Beyond Protection: The Cytotoxic Effect of Anti-Tat Antibodies in People Living with HIV
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. HIV Anti-Tat ELISA
4.3. 8-OHdG Determination
4.4. Nuclear Anomalies Quantitation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, D.E.; Couturier, J.P. Chronic Inflammation in HIV Pathogenesis: Effects on Immune Cells, Organ Systems, and Systemic Consequences. Transl. Inflamm. 2019, 111–131. [Google Scholar] [CrossRef]
- Gutiérrez-Sevilla, J.E.; Cárdenas-Bedoya, J.; Escoto-Delgadillo, M.; Zúñiga-González, G.M.; Pérez-Ríos, A.M.; Gómez-Meda, B.C.; González-Enríquez, G.V.; Figarola-Centurión, I.; Chavarría-Avila, E.; Torres-Mendoza, B.M. Genomic instability in people living with HIV. Mutat. Res.Genet. Toxicol. Environ. Mutagen. 2021, 865, 503336. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Weber, M.S.R.; Ramirez, J.J.D.; Hentzien, M.; Cavassini, M.; Bernasconi, E.; Hofmann, E.; Furrer, H.; Kovari, H.; Stöckle, M.; Schmid, P.; et al. Time Trends in Causes of Death in People with Human Immunodeficiency Virus: Insights from the Swiss HIV Cohort Study. Clin. Infect. Dis. 2024, 79, 177–188. [Google Scholar] [CrossRef]
- Park, B.; Ahn, K.H.; Choi, Y.; Kim, J.H.; Seong, H.; Kim, Y.J.; Choi, J.Y.; Song, J.Y.; Lee, E.; Jun, Y.H.; et al. Cancer Incidence Among Adults with HIV in a Population-Based Cohort in Korea. JAMA Netw. Open 2022, 5, e2224897. [Google Scholar] [CrossRef]
- Lee, S.O.; Lee, J.E.; Lee, S.; Lee, S.H.; Kang, J.S.; Son, H.; Lee, H.; Kim, J. Nationwide population-based incidence of cancer among patients with HIV/AIDS in South Korea. Sci. Rep. 2022, 12, 9974. [Google Scholar] [CrossRef]
- Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J.M. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers 2021, 13, 305. [Google Scholar] [CrossRef]
- Faust, T.B.; Binning, J.M.; Gross, J.D.; Frankel, A.D. Making Sense of Multifunctional Proteins: Human Immunodeficiency Virus Type 1 Accessory and Regulatory Proteins and Connections to Transcription. Annu. Rev. Virol. 2017, 4, 241–260. [Google Scholar] [CrossRef]
- Cafaro, A.; Schietroma, I.; Sernicola, L.; Belli, R.; Campagna, M.; Mancini, F.; Farcomeni, S.; Pavone-Cossut, M.R.; Borsetti, A.; Monini, P.; et al. Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis. Int. J. Mol. Sci. 2024, 25, 1704. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell. Infect. Microbiol. 2020, 10, 61. [Google Scholar] [CrossRef]
- Cafaro, A.; Tripiciano, A.; Picconi, O.; Sgadari, C.; Moretti, S.; Buttò, S.; Monini, P.; Ensoli, B. Anti-tat immunity in HIV-1 infection: Effects of naturally occurring and vaccine-induced antibodies against tat on the course of the disease. Vaccines 2019, 7, 99. [Google Scholar] [CrossRef]
- Rezza, G.; Fiorelli, V.; Dorrucci, M.; Ciccozzi, M.; Tripiciano, A.; Scoglio, A.; Collacchi, B.; Ruiz-Alvarez, M.; Giannetto, C.; Caputo, A.; et al. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: Findings in a cohort of HIV-1 seroconverters. J. Infect. Dis. 2005, 191, 1321–1324. [Google Scholar] [CrossRef]
- Bellino, S.; Tripiciano, A.; Picconi, O.; Francavilla, V.; Longo, O.; Sgadari, C.; Paniccia, G.; Arancio, A.; Angarano, G.; Ladisa, N.; et al. The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: Results of a 3-year cohort study. Retrovirology 2014, 11, 49. [Google Scholar] [CrossRef]
- Nielsen, K.; Kelly, L.; Gall, D.; Smith, P.; Bosse, J.; Nicoletti, P.; Kelly, W. The use of divalent cation chelating agents (EDTA/EGTA) to reduce non-specific serum protein interaction in enzyme immunoassay. Vet. Res. Commun. 1994, 18, 433–437. [Google Scholar] [CrossRef]
- Anani, W.Q.; Zeevi, A.; Lunz, J.G. EDTA Treatment of Serum Unmasks Complement-Mediated Prozone Inhibition in Human Leukocyte Antigen Antibody Testing. Am. J. Clin. Pathol. 2016, 146, 346–352. [Google Scholar] [CrossRef]
- Tripiciano, A.; Picconi, O.; Moretti, S.; Sgadari, C.; Cafaro, A.; Francavilla, V.; Arancio, A.; Paniccia, G.; Campagna, M.; Pavone-Cossut, M.R.; et al. Anti-Tat immunity defines CD4+ T-cell dynamics in people living with HIV on long-term cART. EBioMedicine 2021, 66, 103306. [Google Scholar] [CrossRef]
- Nicoli, F.; Chachage, M.; Clowes, P.; Bauer, A.; Kowour, D.; Ensoli, B.; Cafaro, A.; Maboko, L.; Hoelscher, M.; Gavioli, R.; et al. Association between different anti-Tat antibody isotypes and HIV disease progression: Data from an African cohort. BMC Infect. Dis. 2016, 16, 344. [Google Scholar] [CrossRef]
- Kashi, V.P.; Jacob, R.A.; Paul, S.; Nayak, K.; Satish, B.; Swaminathan, S.; Satish, K.S.; Ranga, U. HIV-1 Tat-specific IgG antibodies in high-responders target a B-cell epitope in the cysteine-rich domain and block extracellular Tat efficiently. Vaccine 2009, 27, 6739–6747. [Google Scholar] [CrossRef]
- McGowan, J.P.; Shah, S.S.; Small, C.B.; Klein, R.S.; Schnipper, S.M.; Chang, C.J.; Rosenstreich, D.L. Relationship of serum immunoglobulin and IgG subclass levels to race, ethnicity and behavioral characteristics in HIV infection. Med. Sci. Monit. 2006, 12, 11–16. [Google Scholar]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef]
- Krone, W.J.A.; Debouck, C.; Epstein, L.G.; Heutink, P.; Meloen, R.; Goudsmit, J. Natural antibodies to HIV-tat epitopes and expression of HIV-1 genes in vivo. J. Med. Virol. 1988, 26, 261–270. [Google Scholar] [CrossRef]
- Shmakova, A.; Tsimailo, I.; Kozhevnikova, Y.; Gérard, L.; Boutboul, D.; Oksenhendler, E.; Tuaillon, E.; Rivault, A.; Germini, D.; Vassetzky, Y.; et al. HIV-1 Tat is present in the serum of people living with HIV-1 despite viral suppression. Int. J. Infect. Dis. 2024, 142, 106994. [Google Scholar] [CrossRef]
- Zamora-Perez, A.L.; Ortiz-García, Y.M.; Lazalde-Ramos, B.P.; Guerrero-Velázquez, C.; Gómez-Meda, B.C.; Ramírez-Aguilar, M.A.; Zúñiga-González, G.M. Increased micronuclei and nuclear abnormalities in buccal mucosa and oxidative damage in saliva from patients with chronic and aggressive periodontal diseases. J. Periodontal Res. 2015, 50, 28–36. [Google Scholar] [CrossRef]
- Kulkarni, A.; Kurle, S.; Shete, A.; Ghate, M.; Godbole, S.; Madhavi, V.; Kent, S.J.; Paranjape, R.; Thakar, M. Indian long-term non-progressors show broad ADCC responses with preferential recognition of V3 region of envelope and a region from tat protein. Front. Immunol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Martino, M.; Cafaro, A.; Torreggiani, E.; Caputo, A.; Tripiciano, A.; Dallan, B.; Proietto, D.; De Laurentis, M.; Tassani, E.; Francavilla, V.; et al. P-193 Evaluation of functional antibodies against HIV Env and Tat proteins: Analysis of antibody-dependent cell-mediated cytotoxicity (ADCC). Sex. Transm. Infect. 2024, 100, A259. [Google Scholar] [CrossRef]
- Forthal, D.N.; Finzi, A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS 2018, 32, 2439–2451. [Google Scholar] [CrossRef]
- Billeskov, R.; Beikzadeh, B.; Berzofsky, J.A. The effect of antigen dose on T cell-targeting vaccine outcome. Hum. Vaccines Immunother. 2019, 15, 407–411. [Google Scholar] [CrossRef]
- Jabea Ekabe, C.; Asaba Clinton, N.; Agyei, E.K.; Kehbila, J. Role of Apoptosis in HIV Pathogenesis. Adv. Virol. 2022, 2022, 8148119. [Google Scholar] [CrossRef]
- Goldberg, B.S.; Ackerman, M.E. Antibody-mediated complement activation in pathology and protection. Immunol. Cell Biol. 2020, 98, 305–317. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Sortino, O.; Verheij, E.; Wit, F.W.; Kootstra, N.A.; Sellers, B.; Schim Van Der Loeff, M.; Belkaid, Y.; Reiss, P.; Sereti, I. The Complement Pathway Is Activated in People with Human Immunodeficiency Virus and Is Associated with Non-AIDS Comorbidities. J. Infect. Dis. 2021, 224, 1405–1409. [Google Scholar] [CrossRef]
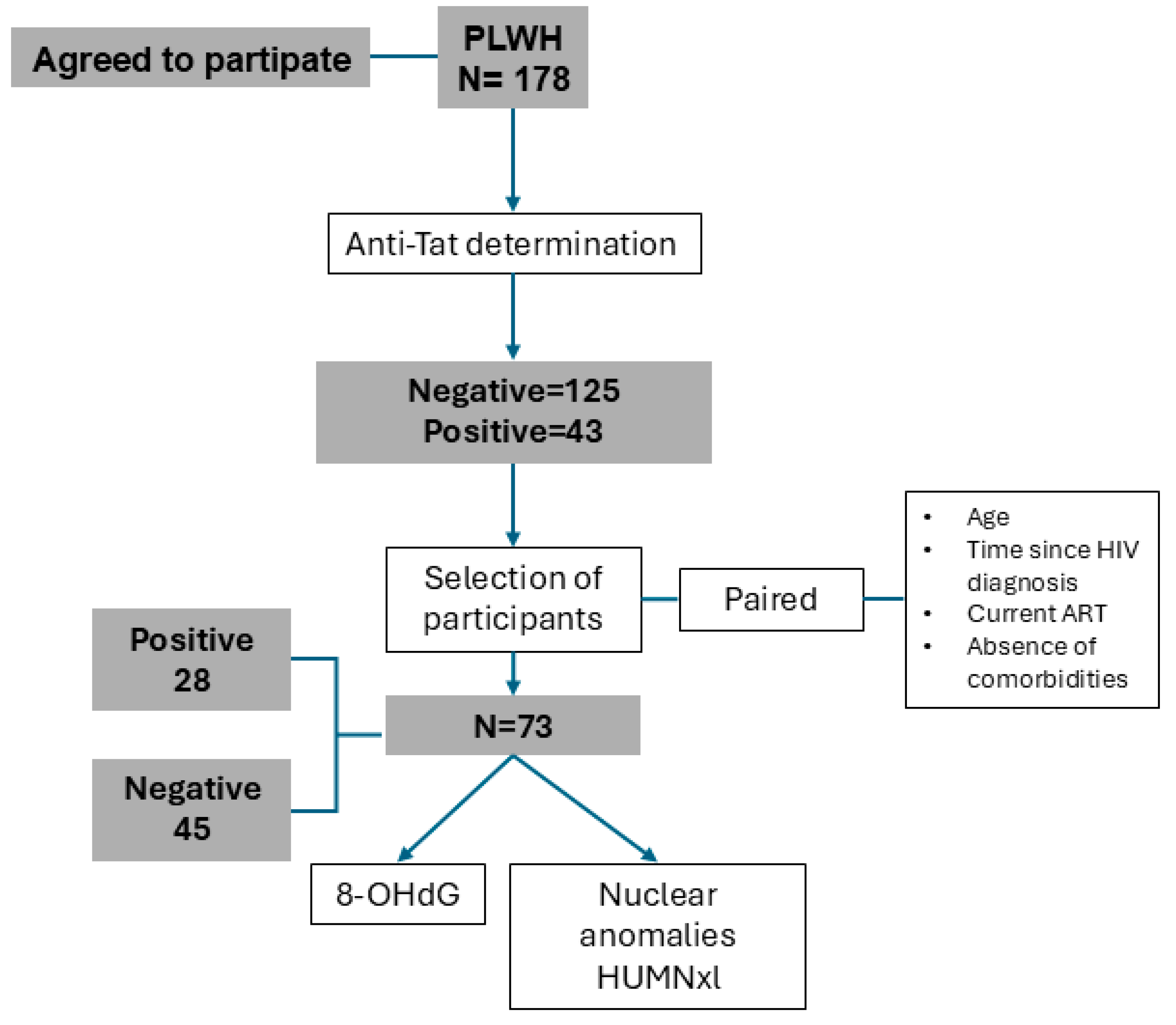

| Variable | Anti-Tat Antibody Status | |||
|---|---|---|---|---|
| Negative (135) | Positive (43) | p-Value | ||
| Sex | Female | 16 (9.0%) | 7 (3.9%) | 0.309 |
| Male | 119 (66.9%) | 36 (20.2%) | ||
| Age (years) | 39.0 (19.0) | 33.0 (17.0) | 0.015 | |
| Time since diagnosis (years) | 5.0 (10.0) | 4.0 (8.0) | 0.519 | |
| ART | Yes | 128 (71.9%) | 43 (24.2%) | 0.198 |
| No | 7 (3.9%) | 0 (0.0%) | ||
| Viral load | Undetectable | 121 (68.0%) | 35 (4.5%) | 0.184 |
| Detectable | 14 (7.9%) | 8 (4.5%) | ||
| Copies/mL * | 103,526.0 (501,549.0) | 44,182.0 (108,148.0) | 0.57 | |
| CD4+ T lymphocytes | Cells/µL | 755.0 (526.0) | 607.0 (435.0) | 0.063 |
| ≥500 cells/µL | 96 (59.3%) | 22 (13.6%) | 0.023 | |
| 0–499 cells/µL | 28 (17.3%) | 16 (9.9%) | ||
| Variable | Anti-Tat Antibody Concentration | |||
|---|---|---|---|---|
| Lower 39 (90.7%) | Higher 4 (9.3%) | p-Value | ||
| Sex | Female | 7.0 (16.3%) | 0 (0.0%) | 0.999 |
| Male | 32.0 (74.4%) | 4 (9.3%) | ||
| Age (years) | 37.5 (20.0) | 33.5 (24.0) | 0.606 | |
| Time since diagnosis (years) | 5.0 (10.0) | 1.0 (4.0) | 0.03 | |
| ART | Yes | 39.0 (9.7%) | 4 (9.3%) | 0.999 |
| No | 0 (0.0%) | 0 (0.0%) | ||
| Viral load | Undetectable | 32.0 (74.4%) | 3 (7.0%) | 0.488 |
| Detectable | 7.0 (16.3%) | 1 (2.3%) | ||
| Copies/mL * | 52,389.0 (336,203.0) | 67,861.0 (N/A) | 0.999 | |
| CD4+ T lymphocytes (cells/µL) | 739.0 (567.0) | 480.5 (83.0) | 0.099 | |
| Variable | Anti-Tat Antibody Status | ||||
|---|---|---|---|---|---|
| Negative (45) | Positive (28) | p-Value | |||
| Sex | Female | 3 (4.1%) | 3 (4.1%) | 0.669 | |
| Male | 42 (57.5%) | 25 (34.2%) | |||
| Age | 32.0 (20.0) | 32.0 (14.0) | 0.654 | ||
| Time since diagnosis (years) | 4.0 (6.0) | 4.0 (4.0) | 0.842 | ||
| Total cholesterol (mg/dL) | 179.0 (48.0) | 170.0 (38.0) | 0.093 | ||
| HDL (mg/dL) | 39.5 (15.0) | 40.0 (11.0) | 0.966 | ||
| LDL (mg/dL) | 100.0 (38.0) | 96.0 (31.0) | 0.204 | ||
| Triglycerides (mg/dL) | 139.0 (87.0) | 130.0 (118.0) | 0.6 | ||
| Viral load | Undetectable | 40 (54.8%) | 23 (31.5%) | 0.492 | |
| Detectable | 5 (6.8%) | 5 (6.8%) | |||
| Copies/mL * | 16,545.0 (103,162.0) | 9761.0 (47,875.0) | 0.841 | ||
| CD4+ T lymphocytes | Cells/µL | 726 (571) | 524 (367) | 0.045 | |
| ≥500 cells/µL | 32 (48.5%) | 14 (21.2%) | 0.031 | ||
| 0–499 cells/µL | 8 (12.1%) | 12 (18.2%) | |||
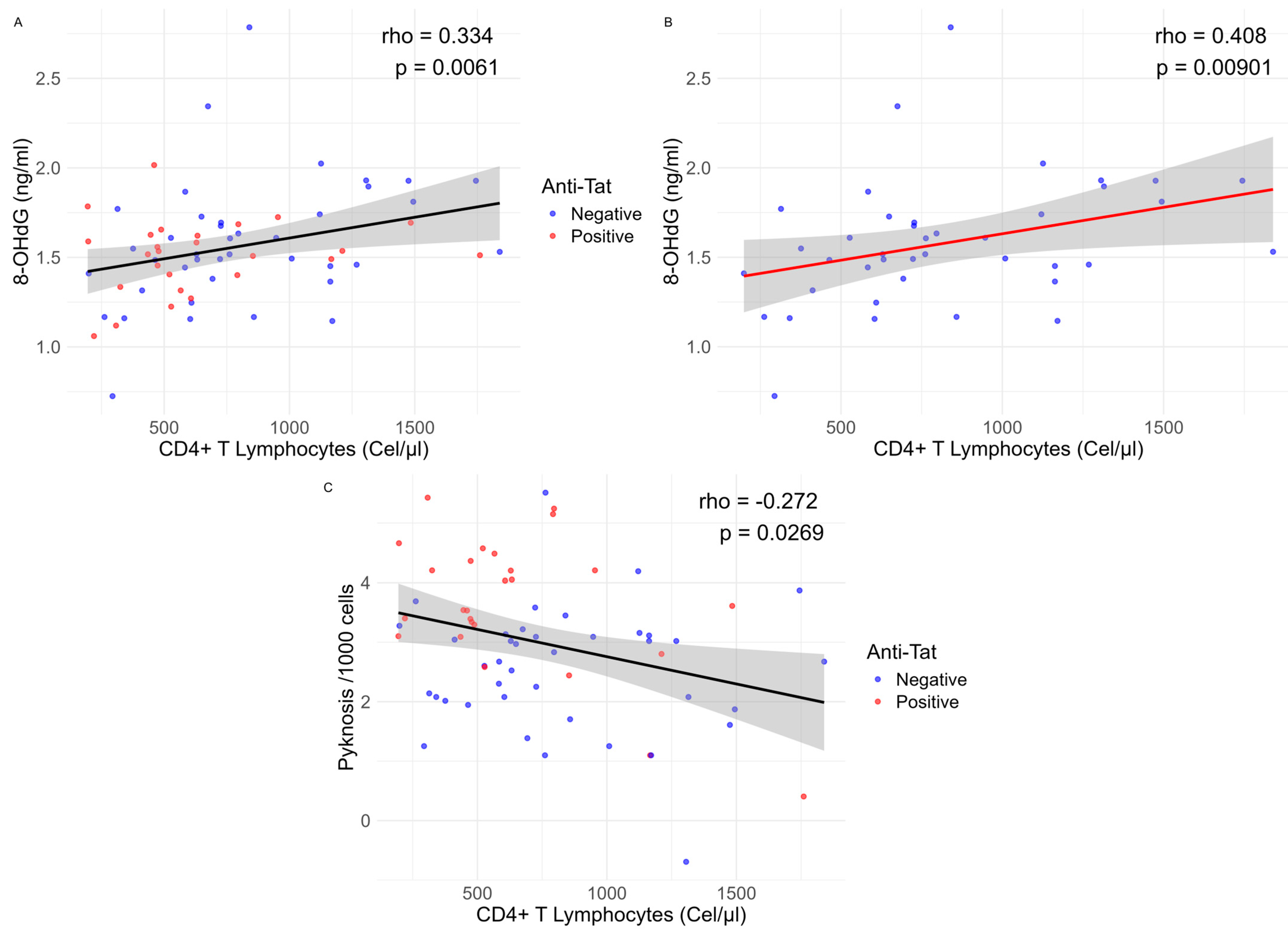
| 8-OHdG (ng/mL) | 4.6 (1.75) | 4.5 (1.21) | 0.326 | ||
| Micronuclei | 0.0 (0.0) | 0.0 (0.25) | 0.026 | ||
| Nuclear buds | 1.0 (1.50) | 1.5 (2.25) | 0.211 | ||
| Binucleated cells | 0.0 (0.0) | 0.0 (0.0) | 0.205 | ||
| Pyknosis | 14.5 (16.0) | 34.37 (57.8) | 0.0001 | ||
| Condensed chromatin | 76.5 (105.0) | 97.0 (143.3) | 0.343 | ||
| Karyorrhexis | 62.0 (197.5) | 58.1 (69.6) | 0.204 | ||
| Karyolysis | 2.25 (3.0) | 0.5 (1.31) | 0.067 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Sevilla, J.E.; Gaona-Bernal, J.; González-Enríquez, G.V.; Escoto-Delgadillo, M.; Zúñiga-González, G.M.; Gómez-Meda, B.C.; Luévano-Gómez, S.G.; Pérez-Ríos, A.M.; Ávila-Morán, M.; García-Arias, V.E.; et al. Beyond Protection: The Cytotoxic Effect of Anti-Tat Antibodies in People Living with HIV. Int. J. Mol. Sci. 2025, 26, 7229. https://doi.org/10.3390/ijms26157229
Gutiérrez-Sevilla JE, Gaona-Bernal J, González-Enríquez GV, Escoto-Delgadillo M, Zúñiga-González GM, Gómez-Meda BC, Luévano-Gómez SG, Pérez-Ríos AM, Ávila-Morán M, García-Arias VE, et al. Beyond Protection: The Cytotoxic Effect of Anti-Tat Antibodies in People Living with HIV. International Journal of Molecular Sciences. 2025; 26(15):7229. https://doi.org/10.3390/ijms26157229
Chicago/Turabian StyleGutiérrez-Sevilla, Juan Ernesto, Jorge Gaona-Bernal, Gracia Viviana González-Enríquez, Martha Escoto-Delgadillo, Guillermo Moisés Zúñiga-González, Belinda Claudia Gómez-Meda, Silvia Gabriela Luévano-Gómez, Alma Minerva Pérez-Ríos, Maribel Ávila-Morán, Víctor Eduardo García-Arias, and et al. 2025. "Beyond Protection: The Cytotoxic Effect of Anti-Tat Antibodies in People Living with HIV" International Journal of Molecular Sciences 26, no. 15: 7229. https://doi.org/10.3390/ijms26157229
APA StyleGutiérrez-Sevilla, J. E., Gaona-Bernal, J., González-Enríquez, G. V., Escoto-Delgadillo, M., Zúñiga-González, G. M., Gómez-Meda, B. C., Luévano-Gómez, S. G., Pérez-Ríos, A. M., Ávila-Morán, M., García-Arias, V. E., Torres-Ríos, J. P., Cárdenas-Bedoya, J., & Torres-Mendoza, B. M. (2025). Beyond Protection: The Cytotoxic Effect of Anti-Tat Antibodies in People Living with HIV. International Journal of Molecular Sciences, 26(15), 7229. https://doi.org/10.3390/ijms26157229






