T Cell Dynamics in COVID-19, Long COVID and Successful Recovery
Abstract
1. Introduction
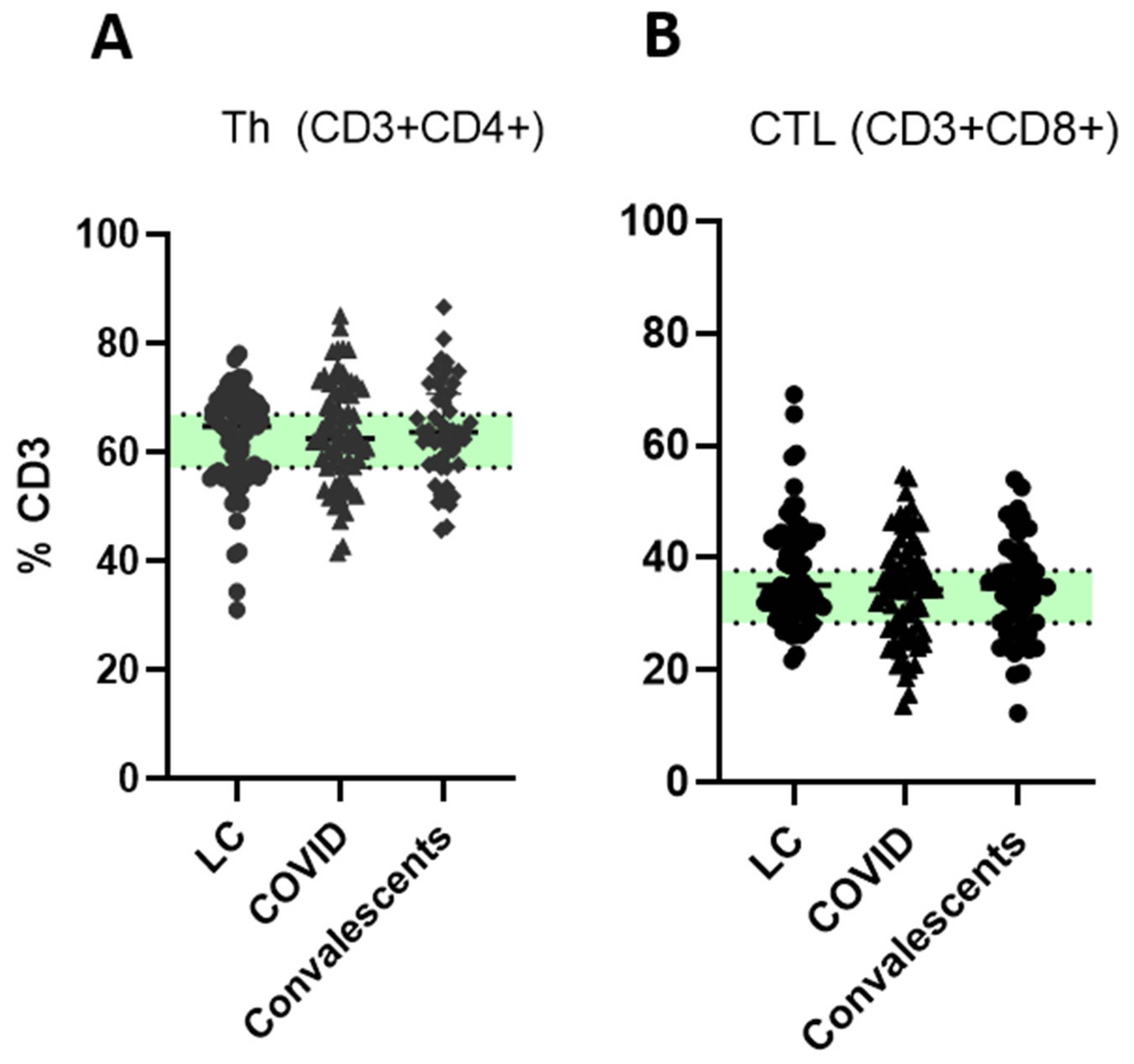
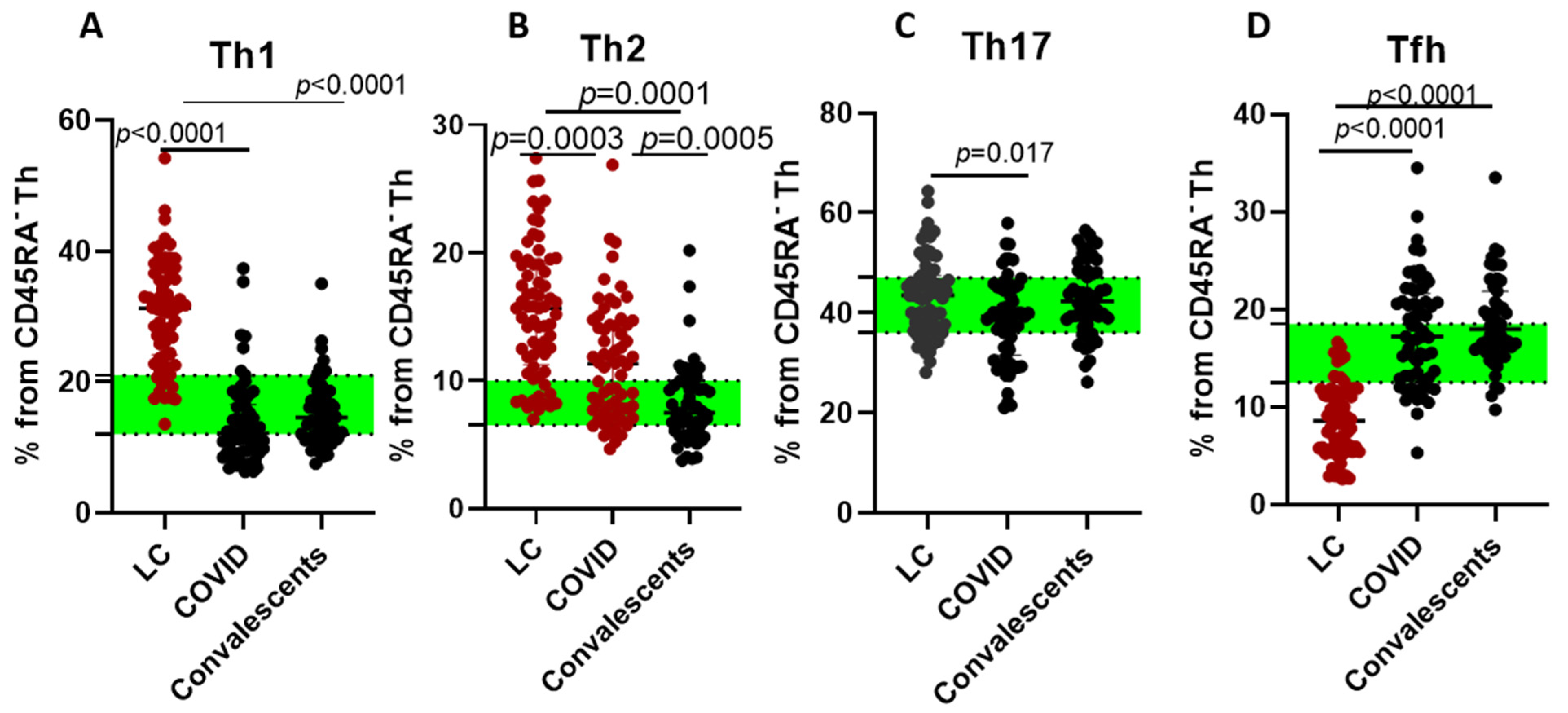
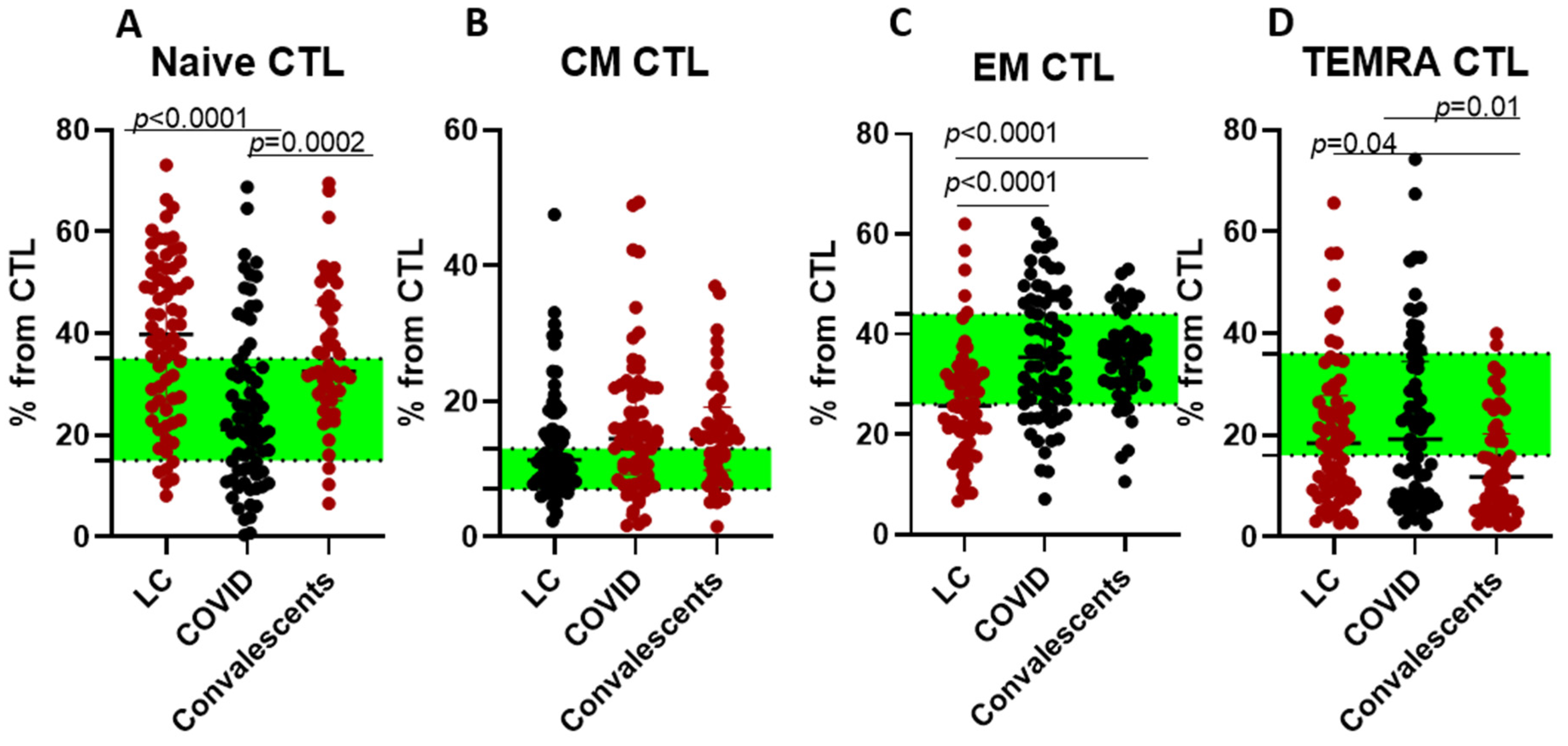
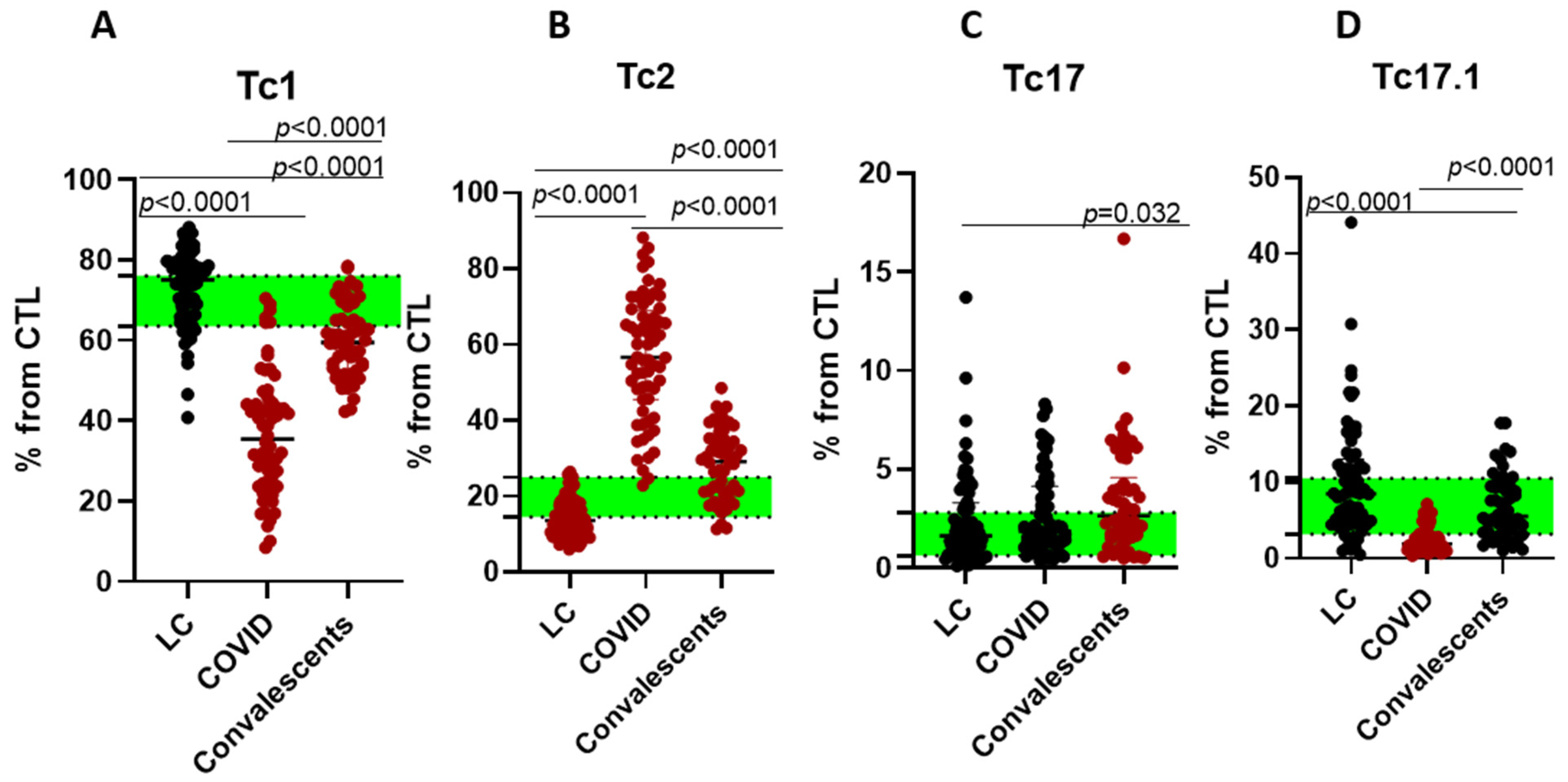
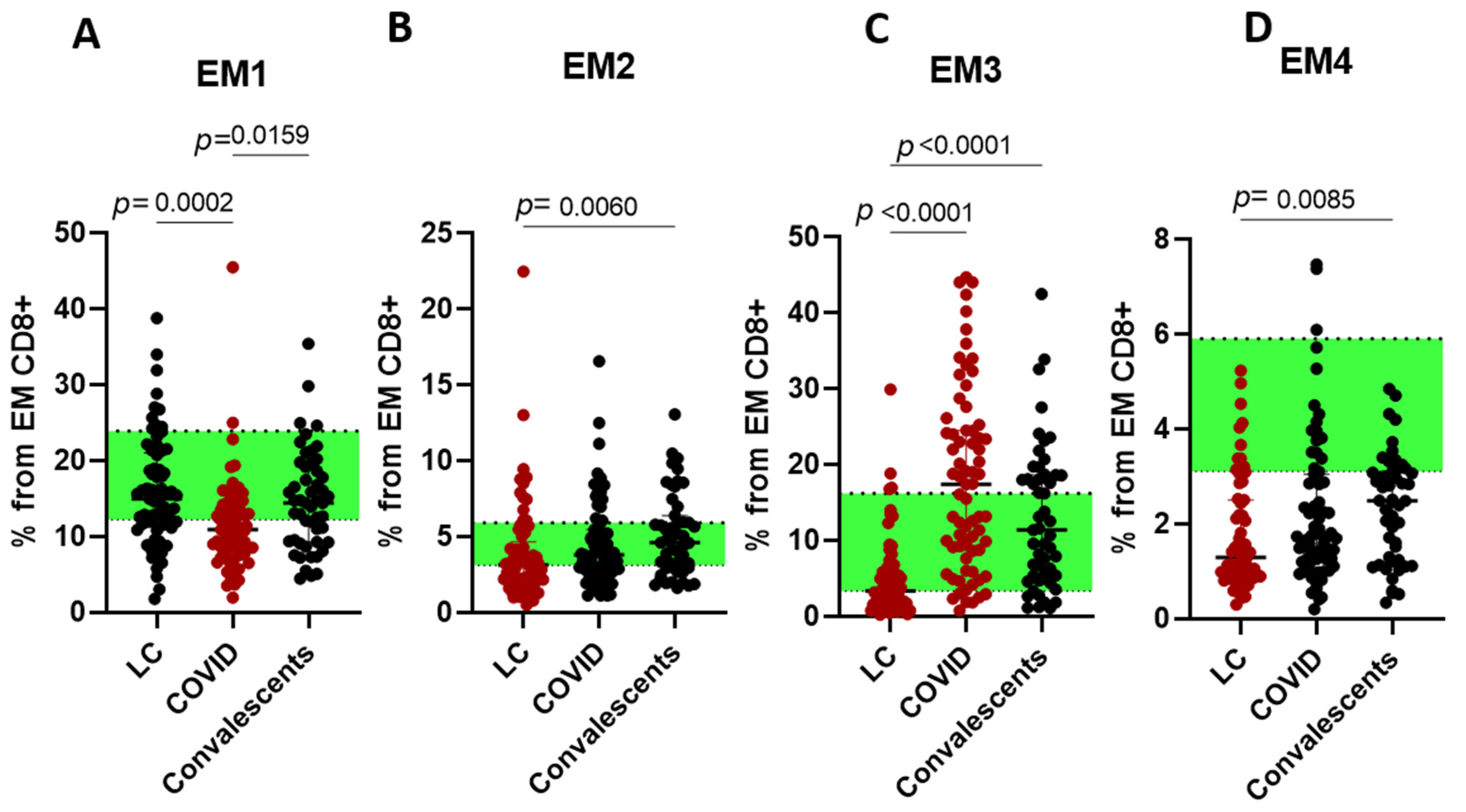
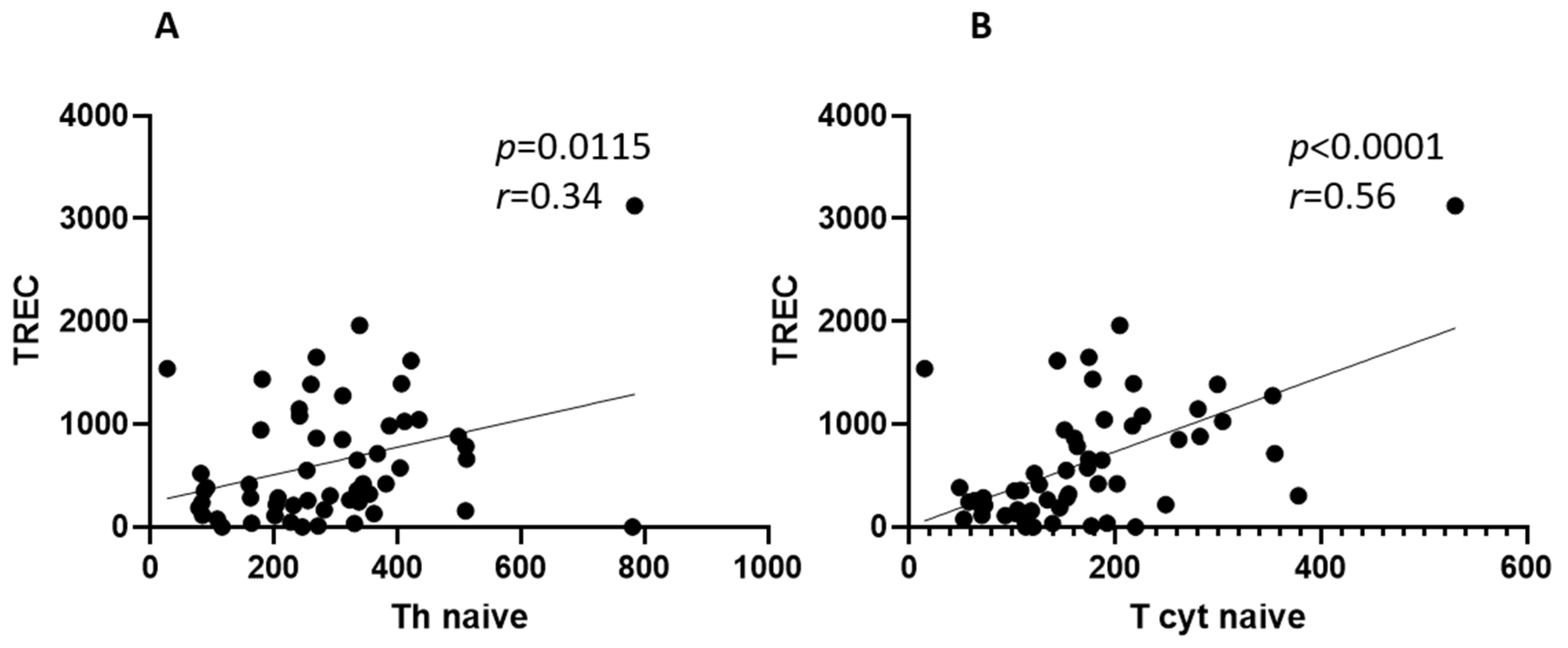
2. Results
3. Discussion
4. Materials and Methods
4.1. Studied Cohorts
4.2. Sample Collection
4.3. T Cell Immunophenotyping by Flow Cytometry
4.4. TREC Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Central memory |
| EM | Effector memory |
| KREC | Kappa-deleting recombination excision circles |
| LC | Long COVID |
| MHC | Major histocompatibility complex |
| Tc | Cytotoxic T cells |
| TCR | T cellular receptor |
| TEMRA | Terminally differentiated effector memory T cells |
| Th | Helper T cells |
| TREC | T cell receptor excision circles |
References
- World Health Organization (WHO). WHO COVID-19 Dashboard; World Health Organization (WHO): Geneva, Switzerland, 2025; Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 6 June 2025).
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- Bull-Otterson, L. Post-COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long Covid in people infected with SARS-CoV-2 after two doses of a COVID-19 vaccine: Community-based, matched cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- FAIR Health. Patients Diagnosed with Post-COVID Conditions: An Analysis of Private Healthcare Claims Using the Official ICD-10 Diagnostic Code; FAIR Health: New York, NY, USA, 2022. [Google Scholar]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.; Lu, P.; Dhodapkar, R.M.; Gehlhausen, J.R.; Tabachnikova, A.; Tabacof, L.; Malik, A.A.; Kamath, K.; et al. Distinguishing features of Long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Peluso, M.J.; Spinelli, M.A.; Deveau, T.M.; Forman, C.A.; Munter, S.E.; Mathur, S.; Tang, A.F.; Lu, S.; Goldberg, S.A.; Arreguin, M.I.; et al. Postacute sequelae and adaptive immune responses in people with HIV recovering from SARS-COV-2 infection. AIDS 2022, 36, F7–F16. [Google Scholar] [CrossRef]
- Zubchenko, S.; Kril, I.; Nadizhko, O.; Matsyura, O.; Chopyak, V. Herpesvirus infections and post-COVID-19 manifestations: A pilot observational study. Rheumatol. Int. 2022, 42, 1523–1530. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Mendes de Almeida, V.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, Í.S.; Alves, D.A.; d’Auriol, M.; Magalhães, J.; Machado, E.C.; Rocha, V.M.; et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar] [CrossRef]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol 2022, 21, 148. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Renz-Polster, H.; Tremblay, M.-E.; Bienzle, D.; Fischer, J.E. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: The case for neuroglial failure. Front. Cell. Neurosci. 2022, 16, 888232. [Google Scholar] [CrossRef]
- Merzon, E.; Weiss, M.D.; Tonetti, A.; Golan-Cohen, A.; Green, I.; Israel, A.; Frenkel-Morgenstern, M.; Magen, E. Clinical and socio-demographic variables associated with the diagnosis of long COVID syndrome in youth: A population-based study. Int. J. Environ. Res. Public Health 2022, 19, 5993. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Takahashi, S.; Wang, T.; Demeter, J.; Wyman, S.K.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Saitgalina, M.A.; Liubimova, N.E.; Ostankova, Y.V.; Kuznetzova, R.N.; Totolian, A.A. Determination of reference values for TREC and KREC in circulating blood of the persons over 18 years. Med. Immunol. 2022, 24, 1227–1236. (In Russian) [Google Scholar] [CrossRef]
- Saitgalina, M.A.; Ostankova, Y.V.; Arsentieva, N.A.; Korobova, Z.R.; Liubimova, N.E.; Kashchenko, V.A.; Kulikov, A.N.; Pevtsov, D.E.; Stanevich, O.V.; Chernykh, E.I.; et al. Assessment of trec and krec levels in COVID-19 patients with varying disease severity. Russ. J. Infect. Immun. 2023, 13, 873–884. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Chi, X.; Gu, J.; Ma, X. Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response. Vaccines 2022, 10, 1623. [Google Scholar] [CrossRef]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Rha, M.S.; Jeong, H.W.; Ko, J.H.; Choi, S.J.; Seo, I.H.; Lee, J.S.; Sa, M.; Kim, A.R.; Joo, E.J.; Ahn, J.Y.; et al. PD-1-Expressing SARS-CoV-2-Specific CD8+ T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity 2021, 54, 44–52.e3. [Google Scholar] [CrossRef]
- Perlman, S.; Sariol, A. Pathogenic T cells in post-viral lung disease in mice. Nat. Immunol. 2024, 25, 1991–1992. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Batsunov, O.K.; Korobova, Z.R.; Khamitova, I.V.; Isakov, D.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2021, 44, 194–205. [Google Scholar] [CrossRef]
- Rubinstein, A.; Kudryavtsev, I.; Arsentieva, N.; Korobova, Z.R.; Isakov, D.; Totolian, A.A. CXCR3−Expressing T Cells in Infections and Autoimmunity. Front. Biosci. 2024, 29, 301. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Dal-Pizzol, F.; Kluwe-Schiavon, B.; Dal-Pizzol, H.R.; da Silveira Prestes, G.; Dominguini, D.; Girardi, C.S.; Santos, L.; Moreira, J.C.F.; Gelain, D.P.; Walz, R.; et al. Association of systemic inflammation and long-term dysfunction in COVID-19 patients: A prospective cohort. Psychoneuroendocrinology 2025, 172, 107269. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Rubinstein, A.; Kudryavtsev, I.; Malkova, A.; Mammedova, J.; Isakov, D.; Isakova-Sivak, I.; Kudlay, D.; Starshinova, A. Sarcoidosis-related autoimmune inflammation in COVID-19 convalescent patients. Front. Med. 2023, 10, 1271198. [Google Scholar] [CrossRef]
- Santopaolo, M.; Gregorova, M.; Hamilton, F.; Arnold, D.; Long, A.; Lacey, A.; Oliver, E.; Halliday, A.; Baum, H.; Hamilton, K.; et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. eLife 2023, 12, e85009. [Google Scholar] [CrossRef]
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 886431. [Google Scholar] [CrossRef]
- Peluso, M.J.; Swank, Z.N.; Goldberg, S.A.; Lu, S.; Dalhuisen, T.; Borberg, E.; Senussi, Y.; Luna, M.A.; Chang Song, C.; Clark, A.; et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect. Dis. 2024, 24, e345–e347. [Google Scholar] [CrossRef]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Krüger, K.; Baumgart, S.; Drube, S.; Lemhöfer, C.; Reuken, P.; Stallmach, A.; Müller, M.; et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients—Indications for their potential involvement in the development of Long COVID? Front. Immunol. 2022, 13, 1070994. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Hu, Z.; Wu, M.; Wang, C.; Feng, Z.; Mao, C.; Tan, Y.; Liu, Y.; Chen, L.; et al. Thymosin Alpha 1 Reduces the Mortality of Severe Coronavirus Disease 2019 by Restoration of Lymphocytopenia and Reversion of Exhausted T Cells. Clin. Infect. Dis. 2020, 71, 2150–2157. [Google Scholar] [CrossRef]
- Khadzhieva, M.B.; Kalinina, E.V.; Larin, S.S.; Sviridova, D.A.; Gracheva, A.S.; Chursinova, J.V.; Stepanov, V.A.; Redkin, I.V.; Avdeikina, L.S.; Rumyantsev, A.G.; et al. TREC/KREC Levels in Young COVID-19 Patients. Diagnostics 2021, 11, 1486. [Google Scholar] [CrossRef]
- Savchenko, A.A.; Tikhonova, E.; Kudryavtsev, I.; Kudlay, D.; Korsunsky, I.; Beleniuk, V.; Borisov, A. TREC/KREC Levels and T and B Lymphocyte Subpopulations in COVID-19 Patients at Different Stages of the Disease. Viruses 2022, 14, 646. [Google Scholar] [CrossRef]
- Zurochka, A.V.; Dobrynina, M.A.; Safronova, E.A.; Zurochka, V.A.; Zuikova, A.A.; Sarapultsev, G.P.; Zabkov, O.I.; Mosunov, A.A.; Verkhovskaya, M.D.; Ducardt, V.V.; et al. Alterations in T cell immunity over 6–12 months post-COVID-19 infection in convalescent individuals: A screening study. Russ. J. Infect. Immun. 2024, 14, 756–768. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Butenko, A.A.; Kokoeva, A.E.; Kucherenko, N.G.; Kashchenko, V.A.; Boeva, E.V.; Norka, A.O.; et al. B Cell Dynamics and Transitional B Cells in Long COVID. Curr. Issues Mol. Biol. 2025, 47, 245. [Google Scholar] [CrossRef]
- Ministry of Health of the Russian Federation. Temporary Guidelines for the Prevention and Treatment of COVID-19 (Version 18); Ministry of Health of the Russian Federation: Moscow, Russian, 2022. Available online: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/064/610/original/ВМР_COVID-19_V18.pdf (accessed on 6 June 2025).
- World Health Organization. Clinical Management of COVID-19: Living Guideline (13 December 2023); World Health Organization (WHO): Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.2 (accessed on 6 June 2025).
- Kudryavtsev, I.V.; Arsentieva, N.A.; Korobova, Z.R.; Isakov, D.V.; Rubinstein, A.A.; Batsunov, O.K.; Khamitova, I.V.; Kuznetsova, R.N.; Savin, T.V.; Akisheva, T.V.; et al. Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients. Viruses 2022, 14, 1906. [Google Scholar] [CrossRef]
- Saitgalina, M.A.; Ostankova, Y.V.; Liubimova, N.E.; Semenov, A.V.; Kuznetsova, R.N.; Totolian, A.A. Modified quantitative approach for assessing peripheral blood TREC and KREC levels in immunodeficient patients. Russ. J. Infect. Immun. 2022, 12, 981–996. [Google Scholar] [CrossRef]



| Age Group | Reference (Me, Q25–Q75) | LC Cohort (Me, Q25–Q75) |
|---|---|---|
| 18–29 | 553.3, 44.9–2135 | 874.4, 528.2–1361 |
| 30–39 | 252.7, 23.6–1597.0 | 612.3, 335–995.5 |
| 40–49 | 191.3, 18.3–1098 | 177, 18.4–336.5 |
| 50–59 | 131.1, 13.9–1543 | 187.8, 76.7–246.1 |
| 60–69 | 74.9, 12.5–1715 | 540.3, 34.5–1046 |
| >70 | 44.7, 11.4–683.1 | n/a |
| COVID-19 | Convalescents | LC | HD | |
|---|---|---|---|---|
| n | 71 | 51 | 63 | 46 |
| Age (Me, Q25–Q75) | 60, 46–70 | 32, 26–38 | 38, 29–48 | |
| Sex distribution (%) | 35.2% males, 64.8% females | 41.2% males, 58.8% females | 21% male, 79% female | 47.8% male, 52.2 female |
| Major symptoms | Fever, fatigue, myalgia, arthralgia, couch, CT-confirmed pneumonia | no | Psychoneurological symptoms (cognitive impairment, anxiety/depression), fatigue, dyspnea | no |
| SARS-CoV-2 RNA PCR | yes | no | no | no |
| SARS-CoV-2 IgG | yes/no | yes | yes | no |
| Subpopulation | Phenotype | % from | Subpopulation | Phenotype | % from |
|---|---|---|---|---|---|
| T Helper Cells (Th), CD45+CD3+CD4+ | Lymphocytes (CD45+) | Cytotoxic T Lymphocytes (CTL) CD45+CD3+CD8+ | |||
| Naïve | CD45RA+CD62L+ | Th (CD4+) | Naïve | CD45RA+CD62L+ | CTL (CD8+) |
| CM | CD45RA−CD62L+ | Th (CD4+) | CM | CD45RA−CD62L+ | CTL (CD8+) |
| EM | CD45RA−CD62L− | Th (CD4+) | EM | CD45RA−CD62L− | CTL (CD8+) |
| TEMRA | CD45RA+CD62L− | Th (CD4+) | TEMRA | CD45RA+CD62L− | CTL (CD8+) |
| Th1 | CXCR5−CXCR3+CCR6−CCR4− | Th (CD4+) | Tc1 | CXCR3+CCR6− | CTL (CD8+) |
| Th2 | CXCR5−CXCR3−CCR6−CCR4+ | Th (CD4+) | Tc2 | CXCR3−CCR6− | CTL (CD8+) |
| Th17 | CXCR5−CCR6+ | Th (CD4+) | Tc17 | CXCR3−CCR6+ | CTL (CD8+) |
| T follicular helpers (Tfh) | CXCR5+ | Th (CD4+) | Tc17.1 | CXCR3+CCR6+ | CTL (CD8+) |
| - | - | - | EM1 | CD27+CD28+ | EM CTL (CD45RA−CD62L) |
| - | - | - | EM2 | CD27+CD28− | EM CTL (CD45RA−CD62L) |
| - | - | - | EM3 | CD27−CD28− | EM CTL (CD45RA−CD62L) |
| - | - | - | EM4 | CD27−CD28+ | EM CTL (CD45RA−CD62L) |
| - | - | - | Pre Effector cells 1 (pE1) | CD27+CD28− | TEMRA (CD45RA+CD62L−) |
| - | - | - | Pre Effector cells 2 (pE2) | CD27+CD28− | TEMRA (CD45RA+CD62L−) |
| Effector cells | CD27−CD28− | TEMRA (CD45RA+CD62L−) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobova, Z.R.; Arsentieva, N.A.; Butenko, A.A.; Kudryavtsev, I.V.; Rubinstein, A.A.; Turenko, A.S.; Ostankova, Y.V.; Boeva, E.V.; Knizhnikova, A.A.; Norka, A.O.; et al. T Cell Dynamics in COVID-19, Long COVID and Successful Recovery. Int. J. Mol. Sci. 2025, 26, 7258. https://doi.org/10.3390/ijms26157258
Korobova ZR, Arsentieva NA, Butenko AA, Kudryavtsev IV, Rubinstein AA, Turenko AS, Ostankova YV, Boeva EV, Knizhnikova AA, Norka AO, et al. T Cell Dynamics in COVID-19, Long COVID and Successful Recovery. International Journal of Molecular Sciences. 2025; 26(15):7258. https://doi.org/10.3390/ijms26157258
Chicago/Turabian StyleKorobova, Zoia R., Natalia A. Arsentieva, Anastasia A. Butenko, Igor V. Kudryavtsev, Artem A. Rubinstein, Anastasia S. Turenko, Yulia V. Ostankova, Ekaterina V. Boeva, Anastasia A. Knizhnikova, Anna O. Norka, and et al. 2025. "T Cell Dynamics in COVID-19, Long COVID and Successful Recovery" International Journal of Molecular Sciences 26, no. 15: 7258. https://doi.org/10.3390/ijms26157258
APA StyleKorobova, Z. R., Arsentieva, N. A., Butenko, A. A., Kudryavtsev, I. V., Rubinstein, A. A., Turenko, A. S., Ostankova, Y. V., Boeva, E. V., Knizhnikova, A. A., Norka, A. O., Rassokhin, V. V., Belyakov, N. A., & Totolian, A. A. (2025). T Cell Dynamics in COVID-19, Long COVID and Successful Recovery. International Journal of Molecular Sciences, 26(15), 7258. https://doi.org/10.3390/ijms26157258







