Albumin Reduces Hepatic Steatosis and Inflammation in High-Fat-Diet-Fed Mice
Abstract
1. Introduction
2. Results
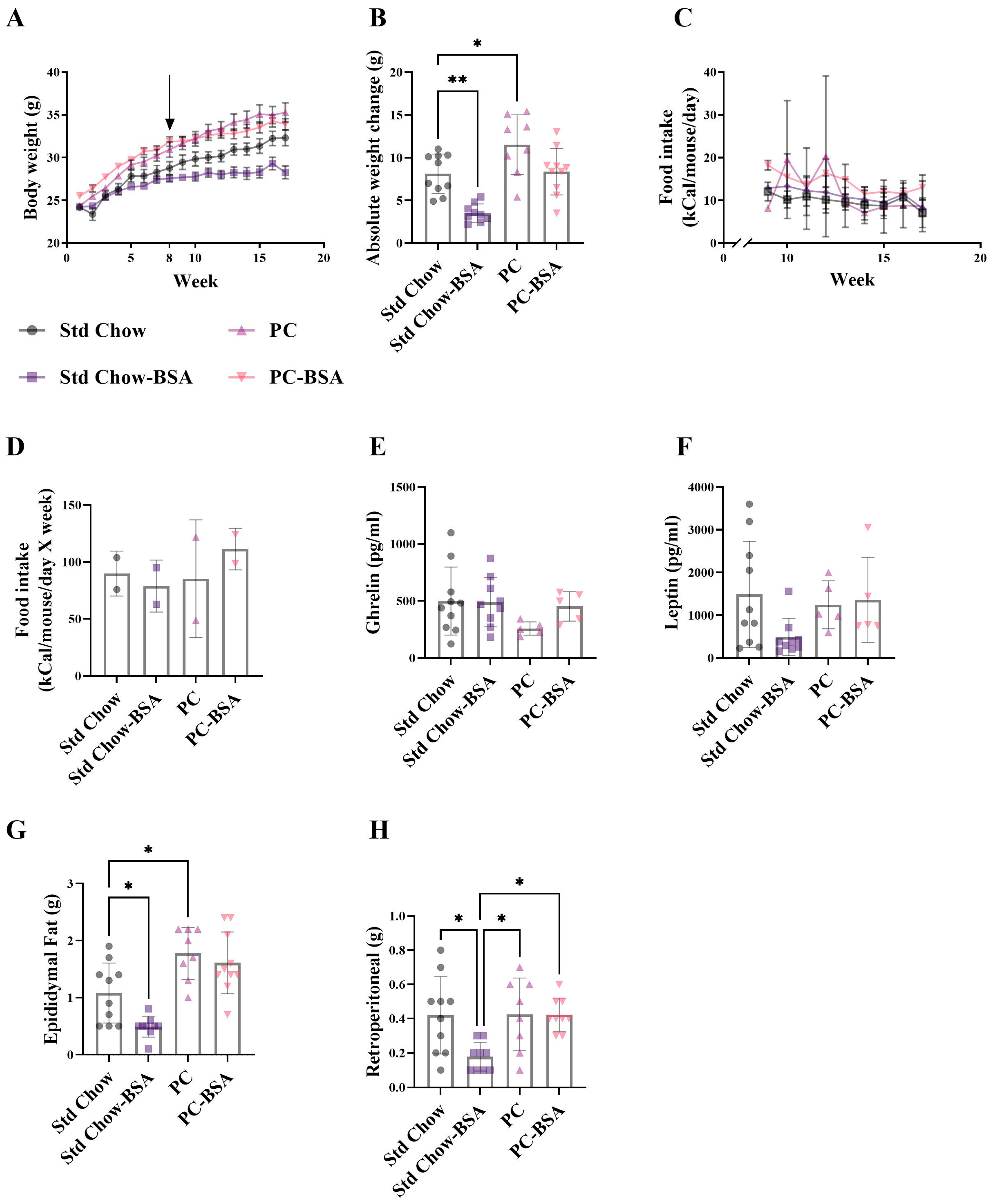
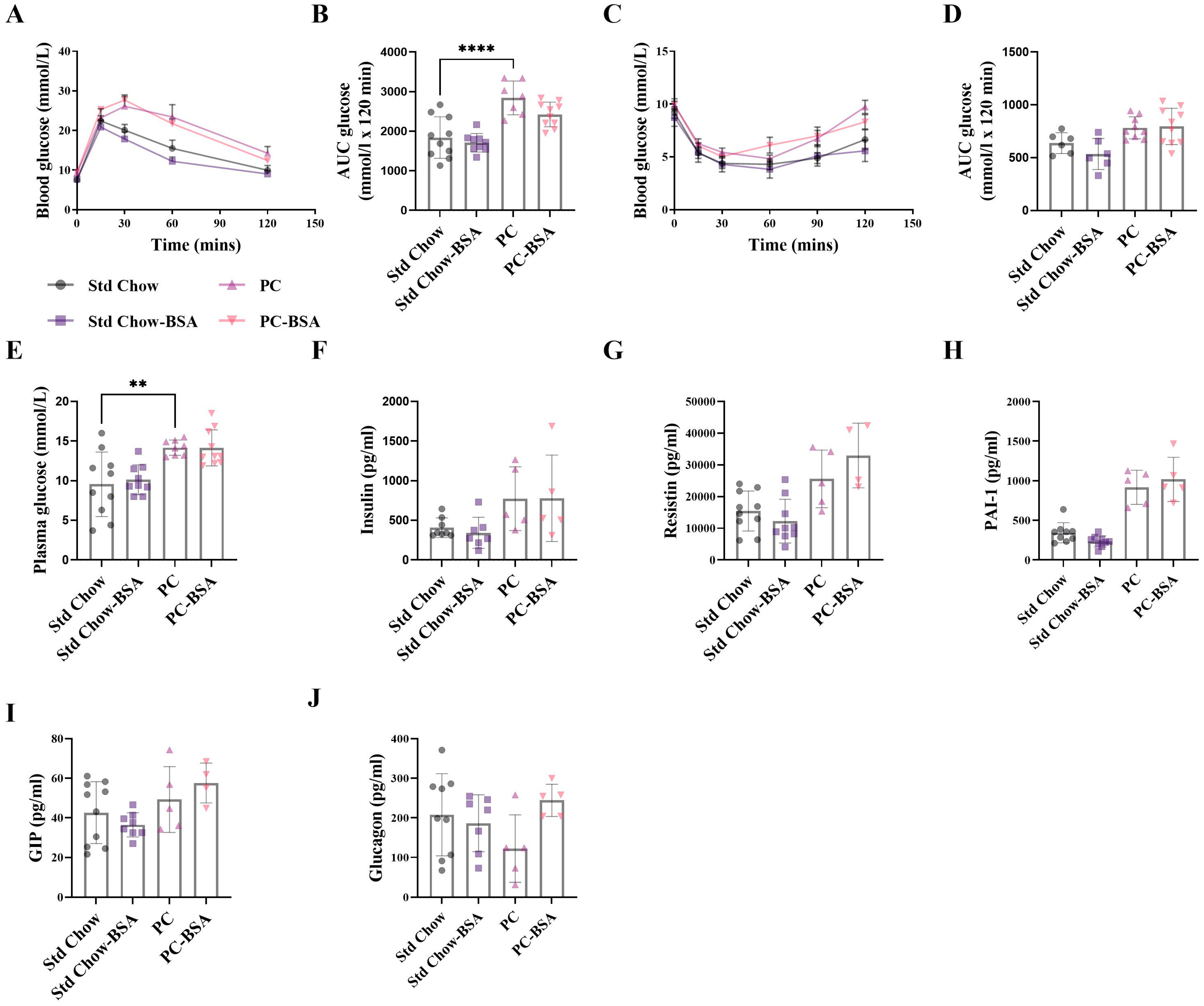
2.1. BSA Treatment Prevented Diet-Induced Weight Gain, However Had No Effect on Glucose Homestasis
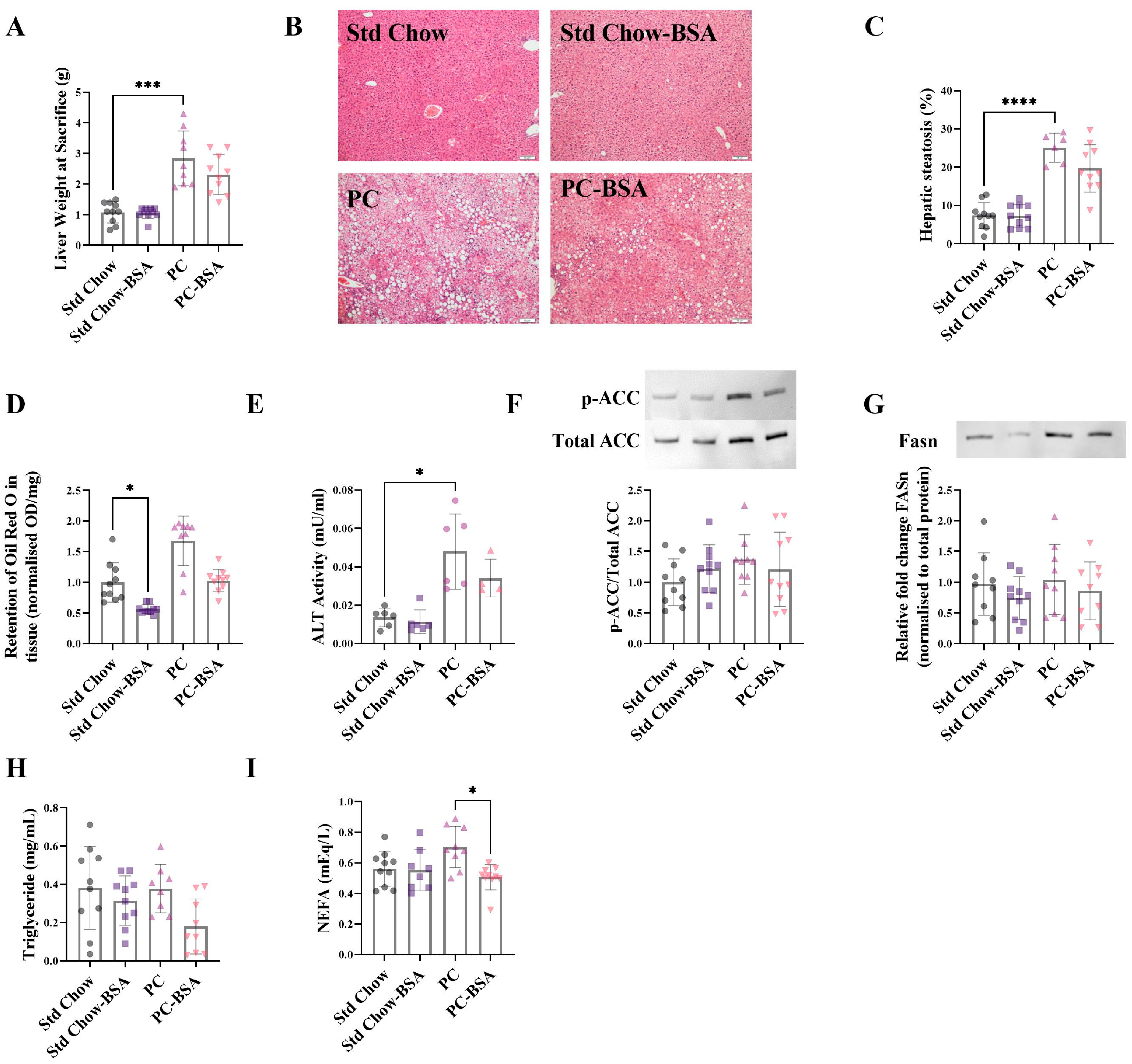
2.2. BSA Treatment Reversed Hepatic Fat Accumulation
2.3. BSA Treatment Decreased Hepatic Inflammation
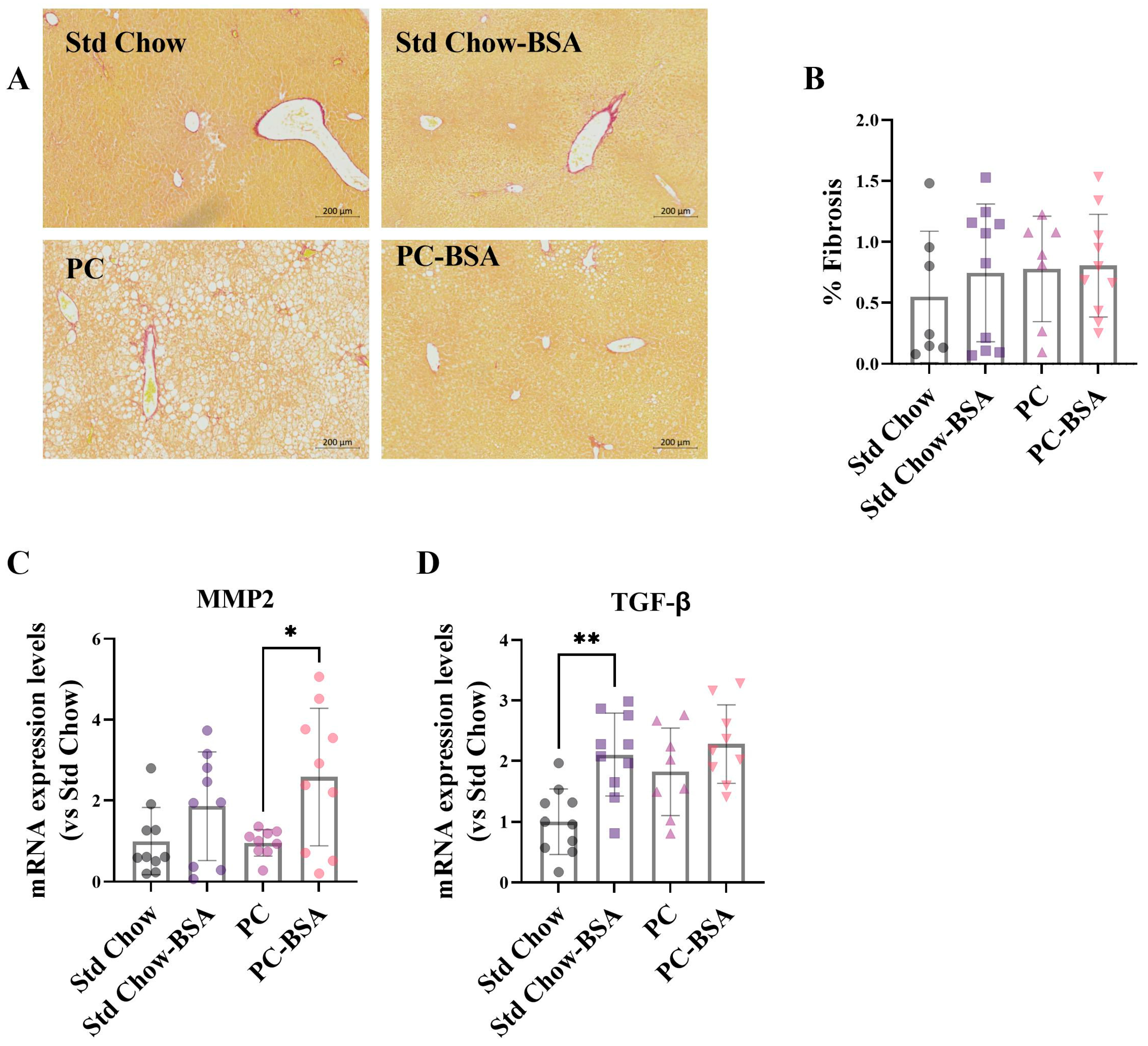
2.4. BSA Treatment of Mice Enhances the Expression of Genes Associated with Fibrotic Pathways
3. Discussion
4. Materials and Methods
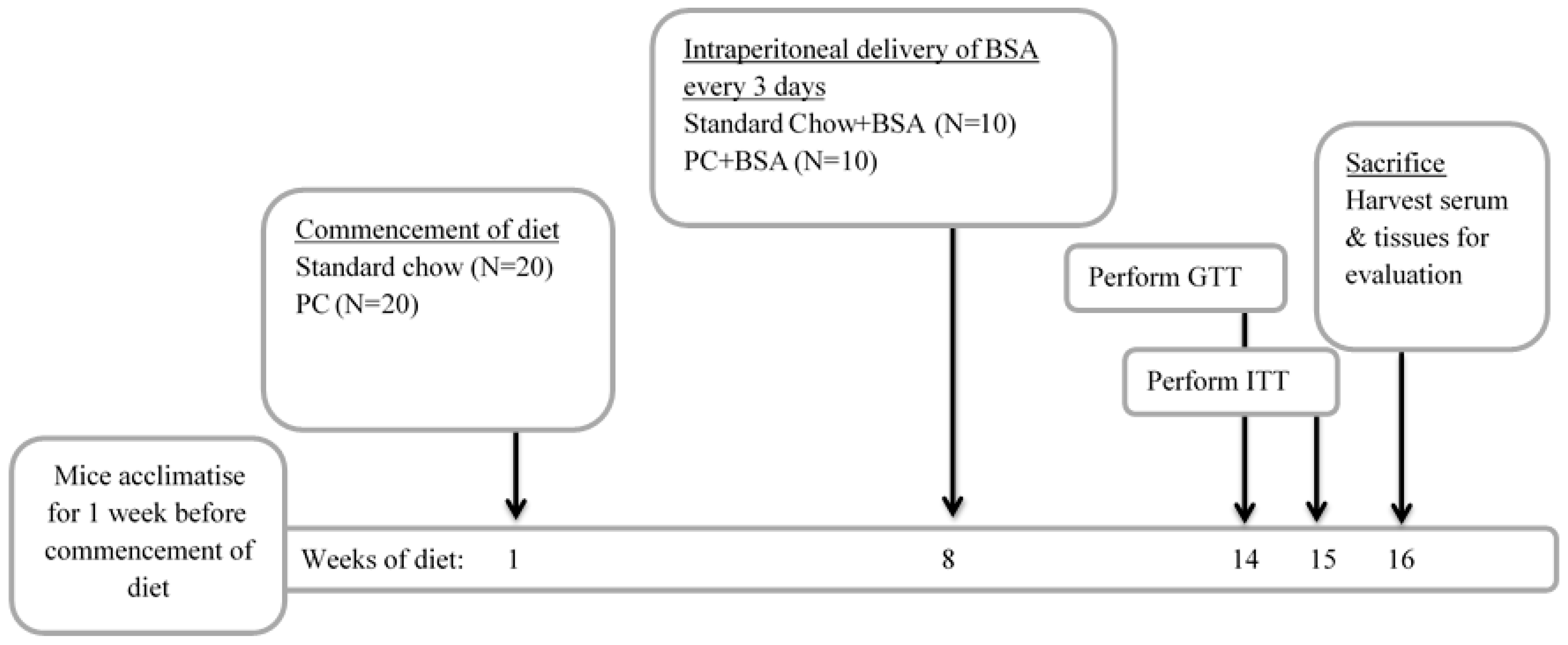
4.1. Animal Husbandry
4.2. Animal Model of Metabolic Dysfunction-Associated Steatotic Liver Disease
4.3. Measurement of the Metabolic Status of Animals
4.4. Biochemical Analysis of Plasma
4.5. Histological Examination of Liver Tissue
4.6. Western Blot Analysis
4.7. Quantification of Gene Expression Using RT-qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Radu, F.; Potcovaru, C.G.; Salmen, T.; Filip, P.V.; Pop, C.; Fierbințeanu-Braticievici, C. The Link between NAFLD and Metabolic Syndrome. Diagnostics 2023, 13, 614. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic steatohepatitis: A review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Lukenda, V.; Racki, S.; Milic, S.; Sladoje-Martinovic, B.; Orlic, L. Nonalcoholic fatty liver disease (NAFLD)—A new factor that interplays between inflammation, malnutrition, and atherosclerosis in elderly hemodialysis patients. Clin. Interv. Aging 2014, 9, 1295–1303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Charatcharoenwitthaya, P.; Lindor, K.D.; Angulo, P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Fierbinteanu-Braticevici, C.; Baicus, C.; Tribus, L.; Papacocea, R. Predictive factors for nonalcoholic steatohepatitis (NASH) in patients with nonalcoholic fatty liver disease (NAFLD). J. Gastrointest. Liver Dis. 2011, 20, 153–159. [Google Scholar]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-term follow-up of patients with nonalcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Gramlich, T.; Matteoni, C.A.; Boparai, N.; McCullough, A.J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. 2004, 2, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakai, Y.; Terashima, T.; Shimode, T.; Seki, A.; Orita, N.; Takeshita, Y.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 2021, 100, e26835. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kawanaka, M.; Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Morishita, A.; Munekage, K.; et al. Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yin, H.; Liu, M.; Xu, G.; Zhou, X.; Ge, P.; Yang, H.; Mao, Y. Impaired albumin function: A novel potential indicator for liver function damage? Ann. Med. 2019, 51, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Loomba, R. Nonalcoholic fatty liver disease: Indications for liver biopsy and noninvasive biomarkers. Clin. Liver Dis. 2012, 1, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Paquette, B.; Richter, M.; Larivee, P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am. J. Respir. Crit. Care Med. 2000, 162, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Taverna, M.; Marie, A.-L.; Mira, J.-P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J. Clin. Med. 2023, 12, 6017. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Ando, T.; Chang, D.C.; Hollstein, T.; Krakoff, J.; Piaggi, P.; Votruba, S. Reduced Albumin Concentration Predicts Weight Gain and Higher Ad Libitum Energy Intake in Humans. Front. Endocrinol. 2021, 12, 642568. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Xu, X.; Ferrante, A.W.; Krakoff, J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol. Metab. Syndr. 2019, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. Trasfus. Sangue 2013, 11 (Suppl. S4), s18–s25. [Google Scholar] [CrossRef]
- Angeli, P.; Labenz, C.; Piano, S.; Juanola, A.; Krag, A.; Caraceni, P.; Trebicka, J.; Maiwall, R.; Singh, V.; Pose, E.; et al. Albumin infusion in hepatorenal syndrome-acute kidney injury: New evidence challenges recent consensus. J. Hepatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, S.; Zhang, J.; Dong, M.; Wang, Y.; Wang, M.; Xin, Y. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.; Baldwin, A.S. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Gustin, J.A.; Korgaonkar, C.K.; Pincheira, R.; Li, Q.; Donner, D.B. Akt Regulates Basal and Induced Processing of NF-κB2 (p100) to p52. J. Biol. Chem. 2006, 281, 16473–16481. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, M.; Liu, J.; Hong, G.; Lin, Q. The preventative effect of Akt knockout on liver cancer through modulating NF-κB-regulated inflammation and Bad-related apoptosis signaling pathway. Int. J. Oncol. 2016, 48, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Dong, L.; Yang, Z.-w.; Zhang, J.; Zhang, S.-l.; Niu, M.-j.; Xia, J.-w.; Gong, Y.; Zhu, N.; et al. Crosstalk between the Akt/mTORC1 and NF-κB signaling pathways promotes hypoxia-induced pulmonary hypertension by increasing DPP4 expression in PASMCs. Acta Pharmacol. Sin. 2019, 40, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Models Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Bagheri, R.; Mesinovic, J.; Ghobadi, H.; Scott, D.; Kargarfard, M.; Dutheil, F. Effects of Resistance Training on Muscular Adaptations and Inflammatory Markers in Overweight and Obese Men. Med. Sci. Sports Exerc. 2025, 57, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J. Effects of Exercise Interventions on Weight, Body Mass Index, Lean Body Mass and Accumulated Visceral Fat in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2635. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.R.; Sallam, S.; Perumpail, B.J.; Iqbal, U.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. When to Initiate Weight Loss Medications in the NAFLD Population. Diseases 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Gudzune, K.A.; Kushner, R.F. Medications for Obesity: A Review. JAMA 2024, 332, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Zaccherini, G.; Tufoni, M.; Bernardi, M. Albumin Administration is Efficacious in the Management of Patients with Cirrhosis: A Systematic Review of the Literature. Hepat. Med. 2020, 12, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.G.; La Villa, G.; Barletta, G.; Vizzutti, F.; Lanini, F.; Arena, U.; Boddi, V.; Tarquini, R.; Pantaleo, P.; Gentilini, P. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: An unblinded randomized trial. World J. Gastroenterol. 2006, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- TGA Consumer Medicine Information (Prescription Medicine) Template: Alburex®20 AU. Available online: https://labeling.cslbehring.com/CMI/AU/Alburex/EN/Alburex-20-AU-Consumer-Medicine-Information.pdf (accessed on 14 July 2025).
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: A systematic review and meta-analysis. Nutr. J. 2013, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Hernandez, L.; Barquera, S. Effect of a high-protein diet versus standard-protein diet on weight loss and biomarkers of metabolic syndrome: A randomized clinical trial. Obes. Facts 2017, 10, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Hsu, K.J.; Chien, K.Y.; Chen, J.J. Effects of Combined High-Protein Diet and Exercise Intervention on Cardiometabolic Health in Middle-Aged Obese Adults: A Randomized Controlled Trial. Front. Cardiovasc. Med. 2021, 8, 705282. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Meziani, F.; Kremer, H.; Tesse, A.; Baron-Menguy, C.; Mathien, C.; Mostefai, H.A.; Carusio, N.; Schneider, F.; Asfar, P.; Andriantsitohaina, R. Human Serum Albumin Improves Arterial Dysfunction during Early Resuscitation in Mouse Endotoxic Model via Reduced Oxidative and Nitrosative Stresses. Am. J. Pathol. 2007, 171, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.A.; Kapus, A.; Khadaroo, R.G.; He, R.; Marshall, J.C.; Lindsay, T.F.; Rotstein, O.D. Twenty-five percent albumin prevents lung injury following shock/resuscitation. Crit. Care Med. 2003, 31, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Aubin, É.; Roberge, C.; Lemieux, R.; Bazin, R. Immunomodulatory effects of therapeutic preparations of human albumin. Vox Sang. 2011, 101, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Castro-Narro, G.; Moctezuma-Velázquez, C.; Male-Velázquez, R.; Trejo-Estrada, R.; Bosques, F.J.; Moreno-Alcántar, R.; Rodríguez-Hernández, H.; Bautista-Santos, A.; Córtez-Hernández, C.; Cerda-Reyes, E.; et al. Position statement on the use of albumin in liver cirrhosis. Ann. Hepatol. 2022, 27, 100708. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, Â.Z.; Simonetto, D.A.; Terra, C.; Farias, A.Q.; Bittencourt, P.L.; Pase, T.H.S.; Toazza, M.R.; de Mattos, A.A. Albumin administration in patients with cirrhosis: Current role and novel perspectives. World J. Gastroenterol. 2022, 28, 4773–4786. [Google Scholar] [CrossRef] [PubMed]
- Kremer, H.; Baron-Menguy, C.; Tesse, A.; Gallois, Y.; Mercat, A.; Henrion, D.; Andriantsitohaina, R.; Asfar, P.; Meziani, F. Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: Concentration-dependent properties. Crit. Care Med. 2011, 39, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Clària, J.; Amorós, A.; Aguilar, F.; Castro, M.; Casulleras, M.; Acevedo, J.; Duran-Güell, M.; Nuñez, L.; Costa, M.; et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology 2019, 157, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Giuliano, J.S.; Lahni, P.M.; Denenberg, A.; Wong, H.R.; Zingarelli, B. The Immunomodulatory Effects of Albumin In Vitro and In Vivo. Adv. Pharmacol. Sci. 2011, 2011, 691928. [Google Scholar] [CrossRef] [PubMed]
- Duran-Guell, M.; Flores-Costa, R.; Casulleras, M.; Lopez-Vicario, C.; Titos, E.; Diaz, A.; Alcaraz-Quiles, J.; Horrillo, R.; Costa, M.; Fernandez, J.; et al. Albumin protects the liver from tumor necrosis factor alpha-induced immunopathology. FASEB J. 2021, 35, e21365. [Google Scholar] [CrossRef] [PubMed]
- Duran-Guell, M.; Garrabou, G.; Flores-Costa, R.; Casulleras, M.; Lopez-Vicario, C.; Zhang, I.W.; Canto-Santos, J.; Contreras, B.J.; Sanchez-Rodriguez, M.B.; Romero-Grimaldo, B.; et al. Essential role for albumin in preserving liver cells from TNFalpha-induced mitochondrial injury. FASEB J. 2023, 37, e22817. [Google Scholar] [CrossRef] [PubMed]
- Utariani, A.; Rahardjo, E.; Perdanakusuma, D.S. Effects of Albumin Infusion on Serum Levels of Albumin, Proinflammatory Cytokines (TNF-α, IL-1, and IL-6), CRP, and MMP-8; Tissue Expression of EGRF, ERK1, ERK2, TGF-β, Collagen, and MMP-8; and Wound Healing in Sprague Dawley Rats. Int. J. Inflam. 2020, 2020, 3254017. [Google Scholar] [CrossRef] [PubMed]
- Abasubong, K.P.; Jiang, G.-Z.; Guo, H.-X.; Wang, X.; Huang, Y.-Y.; Dai, Y.-J.; Li, X.-F.; Dong, Y.-Z.; Gabriel, N.N.; Liu, W.-B. Oral bovine serum albumin administration alleviates inflammatory signals and improves antioxidant capacity and immune response under thioacetamide stress in blunt snout bream fed a high-calorie diet. Fish. Shellfish. Immunol. 2023, 141, 108996. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, T.; Smiljanić, K.; Takić, M.; Šarac, I.; Oggiano, G.; Nikolić, M.; Jovanović, V. The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding. Int. J. Mol. Sci. 2024, 25, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kitani, A.; Fuss, I.; Nakamura, K.; Kumaki, F.; Usui, T.; Strober, W. Transforming Growth Factor (TGF)-β1–producing Regulatory T Cells Induce Smad-mediated Interleukin 10 Secretion That Facilitates Coordinated Immunoregulatory Activity and Amelioration of TGF-β1–mediated Fibrosis. J. Exp. Med. 2003, 198, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Radbill, B.D.; Gupta, R.; Ramirez, M.C.M.; DiFeo, A.; Martignetti, J.A.; Alvarez, C.E.; Friedman, S.L.; Narla, G.; Vrabie, R.; Bowles, R.; et al. Loss of matrix metalloproteinase-2 amplifies murine toxin-induced liver fibrosis by upregulating collagen I expression. Dig. Dis. Sci. 2011, 56, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeon, Y.E.; Jung, J.I.; Kim, S.M.; Hong, S.H.; Lee, J.; Hwang, J.S.; Hwang, M.O.; Kwon, K.; Kim, E.J. Anti-obesity effect of Cydonia oblonga Miller extract in high-fat diet-induced obese C57BL/6 mice. J. Funct. Foods 2022, 89, 104945. [Google Scholar] [CrossRef]
- Collins, S.C.; Hoppa, M.B.; Walker, J.N.; Amisten, S.; Abdulkader, F.; Bengtsson, M.; Fearnside, J.; Ramracheya, R.; Toye, A.A.; Zhang, Q.; et al. Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2(+) channels from secretory vesicles. Diabetes 2010, 59, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.C.; Li, X.H.; Whitworth, P.T.; Kasz, R.; Tan, J.T.; McLennan, S.V.; Celermajer, D.S.; Barter, P.J.; Rye, K.-A.; Heather, A.K. High density lipoproteins improve insulin sensitivity in high-fat diet-fed mice by suppressing hepatic inflammation. J. Lipid Res. 2014, 55, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Brettle, D.; Cockroft, M.; Keating, E.; Revie, C.; Treanor, D. Quantitative assessment of H&E staining for pathology: Development and clinical evaluation of a novel system. Diagn. Pathol. 2024, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xie, Y.; Yao, J.; Li, X. The Dual-Mode Transition of Myofibroblasts Derived from Hepatic Stellate Cells in Liver Fibrosis. Int. J. Mol. Sci. 2023, 24, 15460. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rennie, C.; Donnelly, S.; McGrath, K. Albumin Reduces Hepatic Steatosis and Inflammation in High-Fat-Diet-Fed Mice. Int. J. Mol. Sci. 2025, 26, 7156. https://doi.org/10.3390/ijms26157156
Rennie C, Donnelly S, McGrath K. Albumin Reduces Hepatic Steatosis and Inflammation in High-Fat-Diet-Fed Mice. International Journal of Molecular Sciences. 2025; 26(15):7156. https://doi.org/10.3390/ijms26157156
Chicago/Turabian StyleRennie, Claire, Sheila Donnelly, and Kristine McGrath. 2025. "Albumin Reduces Hepatic Steatosis and Inflammation in High-Fat-Diet-Fed Mice" International Journal of Molecular Sciences 26, no. 15: 7156. https://doi.org/10.3390/ijms26157156
APA StyleRennie, C., Donnelly, S., & McGrath, K. (2025). Albumin Reduces Hepatic Steatosis and Inflammation in High-Fat-Diet-Fed Mice. International Journal of Molecular Sciences, 26(15), 7156. https://doi.org/10.3390/ijms26157156






