Respiratory and Cardiovascular Activity of LENART01, an Analgesic Dermorphin–Ranatensin Hybrid Peptide, in Anesthetized Rats
Abstract
1. Introduction
2. Results
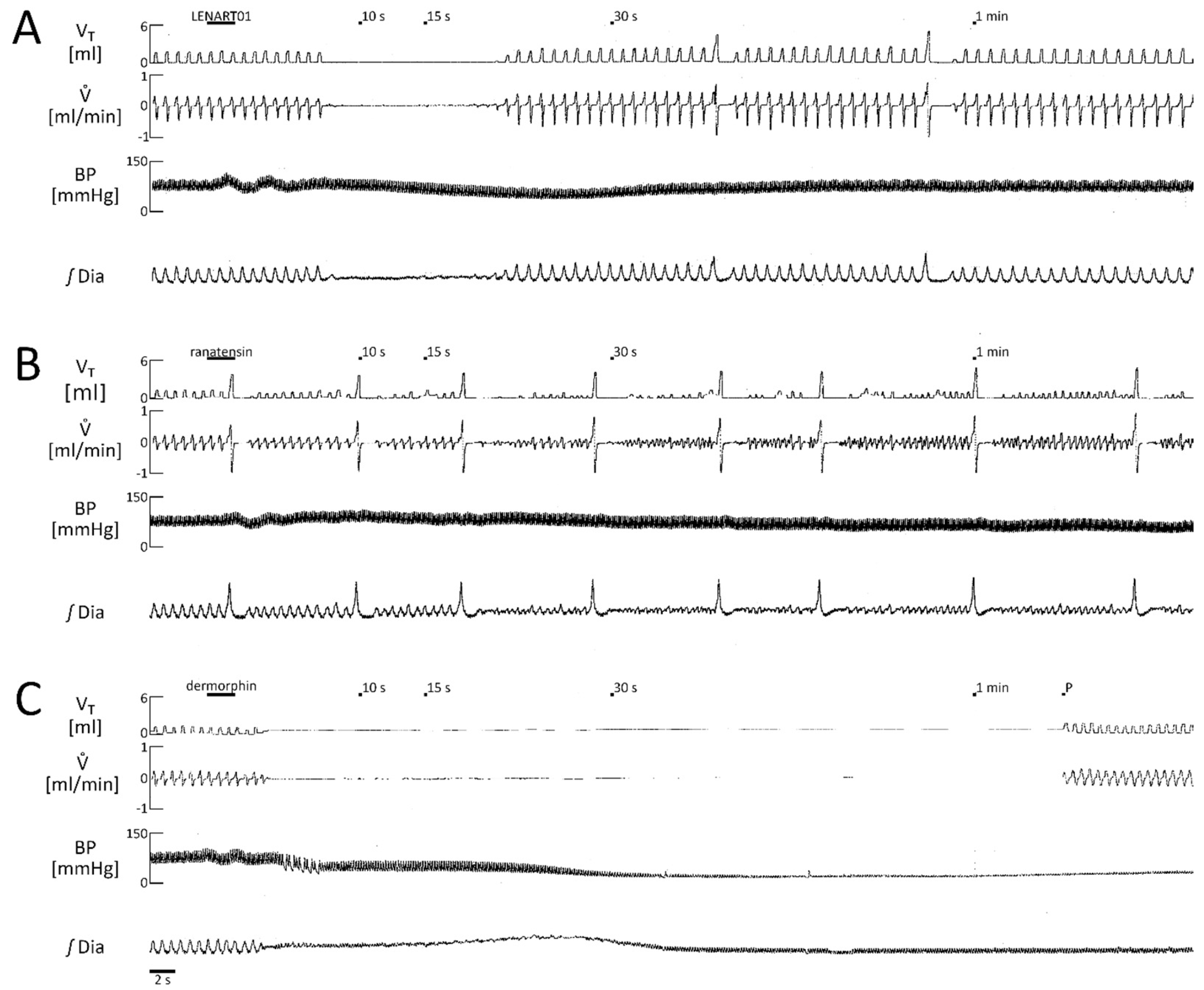
2.1. LENART01 Dose Response
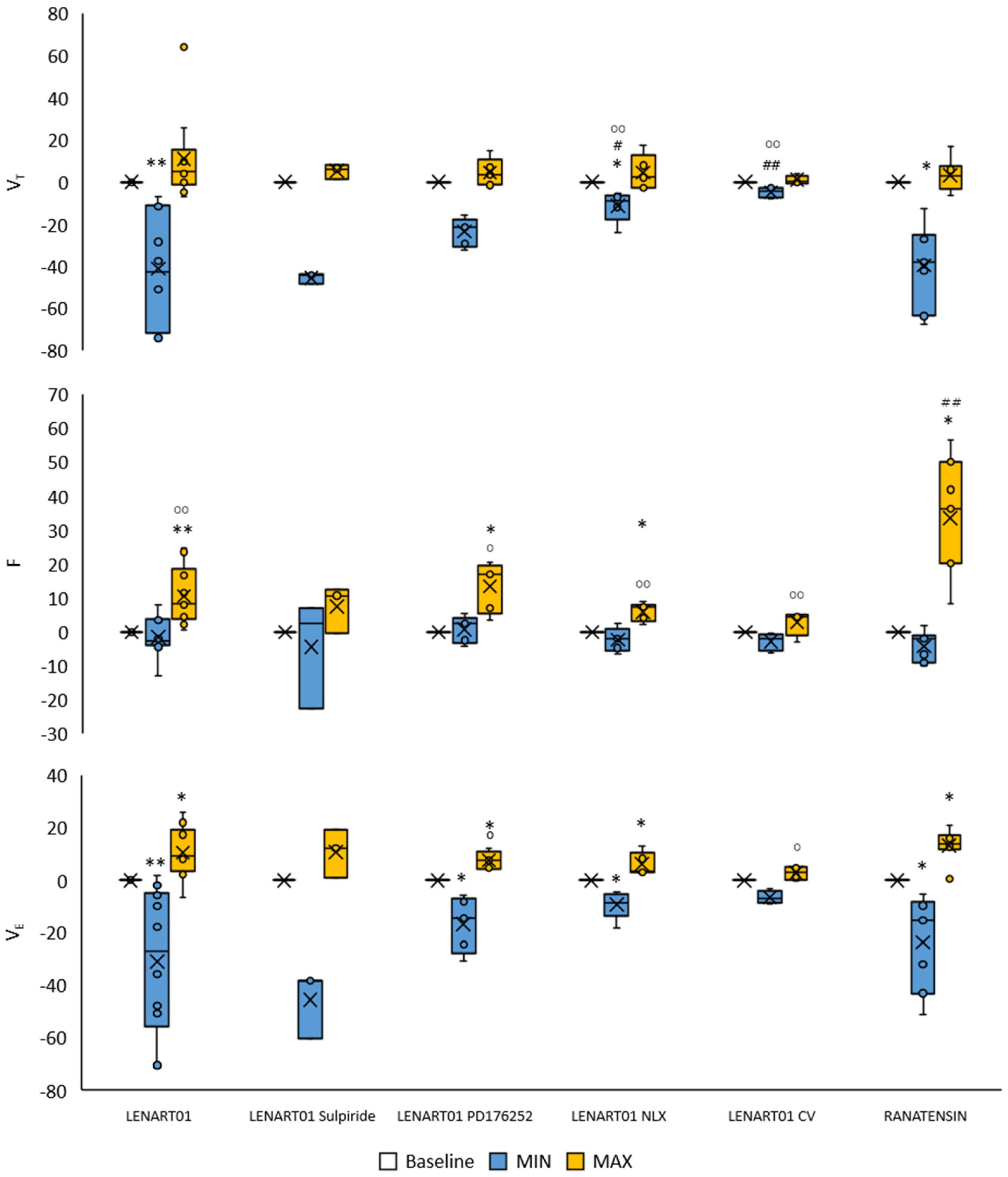
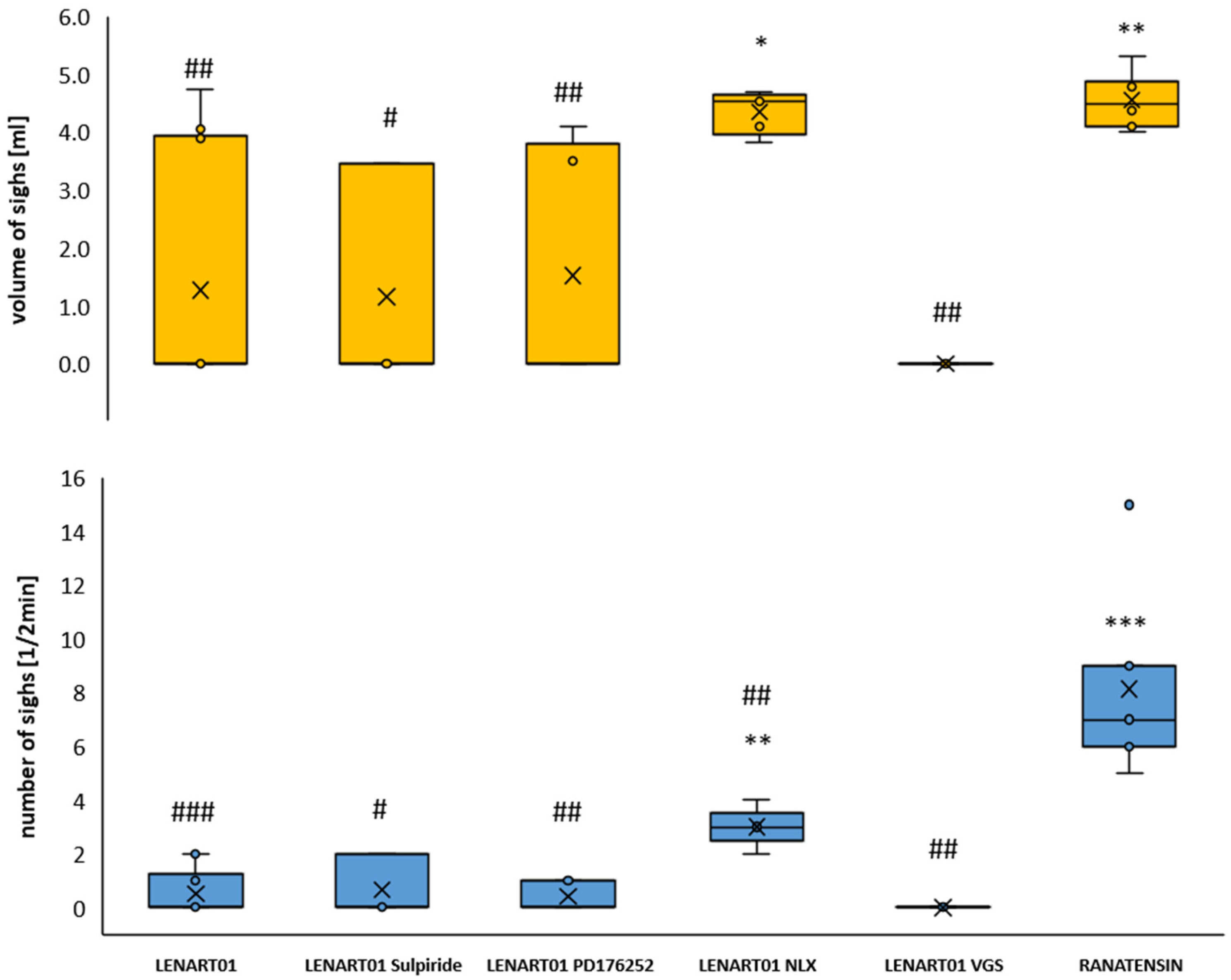
2.2. Respiratory Effects of LENART01: The Effects After Blockade of MOR, BB1/BB2, D2R, and After Cervical Vagotomy
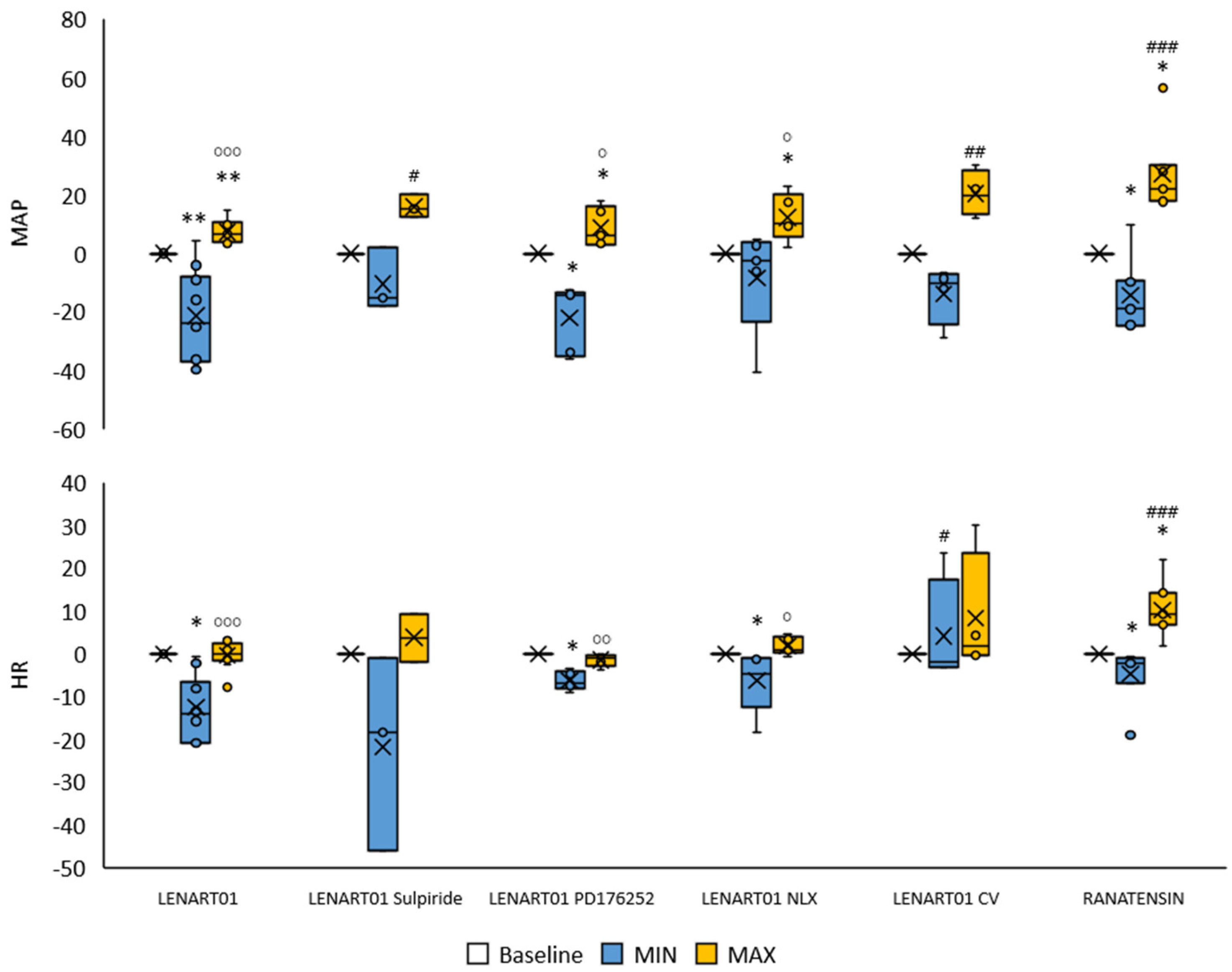
2.3. Cardiovascular Effects of LENART01: The Effects After Blockade of MOR, BB1/BB2, and D2R and After Cervical Vagotomy
2.4. Comparison of the Respiratory Response Mediated by LENART01 with Its Pharmacophores Ranatensin and Dermorphin
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Parameters’ Registration and Counting
4.3. Experimental Groups and Drugs
- LENART01 dose response (10 rats);
- LENART01—naloxone, LENART01 (5 rats);
- LENART01—sulpiride i.v., LENART01 (4 rats);
- LENART01—cervical vagotomy, LENART01 (4 rats);
- LENART01—PD176252 i.p., LENART01 (5 rats);
- Ranatensin (5 rats);
- Dermorphin (5 rats).
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.; Benveniste, H.; McLellan, A.T. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 2018, 69, 451–465. [Google Scholar] [CrossRef]
- Hochrainer, N.; Serafin, P.; D’Ingiullo, S.; Mollica, A.; Granica, S.; Brytan, M.; Kleczkowska, P.; Spetea, M. In Vitro and In Vivo Pharmacological Profiles of LENART01, a Dermorphin–Ranatensin Hybrid Peptide. Int. J. Mol. Sci. 2024, 25, 4007. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Lipkowski, A.W.; Tourwé, D.; Ballet, S. Hybrid Opioid/Non-Opioid Ligands in Pain Research. Curr. Pharm. Des. 2013, 19, 7435–7450. [Google Scholar] [CrossRef]
- Starnowska-Sokół, J.; Przewłocka, B. Multifunctional Opioid-Derived Hybrids in Neuropathic Pain: Preclinical Evidence, Ideas and Challenges. Molecules 2020, 25, 5520. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Chen, Y.-C.; Ma, L.; Huang, K. Rational Design of Hybrid Peptides: A Novel Drug Design Approach. Curr. Med. Sci. 2019, 39, 349–355. [Google Scholar] [CrossRef]
- Broccardo, M.; Erspamer, V.; Falconieri, G.; Improta, G.; Linari, G.; Melchiorri, P.; Montecucchi, P. Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin. Br. J. Pharmacol. 1981, 73, 625–631. [Google Scholar] [CrossRef]
- Montecucchi, P.C.; de Castiglione, R.; Piani, S.; Gozzini, L.; Erspamer, V. Amino Acid Composition and Sequence of Dermorphin, a Novel Opiate-like Peptide from the Skin of Phyllomedusa Sauvagei. Int. J. Pept. Protein Res. 1981, 17, 275–283. [Google Scholar] [CrossRef]
- Geller, R.G.; Govier, W.C.; Pisano, J.J.; Tanimura, T.; Van Clineschmidt, B. The Action of Ranatensin, a New Polypeptide from Amphibian Skin, on the Blood Pressure of Experimental Animals. Br. J. Pharmacol. 1970, 40, 605–616. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Ji, X.Q.; Wu, S.X.; Zou, G. Sulpiride Attenuates Ranatensin-M-Induced Antinociception. Zhongguo Yao Li Xue Bao 1991, 12, 291–293. [Google Scholar] [PubMed]
- Serafin, P.; Szeleszczuk, Ł.; Zhukov, I.; Szűcs, E.; Gombos, D.; Stefanucci, A.; Mollica, A.; Pisklak, D.M.; Kleczkowska, P. Opioid/Dopamine Receptor Binding Studies, NMR and Molecular Dynamics Simulation of LENART01 Chimera, an Opioid-Bombesin-like Peptide. Molecules 2024, 29, 272. [Google Scholar] [CrossRef]
- Serafin, P.; Kowalczyk, P.; Mollica, A.; Stefanucci, A.; Laskowska, A.K.; Zawadzka, M.; Kramkowski, K.; Kleczkowska, P. Evaluation of Antimicrobial Activities against Various E. Coli Strains of a Novel Hybrid Peptide—LENART01. Molecules 2023, 28, 4955. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H.M.; Lim, S.-A.; Yow, J.; Dinkins, M.-L.; Patton, R.; Clemens, S.; Brewer, K.L. Dopamine D1 or D3 Receptor Modulators Prevent Morphine Tolerance and Reduce Opioid Withdrawal Symptoms. Pharmacol. Biochem. Behav. 2020, 194, 172935. [Google Scholar] [CrossRef] [PubMed]
- Portolano, F.; Filippelli, A.; Marrazzo, R.; Susanna, V.; Russo, S.; Stella, L.; Losasso, C.; Budetta, S.N.; Molinario, L.; Angrisani, M. Cardiovascular and Respiratory Effects of Dermorphin in Rats. Res. Commun. Chem. Pathol. Pharmacol. 1991, 71, 131–152. [Google Scholar]
- Wojciechowski, P.; Szereda-Przestaszewska, M.; Lipkowski, A.W. Supranodose Vagotomy Eliminates Respiratory Depression Evoked by Dermorphin in Anaesthetized Rats. Eur. J. Pharmacol. 2007, 563, 209–212. [Google Scholar] [CrossRef]
- Abou Farha, K.; Baljé-Volkers, C.; Tamminga, W.; den Daas, I.; van Os, S. Dopamine D2R Agonist-Induced Cardiovascular Effects in Healthy Male Subjects: Potential Implications in Clinical Settings. ISRN Neurol. 2014, 2014, 956353. [Google Scholar] [CrossRef]
- Kujawa, K.; Leurgans, S.; Raman, R.; Blasucci, L.; Goetz, C.G. Acute Orthostatic Hypotension When Starting Dopamine Agonists in Parkinson’s Disease. Arch. Neurol. 2000, 57, 1461–1463. [Google Scholar] [CrossRef]
- Serafin, P.; Kleczkowska, P. Bombesins: A New Frontier in Hybrid Compound Development. Pharmaceutics 2023, 15, 2597. [Google Scholar] [CrossRef]
- Spindel, E.R. Bombesin Peptides. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2013; pp. 326–330. ISBN 978-0-12-385095-9. [Google Scholar]
- Niewoehner, D.E.; Levine, A.S.; Morley, J.E. Central Effects of Neuropeptides on Ventilation in the Rat. Peptides 1983, 4, 277–281. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Szereda-Przestaszewska, M. Peripheral Cardiorespiratory Effects of Bombesin in Anaesthetized Rats. Eur. J. Pharmacol. 2009, 602, 157–162. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Szereda-Przestaszewska, M. Vasopressor and Heart Rate Responses to Systemic Administration of Bombesin in Anesthetized Rats. Pharmacol. Rep. 2011, 63, 448–454. [Google Scholar] [CrossRef]
- Colman, A.S.; Miller, J.H. Modulation of Breathing by Mu1 and Mu2 Opioid Receptor Stimulation in Neonatal and Adult Rats. Respir. Physiol. 2001, 127, 157–172. [Google Scholar] [CrossRef]
- Paakkari, P.; Paakkari, I.; Sirén, A.L.; Feuerstein, G. Respiratory and Locomotor Stimulation by Low Doses of Dermorphin, a Mu1 Receptor-Mediated Effect. J. Pharmacol. Exp. Ther. 1990, 252, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Eager, K.R.; Robinson, B.J.; Galletly, D.C.; Miller, J.H. Endogenous Opioid Modulation of Hypercapnic-Stimulated Respiration in the Rat. Respir. Physiol. 1994, 96, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Van Dorpe, S.; Adriaens, A.; Polis, I.; Peremans, K.; Van Bocxlaer, J.; De Spiegeleer, B. Analytical Characterization and Comparison of the Blood–Brain Barrier Permeability of Eight Opioid Peptides. Peptides 2010, 31, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Henderson, F.; May, W.J.; Gruber, R.B.; Discala, J.F.; Puscovic, V.; Young, A.P.; Baby, S.M.; Lewis, S.J. Role of Central and Peripheral Opiate Receptors in the Effects of Fentanyl on Analgesia, Ventilation and Arterial Blood-Gas Chemistry in Conscious Rats. Respir. Physiol. Neurobiol. 2014, 191, 95–105. [Google Scholar] [CrossRef]
- Pattinson, K.T.S. Opioids and the Control of Respiration. Br. J. Anaesth. 2008, 100, 747–758. [Google Scholar] [CrossRef]
- Laskowska, A.K.; Szudzik, M.; Ścieżyńska, A.; Komorowski, M.; Szűcs, E.; Gombos, D.; Bączek, B.; Lipka-Miciuk, J.; Benyhe, S.; Kleczkowska, P. The Role of a Natural Amphibian Skin-Based Peptide, Ranatensin, in Pancreatic Cancers Expressing Dopamine D2 Receptors. Cancers 2022, 14, 5535. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Huang, R.; Ning, J.; Bao, X.; Yan, Z.; Chen, H.; Ding, L.; Shu, C. Conjugation of Sulpiride with a Cell Penetrating Peptide to Augment the Antidepressant Efficacy and Reduce Serum Prolactin Levels. Biomed. Pharmacother. 2024, 174, 116610. [Google Scholar] [CrossRef]
- Merali, Z.; Bédard, T.; Andrews, N.; Davis, B.; McKnight, A.T.; Gonzalez, M.I.; Pritchard, M.; Kent, P.; Anisman, H. Bombesin Receptors as a Novel Anti-Anxiety Therapeutic Target: BB 1 Receptor Actions on Anxiety through Alterations of Serotonin Activity. J. Neurosci. 2006, 26, 10387–10396. [Google Scholar] [CrossRef] [PubMed]
- Krumins, S.A. Characterization of Dermorphin Binding to Membranes of Rat Brain and Heart. Neuropeptides 1987, 9, 93–102. [Google Scholar] [CrossRef]
- Melchiorri, P.; Negri, L. The Dermorphin Peptide Family. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Glogowska, M.; Richardson, P.S.; Widdicombe, J.G.; Winning, A.J. The Role of the Vagus Nerves, Peripheral Chemoreceptors and Other Afferent Pathways in the Genesis of Augmented Breaths in Cats and Rabbits. Respir. Physiol. 1972, 16, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Takeda, M.; Saiki, C.; Takahashi, T.; Ojima, K. Effects of Vagal and Carotid Chemoreceptor Afferents on the Frequency and Pattern of Spontaneous Augmented Breaths in Rabbits. Lung 1997, 175, 175–186. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Szereda-Przestaszewska, M. Involvement of Vagal Opioid Receptors in Respiratory Effects of Morphine in Anaesthetized Rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 56, 195–203. [Google Scholar]
- Murphy, M. Dopamine: A Role in the Pathogenesis and Treatment of Hypertension. J. Hum. Hypertens. 2000, 14, S47–S50. [Google Scholar] [CrossRef]
- Polakowski, J.S.; Segreti, J.A.; Cox, B.F.; Hsieh, G.C.; Kolasa, T.; Moreland, R.B.; Brioni, J.D. Effects of Selective Dopamine Receptor Subtype Agonists on Cardiac Contractility and Regional Haemodynamics in Rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 837–841. [Google Scholar] [CrossRef]
- Saari, T.I.; Strang, J.; Dale, O. Clinical Pharmacokinetics and Pharmacodynamics of Naloxone. Clin. Pharmacokinet. 2024, 63, 397–422. [Google Scholar] [CrossRef]
- Jose, P.A.; Eisner, G.M.; Felder, R.A. Role of Dopamine in the Pathogenesis of Hypertension. Clin. Exp. Pharmacol. Physiol. Suppl. 1999, 26, S10–S13. [Google Scholar]
- Wojciechowski, P.; Szereda-Przestaszewska, M.; Lipkowski, A.W. Cardiorespiratory Action of Opioid/Tachykinin Agonist Peptide Hybrid in Anaesthetized Rats: Transduction Pathways. Eur. J. Pharmacol. 2017, 810, 9–14. [Google Scholar] [CrossRef]
- Bazzani, C.; Nardi, M.G.; Ferrante, F.; Bertolini, A.; Guarini, S. Dopamine D1 Receptors Are Involved in the ACTH-Induced Reversal of Hemorrhagic Shock. Eur. J. Pharmacol. 1994, 253, 303–306. [Google Scholar] [CrossRef]

| LENART01 Dose | n | Number of Apnea Episodes Exceeding Duration of 30 s | Number of Apnea Episodes Shorter than 30 s | Apnea Duration [s] (Median (Q1, Q3) IQR) | TE test/TE baseline (Median (Q1, Q3) IQR |
|---|---|---|---|---|---|
| 5 μg/kg | 5 | 0 | 0 | 0 | 1.0 (0.9, 1.0) 0.1 * |
| 10 μg/kg | 7 | 1 | 2 | 1.1 | 1.0 (0.9, 1.8) 1.0 |
| 15 μg/kg | 7 | 1 | 3 | 6.1 (2.4, 12.2) 9.8 | 3.3 (1.0, 10.2) 9.2 |
| 20 μg/kg | 9 | 6 | 2 | 1.7 | 2.4 (0.8, 3.9) 3.1 |
| Treatment | LENART01 | LENART01 Sulpiride (D2 R Antagonist) | LENART01 PD176252 (BB1/BB2 R Antagonist) | LENART01 Naloxone (MOR Antagonist) | LENART01 Cervical Vagotomy |
|---|---|---|---|---|---|
| N | 14 | 4 | 5 | 5 | 4 |
| Number of apnea episodes exceeding duration of 30 s | 4 | 1 | 0 | 0 | 0 |
| Number of apnea episodes shorter than 30 s | 6 | 1 | 1 | 0 | 0 |
| Apnea duration [s] (median (Q1, Q3) IQR) | 11.2 (2.7, 13.5) 10.8 | 5.7 | 12.8 | 0 | 0 |
| TE test/TE baseline (median(Q1, Q3) IQR | 3.8 (0.9, 20.9) 20.0 | 1.2 (0.9, 9.2) 8.3 | 0.8 (0.7, 16.5) 15.8 | 1.0 (0.9, 1.0) 0.1 | 1.0 (0.9, 1.0) 0.1 |
| Tidal volume (VT) [ml] (median (Q1, Q3) IQR) Baseline | 1.47 (1.15, 1.62) 0.47 | 1.31 | 1.42 (1.41, 1.62) 0.21 | 1.84 (1.56, 2.12) 0.54 | 2.02 (1.99, 2.15) 0.16 |
| Tidal volume at 10 s post injection (VT) [mL] (median (Q1, Q3) IQR) | 1.06 (0.54, 1.29) 0.75 | 1.02 | 1.17 (1.14, 1.23) 0.09 | 1.84 (1.51, 2.03) 0.52 | 2.05 (1.99, 2.12) 0.13 |
| VT % of median change | 28 | 22 | 18 | 0 | −1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowski, P.; Zając, D.; Górski, A.; Kamysz, W.; Kleczkowska, P.; Kaczyńska, K. Respiratory and Cardiovascular Activity of LENART01, an Analgesic Dermorphin–Ranatensin Hybrid Peptide, in Anesthetized Rats. Int. J. Mol. Sci. 2025, 26, 7188. https://doi.org/10.3390/ijms26157188
Wojciechowski P, Zając D, Górski A, Kamysz W, Kleczkowska P, Kaczyńska K. Respiratory and Cardiovascular Activity of LENART01, an Analgesic Dermorphin–Ranatensin Hybrid Peptide, in Anesthetized Rats. International Journal of Molecular Sciences. 2025; 26(15):7188. https://doi.org/10.3390/ijms26157188
Chicago/Turabian StyleWojciechowski, Piotr, Dominika Zając, Adrian Górski, Wojciech Kamysz, Patrycja Kleczkowska, and Katarzyna Kaczyńska. 2025. "Respiratory and Cardiovascular Activity of LENART01, an Analgesic Dermorphin–Ranatensin Hybrid Peptide, in Anesthetized Rats" International Journal of Molecular Sciences 26, no. 15: 7188. https://doi.org/10.3390/ijms26157188
APA StyleWojciechowski, P., Zając, D., Górski, A., Kamysz, W., Kleczkowska, P., & Kaczyńska, K. (2025). Respiratory and Cardiovascular Activity of LENART01, an Analgesic Dermorphin–Ranatensin Hybrid Peptide, in Anesthetized Rats. International Journal of Molecular Sciences, 26(15), 7188. https://doi.org/10.3390/ijms26157188







