.; Kwon, M.S.; Cho, S.S.; Park, D.H. Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4 pathway and its effective and major component is oleic acid. Phytomedicine 2019, 57, 84–94. [Google Scholar] [CrossRef]
- Akanda, M.R.; Park, B.Y. Involvement of MAPK/NF-κB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed. Pharmacother. 2017, 95, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, J.H.; Cui, L.; Li, Y.; Yang, J.M.; Yun, J.J.; Jung, J.E.; Choi, W.; Yoon, K.C. Anti-Inflammatory and Antioxidative Effects of Camellia japonica on Human Corneal Epithelial Cells and Experimental Dry Eye: In Vivo and In Vitro Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, E.A.; Peters-Kennedy, J. Integumentary system. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Saunders Ltd.: Philadelphia, PA, USA, 2016; Volume 1, p. 509. [Google Scholar]
- Majumder, S.; Ghosh, A.; Bhattacharya, M. Natural anti-inflammatory terpenoids in Camellia japonica leaf and probable biosynthesis pathways of the metabolome. Bull. Natl. Res. Cent. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.-G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Ckless, K.; Hodgkins, S.R.; Ather, J.L.; Martin, R.; Poynter, M.E. Epithelial, dendritic, and CD4+ T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization. Biochim. Et. Biophys. Acta 2011, 1810, 1025–1034. [Google Scholar] [CrossRef]
- Pellefigues, C. IgE Autoreactivity in Atopic Dermatitis: Paving the Road for Autoimmune Diseases? Antibodies 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.W.; Ko, N.Y.; Mun, S.H.; Kim, D.K.; Kim, J.D.; Kim, H.S.; Lee, K.R.; Kim, Y.K.; Radinger, M.; et al. Camellia japonica suppresses immunoglobulin E-mediated allergic response by the inhibition of Syk kinase activation in mast cells. Clin. Exp. Allergy 2008, 38, 794–804. [Google Scholar] [CrossRef]
- Oettgen, H.C. Fifty years later: Emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J. Allergy Clin. Immunol. 2016, 137, 1631–1645. [Google Scholar] [CrossRef]
- MacGlashan, D. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kurashima, Y. Two sides of the coin: Mast cells as a key regulator of allergy and acute/chronic inflammation. Cells 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2024, 24, 846. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.t.; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef]
- Murakami, T.; Yamanaka, K.; Tokime, K.; Kurokawa, I.; Tsutsui, H.; Nakanishi, K.; Mizutani, H. Topical suplatast tosilate (IPD) ameliorates Th2 cytokine-mediated dermatitis in caspase-1 transgenic mice by downregulating interleukin-4 and interleukin-5. Br. J. Dermatol. 2006, 155, 27–32. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jiménez, M.; Ventura-Juárez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the treatment of atopic dermatitis. ImmunoTargets Ther. 2020, ume 9, 151–156. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Rodriguez, E.; Stölzl, D.; Wehkamp, U.; Sun, J.; Gerdes, S.; Sarkar, M.K.; Hübenthal, M.; Zeng, C.; Uppala, R.; et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J. Allergy Clin. Immunol. 2023, 151, 1413. [Google Scholar] [CrossRef]
- Alshevskaya, A.A.; Lopatnikova, J.A.; Krugleeva, O.L.; Nepomnyschih, V.M.; Lukinov, V.L.; Karaulov, A.V.; Sennikov, S.V. Expression density of receptors to IL-1β in atopic dermatitis. Mol. Immunol. 2016, 75, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, S.; Kawai, M.; Kasajima, Y.; Hamamoto, T. Increased plasma tumour necrosis factor-alpha concentration in atopic dermatitis. Arch. Dis. Child. 1992, 67, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, N.J.; Jegal, J.; Jo, B.G.; Choi, S.; Lee, S.W.; Uddin, M.S.; Kim, S.N.; Yang, M.H. Suppression of DNCB-Induced Atopic Skin Lesions in Mice by Wikstroemia indica Extract. Nutrients 2020, 12, 173. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.; Rahman, M.H.; Pham, T.T.; Kim, C.-S.; Bajgai, J.; Lee, K.-J. Protective Effect of Camellia japonica Extract on 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis in an SKH-1 Mouse Model. Int. J. Mol. Sci. 2025, 26, 7286. https://doi.org/10.3390/ijms26157286
Mo C, Rahman MH, Pham TT, Kim C-S, Bajgai J, Lee K-J. Protective Effect of Camellia japonica Extract on 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis in an SKH-1 Mouse Model. International Journal of Molecular Sciences. 2025; 26(15):7286. https://doi.org/10.3390/ijms26157286
Chicago/Turabian StyleMo, Chaodeng, Md. Habibur Rahman, Thu Thao Pham, Cheol-Su Kim, Johny Bajgai, and Kyu-Jae Lee. 2025. "Protective Effect of Camellia japonica Extract on 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis in an SKH-1 Mouse Model" International Journal of Molecular Sciences 26, no. 15: 7286. https://doi.org/10.3390/ijms26157286
APA StyleMo, C., Rahman, M. H., Pham, T. T., Kim, C.-S., Bajgai, J., & Lee, K.-J. (2025). Protective Effect of Camellia japonica Extract on 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis in an SKH-1 Mouse Model. International Journal of Molecular Sciences, 26(15), 7286. https://doi.org/10.3390/ijms26157286







