Antioxidant Enzyme Activity and mRNA Expression in the Reproductive Tissues of Male European Red Deer (Cervus elaphus elaphus)
Abstract
1. Introduction
2. Results
2.1. Activity of Antioxidant Enzymes
2.2. Relative Abundance of Analyzed Enzymes
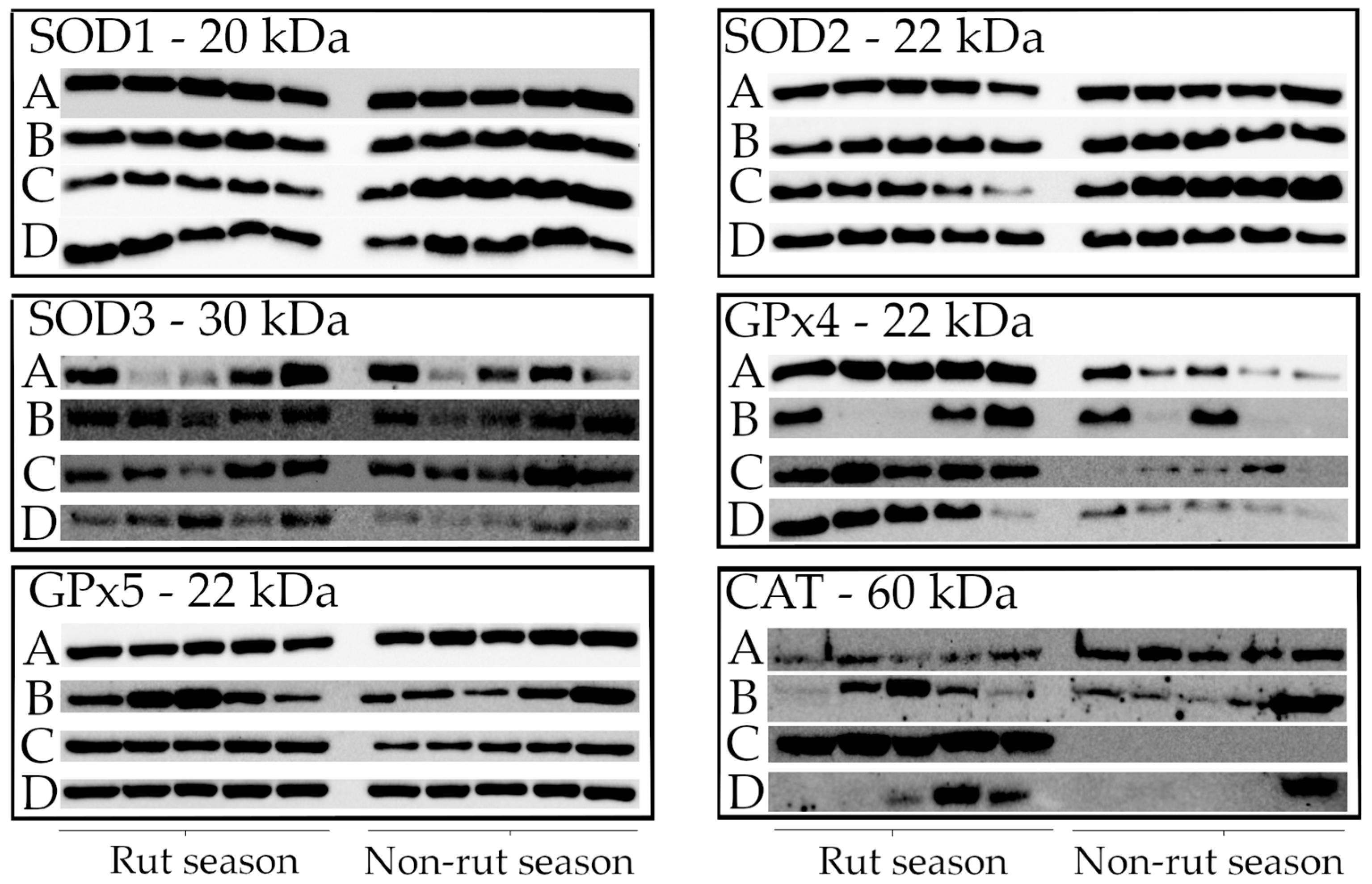
2.3. Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Tissue Preparation
4.2.1. Antioxidant Enzyme Activity
4.2.2. Real-Time PCR
4.2.3. Western Blot
4.3. Sample Analyses
4.3.1. Antioxidant Enzyme Activity
- Superoxide dismutase activity
- Glutathione peroxidase activity
- Catalase activity
4.3.2. Gene Expression
Reverse Transcription Polymerase Chain Reaction
Real-Time PCR
4.3.3. Western Blot
SDS-PAGE
Semi-Dry Protein Transfer
Antibody Incubation
- Rabbit polyclonal anti-SOD1 antibody (1:10,000; PA5-23245; Thermo Fisher Scientific, Waltham, MA, USA);
- Rabbit anti-SOD2 polyclonal antibody (1:1000; PA1-31072; Thermo Fisher Scientific, Waltham, MA, USA);
- Rabbit polyclonal anti-SOD3 antibody (1:1000; PA5-93329; Thermo Fisher Scientific, Waltham, MA, USA);
- Rabbit polyclonal anti-GPx4 antibody (1:1000; ab41787; Abcam, Cambridge, UK);
- Rabbit polyclonal anti-GPx5 antibody (1:500; PA5-102342; Thermo Fisher Scientific, Waltham, MA, USA);
- Rabbit polyclonal anti-CAT antibody (1:15,000; 200-401-051; Thermo Fisher Scientific, Waltham, MA, USA);
- Mouse monoclonal anti-beta actin (C4) antibody (1:200; sc-47778; Santa Cruz Biotechnology, Dallas, TX, USA).
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Saez, F.; Drevet, J.R. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J. Anim. Sci. 2010, 88, 1321–1331. [Google Scholar] [CrossRef]
- Łuszczewski, A.; Matyska-Piekarska, E.; Trefler, J.; Wawer, I.; Łącki, J.; Śliwińska-Stańczyk, P. Reaktywne formy tlenu—Znaczenie w fizjologii i stanach patologii organizmu. Reumatologia 2007, 45, 284–289. [Google Scholar]
- Frączek, M.; Kurpisz, M. The redox system in human semen and peroxidative damage of spermatozoa. Postępy Hig. Med. Dośw. 2005, 59, 523–534. [Google Scholar]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Gilun, P.; Fraser, L.; Koziorowski, M.; Kordan, W.; Stefanczyk-Krzymowska, S. Antioxidant enzyme activity and mRNA expression in reproductive tract of adult male European bison (Bison bonasus, Linnaeus 1758). Reprod. Domest. Anim. 2013, 48, 7–14. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A.; Coy, P.; Matás, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef]
- Perumal, P. Effect of Superoxide Dismutase on Semen Parameters and Antioxidant Enzyme Activities of Liquid Stored (5 °C) Mithun (Bos frontalis) Semen. J. Anim. 2014, 2014, 821954. [Google Scholar] [CrossRef]
- Peeker, R.; Abramsson, L.; Marklund, S.L. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol. Hum. Reprod. 1997, 3, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Silvestini, B.; Mo, M.Y.; Cheng, C.Y. Antioxidant superoxide dismutase—A review: Its function, regulation in the testis, and role in male fertility. Contraception 2002, 65, 305–311. [Google Scholar] [CrossRef]
- Castellini, C.; D’Andrea, S.; Cordeschi, G.; Totaro, M.; Parisi, A.; Di Emidio, G.; Tatone, C.; Francavilla, S.; Barbonetti, A. Pathophysiology of Mitochondrial Dysfunction in Human Spermatozoa: Focus on Energetic Metabolism, Oxidative Stress and Apoptosis. Antioxidants 2021, 10, 695. [Google Scholar] [CrossRef]
- Gotowiecka, M.; Niżański, W.; Partyka, A.; Strzeżek, R.; Koziorowska-Gilun, M. Ocena wpływu stresu oksydacyjnego na nasienie zwierząt. Med. Weter. 2015, 71, 743–747. [Google Scholar]
- Ścibor, D.; Czeczot, H. Katalaza-budowa, właściwości, funkcje. Postępy Hig. Med. Dośw. 2006, 60, 170–180. [Google Scholar]
- Kankofer, M.; Kolm, G.; Aurich, J.; Aurich, C. Activity of glutathione peroxidase, superoxide dismutase and catalase and lipid peroxidation intensity in stallion semen during storage at 5 degrees C. Theriogenology 2005, 63, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Koziorowska-Gilun, M.; Fraser, L.; Gilun, P.; Koziorowski, M.; Kordan, W. Activity of antioxidant enzymes and their mRNA expression in different reproductive tract tissues of the male roe deer (Capreolus capreolus) during the pre-rut and rut season. Small Rumin. Res. 2015, 129, 97–103. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Gilun, P.; Mietelska, K.; Kordan, W. Determination of the activity and relative abundance of mRNA for antioxidant enzymes in stallion testicular and epididymal tissues: A comparison between two breeding seasons. Anim. Reprod. Sci. 2018, 196, 230–238. [Google Scholar] [CrossRef]
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef]
- Drevet, J.R. The antioxidant glutathione peroxidase family and spermatozoa: A complex story. Mol. Cell. Endocrinol. 2006, 250, 70–79. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta (BBA)-Gen. Subjects 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Suzuki, K.; Ishizaka, K.; Ichinose, S.; Oshima, H.; Okayasu, I.; Emoto, K.; Umera, M.; Nakagawa, Y. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol. Reprod. 2001, 64, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Grignard, E.; Morin, J.; Vernet, P.; Drevet, J.R. GPx5 orthologs of the mouse epididymis-restricted and sperm-bound selenium-independent glutathione peroxidase are not expressed with the same quantitative and spatial characteristics in large domestic animals. Theriogenology 2005, 64, 1016–1033. [Google Scholar] [CrossRef]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Vicente-Carrillo, A.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione Peroxidase 5 Is Expressed by the Entire Pig Male Genital Tract and Once in the Seminal Plasma Contributes to Sperm Survival and In Vivo Fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef] [PubMed]
- Kuba, J.; Landete-Castillejos, T.; Udala, J. Red deer farming: Breeding practice, trends and potential in Poland—A review. Ann. Anim. Sci. 2015, 15, 591–599. [Google Scholar] [CrossRef]
- Garcia, F.; Silva, A.A.; Freitas, H.; Sousa, J.P.; Alves, J. The essential, but complex, role of red deer as an ecosystem service provider: A comprehensive review across Europe. Eur. J. Wildl. Res. 2025, 71, 46. [Google Scholar] [CrossRef]
- Millán, M.; Carranza, J.; Seoane, J.M.; Pérez-González, J. Forage quality of consecutive years interact to affect body condition, reproductive rate and rut phenology in Iberian red deer. PLoS ONE 2022, 17, e0278367. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Hameed, J.; Kumar, V.; Segu, H.; Narayan, S.; John, M.; Vasudevan, K.; Umapathy, G. Establishing reproductive seasons for the conservation of the critically endangered Kashmir red deer Cervus hanglu. Sci. Rep. 2025, 15, 4955. [Google Scholar] [CrossRef]
- Giżejewski, Z.; Snochowski, M.; Mayntz, M. Fractions of the semen of red deer (Cervus elaphus)—Their occurrence and characteristics in different periods of season. Pol. J. Vet. Sci. 2003, 6, 219–223. [Google Scholar]
- Marti, E.; Mara, L.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Seasonal variations in antioxidant enzyme activity in ram seminal plasma. Theriogenology 2007, 67, 1446–1454. [Google Scholar] [CrossRef]
- Zini, A.; Schlegel, P.N. Catalase mRNA expression in the male rat reproductive tract. J. Androl. 1996, 17, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Dziekońska, A.; Fraser, L.; Koziorowska-Gilun, M.; Strzezek, J.; Koziorowski, M.; Kordan, W. Seasonal-dependent variations in metabolic status of spermatozoa and antioxidant enzyme activity in the reproductive tract fluids of wild boar/domestic pig hybrids. Pol. J. Vet. Sci. 2014, 17, 307–313. [Google Scholar] [CrossRef]
- Marchlewicz, M.; Szypulska-Koziarska, D.; Grzegrzółka, A.; Kruk, J.; Duchnik, E.; Wiszniewska, B. Ochrona przed stresem oksydacyjnym w męskim układzie płciowym. Pomeranian J. Life Sci. 2016, 62, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fan, X.; Zhang, T.; Song, H.; Bian, X.; Nai, R.; Li, J.; Zhang, J. Expression of selenium-independent glutathione peroxidase 5 (GPx5) in the epididymis of Small Tail Han sheep. Asian-Australas. J. Anim. Sci. 2018, 31, 1591–1597. [Google Scholar] [CrossRef]
- Lecewicz, M.; Majewska, A.; Kordan, W.; Wysocki, P.; Koziorowska-Gilun, M. Changes in the expression of selected antioxidative proteins in roe deer (Capreolus capreolus) epididymis in different periods of the rutting season. Ann. Anim. Sci. 2017, 17, 423–431. [Google Scholar] [CrossRef]
- Nonogaki, T.; Noda, Y.; Narimoto, K.; Shiotani, M.; Mori, T.; Matsuda, T.; Yoshida, O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum. Reprod. 1992, 7, 81–85. [Google Scholar] [CrossRef]
- Williams, K.; Frayne, J.; McLaughlin, E.; Hall, L. Expression of extracellular superoxide dismutase in the human reproductive tract, detected using antisera against a recombinant protein. Mol. Hum. Reprod. 1998, 4, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T. Antioxidants and reactive oxygen species in human fertility. Env. Toxic. Pharmac. 2001, 10, 189–198. [Google Scholar] [CrossRef]
- Aitken, R.J.; Vernet, P. Maturation of redox regulatory mechanisms in the epididymis. J. Reprod. Fertil. Suppl. 1998, 53, 109–118. [Google Scholar] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous Lipid Peroxidation and Production of Hydrogen Peroxide and Superoxide in Human Spermatozoa Superoxide Dismutase as Major Enzyme Protectant against Oxygen Toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Kucera, G.; Gordon, H.; Boss, J.M. Cloning and characterization of the murine manganous superoxide dismutase-encoding gene. Gene 1995, 153, 155–161. [Google Scholar] [CrossRef]
- Partyka, A.; Łukaszewicz, E.; Niżański, W. Effect of cryopreservation on sperm parameters, lipid peroxidation and antioxidant enzymes activity in fowl semen. Theriogenology 2012, 77, 1497–1504. [Google Scholar] [CrossRef]
- Reglero, M.M.; Taggart, M.A.; Castellanos, P.; Mateo, R. Reduced sperm quality in relation to oxidative stress in red deer from a lead mining area. Environ. Pollut. 2009, 157, 2209–2215. [Google Scholar] [CrossRef]
- Peltola, V.; Huhtaniemi, I.; Metsa-Ketela, T.; Ahotupa, M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrin. 1996, 137, 105–112. [Google Scholar] [CrossRef][Green Version]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing. Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Lim, S.-O.; Jung, G. Reactive oxygen species downregulate catalase expression via methylation of a CpG Island in the Oct-1 promoter. FEBS Lett. 2011, 585, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Noblanc, A.; Peltier, M.; Damon-Soubeyrand, C.H.; Kerchkove, N.; Chabory, E.; Vernet, P.; Saez, F.; Cadet, R.; Janny, L.; Pons-Rejraji, H.; et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS ONE 2012, 7, 38565. [Google Scholar] [CrossRef]
- Foresta, C.; Flohe, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Iwaki, Y.; Hiramoto, S.; Tamba, M.; Bannai, S.; Sugita, Y.; Syntin, P.; Dacheux, F.; Dacheux, J.L. Molecular cloning and characterization of the epididymis-specific glutathione peroxidase-like protein secreted in the porcine epididymal fluid. Biochim. Biophys. Acta 1997, 1336, 99–109. [Google Scholar] [CrossRef]
- Williams, K.; Frayne, J.; Hall, L. Expression of extracellular glutathione peroxidase type 5 (GPX5) in the rat male reproductive tract. Mol. Hum. Reprod. 1998, 4, 841–848. [Google Scholar] [CrossRef]
- Belleannée, C.; Labas, V.; Teixeira-Gomes, A.P.; Gatti, J.L.; Dacheux, J.L.; Dacheux, F. Identification of luminal and secreted proteins in bull epididymis. J. Proteom. 2011, 74, 59–78. [Google Scholar] [CrossRef]
- Gerlach, T.; Aurich, J. Regulation of seasonal reproductive activity in the stallion, ram and hamster. Anim. Reprod. Sci. 2000, 58, 197–213. [Google Scholar] [CrossRef]
- Martinez-Pastor, F.; Guerra, C.; Kaabi, M.; Garcia-Macias, V.; de Paz, P.; Alvarez, M.; Herraez, P.; Anel, L. Season effect on genitalia and epididymal sperm from Iberian red deer, roe deer and Cantabrian chamois. Theriogenology 2005, 63, 1857–1875. [Google Scholar] [CrossRef]
- Lincoln, G. The seasonal reproductive changes in the red deer stag (Cervus elaphus). J. Zool. 2010, 163, 105–123. [Google Scholar] [CrossRef]
- Barrell, G.K.; Muir, P.D.; Sykes, A.R. Seasonal Profiles of Plasma Testosterone, Prolactin and Growth Hormone in Red Deer Stags. In Biology of Deer Production; Bulletin 22; The Royal Society of New Zealand: Wellington, New Zealand, 1985; pp. 135–190. [Google Scholar]
- Roelants, H.; Schneider, F.; Göritz, F.; Streich, J.; Blottner, S. Seasonal changes of spermatogonial proliferation in roe deer, demonstrated by flow cytometric analysis of c-kit receptor, in relation to follicle-stimulating hormone, luteinizing hormone, and testosterone. Biol. Reprod. 2002, 67, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Goeritz, F.; Quest, M.; Wagener, A.; Fassbender, M.; Broich, A.; Hildebrandt, T.B.; Hofmann, R.R.; Blottner, S. Seasonal timing of sperm production in roe deer: Interrelationship between changes in ejaculate parameters, morphology and function of testis and accessory glands. Theriogenology 2003, 59, 1487–1502. [Google Scholar] [CrossRef]
- Kozioł, K.; Koziorowski, M. Steroid hormones in peripheral blood plasma and androgen receptors in testis and epididymis of roe deer male (Capreolus capreolus) during the reproduction season. Small Rumin. Res. 2013, 115, 86–93. [Google Scholar] [CrossRef]
- Belleannèe, C.; Thimon, V.; Sullivan, R. Region-specific gene expression in the epididymis. Cell. Tissue Res. 2012, 349, 717–731. [Google Scholar] [CrossRef]
- Luan, Z.; Fan, X.; Song, H.; Li, R.; Zhang, W.; Zhang, J. Testosterone promotes GPX5 expression of goat epididymal epithelial cells cultured in vitro. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1045–1062. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sip Lex. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/ochrona-zwierzat-wykorzystywanych-do-celow-naukowych-lub-edukacyjnych-18169618 (accessed on 9 July 2025).


| Enzyme Activity | Season | Tissue | Season × Tissue | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| SOD activity | 19.968 | <0.001 | 10.323 | <0.001 | 7.780 | <0.001 |
| GPx activity | 7.437 | 0.007 | 7.868 | <0.001 | 4.285 | 0.006 |
| CAT activity | 7.803 | 0.006 | 5.940 | <0.001 | 3.160 | 0.026 |
| Gene Expression | Season | Tissue | Season × Tissue | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| SOD1 expression | 0.610 | n.s. | 11.576 | <0.001 | 3.330 | 0.020 |
| SOD2 expression | 1.280 | n.s. | 137.558 | <0.001 | 4.700 | 0.003 |
| SOD3 expression | 16.495 | <0.001 | 12.885 | <0.001 | 2.455 | n.s. |
| GPx4 expression | 6.442 | 0.012 | 254.887 | <0.001 | 2.712 | 0.045 |
| GPx5 expression | 34.054 | <0.001 | 100.495 | <0.001 | 6.300 | <0.001 |
| CAT expression | 5.639 | 0.018 | 53.686 | <0.001 | 12.883 | <0.001 |
| Gene | Forward Primer 5′–3′ | Reverse Primer 5′–3′ | Accession Number |
|---|---|---|---|
| SDHA | GACAGGAGCCCGCAGTTTT | CACGGCATCAAACTCATGGT | XM_043887904.1 |
| SOD1 | CTCCTTTTCCCCGAGTCATG | CGACGACTGTATTTCCCTTTGC | XM_043892610.1 |
| SOD2 | TCTGCAGCCTGCGTTAAAGTT | CTTCCAGCAATTCCCCTTTG | XM_043888243.1 |
| SOD3 | CGCTGCTCTGTGCCTATCTG | TGTGCATGTCGCGGATCT | XM_043870913.1 |
| GPx4 | TACGCCGAGTGTGGTTTACG | GCGGCGAACTCTTTGATCTC | XM_043912658.1 |
| GPx5 | TGACATCCGCTGGAATTTTG | ACTGACGGGAGTCCGATGAA | XM_043908264.1 |
| CAT | TGCCCTATTGTTTCCGTCCTT | ACCATGTCCGGATCCTTCAG | XM_043909065.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuman, N.M.; Gilun, P.; Koziorowska-Gilun, M.; Janiszewski, P.; Dziekońska, A. Antioxidant Enzyme Activity and mRNA Expression in the Reproductive Tissues of Male European Red Deer (Cervus elaphus elaphus). Int. J. Mol. Sci. 2025, 26, 7221. https://doi.org/10.3390/ijms26157221
Neuman NM, Gilun P, Koziorowska-Gilun M, Janiszewski P, Dziekońska A. Antioxidant Enzyme Activity and mRNA Expression in the Reproductive Tissues of Male European Red Deer (Cervus elaphus elaphus). International Journal of Molecular Sciences. 2025; 26(15):7221. https://doi.org/10.3390/ijms26157221
Chicago/Turabian StyleNeuman, Nicoletta M., Przemysław Gilun, Magdalena Koziorowska-Gilun, Paweł Janiszewski, and Anna Dziekońska. 2025. "Antioxidant Enzyme Activity and mRNA Expression in the Reproductive Tissues of Male European Red Deer (Cervus elaphus elaphus)" International Journal of Molecular Sciences 26, no. 15: 7221. https://doi.org/10.3390/ijms26157221
APA StyleNeuman, N. M., Gilun, P., Koziorowska-Gilun, M., Janiszewski, P., & Dziekońska, A. (2025). Antioxidant Enzyme Activity and mRNA Expression in the Reproductive Tissues of Male European Red Deer (Cervus elaphus elaphus). International Journal of Molecular Sciences, 26(15), 7221. https://doi.org/10.3390/ijms26157221







