Abstract
Lifileucel, a tumor-infiltrating lymphocyte (TIL) cell therapy approved for advanced melanoma, demonstrates promise for treating other solid tumors, including endometrial cancer (EC). The current study evaluates the feasibility of manufacturing TILs from EC tumors using Iovance’s proprietary 22-day Gen2 manufacturing process. Key parameters, including TIL yield, viability, immune phenotype, T-cell receptor clonality, and cytotoxic activity, were assessed. Of the 11 EC tumor samples processed at research scale, 10 (91%) successfully generated >1 × 109 viable TIL cells, with a median yield of 1.1 × 1010 cells and a median viability of 82.8%. Of the four EC tumor samples processed at full scale, all achieved the pre-specified TVC and viability targets. Putative tumor-reactive T-cell clones were maintained throughout the manufacturing process. Functional reactivity was evidenced by the upregulation of 4-1BB in CD8+ T cells, OX40 in CD4+ T cells, and increased production of IFN-γ and TNF-α upon autologous tumor stimulation. Furthermore, antitumor activity was confirmed using an in vitro autologous tumor organoid killing assay. These findings demonstrate the feasibility of ex vivo TIL expansion from EC tumors. This study provides a rationale for the initiation of the phase II clinical trial IOV-END-201 (NCT06481592) to evaluate lifileucel in patients with advanced EC.
1. Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries, with rising incidence rates globally [1]. While early-stage EC is often curable with surgery and adjuvant therapies, advanced or recurrent disease remains a significant clinical challenge, with limited treatment options and poor survival outcomes [1]. Immune checkpoint inhibitors (ICIs) have shown promise in a subset of patients with EC, particularly those with mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) tumors [2]. However, even in this subgroup, at least 20% of patients experience recurrence within 12 months and others may experience recurrences later [2]. Furthermore, among patients with the more common proficient MMR (pMMR) tumors, the addition of ICI to chemotherapy and following chemotherapy has modestly improved progression-free survival, but there is no sign of overall survival benefit [3,4]. Both of these clinical scenarios point to the need for continued therapeutic development in this space.
Tumor-infiltrating lymphocytes (TILs) represent a promising avenue for adoptive cell therapy (ACT) in solid tumors [5,6]. TILs are naturally occurring T cells that infiltrate tumors and possess intrinsic tumor-specific reactivity. Preclinical and clinical studies have demonstrated that TILs can recognize and eliminate tumor cells through diverse mechanisms, including the release of cytotoxic molecules and cytokines, as well as direct cell-to-cell contact [7,8,9]. The success of TIL therapy in advanced melanoma, exemplified by the recent FDA approval of lifileucel [10], has sparked interest in extending this approach to other solid tumors, including EC. However, the feasibility of generating TILs from EC tumors and their functional characterization remain underexplored.
The manufacturing of TILs for therapeutic use involves the isolation, ex vivo expansion, and reinfusion of autologous TILs into patients. Iovance’s proprietary 22-day Generation 2 (Gen2) manufacturing process has been implemented to produce large quantities of viable, tumor-reactive TILs while maintaining their functional potency [11,12]. This process has been applied in melanoma and other solid tumors, including NSCLC, head and neck cancer, and cervical cancer. Key considerations in TIL therapy for EC include the heterogeneity of the tumor microenvironment, the variability in TIL infiltration across tumor subtypes, and the need to preserve tumor-reactive T-cell clones during expansion [13,14].
The current study aims to evaluate the feasibility of manufacturing TILs from EC tumors using the Gen2 process and to characterize their phenotypic and functional properties. Critical parameters were assessed, including TIL yield, viability, immune phenotype, and T-cell receptor (TCR) clonality. Additionally, the tumor-reactive activity of TILs was investigated through activation marker expression and cytokine production. Through addressing the challenges of TIL manufacturing and demonstrating robust antitumor activity, this study lays the groundwork for advancing TIL therapy as a viable treatment option for EC patients.
2. Results
2.1. Successful Manufacturing of Endometrial TIL
To study the feasibility of manufacturing endometrial TILs, 11 distinct patients’ EC tumor samples, comprising five dMMR and six pMMR cases (Table A1), were processed. The tumor samples were from patients with EC aged 45–84 years, predominantly White (n = 10), with one Hispanic patient. Histopathology revealed endometrioid adenocarcinoma/carcinoma in 10 cases with FIGO grading ranging from I to III, with 73% (8/11) classified as grade II and one case as high-grade serous carcinoma (Table A1).
The tumor manufacturing outcomes (Table 1) demonstrated robust viability and scalability: 10 of 11 samples (91%) yielded >1 × 109 extrapolated total viable cells (TVC), with a median TVC rate of 1.1 × 1010 and a median viability of 82.8%. High CD45+CD3+ T cells were enriched in the final TIL product (median: 97%, range: 90.2–98.3%). Notably, CD8+ T-cell proportions varied significantly across samples (6.4–63.1%), with the highest CD8+ infiltration (63.1%) observed in END22098 (the Hispanic patient’s tumor). Conversely, CD4+ T-cell dominance (>75%) characterized four cases. Only one sample (END22063) failed viability testing, highlighting occasional processing challenges. The data also indicate that manufacturing can be successfully achieved regardless of MMR status (Table 1 and Table A1). These results underscore the feasibility of generating TIL-based immunotherapies from EC tumors.

Table 1.
Summary of product attributes.
2.2. Phenotypic Characterization of Endometrial TILs
Flow cytometry analyses were performed to characterize TIL phenotype. As shown in Figure 1, the data revealed immune profiles consistent with historical data of TILs derived from other solid tumor types [15]. Analysis of five EC-derived TILs revealed that both the CD4+ and CD8+ subsets expressed markers associated with co-stimulation (CD27 and CD28), memory (CD45RA, CD62L, CD127), activation (CD25 and CD69), migration (CXCR3), exhaustion (LAG3, PD-1, TIM3, CD38, CD39), and transcription factors known to regulate these molecules and effector functions (EOMES, Tbet, and TOX). Notably, CD8+ TILs, in comparison to CD4+ TILs, had different expressions of various markers, including increased CD27, LAG3, TIM3, and EOMES. The expression of these markers in conjunction with Tbet, TOX, memory markers, and exhaustion markers are suggestive of an effector memory, T progenitor exhausted, or terminal exhaustion phenotype, all of which are critical for antitumor immunity [16,17].
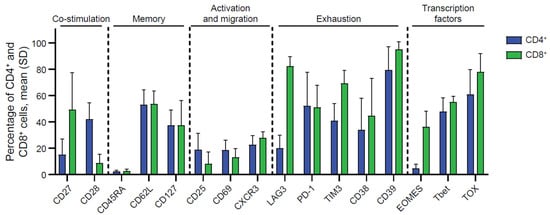
Figure 1.
Phenotypes of endometrial TIL were characterized by flow cytometry. Cellular phenotypic analysis expression markers related to co-stimulation, memory, activation, migration, exhaustion, and transcription factors in both CD4+ T and CD8+ T subsets. Data generated from EC TIL samples were presented as expression frequency (n = 5, biological donors). See Figure S1 for gating strategy.
2.3. Single-Cell Analysis of Endometrial TILs
With the end goal of assessing the neoantigen-reactive T-cell population of EC tumors, single cell RNA+TCR sequencing was performed on six CD45+ enriched EC tumor digest samples. After QC and filtering, a total of 37,054 single cells remained for downstream analysis (Table A2). Using a pan-cancer automated cell type annotation tool, a notable proportion of cells were annotated as macrophage, cDC2, and T-regulatory (Treg) cells (Figure 2A,B), suggesting an immunosuppressive tumor microenvironment consistent with previous reports for EC [18]. T cells were then subgrouped and manual annotation of clusters resulted in 19 unique tumor-infiltrating T-cell subsets (Figure 2C,D). Naive/memory-like (marked by CCR7, KLF2, and IL7R) and Treg (marked by FOXP3 and IL2RA) were among the most prevalent cell types among T cells (Figure 2D,E). Interestingly, these two populations mainly expressed TCRs that fell into the small- or medium-proportion scTCR clone group, whereas clusters of CD8+ T cells that exhibited an exhausted, progenitor exhausted, activated, or effector-like phenotype appeared to express the higher frequency (Hyperexpanded) TCR clones found in the tumor (Figure 2F). Using the NeoTCR gene set scoring method of putative neoantigen-specific T-cell identification [19], NeoTCR4 and NeoTCR8 clusters were annotated (Figure 2G). To track these putative neoantigen-specific T cells in the corresponding TIL drug products (DPs), bulk TCR-seq was performed on the TIL DP. The TCRβ CDR3 clonotypes from the NeoTCR clusters were then identified and quantified in the TIL DP bulk TCR-seq data. Notably, the majority of these putative neoantigen-reactive T-cell clonotypes detected in the tumor digest were preserved in the final TIL product, as demonstrated by proportional and quantitative tracking of TCR clonotypes (Figure 2H–K). While the proportion of neoantigen-specific TCRs varied between the tumor and TIL samples, their consistent presence in expanded products underscores the fidelity of TIL expansion protocols in maintaining tumor-reactive populations. These findings demonstrate that EC-derived TILs retain clinically relevant neoantigen-specific T cells through ex vivo processing, supporting their utility for ACT. Meanwhile, the heterogeneity in clonotype abundance across samples may reflect individual tumor immunogenicity or expansion efficiency, warranting further investigation in a larger population.
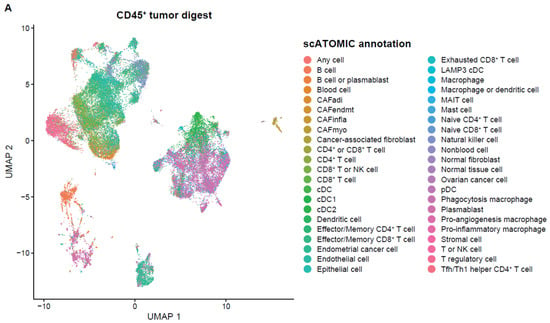
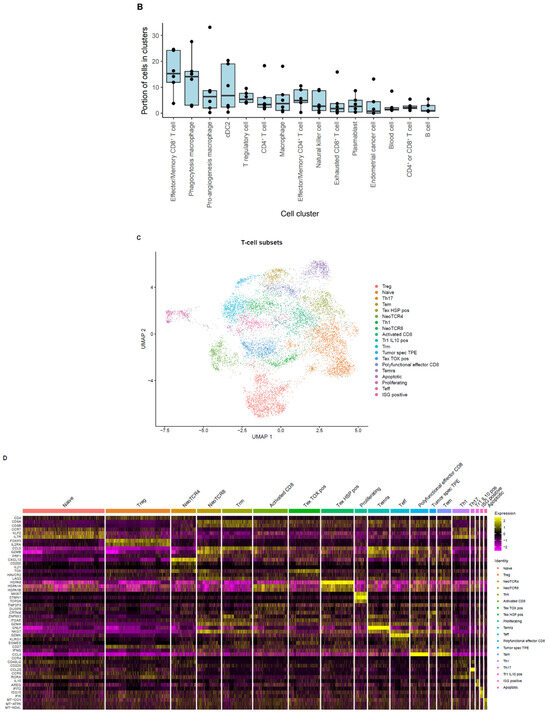
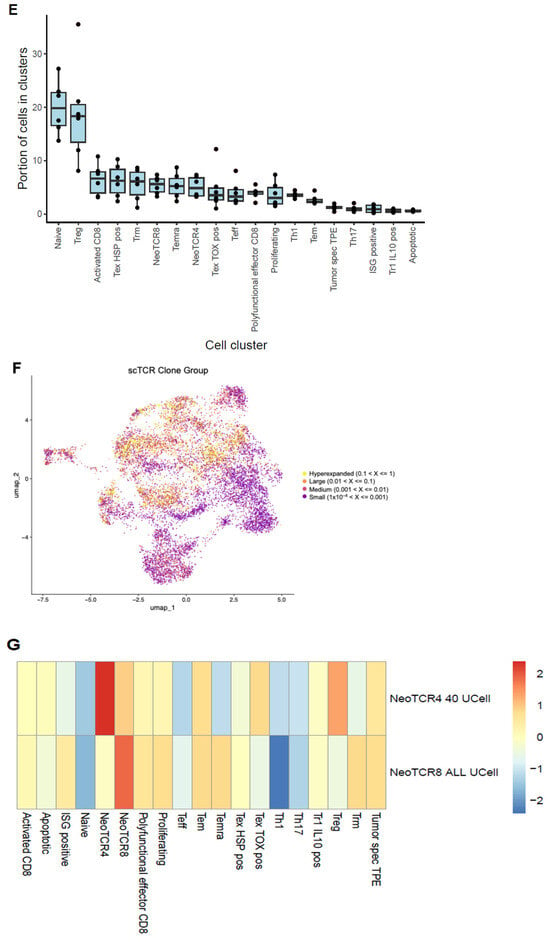
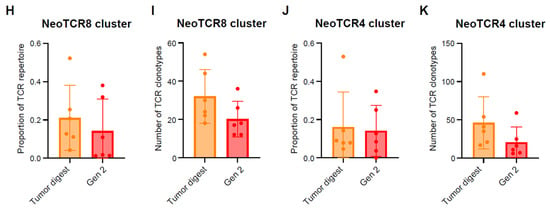
Figure 2.
Single cell analysis of endometrial tumor infiltrating leukocytes and tracking of putative neoantigen-reactive T cells. (A) UMAP dimensionality reduction visualization projecting cell type annotations using the scAtomic package (v2.0.2) on integrated single-cell data consisting of 6 EC CD45+ tumor samples (B) Proportion of cells in the top 15 cell types ordered based on the average value across the 6 EC CD45+ tumor samples. (C) Integrated single-cell T-cell subset with 19 manually annotated cell types. (D) Heatmap of the normalized gene expression for the top marker genes detected in each cell type. (E) Proportion of cells in the T cell integrated single-cell data ordered based on the average value across 6 samples. (F) Integrated T-cell subset UMAP dimensionality reduction visualization projecting single cell TCR clone group, labeled based on the frequency of clones (X): hyperexpanded (0.1 < X < 1), large (0.01 < X < 0.1), medium (0.001 < X < 0.01) and small (1 × 10−4 < X < 0.001). (G) Expression level of the NeoTCR8 and NeoTCR4 gene set scores for all clusters. Clusters with the highest score for the NeoTCR8 and NeoTCR4 gene sets were annotated as NeoTCR8 and NeoTCR4 cell clusters, respectively. TCR clonotypes from the NeoTCR8 cluster were identified and tracked from tumor digest to TIL drug product via bulk TCR-seq. The clonotype proportion (H) and number (I) of unique clonotypes for each sample are summarized. TCR clonotypes from the NeoTCR4 cluster were identified and tracked from tumor digest to Gen2 TIL drug product via bulk TCR-seq. The clonotype proportion (J) and number (K) of unique clonotypes for each sample are summarized. Six biological replicates were used for the single-cell analysis.
2.4. Functional Evaluation of Endometrial TIL Reactivity
In addition to identifying putative tumor-reactive TIL clonotypes, the presence of tumor-reactive T cells was confirmed by in vitro co-culture assays. Robust upregulation of 4-1BB within CD8+ TILs and OX40 upregulation within CD4+ TIL upon co-culture with autologous tumor digests were observed. The upregulation of 4-1BB and OX40 was diminished in the TIL-digest co-cultures when HLA blocking antibodies were added, signifying that HLA-peptide recognition by the TIL triggered the expression of 4-1BB and OX40 (Figure 3A–C). Moreover, recognition of autologous tumor digest by TIL induced the production of IFN-γ, TNF-α, and MIP-1β in comparison to TIL cultured without stimulus (TILs only) (Figure 4A). The production of cytokines was 2- to 6.3-fold higher in comparison to cultures with HLA blocking antibodies (Figure 4B; see Table S1). Together, these data establish that expanded EC TIL products retain autologous tumor reactivity in an HLA-dependent manner, with both CD8+ and CD4+ subsets contributing to effector responses through distinct activation pathways (4-1BB vs. OX40) and potent Th1 cytokine production.
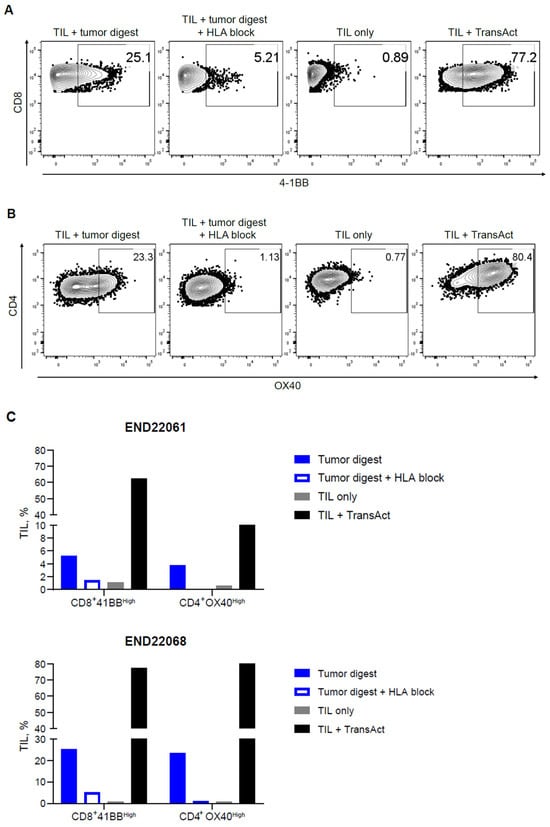
Figure 3.
Endometrial drug product TILs are activated when cultured with autologous tumor digest. Endometrial TILs (derived from two donors) were co-cultured with a matched tumor digest with or without HLA-blocking antibodies to block the interaction of TCRs with cognate peptide-MHC tumor antigens. TILs were cultured alone (TILs only) or with TransAct, functioning as negative and positive controls, respectively, for 4-1BB expression in CD8+ TILs, and OX40 expression in CD4+ TILs. (A) Representative dot plot for 4-1BB upregulation in CD8+ T cells and (B) OX40 upregulation in CD4+ T cells after 24 h of co-culture. (C) Summarized data for both TIL donors (END22061 and END22068) demonstrating 4-1BB or OX40 upregulation in an HLA-dependent manner within CD8+ and CD4+ TILs, respectively.
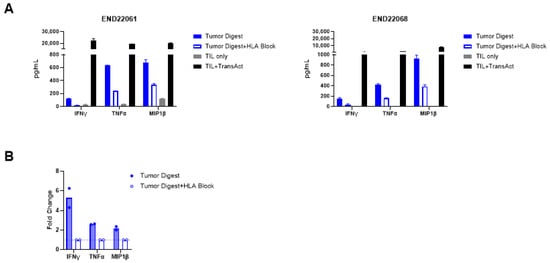
Figure 4.
Endometrial drug product TILs produce effector cytokines in response to tumor digest. (A) Production of IFN-γ, TNF-α, and MIP-1β by TILs from two donors, END22061 (left) and END22068 (right), in response to autologous tumor digest stimulation, and the abrogation of cytokine production upon HLA block. TILs were stimulated with TransAct as a positive control, demonstrating robust cytokine production. (B) Fold change in cytokine production by TILs cultured with autologous tumor digest in comparison to co-cultures of tumor digest and HLA-blocking antibodies. See also Table S1 for the cytokine values of each patient’s TILs in technical replicates of two.
2.5. In Vitro Antitumor Activity of Endometrial TIL
After identifying that EC TILs can increase the expression of activation markers and produce effector cytokines upon recognition of primary tumor cells within tumor digests, autologous tumor killing in vitro was evaluated. A patient-derived endometrial tumor organoid (tumoroid; END22098) was established. Upon co-culture, the TILs swarmed the tumoroids and mediated the destruction of these cells over multiple days of culture (Figure 5A,B). Moreover, the magnitude of tumor killing was dependent on the effector-to-target (ET) ratio, as demonstrated by strong activity at high ET ratios, which diminished at low ET ratios (Figure 5C). However, the TILs still maintained a level of killing at the lowest ET ratio (0.5:1) conferring a cytostatic effect, preventing the expansion of tumoroids. These data demonstrate that EC TILs can mount robust killing of three-dimensional tumors in vitro over the course of multiple days, recapitulating critical in vivo mechanisms observed in tumor beds required for mediating clinical responses.
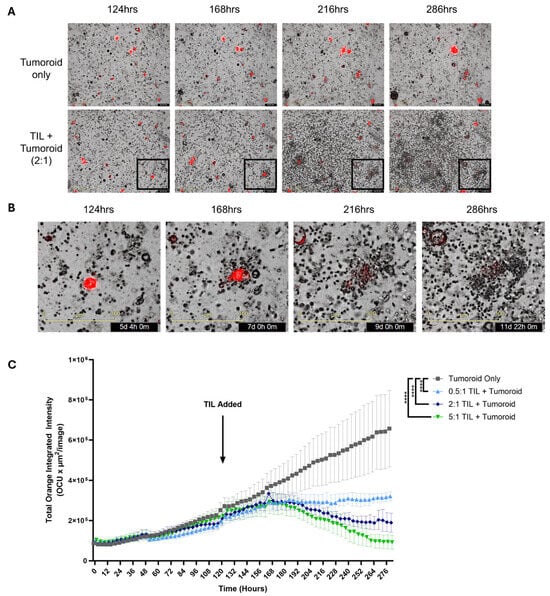
Figure 5.
TIL-mediated autologous killing of patient-derived tumoroid. An endometrial tumoroid from a single donor (END22098) stably expressing mCherry was grown for 124 h prior to adding autologous TILs to monitor and assess killing using the Incucyte® system (Sartorius AG, Göttingen, Germany). (A) Representative images of the growth of an endometrial tumoroid and its killing of the tumoroid by autologous TILs between 124 and 286 h at 100× magnification. Tumoroid only (top row); TILs+Tumoroid at a 2:1 ET ratio (bottom row). (B) Lower right boxed area in A is magnified, demonstrating TIL swarming, tumoroid contact, and the destruction of the tumoroid. (C) Incucyte assay comparing tumor cell killing by increasing doses of TILs. Summarized data representing autologous TILs killing of tumoroids, which is dependent on the effector to target (TILs/Tumoroid) ratio. Tumoroids were grown without TILs (gray), 0.5:1 (pale blue), 2:1 (dark blue), and 5:1 (green) TILs/Tumoroid ratios. Data for each group are represented as the mean ± SEM. Statistical analysis was performed using a Friedman test, with Dunn’s multiple comparisons test, **** p < 0.0001. Scale bar in (A) 400 µm, (B) 300 µm.
2.6. Full-Scale Manufacturing of the Endometrial TIL Drug Products
To further validate the manufacturing feasibility, an additional four endometrial tumors from patients were processed at full scale using Gen2 manufacturing close to the clinical manufacturing process. The baseline characteristics of all patients included in this study are presented in Table A1. Full-scale TIL products were successfully achieved for all four EC tumor samples (END22111, END22133, END22134 and END22135), meeting or exceeding critical quality benchmarks (Table 2). All TIL products demonstrated high viability (88.5–95.5%, exceeding the ≥70% target) and purity, with CD45+/CD3+ T-cell enrichment ≥96% (surpassing the ≥90% threshold). The TVC yields ranged from 2.7 × 109 to 77.1 × 109 cells, encompassing the expected target dose range (1 × 109–150 × 109 cells), while tumor cell impurities (TROP2+/EPCAM+) remained minimal (<0.1%). Functional potency, assessed via Dynabead-stimulated IFN-γ release, varied between 3940 and 12,620 pg/mL, reflecting inherent biological variability in TIL effector function. These results confirm the robustness of the manufacturing process across diverse EC samples, consistently generating TIL products with attributes suitable for clinical application. The successful ex vivo expansion of TILs from EC tumors supports the feasibility of TIL manufacturing from EC tumors for use in the adoptive transfer setting.

Table 2.
Final product attributes of endometrial TILs manufactured at full scale.
3. Discussion
The current study demonstrates the feasibility of manufacturing TILs from EC tumors using Iovance’s proprietary 22-day Gen2 process at both the research scale and full-scale levels and provides evidence of their antitumor activity. Of the 11 EC tumor samples processed at research scale, 10 (91%) successfully generated a TIL drug product exceeding the target yield of 1 × 109 viable cells, with a median yield of 1.1 × 1010 cells and high viability (82.8%). Of the four EC tumor samples processed at full scale, all (100%) achieved the pre-specified TVC and viability targets. These results align with the manufacturing success rates observed in melanoma and other solid tumors, underscoring the robustness of the Gen2 process for TIL expansion in EC.
Neoantigen-driven recognition and T-cell-mediated killing contribute to tumor clearance following ACT with TILs. Accumulating clinical trial data support that the absolute number and frequency of tumor-reactive lymphocytes in TIL drug products from patients with metastatic melanoma highly correlate with clinical efficacy and increased survival [20,21]. In this study, the tumor-reactive lymphocytes in TIL products were assessed using Lowery’s NeoTCR signature [19], confirming the presence of putative tumor-specific TILs in the drug product. The upregulation of activation markers such as 4-1BB on CD8+ T cells [22] and OX40 on CD4+ T cells [23] upon autologous tumor stimulation provides functional evidence of tumor reactivity. These markers are associated with T-cell activation, survival, and memory formation, which are essential for sustained antitumor responses. The increased production of IFN-γ and TNF-α by TILs in response to tumor stimulation highlights their capacity to mount a robust cytokine-mediated anti-tumor response, a hallmark of effective TIL therapy. Furthermore, the killing of tumor cells grown as three-dimensional tumoroids provides evidence that tumor-specific TILs are present in the drug product, supporting their potential to mount cytotoxic activity in vivo. The combination of phenotypic transcriptional, TCR clonotypic, and functional data supports the hypothesis that TIL therapy could be a treatment option for advanced or recurrent EC.
The success of TIL therapy in melanoma has paved the way for its potential application in other solid tumors, including EC. However, several challenges remain. The heterogeneity of EC, both in terms of molecular subtypes and immune infiltration, may influence the efficacy of TIL therapy [14,24]. For example, tumors with high levels of immune cell infiltration, such as those with a POLE mutation or MSI-H status, are hypothesized to be more responsive to TIL therapy and warrant further investigation.
In conclusion, this study demonstrates the feasibility of generating functional TILs from endometrial cancer (EC) using the Gen2 manufacturing process, with robust anti-tumor activity supporting therapeutic potential. The findings support the ongoing phase II trial IOV-END-201 (NCT06481592) evaluating the safety and efficacy of lifileucel in patients with advanced EC. As ACT advances, EC tumor-derived TILs represent a promising strategy to harness the immune system against cancer, offering new hope for patients with advanced or recurrent EC.
4. Materials and Methods
4.1. Tumor Processing for Expansion of TILs and Immunophenotyping
To evaluate the feasibility of TIL expansion from endometrial carcinomas, fresh tumor specimens weighing more than 1 g were obtained from certified tumor biospecimen providers. All tumor samples were collected under protocols requiring documented informed consent from participating patients. Each tumor sample was accompanied by detailed clinical information, enabling comprehensive analysis. Tumor material was collected irrespective of origin, histologic classification (based on morphology and grade), molecular classification, or prior treatment status. Specifically, patient information contained patient age, race/ethnicity, tumor staging, and molecular profiles. The baseline characteristics of all patients included in this study are presented in Table A1.
The Gen2 TIL manufacturing process used to generate the endometrial TIL final product included a pre-rapid expansion protocol (REP) phase (days 0–11) and REP phase (days 11–22). During pre-REP (days 11–22), performed at 1/10th small-scale and full-scale, 1- to 3 mm tumor fragments were placed in media containing high-dose interleukin-2 (IL-2) for 11 days, allowing TILs to migrate out from the tumor. To further promote TIL growth, TILs were expanded by REP (performed at 1/50th and full-scale) that included irradiated PBMC, IL-2, and anti-CD3 for 11 days. Final harvested REP and in-process samples were assayed for total nucleated cells, TVCs, and viability.
Frozen TILs were thawed and stained with various markers related to exhaustion, activation, memory, and function. Three different panels were used, including a surface staining only panel, an intracellular staining panel using eBioscience™ Foxp3/Transcription Factor Staining Buffer Set (ThermoFisher Scientific, Waltham, MA, USA), and an intracellular staining panel using Monesin and Brefeldin A with anti-CD3 stimulated TILs. Stained cells were analyzed using a BioRad Ze5 flow cytometer (Hercules, CA, USA). Data were analyzed using FlowJo v10.8.1.
4.2. TIL Reactivity Assay
REP TILs were labeled with CellTrace Violet (C34557, Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Cryopreserved autologous tumor digest cells (1 × 105) were added to 96-well U bottom plates. Where applicable, HLA-blocking antibodies, Ultra-LEAF™ Purified anti-human HLA-A,B,C Antibody (BioLegend, San Diego, CA, USA) and Purified anti-human HLA-DR, DP, DQ Antibody (BioLegend), were added at a final concentration of 10 µg/mL each to tumor digest cultures and incubated for 10 min at room temperature. Then, CellTrace Violet-labeled TILs were added to the culture, followed by an overnight incubation. Each culture consisted of media containing IL-2 (300 IU/mL). For a negative control, TILs were cultured without stimulation (TILs only). For a positive control, TILs were cultured with GMP TransAct (MiltenyiBiotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. After overnight culturing, the supernatants were harvested for cytokine measurements of IFN-γ, TNF-α, and MIP-1β. Supernatants were assayed on the Bio-Plex 200 (Bio-Rad, Hercules, CA, USA) using Bio-Plex Pro Human Cytokine IFN-γ Set (171B5019M, Bio-Rad), Bio-Plex Pro Human Cytokine TNF-α Set (171B5026M, Bio-Rad), and Bio-Plex Pro Human Cytokine MIP-1β Set (171B5023M, BioRad). For flow cytometry analysis, TILs were harvested after overnight culturing and labeled with flow cytometry antibodies: CD3 BUV 395 (563546, BD Biosciences, San Jose, CA, USA), CD4 BV 510 (300546, BioLegend), CD8 BV 785 (344740, BioLegend), 4-1BB Alexa Fluor 647 (309824, BioLegend), and OX40 PE-Cy7 (119416, BioLegend). Viability was determined by staining with a LIVE/DEAD™ Fixable Near-IR Dead Cell Stain Kit (L10119, Invitrogen). For analysis, REP TILs were identified by CellTrace Violet staining followed by gating on CD3, CD4, and CD8 to identify upregulation of 4-1BB in CD8+ REP TILs and OX40 in CD4+ REP TILs.
4.3. Single-Cell RNA and Bulk TCR Sequencing
Human endometrial tumor material (n = 6, biological donors) was digested using the Human Tumor Dissociation kit (Miltenyi PN 130-095-929, Bergisch Gladbach, Germany) and the Miltenyi gentleMACS™ Octo Dissociator instrument according to the manufacturer’s protocols. Digested cell suspensions were filtered through 70 µm cell strainers and washed using RPMI medium. CD45+ cells were isolated from the day 0 tumor digest using the StemCell Technologies EasySep™ Release Human CD45 Positive Selection kit (PN 100-0105, Vancouver, BC, Canada) and then cryopreserved. Endometrial tumor digest samples (n = 6) were obtained from cryopreservation, thawed, and washed twice with PBS + 0.04% BSA. Cell viability was assessed using a Nexcelom Cellaca (Lawrence, MA, USA) automated cell counter with AOPI. Dead cell removal was performed on any sample containing fewer than 75% viable cells using the Miltenyi Dead Cell Removal kit, according to manufacturer guidelines. Single cell Gel-bead emulsions were prepared using 10× Genomics Chromium X controller (Pleasanton, CA, USA). Sequencing libraries were prepared using 10× 5′ Immune profiling v2 reagents following the manufacturer’s guidelines and sequenced on an Illumina NextSeq2000 instrument (San Diego, CA, USA) with an insert read length of 90 base pairs. Primary and secondary data analysis were performed using Cell Ranger (v7.0.0, 10× Genomics) with default parameters. The R package Seurat (v5.0.1) was used for tertiary analysis and data visualization [25]. Genes detected in less than 3 cells were discarded and TCRA and TCRB family genes were removed to avoid clonotype clustering bias. Dead cells, doublets, or low-quality cells were discarded based on the following empirically determined filter: percent mitochondria reads less than 8% and number of genes detected greater than 500 but less than 6000. Single cell TCR-seq data were added to the single cell meta data, and visualizations were generated using scRepertoire (v1.8.0) [26]. Cells where a TCR was detected and gene expression was consistent with a T cell (e.g., CD3E) were kept for downstream analysis of T-cell subsets. sctransform (v0.4.1) [27] was used for normalization and feature selection, followed by PCA, and integration using harmony (v1.2.0) [28] with default parameters. Clustering was performed at resolution = 2 and UMAP visualizations were generated using Seurat. Automated cell type annotation was performed using scATOMIC (v2.0.2) [29]. For tumor digest T-cell subsets, manual annotation of clusters was performed using cluster marker genes, scATOMIC annotations, and cell-type–specific gene signatures with the R package VISION (v3.0.1) [30]. For gene set analysis, the R packages escape (v1.9.0) [31] and UCell (v2.2.0) [32] were used to quantify the gene set scores for the NeoTCR8 gene signature from Lowrey et al. [19]. TCRβ clonotypes expressed by the NeoTCR8 cluster were identified and exported for further analysis. Heatmap and boxplot visualizations were generated using pheatmap (v1.0.12) [33] and ggplot2 (v3.4.4) [34], respectively.
Matching endometrial TIL drug product samples (n = 6) were obtained from cryopreservation, thawed, and washed twice with PBS + 0.04% BSA. Total RNA was extracted from approximately 1 million cells using an NEB Monarch Total RNA Miniprep Kit (Ipswich, MA, USA). Purified total RNA (500 ng) was used as template for preparing TCR beta-chain libraries using Takara SMARTer Human TCR a/b Profiling Kit v2 (Kusatsu, Japan), according to the manufacturer’s guidelines. Equimolar quantities of each library were pooled together and sequenced on a NextSeq2000 instrument (San Diego, CA, USA) with paired end 150 bp reads. High base level sequencing quality of reads was confirmed using DRAGEN’s FastQC module (Illumina DRAGEN v3.10.12). Primary analysis of fastq files to identify and quantify productive rearrangements (clonotypes) was performed using RTCR (v0.5.1) with default parameters [35]. Clonotypes with a less than perfect average base quality score in the CDR3 region were filtered out. Tertiary analysis was performed using the R package immunarch (v0.9.1) [36]. Clonotypes from the NeoTCR8 cluster were identified in the TIL Bulk TCR-Seq and their number and proportion of the total TCR repertoire exported for visualization in GraphPad Prism (v10.0.3).
4.4. Tumor Organoid Establishment and TIL Co-Culture Assay
To generate tumoroids, digested cell suspensions were generated as described above and cultured in Cultrex RGF BME Type II, w/o phenol red (R&D systems, Minneapolis, MN, USA, 3536-005-02) in the media listed in Table A3. When a tumoroid grew beyond 2 passages, the cells were transduced with a recombinant lentivirus, pLV[Exp]-Puro-EF1A > mCherry (VectorBuilder, Chicago, IL, USA). mCherry-labeled tumoroids were seeded in 96 well plates for live cell imaging and killing assay using the Incucyte SX5 Live-Cell Analysis imaging system (Sartorius, Göttingen, Germany). After 5 days of growth, autologous TILs were added to tumoroid cultures at various ET ratios. The final media formulation was 50% tumoroid media and 50% TIL media containing IL-2 (300 IU/mL). Tumoroid growth was quantified by the increase in mCherry fluorescence in the orange channel, and killing was quantified by the reduction in mCherry fluorescence over time.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26157151/s1.
Author Contributions
Conceptualization, A.A.J. and H.Y.; methodology, P.I., I.P., A.Y., J.F., S.R.R.H. and J.W.D.; validation, I.P.; formal analysis, P.I., I.P., J.W.D. and S.R.R.H.; investigation, J.W.D.; data curation, I.P. and J.W.D.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z., K.N.M., J.F., I.P., A.Y., P.I., N.G., J.W.D., B.D., J.Y., R.Q., M.R.S.-A., E.C., S.R.R.H. and H.Y.; writing—review and editing and final approval, H.Y.; visualization, Y.Z., P.I., I.P. and J.W.D.; supervision, Y.Z., S.R.R.H. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study is sponsored by Iovance Biotherapeutics (San Carlos, CA, USA).
Institutional Review Board Statement
This study was approved by the Biomedical Sciences Institutional Review Board of The Ohio State. (Study Number: 2014H0130, approved on 18 February 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Sequencing data generated during the study have not been made publicly available in a repository due to proprietary considerations and to protect the privacy and confidentiality of the participating patients. Upon reasonable request and authorization by Iovance Biotherapeutics, and after appropriate data-sharing and use agreements have been agreed upon, eligible academic researchers in the field may be provided access to de-identified gene-limited datasets. Requesters should submit a proposal outlining the objective, data format and features, hypothesis and specific rationale to the corresponding authors.
Acknowledgments
Medical writing and editorial support were provided by Peloton Advantage, LLC, an OPEN Health company.
Conflicts of Interest
Authors Yongliang Zhang, Judy Fang, Ilabahen Patel, Andrew Yuhas, Patrick Innamarato, Nathan Gilbert, Joseph W. Dean, Behzad Damirchi, Joe Yglesias, Rongsu Qi, Michelle R. Simpson-Abelson, Erwin Cammaart, Sean R. R. Hall, and Hequn Yin were employed by Iovance Biotherapeutics. Author Yongliang Zhang is employed by Immunebro Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Iovance Biotherapeutics. The funder and its employees conducted the experiments and collected the data.
Abbreviations
The following abbreviations are used in this manuscript:
| ACT | Adoptive cell therapy |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| CDR3 | Complementarity determining region 3 |
| cDC | Conventional dendritic cell |
| cDC1 | Conventional dendritic cell type 1 |
| cDC2 | Conventional dendritic cell type 2 |
| dMMR | Mismatch repair-deficient |
| DP | Drug product |
| EC | Endometrial cancer |
| ET | Effector-to-target |
| FDA | US Food and Drug Administration |
| FIGO | International Federation of Gynecology and Obstetrics |
| Gen2 | Generation 2 |
| HLA | Human leukocyte antigen |
| ICI | Immune checkpoint inhibitor |
| ICOS | Inducible T-cell costimulator |
| IFN-γ | Interferon-gamma |
| IL-2 | Interleukin-2 |
| ISG | Interferon-stimulated gene |
| IU | International units |
| LAG3 | Lymphocyte activation gene-3 |
| LAMP3 | Lysosome-associated membrane protein 3 |
| MHC | Major histocompatibility complex |
| MIP-1β | Macrophage inflammatory protein beta |
| MMR | Mismatch repair |
| MSI-H | Microsatellite instability-high |
| N/A | Not applicable |
| NeoTCR | Neoantigen-reactive T cells |
| NIR | Near-infrared spectroscopy |
| NK | Natural killer |
| NSCLC | Non-small cell lung cancer |
| PBMC | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| pDC | Plasmacytoid dendritic cell |
| PD-1 | Programmed cell death protein-1 |
| pMMR | Mismatch repair-proficient |
| pNx | No lymph node was collected |
| pos | Positive |
| QC | Quality control |
| REP | Rapid expansion protocol |
| scTCR | Single cell T-cell receptor |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| TCR | T-cell receptor |
| Tem | Effector memory T cells |
| Tfh | Follicular helper T cells |
| Th1 | T helper 1 cells |
| TIL | Tumor-infiltrating lymphocytes |
| TIM3 | T-cell immunoglobulin and mucin domain-containing protein 3 |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor-alpha |
| TNM | Tumor, node, and metastasis |
| Treg | Regulatory T cells |
| TRM | Tissue-resident memory T cells |
| TVC | Total viable cells |
| UMAP | Uniform Manifold Approximation and Projection |
Appendix A

Table A1.
Clinical and pathological characteristics of all patients are included in this study.
Table A1.
Clinical and pathological characteristics of all patients are included in this study.
| Tumor ID | Tumor Site | Age | Race/ Ethnicity | Pathology | FIGO Grade | TNM Classification | MMR | MLH1 Promoter Methylation |
|---|---|---|---|---|---|---|---|---|
| END22061 | Uterus | 61 | White | Endometrioid adenocarcinoma | FIGO grade II | pT3a, pN0 | pMMR | |
| END22062 | Uterus | 68 | White | Endometrioid adenocarcinoma | FIGO grade II | pT1b, pN0 | dMMR | 81% |
| END22063 | Uterus | 67 | White | Endometrioid adenocarcinoma | FIGO grade II | pT1b, pN0 | dMMR | 97% |
| END22091 | Uterus | 83 | White | Endometrioid carcinoma | FIGO grade II | pT1a, pN0 | pMMR | |
| END22093 | Uterus | 63 | White | Endometrioid carcinoma | FIGO grade II | pT1b, pN0 | pMMR | |
| END22094 | Uterus | 66 | White | Endometrioid adenocarcinoma | FIGO grade I | pT1b, pN0 | pMMR | |
| END22095 | Uterus | 81 | White | Serous carcinoma | High grade (grade III) | pT3a, pN0 | pMMR | |
| END22096 | Uterus | 45 | White | Endometrioid carcinoma | FIGO grade II | pT3b, pNx | dMMR | Positive |
| END22098 | Uterus | 67 | Hispanic | Endometrioid carcinoma | FIGO grade II | pT1a, pN0 | dMMR | Positive |
| END22100 | Uterus | 69 | White | Endometrioid carcinoma | FIGO grade II | pT1a, pN0 | dMMR | MLH1 intact |
| END22102 | Uterus | 84 | White | Endometrioid carcinoma | FIGO grade III | pT1a, pNx | pMMR | |
| END22111 | Uterus | 69 | Not available | Endometrioid | FIGO grade I | pT1a, pN0 | pMMR | |
| END22133 | Uterus | 49 | Not available | Endometrioid carcinoma | FIGO grade II | pT1a, pN0 | dMMR | MLH1 intact |
| END22134 | Uterus | 35 | Not available | Endometrioid adenocarcinoma | FIGO grade II | pT1a, pN0 | pMMR | |
| END22135 | Uterus | 49 | White | Adenocarcinoma | Poorly differentiated | T1bN1M0 | Not available |

Table A2.
Count of single cells from EC samples before and after filtering and sub-setting.
Table A2.
Count of single cells from EC samples before and after filtering and sub-setting.
| Sample | Cell Count Before Filter | Cell Count After Filter | After T-Cell Subset |
|---|---|---|---|
| END22094 | 6106 | 5408 | 1830 |
| END22095 | 6413 | 5878 | 1655 |
| END22096 | 7722 | 6612 | 1705 |
| END22098 | 12,495 | 10,544 | 4761 |
| END22100 | 5498 | 4798 | 732 |
| END22102 | 4376 | 3814 | 667 |

Table A3.
Media formulation for endometrial tumoroid.
Table A3.
Media formulation for endometrial tumoroid.
| Media Component | Working Concentration | Manufacturer | Catalog # |
|---|---|---|---|
| 17B-Estradiol-Water Soluble | 10 mg/mL | Sigma Aldrich (St. Louis, MO, USA) | E4389 |
| A83-01 | 5 mM | Sigma Aldrich | SML0788-5MG |
| B27 (50X) | 1X | Gibco (Waltham, MA, USA) | 17504-044 |
| Chemically defined lipid concentrate | 1% | Life technologies (Carlsbad, CA, USA) | 11905031 |
| EGF | 50 ng/mL | Peprotech (Cranbury, NJ, USA) | AF-100-15 |
| GlutaMAX™ (100X) | 1X | Life technologies | 35050061 |
| HGF | 20 ng/mL | Peprotech | 100-39 |
| IGF-1 | 40 ng/mL | Peprotech | 100-11 |
| N2 (100X) | 1X | Life technologies | 17502001 |
| N-acetyl-cysteine | 1.25 mM | Sigma Aldrich | A7250-50G |
| Nicotinamide | 5 mM | Sigma Aldrich | 73240-100G |
| Noggin | 10 ng/mL | Peprotech | 120-10C |
| RH R-Spondin 3 | 25 ng/mL | R&D Systems | 3500-RS-025/CF |
| SB202190 | 100 nM | Sigma Aldrich | S7067-5MG |
| Y-27632 | 10 µM | Selleckchem (Houston, TX, USA) | S1049 |
| Anti-Anti (100X) | 1X | Gibco | 15-240-062 |
| Advance DMEM/F12 | n/a | Invitrogen | 12634028 |
#, number.
References
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; de Pont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: The phase III DUO-E trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Lee, S.M.; Doger de Spéville, B.; Gettinger, S.N.; Häfliger, S.; Sukari, A.; Papa, S.; Rodríguez-Moreno, J.F.; Graf Finckenstein, F.; Fiaz, R.; et al. Lifileucel, an Autologous Tumor-Infiltrating Lymphocyte Monotherapy, in Patients with Advanced Non-Small Cell Lung Cancer Resistant to Immune Checkpoint Inhibitors. Cancer Discov. 2024, 14, 1389–1402. [Google Scholar] [CrossRef]
- Muul, L.M.; Spiess, P.J.; Director, E.P.; Rosenberg, S.A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol. 1987, 138, 989–995. [Google Scholar] [CrossRef]
- Pockaj, B.A.; Sherry, R.M.; Wei, J.P.; Yannelli, J.R.; Carter, C.S.; Leitman, S.F.; Carasquillo, J.A.; Steinberg, S.M.; Rosenberg, S.A.; Yang, J.C. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer 1994, 73, 1731–1737. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-lifileucel-unresectable-or-metastatic-melanoma (accessed on 20 June 2025).
- Wardell, S.; Frank, I.; Karyampudi, L.; Veerapathran, A.; Simpson-Abelson, M.; Lienlaf Moreno, M.; Blaskovich, M.; Wypych, J.; Fardis, M. A cryopreserved tumor infiltrating lymphocyte (TIL) product for LN-144, generated with an abbreviated method suitable for high throughput commercial manufacturing exhibits favorable quality attributes for adoptive cell transfer [poster]. In Proceedings of the Annual Meeting of the Society for Immunotherapy of Cancer, National Harbor, MD, USA, 8–12 November 2017. [Google Scholar]
- Wardell, S.; Lienlaf-Moreno, M.; Blaskovich, M.; Frank, I.; Van Grod, V.; Fardis, M.; Wypych, J. Iovance gen 2 TIL manufacturing process produces drug products that exhibit favorable quality attributes for adoptive cell transfer across 5 solid tumor indications [poster]. In Proceedings of the Annual Meeting of the Society for Immunotherapy of Cancer, National Harbor, MD, USA, 6–10 November 2019. [Google Scholar]
- de Jong, R.A.; Leffers, N.; Boezen, H.M.; ten Hoor, K.A.; van der Zee, A.G.; Hollema, H.; Nijman, H.W. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol. Oncol. 2009, 114, 105–110. [Google Scholar] [CrossRef]
- Son, J.; George, G.C.; Nardo, M.; Krause, K.J.; Jazaeri, A.A.; Biter, A.B.; Hong, D.S. Adoptive cell therapy in gynecologic cancers: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 165, 664–670. [Google Scholar] [CrossRef]
- Cubas, R.; Yuhas, A.; Machin, M.; Zhang, Y. Decitabine treatment of tumor-infiltrating lymphocytes (TIL) during ex vivo expansion induces a more memory-like phenotype, reduces inhibitory receptor expression, and increases functionality [poster 270]. In Proceedings of the Annual Transplantation and Cellular Therapy Meetings of ASTCT and CIBMTR, Salt Lake City, UT, USA, 23–26 April 2022. [Google Scholar]
- Beltra, J.C.; Manne, S.; Abdel-Hakeem, M.S.; Kurachi, M.; Giles, J.R.; Chen, Z.; Casella, V.; Ngiow, S.F.; Khan, O.; Huang, Y.J.; et al. Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 2020, 52, 825–841.e828. [Google Scholar] [CrossRef]
- Chiffelle, J.; Barras, D.; Pétremand, R.; Orcurto, A.; Bobisse, S.; Arnaud, M.; Auger, A.; Rodrigo, B.N.; Ghisoni, E.; Sauvage, C.; et al. Tumor-reactive T cell clonotype dynamics underlying clinical response to TIL therapy in melanoma. Immunity 2024, 57, 2466–2482.e2412. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Liu, X.; Zhang, J.; Cao, Y.; Wei, B. Immune disorder in endometrial cancer: Immunosuppressive microenvironment, mechanisms of immune evasion and immunotherapy. Oncol. Lett. 2020, 20, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Lowery, F.J.; Krishna, S.; Yossef, R.; Parikh, N.B.; Chatani, P.D.; Zacharakis, N.; Parkhurst, M.R.; Levin, N.; Sindiri, S.; Sachs, A.; et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 2022, 375, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Donia, M.; Ellebaek, E.; Borch, T.H.; Kongsted, P.; Iversen, T.Z.; Hölmich, L.R.; Hendel, H.W.; Met, Ö.; Andersen, M.H.; et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin. Cancer Res. 2016, 22, 3734–3745. [Google Scholar] [CrossRef]
- Kristensen, N.P.; Heeke, C.; Tvingsholm, S.A.; Borch, A.; Draghi, A.; Crowther, M.D.; Carri, I.; Munk, K.K.; Holm, J.S.; Bjerregaard, A.M.; et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J. Clin. Investig. 2022, 132, e150535. [Google Scholar] [CrossRef]
- Ye, Q.; Song, D.G.; Poussin, M.; Yamamoto, T.; Best, A.; Li, C.; Coukos, G.; Powell, D.J., Jr. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin. Cancer Res. 2014, 20, 44–55. [Google Scholar] [CrossRef]
- Vetto, J.T.; Lum, S.; Morris, A.; Sicotte, M.; Davis, J.; Lemon, M.; Weinberg, A. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am. J. Surg. 1997, 174, 258–265. [Google Scholar] [CrossRef]
- Marín-Jiménez, J.A.; García-Mulero, S.; Matías-Guiu, X.; Piulats, J.M. Facts and Hopes in Immunotherapy of Endometrial Cancer. Clin. Cancer Res. 2022, 28, 4849–4860. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e3529. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, N.; Bormann, N.L.; Kraus, G. scRepertoire: An R-based toolkit for single-cell immune receptor analysis. F1000Research 2020, 9, 47. [Google Scholar] [CrossRef]
- Hafemeister, C.; Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019, 20, 296. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.R.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Nofech-Mozes, I.; Soave, D.; Awadalla, P.; Abelson, S. Pan-cancer classification of single cells in the tumour microenvironment. Nat. Commun. 2023, 14, 1615. [Google Scholar] [CrossRef]
- Jones, M.; Detomaso, D.; Ashuach, T.; Rosen, Y. VISION: Functional Interpretation of Single Cell RNA-seq Latent Manifolds. Available online: https://yoseflab.github.io/VISION/authors.html#citation (accessed on 21 May 2025).
- Borcherding, N.; Andrews, J. Package ‘Escape’. Easy Single Cell Analysis Platform for Enrichment. Available online: https://git.bioconductor.org/packages/escape (accessed on 21 May 2025).
- Andreatta, M.; Carmona, S.J. UCell: Robust and scalable single-cell gene signature scoring. Comput. Struct. Biotechnol. J. 2021, 19, 3796–3798. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. Available online: https://raivokolde.r-universe.dev/pheatmap (accessed on 21 May 2025).
- Wickham, H.; Navarro, D.; Pedersen, T.L. ggplot2: Elegant Graphics for Data Analysis; Springer Nature: New York, NY, USA, 2016. [Google Scholar]
- Gerritsen, B.; Pandit, A.; Andeweg, A.C.; de Boer, R.J. RTCR: A pipeline for complete and accurate recovery of T cell repertoires from high throughput sequencing data. Bioinformatics 2016, 32, 3098–3106. [Google Scholar] [CrossRef]
- Popov, A.; Samokhina, M.; Balashov, I.; immunarch.bot; Rumynskiy, E.; Nazarov, V.I.; gracecodeadventures; tsvvas; Zarodniuk, M. Immunomind/Immunarch: Immunarch 0.9.1 (0.9.1). Available online: https://doi.org/10.5281/zenodo.10840553 (accessed on 21 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).