Toxic Threats from the Fern Pteridium aquilinum: A Multidisciplinary Case Study in Northern Spain
Abstract
1. Introduction
2. Results
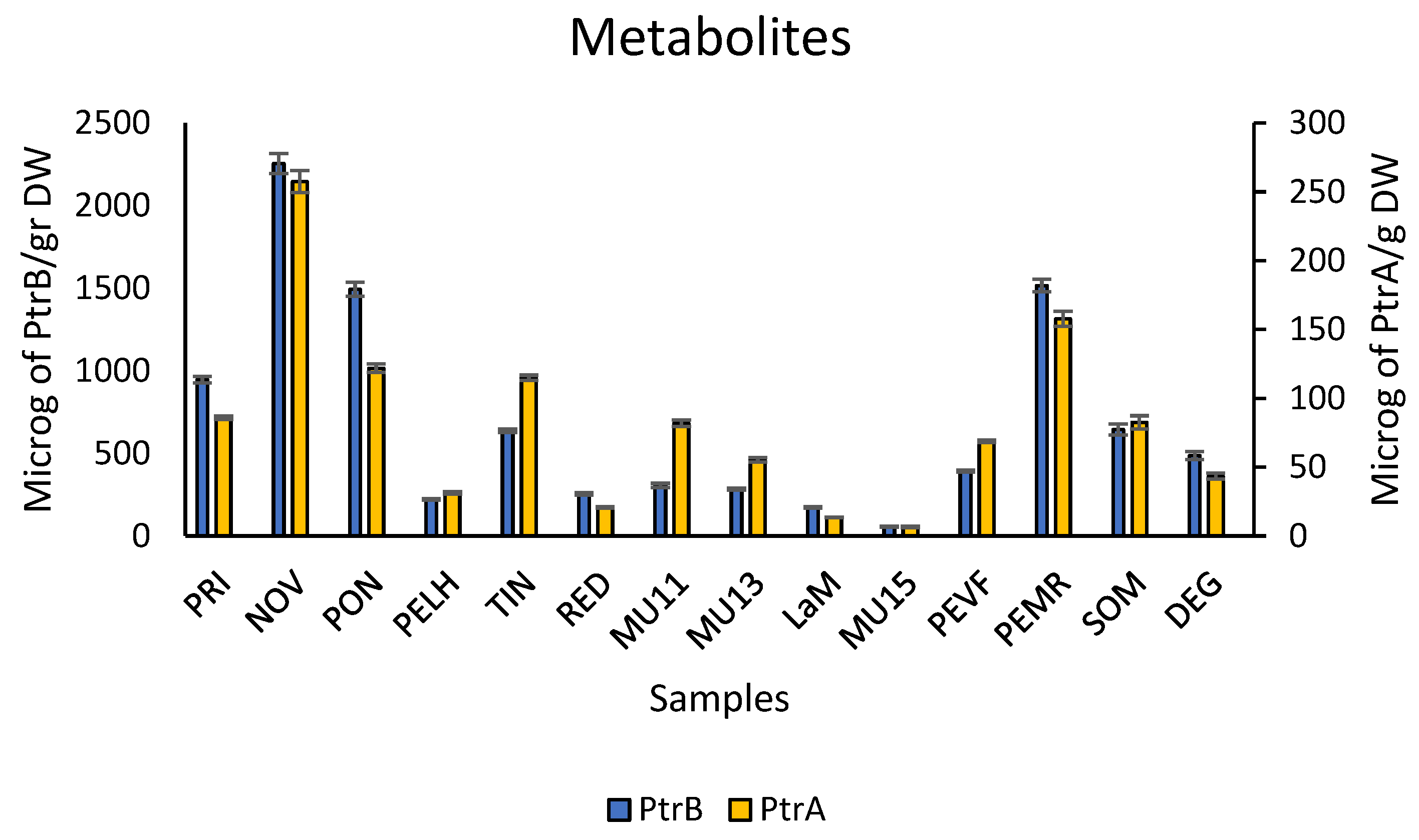
2.1. Quantification of Toxic Metabolites in Croziers Samples
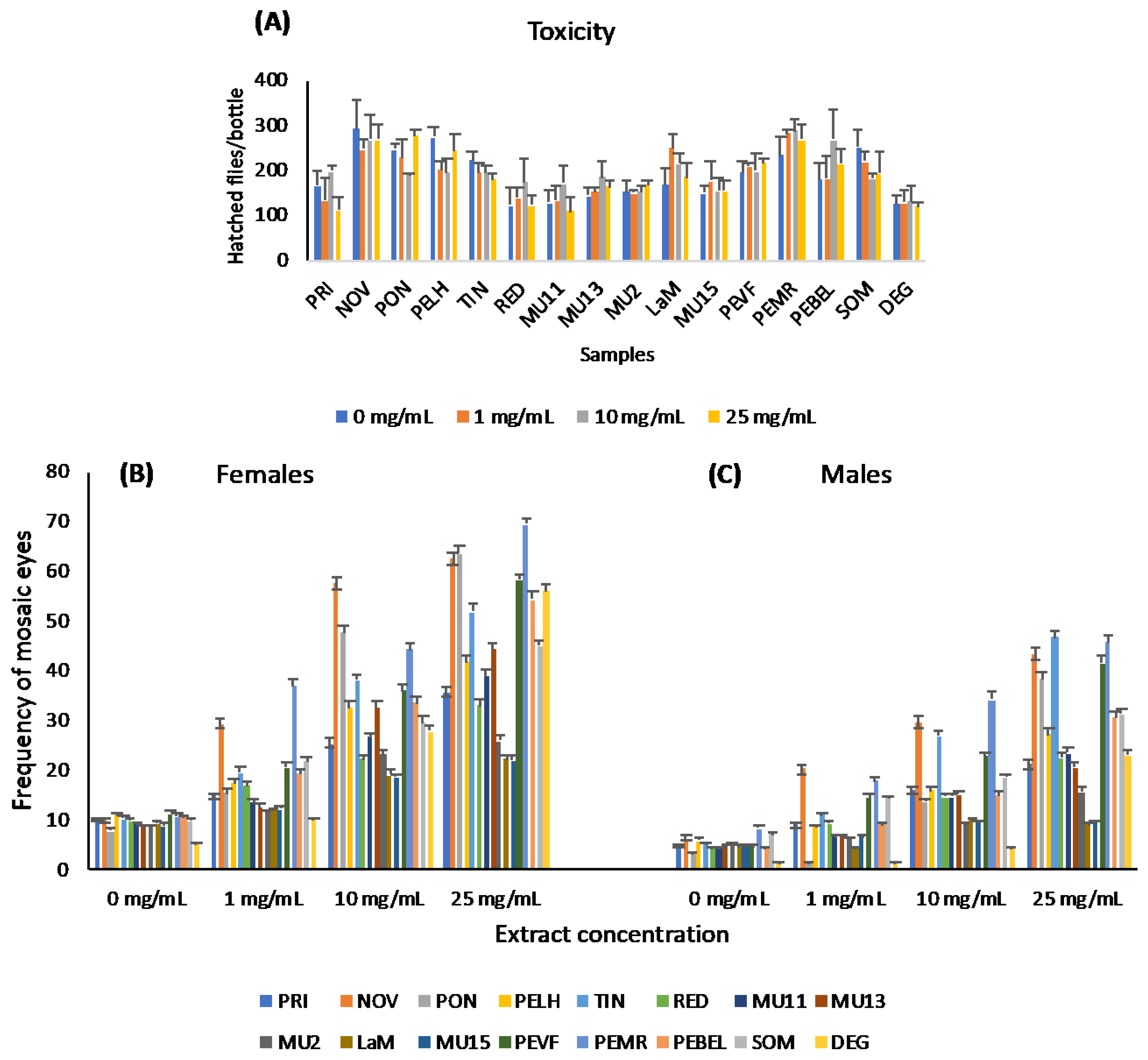
2.2. Genotoxicity Analysis of Aqueous Plant Extracts
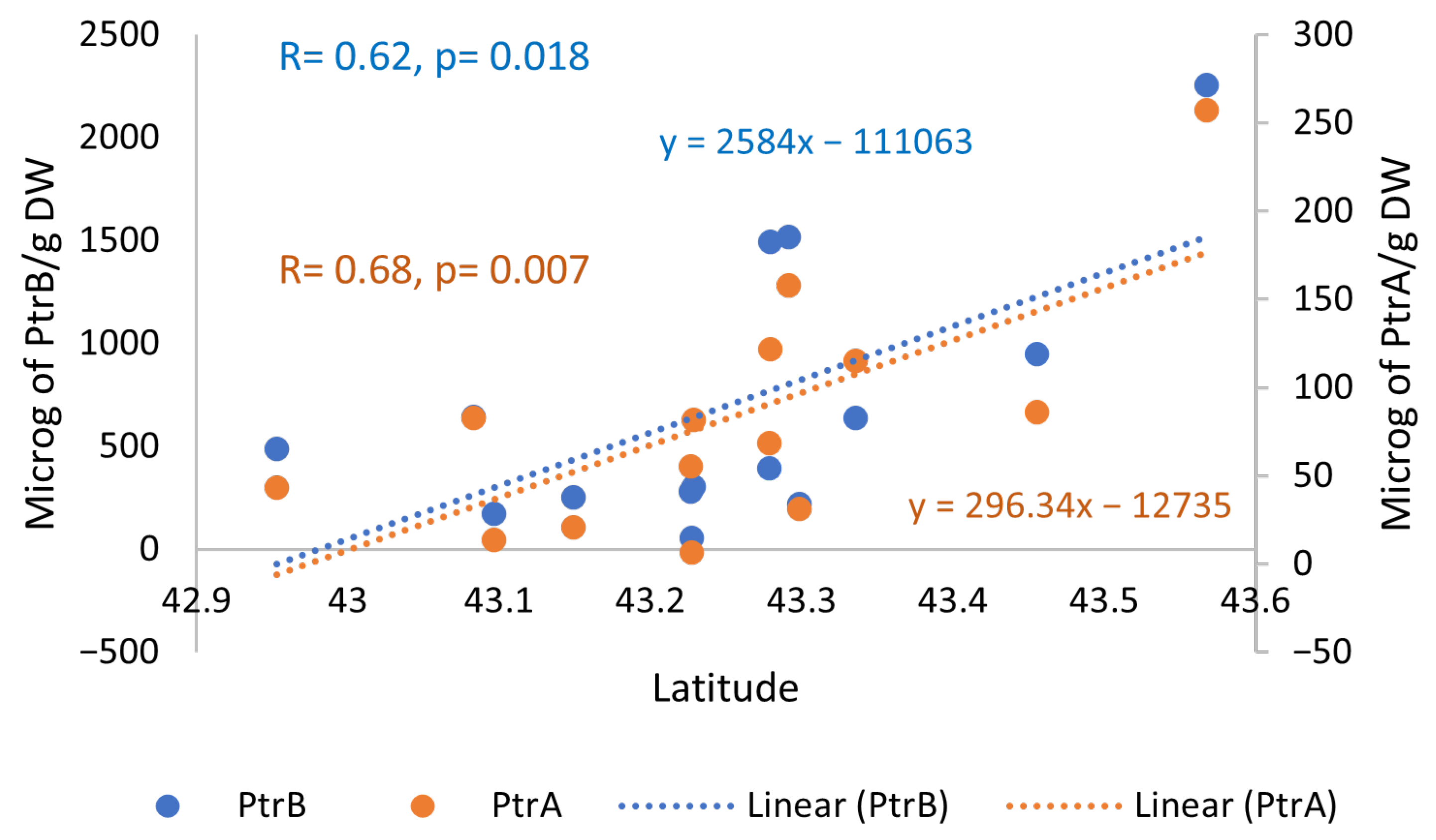
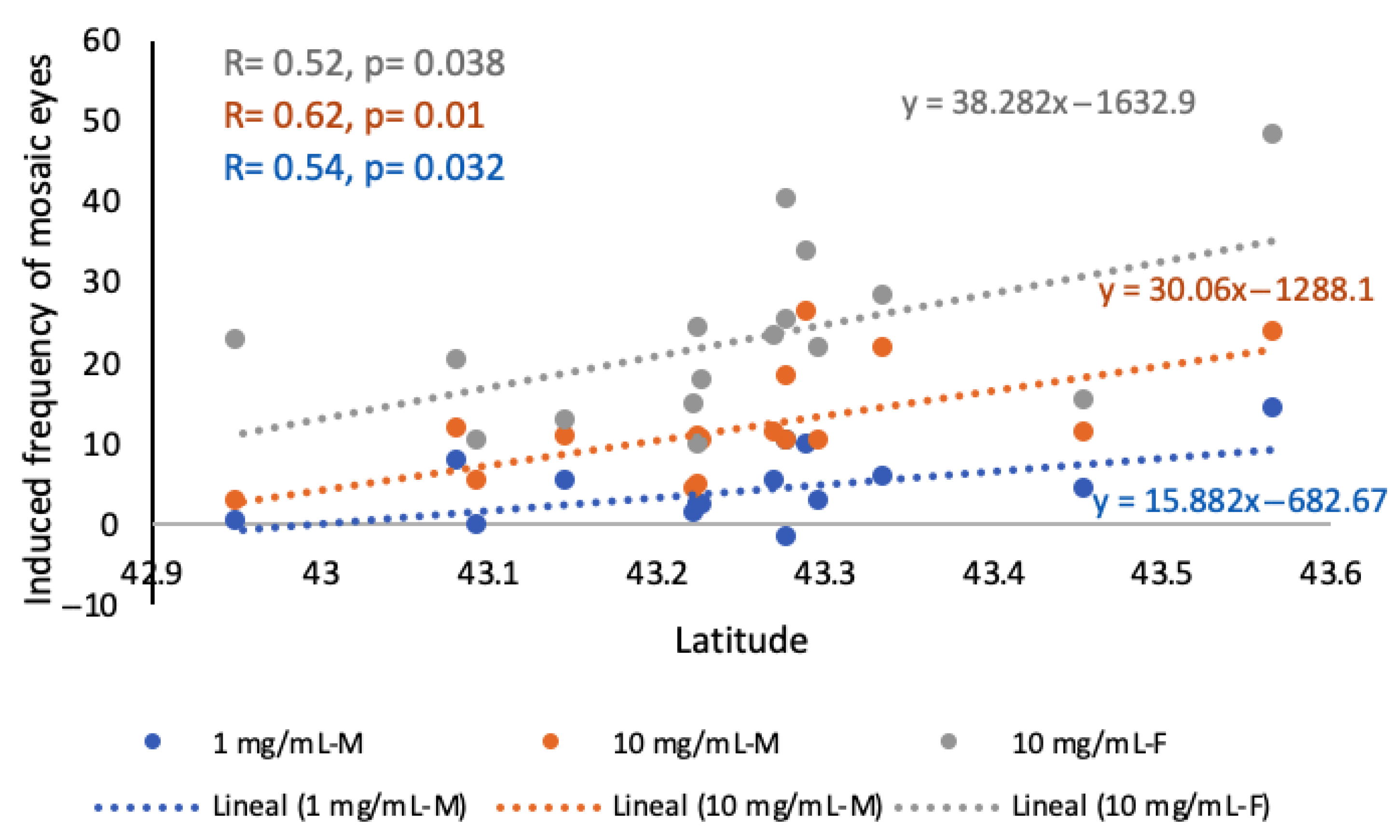
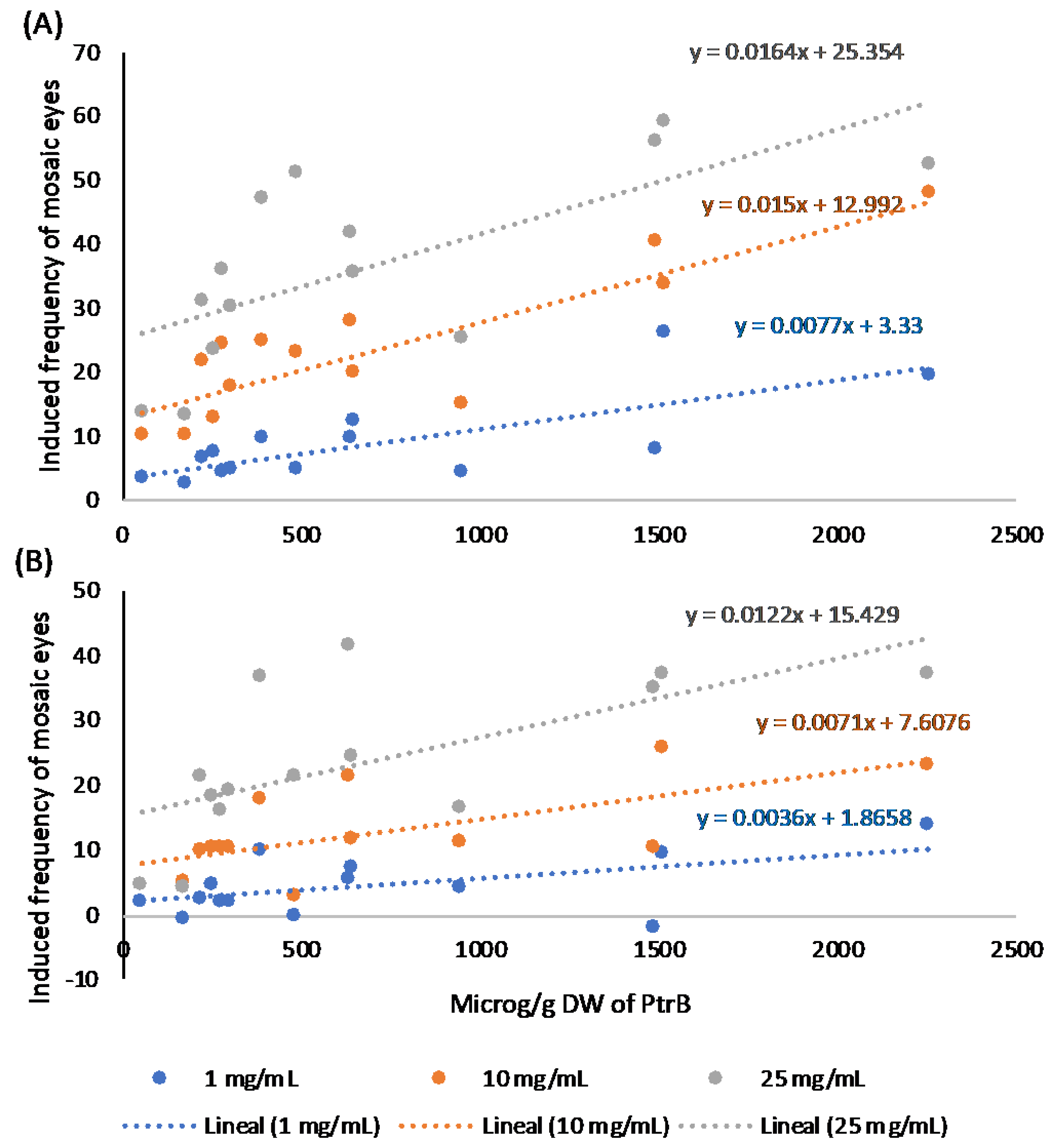
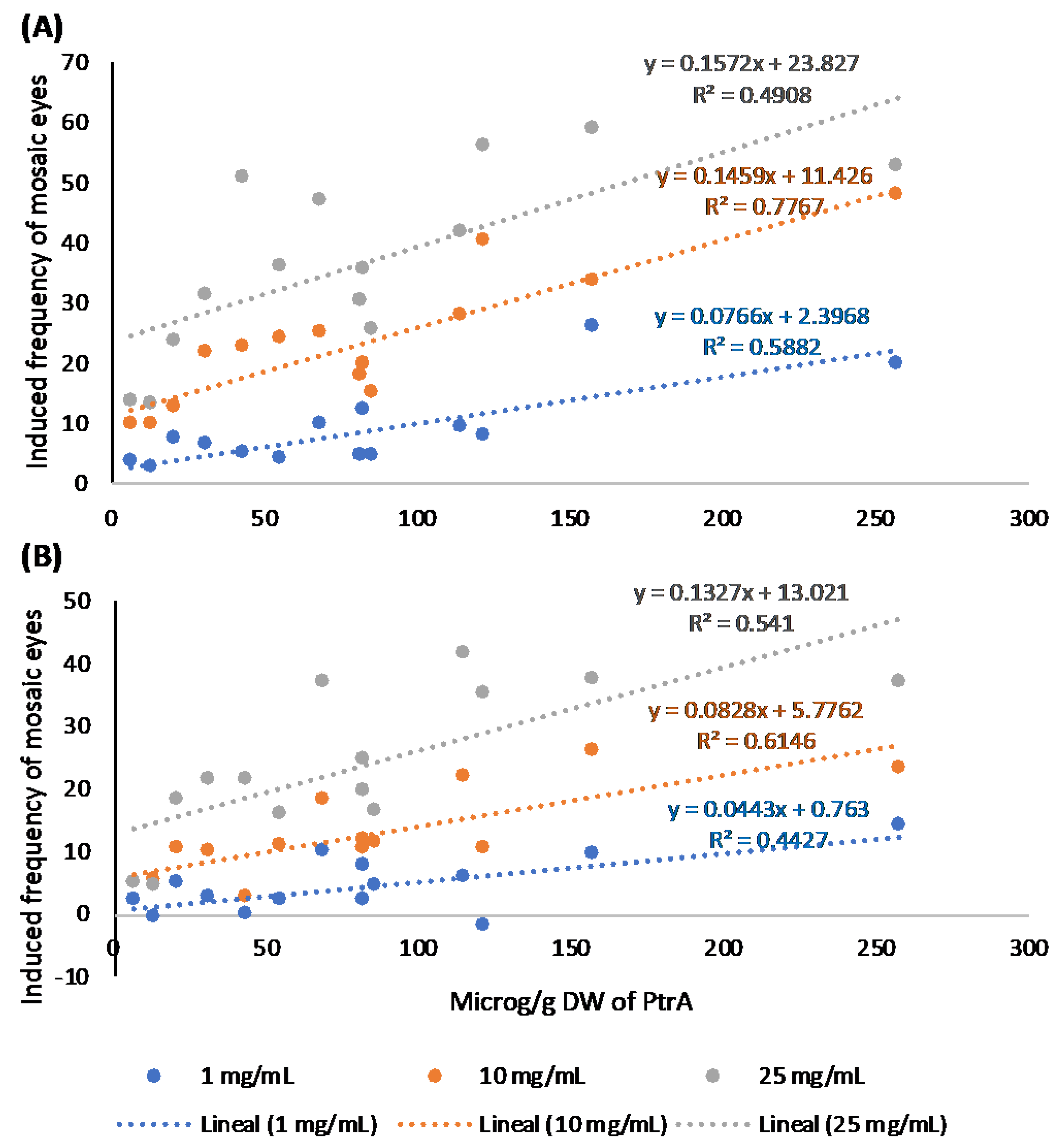
2.3. Relationships Between Pterosin Levels and Genotoxic Activities
2.4. Results of Farmer Surveys
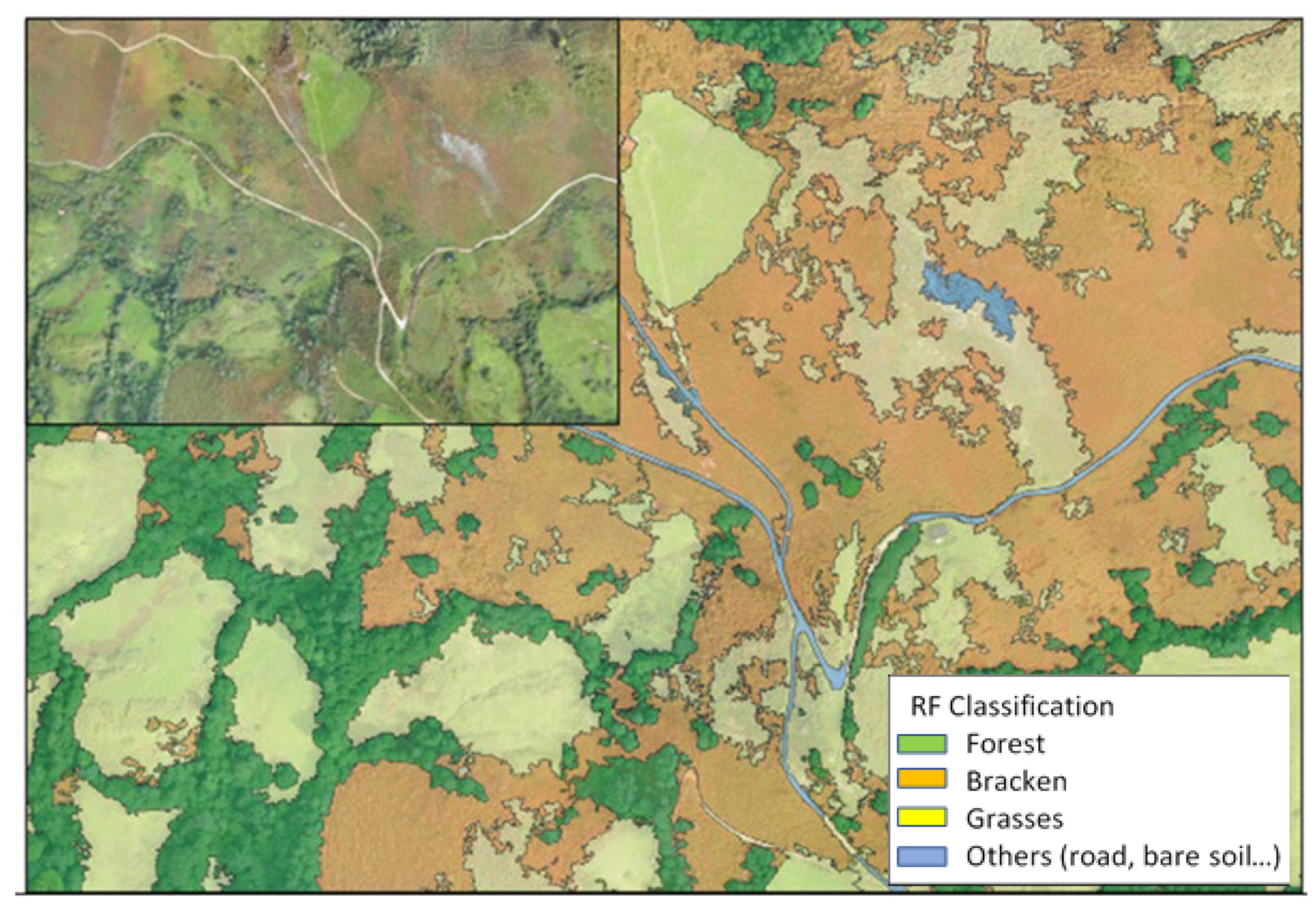
2.5. Implementing a Tool to Map Bracken
3. Discussion
4. Materials and Methods
4.1. Sampling Sites and Pteridium Plant Collection
4.2. Metabolite Analysis: Determination of Pterosins in Croziers from Bracken
4.3. Genotoxicity Analysis: SMART Assay
4.4. Surveys Obtained from Farmers
4.5. Implementing a Tool to Map Bracken
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuettpelz, E.; Schneider, H.; Smith, A.R.; Hovenkamp, P.; Prado, J.; Rouhan, G.; Salino, A.; Sundue, M.; Almeida, T.E.; Parris, B.; et al. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Smith, B.L.; Seawright, A.A. Bracken fern (Pteridium spp.). Carcinogenicity and human health—A brief review. Nat. Toxins 1995, 3, 1–5. [Google Scholar] [CrossRef]
- Marrs, R.H.; Watt, A.S. Biological Flora of the British Isles: Pteridium aquilinum (L.) Kuhn. J. Ecol. 2006, 4, 1272–1321. [Google Scholar] [CrossRef]
- Rasmussen, L.H.; Donnelly, E.; Strobel, B.W.; Holm, P.E.; Hansen, H.C.B. Land management of bracken needs to account for bracken carcinogens. A case study from Britain. J. Environ. Manag. 2015, 151, 258–266. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Marrs, R.H.; Howard, D.C.; Barr, C.J.; Fuller, R.M. The bracken problem in Great Britain: Its present extent and future changes. Appl. Geogr. 1996, 16, 65–86. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Le Duc, M.G.; Marrs, R. A review of current bracken control and associated vegetation strategies in Great Britain. Web Ecol. 2002, 3, 6–11. [Google Scholar] [CrossRef]
- Holland, J.; Aplin, P. Super-resolution image analysis as a means of monitoring bracken (Pteridium aquilinum) distributions. ISPRS J. Photogramm. Remote Sens. 2013, 75, 48–63. [Google Scholar] [CrossRef]
- Cao, H.; Chai, T.T.; Wang, X.; Morais-Braga, M.F.B.; Yang, J.H.; Wong, F.C.; Wang, R.; Yao, H.; Cao, J.; Cornara, L.; et al. Phytochemicals from fern species: Potential for medicine applications. Phytochem. Rev. pPSE 2017, 16, 379–440. [Google Scholar] [CrossRef]
- Matongera, T.N.; Mutanga, O.; Dube, T.; Sibanda, M. Detection and mapping the spatial distribution of bracken fern weeds using the Landsat 8 OLI new generation sensor. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 93–103. [Google Scholar] [CrossRef]
- Milligan, G.; Booth, K.E.; Cox, E.S.; Pakeman, R.J.; Le Duc, M.G.; Connor, L.; Blackbird, S.; Marrs, R.H. Change to ecosystem properties through changing the dominant species: Impact of Pteridium aquilinum-Control and heathland restoration treatments on selected soil properties. J. Environ. Manag. 2018, 207, 1–9. [Google Scholar] [CrossRef]
- Schneider, L.C. Bracken fern invasion in southern yucatán: A case for land-change science. Geogr. Rev. 2004, 94, 229–241. [Google Scholar] [CrossRef]
- Den Ouden, J. The Role of Bracken (Pteridium aquilinum) in Forest Dynamics. Doctoral Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2000. Available online: https://edepot.wur.nl/197082 (accessed on 20 July 2025).
- Senyanzobe, J.M.V.; Mulei, J.M.; Bizuru, E.; Nsengimuremyi, C. Impact of Pteridium aquilinum on vegetation in Nyungwe forest, Rwanda. Heliyon 2020, 6, e04806. [Google Scholar] [CrossRef] [PubMed]
- Fernández, H.; Sierra, L.M. Pteridium aquilinum: A Threat to Biodiversity and Human and Animal Health. In Ferns; Marimuthu, J., Fernández, H., Kumar, A., Thangaiah, S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Vetter, J. A biological hazard of our age: Bracken fern [Pteridium aquilinum (L.) Kuhn]. A review. Acta Vet. Hung. 2009, 57, 183–196. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Bastos, M.M.S.M.; Oliveira, P.A.; Lopes, C. Bracken-associated human and animal health hazards: Chemical, biological and pathological evidence. J. Hazard. Mater. 2012, 203–204, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aranha, P.C.R.; Rasmussen, L.H.; Wolf-Jäckel, G.A.; Elvang Jensen, H.M.; Bruun Hansen, H.C.; Christian Frits, C. Fate of ptaquiloside—A bracken fern toxin—In cattle. PLoS ONE 2019, 14, e0218628. [Google Scholar] [CrossRef] [PubMed]
- Malík, M.; Mika, O.J.; Navrátilová, Z.; Killi, U.K.; Tlustoš, P.; Patočka, J. Health and environmental hazards of the toxic Pteridium aquilinum (L.) Kuhn (bracken fern). Plants 2023, 13, 18. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Avendaño, M. Human carcinogenesis and bracken fern: A review of the evidence. Curr. Med. Chem. 2002, 9, 675–686. [Google Scholar] [CrossRef]
- Rasmussen, L.H.; Kroghsbo, S.; Frisvad, J.C.; Hansen, H.C.B. Occurrence of the carcinogenic bracken constituent ptaquiloside in fronds, topsoils and organic soil layers in Denmark. Chemosphere 2003, 51, 117–127. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Hayes, P.Y.; Somerville, M.J.; De Voss, J.J. Ptesculentoside, a novel sesquiterpene glucoside from the australian bracken fern Pteridium esculentum. Tetrahedron Lett. 2010, 5, 1997–1999. [Google Scholar] [CrossRef]
- Povey, A.; Potter, D.; O’Connor, P. 32P-Post-labelling analysis of DNA adducts formed in the upper gastrointestinal tissue of mice fed bracken extract or bracken spores. Br. J. Cancer 1996, 74, 1342–1348. [Google Scholar] [CrossRef]
- Shahin, M.; Smith, B.L.; Prakash, A.S. Bracken carcinogens in the human diet. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 1999, 44, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.M.; Baird, M.S. Carcinogenic effects of ptaquiloside in bracken fern and related compounds. Br. J. Cancer 2000, 83, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.N.; O’Connor, P.J.; Prakash, A.S.; Shahin, M.; Povey, A.C. Bracken (Pteridium aquilinum)-Induced DNA adducts in mouse tissues are different from the adduct induced by the activated form of the bracken carcinogen ptaquiloside. Biochem. Biophys. Res. Commun. 2001, 281, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Dawra, R.K.; Kurade, N.P.; Sharma, O.P. Carcinogeniciy of the fern Pteridium aquilinum collected from an enzootic bovine haematuria-free hilly area in India. Curr. Sci. 2002, 83, 1005–1009. [Google Scholar]
- Alonso-Amelot, M.E. The chemistry and toxicology of bioactive compounds in bracken fern (Pteridium ssp) with special reference to chemical ecology and carcinogenesis. In Studies in Natural Product Chemistry; Atta-Uhr-Rahman, Ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; Volume 26, pp. 685–740. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Coelho, P.; Sousa, R.; Bastos, M.M.S.M.; Porto, B.; Teixeira, J.P.; Malheiro, I.; Lopes, C. Multiple genotoxic activities of ptaquiloside in human lymphocytes: Aneugenesis, clastogenesis and induction of sister chromatid exchange. Mutat. Res. 2012, 747, 77–81. [Google Scholar] [CrossRef]
- Oliveros-Bastidas, A.; Calcagno-Pissarelli, M.P.; Naya, M.; Ávila-Núñez, J.L.; Alonso-Amelot, M.E. Human gastric cancer, Helicobacter pylori and bracken carcinogens: A connecting hypothesis. Med. Hypotheses 2016, 88, 91–99. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Alonso-Amelot, M.E.; Roberts, S.A.; Povey, A.C. The role of bracken fern iludanes in bracken fern-induced toxicities. Mut. Res. Rev. Mut. Res. 2019, 782, 108276. [Google Scholar] [CrossRef]
- Kisielius, V.; Markusen, B.; Hansen, H.C.B.; Rasmussen, L.H. Geographical distribution of caudatoside and ptaquiloside in bracken ferns in northern europe. Environ. Sci. Eur. 2024, 36, 186. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Avendaño, M. Possible association between gastric cancer and bracken fern in Venezuela: An epidemiologic study. Int. J. Cancer 2001, 91, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.N.; Brasileiro-Filho, G.; Silva, M.E.; Pena, S.D.J. Bracken fern-induced malignant tumours in rats: Absence of mutations in p53, h-ras and k-ras and no microsatellite instability. Mutat. Res. 2002, 499, 189–196. [Google Scholar] [CrossRef]
- Schmidt, B.; Rasmussen, L.H.; Svendsen, G.W.; Ingerslev, F.; Hansen, H.C.B. Genotoxic activity and inhibition of soil respiration by ptaquiloside, a bracken fern carcinogen. Environ. Toxicol. Chem. 2005, 24, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Almeida Santos, M.; Dórea, J.; Luna, H. Bracken fern can be clastogenic or aneugenic depending on the tissue cell assay. Food Chem. Toxicol. 2006, 44, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Bracken fern (Pteridium aquilinum) and some of its constituents. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1986, 40, 47–65. [Google Scholar]
- Niwa, H.; Ojika, M.; Wakamatsu, K.; Yamada, K.; Hirono, I.; Matsushita, K. Ptaquiloside, a novel norsesquiterpene glucoside from bracken, Pteridium aquilinum var. latiusculum. Tetrahedron Lett. 1983, 24, 4117–4120. [Google Scholar] [CrossRef]
- van der Hoeven, J.C.M.; Lagerweij, W.J.; Posthumus, M.A.; van Veldhuizen, A.; Holterman, H.A.J. Aquilide A, a new mutagenic compound isolated from bracken fern (Pteridium aquilinum (L.) Kuhn). Carcinogenesis 1983, 4, 1587–1590. [Google Scholar] [CrossRef]
- Ojika, M.; Wakamatsu, K.; Niwa, H.; Yamada, K. Ptaquiloside, a potent carcinogen isolated from bracken fern Pteridiumaquilinum var. latiusculum: Structure elucidation based on chemical and spectral evidence, and reactions with amino acids, nucleosides, and nucleotides. Tetrahedron 1987, 43, 5261–5274. [Google Scholar] [CrossRef]
- Matsuoka, A.; Hirosawa, A.; Natori, S.; Iwasaki, S.; Sofuni, T.; Ishidate, M., Jr. Mutagenicity of ptaquiloside, the carcinogen in bracken, and its related iludane-type sesquiterpenes: II. Chromosomal aberration tests with cultured mammalian cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1989, 215, 179–185. [Google Scholar] [CrossRef]
- Yamada, K.; Ojika, M.; Kigoshi, H. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, Biology and Ecology. Nat. Prod. Rep. 2007, 24, 798–813. [Google Scholar] [CrossRef]
- Kushida, T.; Uesugi, M.; Sugiura, Y.; Kigoshi, H.; Tanaka, H.; Hirokawa, J.; Ojika, M.; Yamada, K. DNA damage by ptaquiloside, a potent bracken carcinogen: Detection of selective strand breaks and identification of DNA cleavage products. J. Am. Chem. Soc. 1994, 116, 479–486. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chang, F.-R.; Lu, M.-C.; Hsieh, P.-W.; Wu, M.-J.; Du, Y.-C.; Wu, Y.-C. New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm. Molecules 2008, 13, 255–266. [Google Scholar] [CrossRef]
- Rasmussen, L.H.; Bruun Hansen, H.C.; Lauren, D. Sorption, degradation and mobility of ptaquiloside, a carcinogenic bracken (Pteridium sp.) Constituent, in the soil environment. Chemosphere 2005, 58, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Lauren, D.R.; Smith, B.L. Variation in ptaquiloside content in bracken (Pteridium esculentum (Forst. F) Cockayne) in New Zealand. N. Z. Vet. J. 2008, 56, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.H.; Schmidt, B.; Sheffield, E. Ptaquiloside in bracken spores from Britain. Chemosphere 2013, 90, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Siman, S.E.; Povey, A.C.; Ward, T.H.; Margison, G.P.; Sheffield, E. Fern spore extracts can damage DNA. Br. J. Cancer 2000, 63, 69–73. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E.; Avendaño, M. Conglomerados de cáncer gástrico en el estado de Mérida, Venezuela. Interciencia 2009, 34, 617–622. [Google Scholar]
- Rasmussen, L.H. Presence of the carcinogen ptaquiloside in fern-based food products and traditional medicine: Four cases of human exposure. Curr. Res. Food Sci. 2021, 4, 557–564. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.; Castillo, U.; De Jongh, F. Passage of the bracken fern carcinogen ptaquiloside into bovine milk. Le Lait 1993, 73, 323–332. [Google Scholar] [CrossRef]
- Galpin, O.P.; Whitaker, C.J.; Whitaker, R.; Kassab, J.Y. Gastric cancer in Gwynedd. Possible links with bracken. Br. J. Cancer 1990, 61, 737–740. [Google Scholar] [CrossRef]
- Jensen, P.H.; Jacobsen, O.S.; Hansen, H.C.B.; Juhler, R.K. Quantification of ptaquiloside and pterosin b in soil and groundwater using liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 2008, 56, 9848–9854. [Google Scholar] [CrossRef]
- Ovesen, R.G.; Rasmussen, L.H.; Hansen, H.C.B. Degradation kinetics of ptaquiloside in soil and soil solution. Environ. Toxicol. Chem. 2008, 27, 252–259. [Google Scholar] [CrossRef]
- Clauson-Kaas, F.; Ramwell, C.; Hansen, H.C.B.; Strobel, B.W. Ptaquiloside from bracken in stream water at base flow and during storm events. Water Res. 2016, 106, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Skrbic, N.; Kisielius, V.; Pedersen, A.K.; Christensen, S.C.B.; Hedergaad, M.J.; Hansen, H.C.B.; Rasmussen, L.H. Occurrence of carcinogenic iludane glycosides in drinking water wells. Environ. Sci. Eur. 2021, 33, 44. [Google Scholar] [CrossRef]
- Kisielius, V.; Lindqvist, D.N.; Thygesen, M.B.; Rodamer, M.; Hansen, H.C.B.; Rasmussen, L.H. Fast LC-MS. Quantification of ptesculentoside, caudatoside, ptaquiloside and corresponding pterosins in bracken ferns. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1138, 121966. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Pescador, A.; Gutiérrez Romero, L.; Blanco-González, E.; Montes-Bayón, M.; Sierra, L.M. Intracellular biotransformation of ultrasmall iron oxide nanoparticles and their effect in cultured human cells and in Drosophila larvae in vivo. Int. J. Mol. Sci. 2022, 23, 8788. [Google Scholar] [CrossRef]
- Vogel, E.W.; Nivard, M.J.M. Performance of 181 chemicals in a Drosophila assay predominantly monitoring interchromosomal mitotic recombination. Mutagenesis 1993, 8, 57–81. [Google Scholar] [CrossRef]
- Marcos, R.; Sierra, L.M.; Gaivão, I. The SMART assays of Drosophila: Wings and eyes as target tissues. In Genotoxicity and DNA Repair: A Practical Approach; Sierra, L.M., Gaivão, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 283–295. ISBN 978-1-4939-1068-7. [Google Scholar] [CrossRef]
- Gaivão, I.; Ferreira, J.; Sierra, L.M. The w/w + somatic mutation and recombination test (smart) of Drosophila melanogaster for detecting antigenotoxic activity in genotoxicity and mutagenicity—Mechanisms and test methods. In Genotoxicity and Mutagenicity-Mechanisms and Test Methods; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83880-042-0. [Google Scholar] [CrossRef]
- Vogel, E.W.; Nivard, M.J.M. A novel method for the parallel monitoring of mitotic recombination and clastogenicity in somatic cells in vivo. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 431, 141–153. [Google Scholar] [CrossRef]
- García Sar, D.; Aguado, L.; Montes Bayón, M.; Comendador, M.A.; Blanco González, E.; Sanz-Medel, A.; Sierra, L.M. Relationships between cisplatin-induced adducts and DNA strand-breaks, mutation and recombination in vivo in somatic cells of Drosophila melanogaster, under different conditions of nucleotide excision repair. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 741, 81–88. [Google Scholar] [CrossRef]
- Rasmussen, L.H.; Hansen, H.C.B. Growth of bracken in Denmark and the content of ptaquiloside in fronds, rhizomes and roots. In Poisonous Plants and Related Toxins; Stewart, C.S., Pennycott, T.W., Acamovic, T., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 354–360. [Google Scholar] [CrossRef]
- Vetter, J. Toxicological and medicinal aspects of the most frequent fern species, Pteridium aquilinum (L.) Kuhn. In Working with Ferns: Issues and Applications; Fernández, H., Revilla, M.A., Kumar, A., Eds.; Springer: New York, NY, USA, 2011; pp. 361–375. ISBN 978-1-4419-7161-6. [Google Scholar] [CrossRef]
- Rincón, D.F.; Díaz, G.J.; Gardner, D.R. Detección de ptaquilósido en diferentes estados fenológicos de “helecho macho” (Pteridium aquilinum) y análisis de muestras de leche en granjas con hematuria en Tolima, Colombia. CES Med. Vet. Zootec. 2016, 11, 72–77. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Brock, I.J.; Reichmann, K.G.; McKenzie, R.A.; Blaney, B.J. Norsesquiterpene glycosides in bracken ferns (Pteridium esculentum and Pteridium aquilinum subsp. wightianum) from eastern Australia: Reassessed poisoning risk to animals. J. Agric. Food Chem. 2011, 59, 5133–5138. [Google Scholar] [CrossRef]
- García-Jorgensen, D.B.; Diamantopoulos, E.; Kisielius, V.; Rosenfjeld, M.; Rasmussen, L.H.; Strobel, B.W.; Hansen, H.C.B. Bracken growth, toxin production and transfer from plant to soil: A 2-year monitoring study. Environ. Sci. Eur. 2021, 33, 45. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Povey, A.; Medeiros-Fonseca, B.; Ramwell, C.; O’Driscoll, C.; Williams, D.; Hansen, H.C.B.; Rasmussen, L.H.; Fletcher, M.T.; O’Connor, P.; et al. Sixty years of research on bracken fern (Pteridium spp.) toxins: Environmental exposure, health risks and recommendations for bracken fern control. Environ. Res. 2024, 257, 119274. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L.; Esteban, B.; Sánchez, J.; Checa, M.; Mosquera, F.; Romero, L. Principado de Asturias, Tomo 7. In Caracterización de las Comarcas Agrarias de España; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011; ISBN 978-84-491-1175-4. Available online: https://comarcasagrarias.chil.me/post/tomo-7-Principado-de-Asturias-93415 (accessed on 20 April 2025).
- Zaccone, C.; Cavoski, I.; Costi, R.; Sarais, G.; Caboni, P.; Traversa, A.; Miano, T.M. Ptaquiloside in Pteridium aquilinum subsp. aquilinum and corresponding soils from the South of Italy: Influence of physical and chemical features of soils on its occurrence. Sci. Total Environ. 2014, 496, 365–372. [Google Scholar] [CrossRef]
- Skrbic, N.; Pedersen, A.-K.; Christensen, S.C.B.; Hansen, H.C.B.; Rasmussen, L.H. A Novel method for determination of the natural toxin ptaquiloside in ground and drinking water. Water 2020, 12, 2852. [Google Scholar] [CrossRef]
- Vetter, J. The norsesquiterpene glycoside ptaquiloside as a poisonous, carcinogenic component of certain ferns. Molecules 2022, 27, 6662. [Google Scholar] [CrossRef]
- Campos-da-Paz, M.; Pereira, L.O.; Bicalho, L.S.; Dorea, J.G.; Pocas-Fonseca, M.J.; Santos, M. de F. Interaction of bracken-fern extract with vitamin C in human sub-mandibular gland and oral epithelium cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 652, 158–163. [Google Scholar] [CrossRef]
- Pereira, L.O.; Bicalho, L.S.; Campos-da-Paz Lopes, M.; de Sousa, T.M.; Báo, S.N.; de Fátima Menezes Almeida Santos, M.; Fonseca, M.J. DNA damage and apoptosis induced by Pteridium aquilinum aqueous extract in the oral cell lines HSG and OSCC-3. J. Oral Pathol. Med. 2009, 38, 441–447. [Google Scholar] [CrossRef]
- Gomes, J.; Magalhães, A.; Michel, V.; Amado, I.F.; Aranha, P.; Ovesen, R.G.; Hansen, H.C.B.; Gärtner, F.; Reis, C.A.; Touati, E. Pteridium aquilinum and its ptaquiloside toxin induce DNA damage response in gastric epithelial cells, a link with gastric carcinogenesis. Toxicol. Sci. 2012, 126, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.W.; Stocco dos Santos, R.C.; Dagli, M.L.; D’Angelino, J.L.; Birgel, E.H.; Becak, W. Chromosome aberrations in cattle raised on bracken fern pasture. Experientia 1988, 44, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.B.; Barbieri, R.; Borzacchiello, G.; Dezzi, S.; Roperto, S.; Santoro, A.; Russo, V.; Roperto, F. Chromosome aberrations in cattle with chronic enzootic haematuria. J. Comp. Pathol. 2004, 131, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Peretti, V.; Ciotola, E.; Albarella, S.; Russo, V.; Di Meo, G.P.; Iannuzzi, L.; Roperto, F.; Barbieri, V. Chromosome fragility in cattle with chronic enzootic haematuria. Mutagenesis 2007, 22, 317–320. [Google Scholar] [CrossRef]
- Shahin, M.; Smith, B.L.; Worral, S.; Moore, M.R.; Seawright, A.A.; Prakash, A.S. Bracken fern carcinogenesis: Multiple intravenous doses of activated ptaquiloside induce DNA adducts, monocytosis, increased TNF alpha levels, and mammary gland carcinoma in rats. Biochem. Biophys. Res. Commun. 1998, 244, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Matoba, M.; Saito, E.; Saito, K.; Koyama, K.; Natori, S.; Matsushima, T.; Takimoto, M. Assay of ptaquiloside, the carcinogenic principle of bracken, Pteridium aquilinum, by mutagenicity testing in Salmonella typhimurium. Mutagenesis 1987, 2, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Saito, K.; Hirayama, E.; Uchikoshi, K.; Koyama, K.; Natori, S.; Morisaki, N.; Iwasaki, S.; Matsushima, T. Mutagenicity of ptaquiloside, the carcinogen in bracken, and its related iludane-type sesquiterpenes I. Mutagenicity in Salmonella typhimurium. Mutat. Res. 1989, 215, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.S.; Pereira, T.N.; Smith, B.L.; Shaw, G.; Seawright, A.A. Mechanism of bracken fern carcinogenesis: Evidence for H-ras activation via initial adenine alkylation by ptaquiloside. Nat. Toxins 1996, 4, 221–227. [Google Scholar] [CrossRef]
- Shahin, M.; Moore, M.R.; Worrall, S.; Smith, B.L.; Seawright, A.A.; Prakash, A.S. H-ras activation is an early event in the ptaquiloside-induced carcinogenesis: Comparison of acute and chronic toxicity in rats. Biochem. Biophys. Res. Commun. 1998, 250, 491–497. [Google Scholar] [CrossRef]
- Sardón, D.; de la Fuente, I.; Calonge, E.; Pérez-Alenza, M.D.; Castaño, M.; Dunner, S.; Peña, L. H-ras Immunochemical expression and molecular analysis of urinary bladder lesions in grazing adult cattle exposed to bracken fern. J. Comp. Pathol. 2005, 132, 195–202. [Google Scholar] [CrossRef]
- Sasani, F.; Baghban, F.; Brujeni, G.H.N.; Kazemi, M. Tp53 intronic mutations in bovine enzootic hematuria-associated urinary bladder tumours. Vet. Pathol. 2012, 50, 543–547. [Google Scholar] [CrossRef]
- Wong, K.; Abascal, F.; Ludwig, L.; Aupperle-Lellbach, H.; Grassinger, J.; Wright, C.W.; Allison, S.J.; Pinder, E.; Phillips, R.M.; Romero, L.P.; et al. Cross-Species oncogenomics offers insight into human muscle- invasive bladder cancer. Genome Biol. 2023, 24, 191. [Google Scholar] [CrossRef]
- Kisielius, V.; Drejer, M.; Dornhoff, J.K.; Mrkajic, N.S.; Lindqvist, D.N.; Hansen, H.C.B.; Rasmussen, L.H. Occurrence and stability of ptesculentoside, caudatoside and ptaquiloside in surface waters. Environ. Sci. Process. Impacts 2022, 24, 277–289. [Google Scholar] [CrossRef]
- Sheaves, B.J.; Brown, R.W. Densities of Ixodes ricinus ticks (Acari: Ixodidae) on moorland vegetation communities in the UK. Exp. Appl. Acarol. 1995, 19, 489–497. [Google Scholar] [CrossRef]
- Gray, J.S. The Development and Seasonal Activity of the Tick Ixodes ricinus: A vector of Lyme borreliosis and other tick-borne diseases. Rev. Med. Vet. Entomol. 1991, 79, 323–333. [Google Scholar]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, 10–128. [Google Scholar] [CrossRef]
- Matongera, T.N.; Mutanga, O.; Dube, T.; Lottering, R.T. Detection and mapping bracken fern weeds using multispectral data: A review of progress and challenges. Geocarto Int. 2016, 33, 209–224. [Google Scholar] [CrossRef]
- Takeshige, R.; Onishi, M.; Aoyagi, R.; Sawada, Y.; Imai, N.; Ong, R.; Kitayama, K. Mapping the spatial distribution of fern thickets and vine-laden forests in the landscape of bornean logged-over tropical secondary rainforests. Remote Sens. 2022, 14, 3354. [Google Scholar] [CrossRef]
- Rai, S.K.; Sharma, R.; Kumari, A.; Rasmussen, L.H.; Patil, R.D.; Bhar, R. Survey of ferns and clinic-pathological studies on the field cases of enzootic bovine haematuria in Himachal Pradesh, a North-western Himalayan state of India. Toxicon 2017, 138, 31–36. [Google Scholar] [CrossRef]
- Henrich, V. IDB—Index-Database, Development of a Database for Remote Sensing Indices; ZFL-Colloquium: Bonn, Germany, 2012; Available online: https://www.50northspatial.org.ua/idb-remote-sensing-indices-database/ (accessed on 20 July 2025).
| Chemical | μgL−1 Min | μgL−1 Max | R2 | LOD (μgL−1) | LOQ (μgL−1) |
|---|---|---|---|---|---|
| PtrB | 5 | 160 | 0.998 | 2.5 | 5 |
| PtrA | 0.75 | 80 | 0.994 | 0.046 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra, L.M.; Feito, I.; Rodríguez, M.L.; Velázquez, A.; Cué, A.; San-Juan-Guardado, J.; Martín, M.; López, D.; Peña, A.E.; Canga, E.; et al. Toxic Threats from the Fern Pteridium aquilinum: A Multidisciplinary Case Study in Northern Spain. Int. J. Mol. Sci. 2025, 26, 7157. https://doi.org/10.3390/ijms26157157
Sierra LM, Feito I, Rodríguez ML, Velázquez A, Cué A, San-Juan-Guardado J, Martín M, López D, Peña AE, Canga E, et al. Toxic Threats from the Fern Pteridium aquilinum: A Multidisciplinary Case Study in Northern Spain. International Journal of Molecular Sciences. 2025; 26(15):7157. https://doi.org/10.3390/ijms26157157
Chicago/Turabian StyleSierra, L. María, Isabel Feito, Mª Lucía Rodríguez, Ana Velázquez, Alejandra Cué, Jaime San-Juan-Guardado, Marta Martín, Darío López, Alexis E. Peña, Elena Canga, and et al. 2025. "Toxic Threats from the Fern Pteridium aquilinum: A Multidisciplinary Case Study in Northern Spain" International Journal of Molecular Sciences 26, no. 15: 7157. https://doi.org/10.3390/ijms26157157
APA StyleSierra, L. M., Feito, I., Rodríguez, M. L., Velázquez, A., Cué, A., San-Juan-Guardado, J., Martín, M., López, D., Peña, A. E., Canga, E., Ramos, G., Majada, J., Alvarez, J. M., & Fernández, H. (2025). Toxic Threats from the Fern Pteridium aquilinum: A Multidisciplinary Case Study in Northern Spain. International Journal of Molecular Sciences, 26(15), 7157. https://doi.org/10.3390/ijms26157157








