Functionalised Mesoporous Silica Thin Films as ROS-Generating Antimicrobial Coatings
Abstract
1. Introduction
2. Results and Discusion
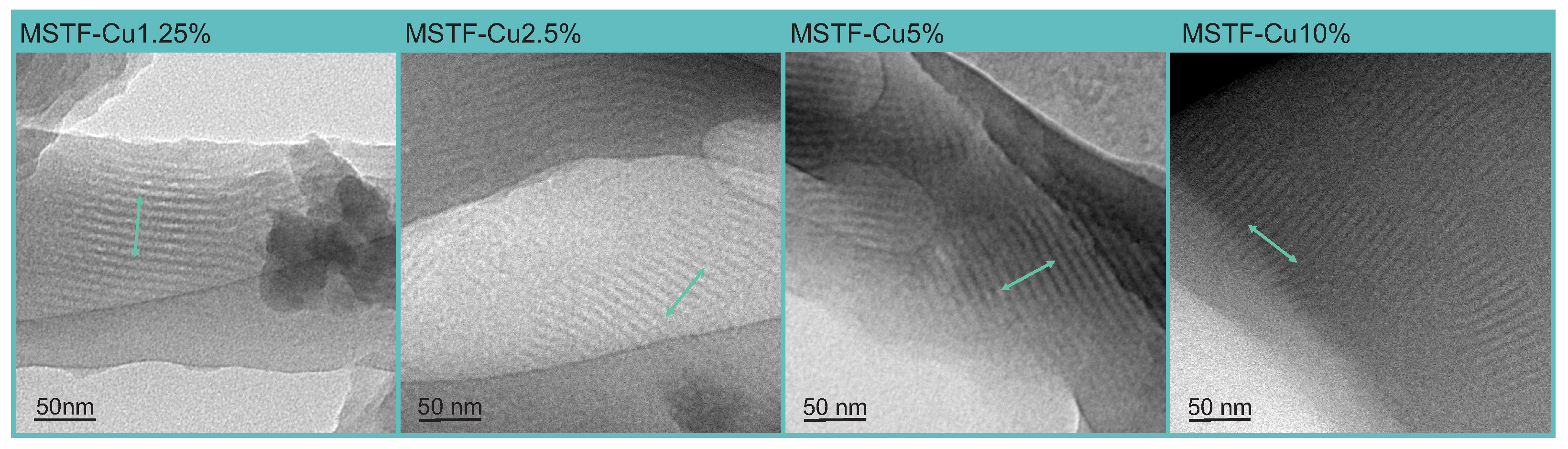
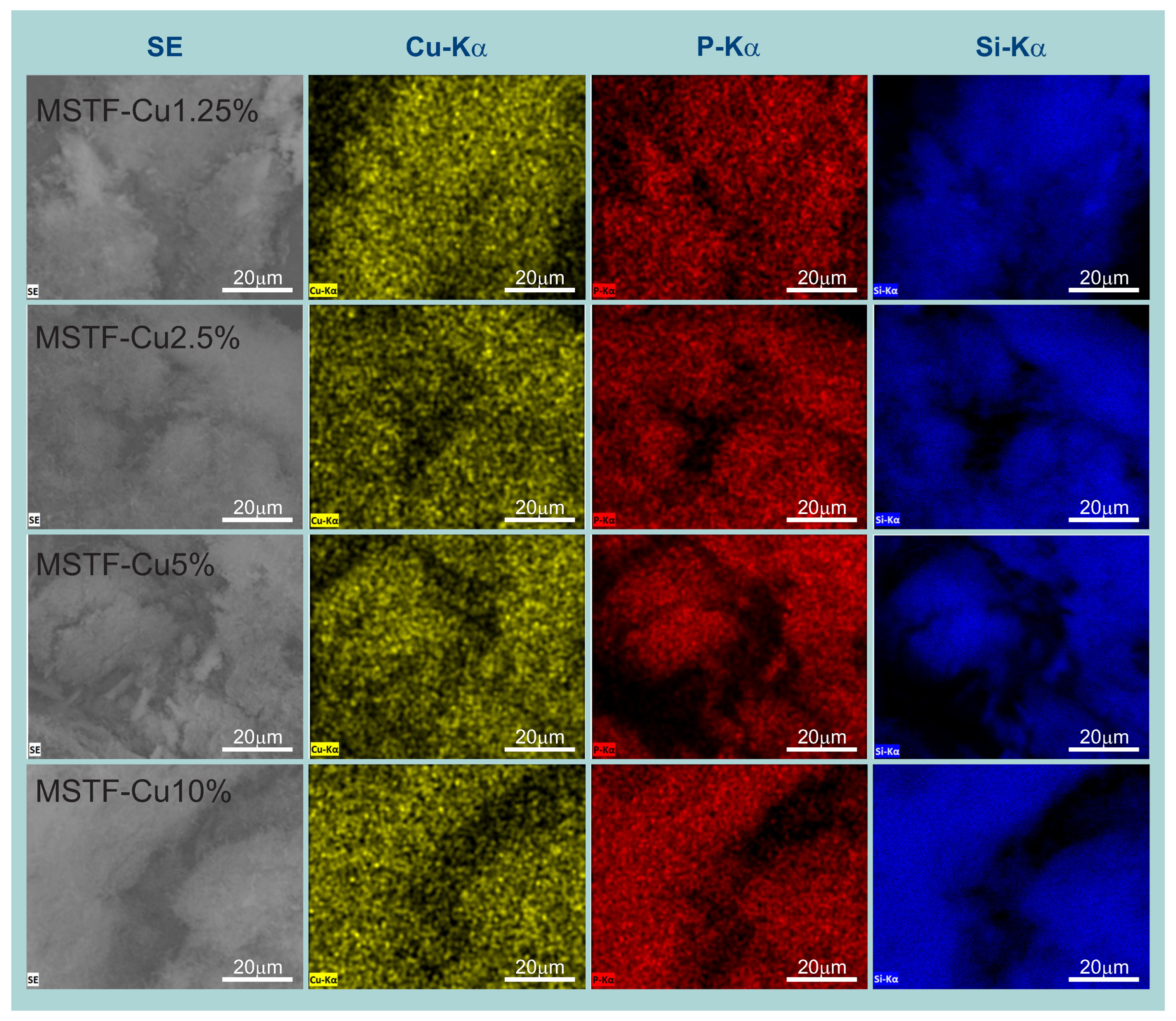
2.1. Physico-Chemical Analysis
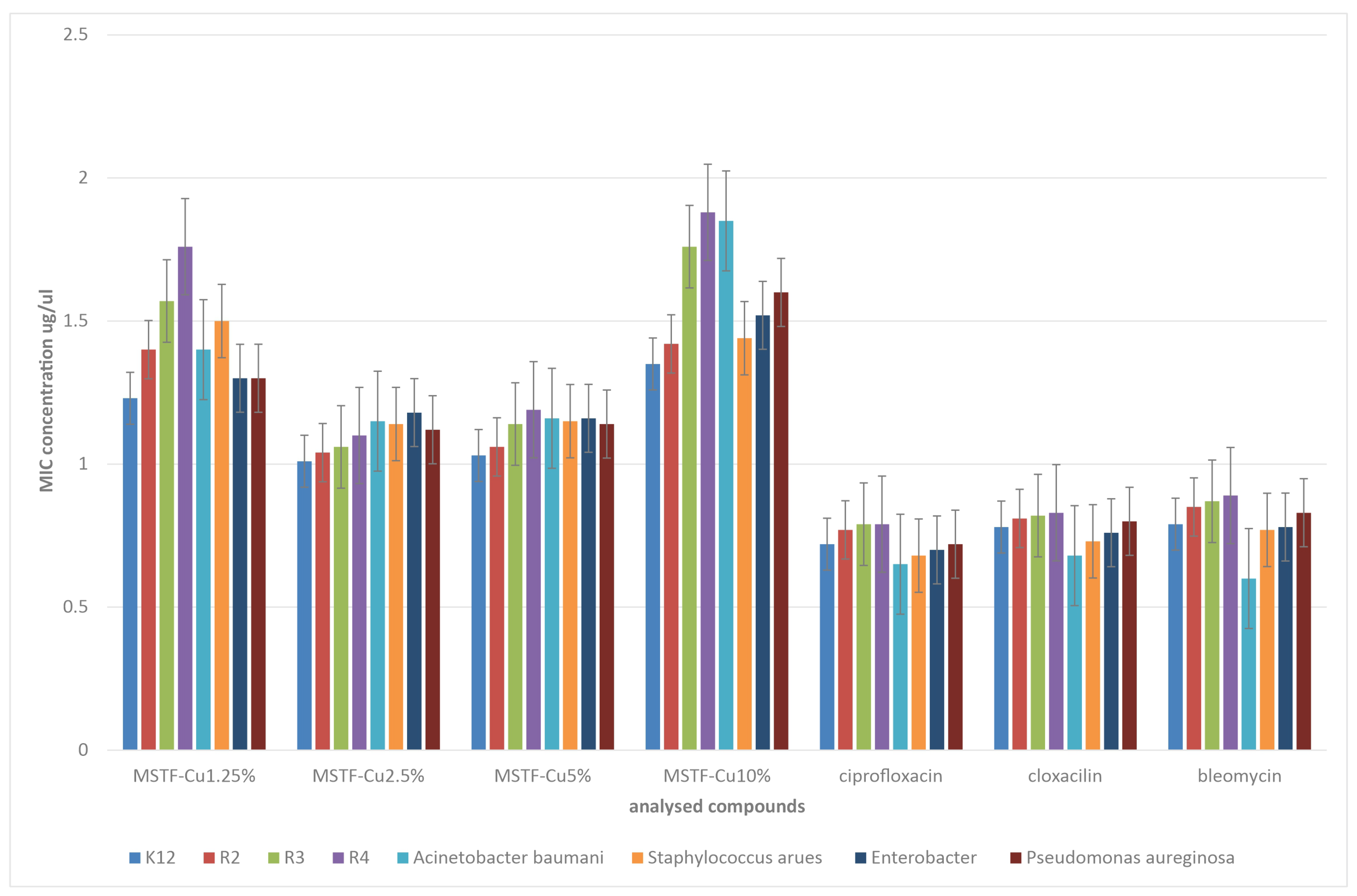
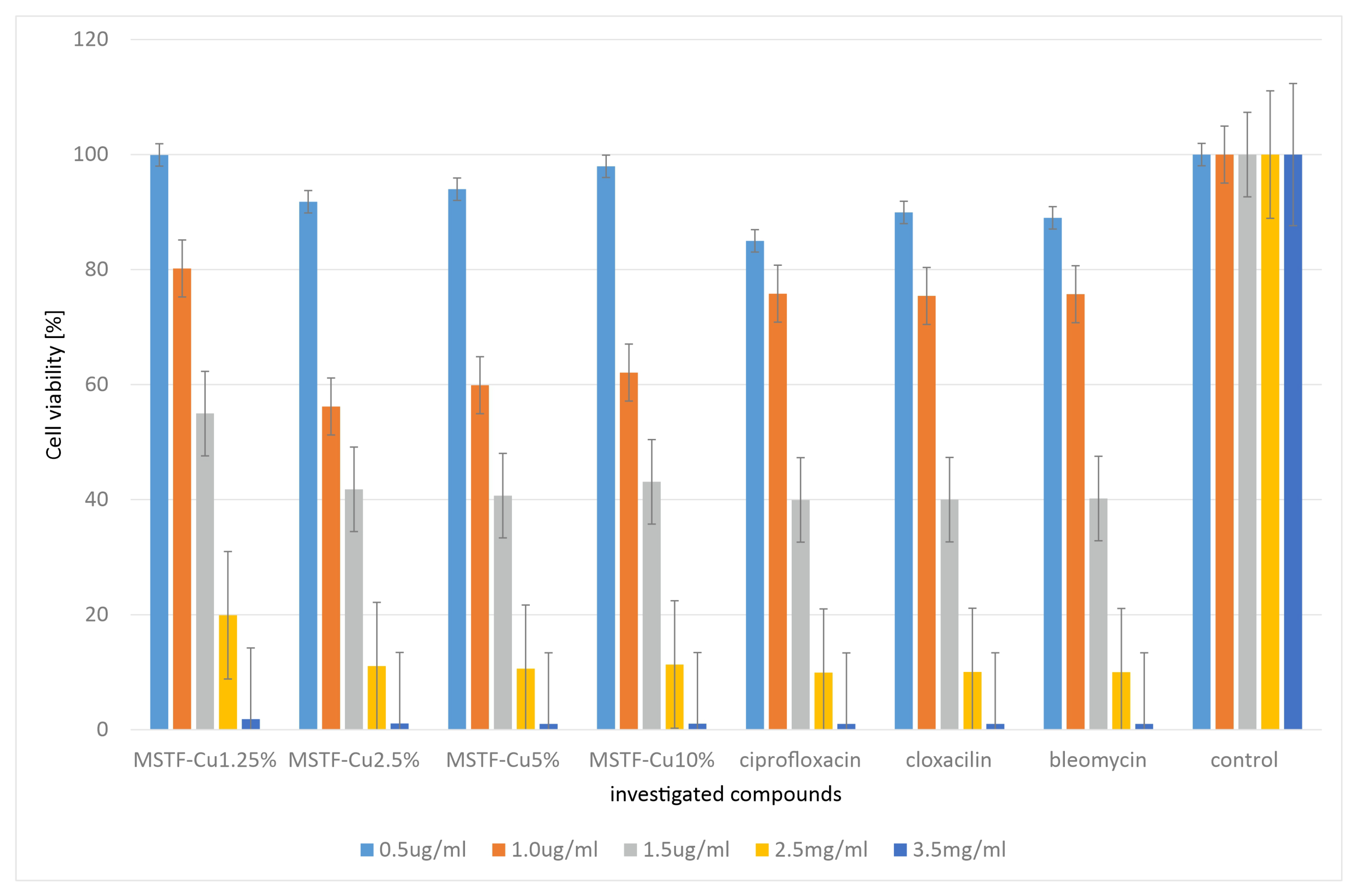
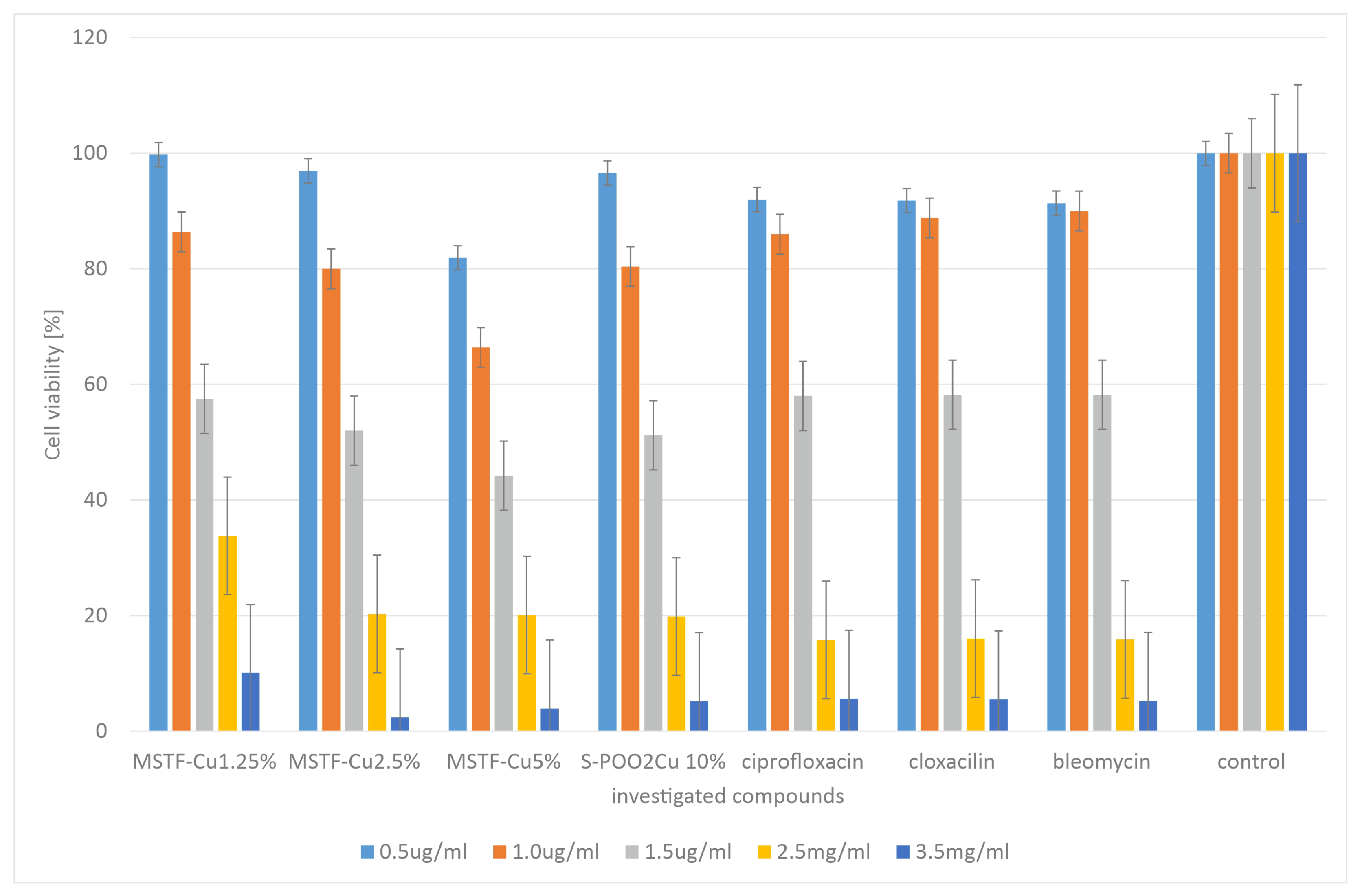
2.2. Microbiological Tests
3. Materials and Methods
3.1. Samples Synthesis
3.2. Characterisation Methods
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| TEOS | Tetraethoxysilane |
| PPTES | Phosphonate propyl dietyltriethoxysilane |
| TMS | Trimethylsilyl groups |
References
- Friedman, H.; Newton, C.; Klein, T.W. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 2003, 16, 209–219. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Cavaillon, J.M. Septic shock. Lancet 2005, 365, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Barupal, T.; Sharma, K. A review on infectious pathogens and mode of transmission. J. Plant Pathol. Microbiol. 2019, 10, 472. [Google Scholar]
- Volz, E.M.; Miller, J.C.; Galvani, A.; Ancel Meyers, L. Effects of heterogeneous and clustered contact patterns on infectious disease dynamics. PLoS Comput. Biol. 2011, 7, e1002042. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Kumar, R.; Masand, R.; Rana, J.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Mohapatra, R.K.; Natesan, S.; et al. The role of disinfectants and sanitizers during COVID-19 pandemic: Advantages and deleterious effects on humans and the environment. Environ. Sci. Pollut. Res. 2021, 28, 34211–34228. [Google Scholar] [CrossRef]
- Marzoli, F.; Bortolami, A.; Pezzuto, A.; Mazzetto, E.; Piro, R.; Terregino, C.; Bonfante, F.; Belluco, S. A systematic review of human coronaviruses survival on environmental surfaces. Sci. Total Environ. 2021, 778, 146191. [Google Scholar] [CrossRef]
- Chin, A.W.; Chu, J.T.; Perera, M.R.; Hui, K.P.; Yen, H.L.; Chan, M.C.; Peiris, M.; Poon, L.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Ning, Y.; Hu, X.; Ling, J.; Du, Y.; Liu, J.; Liu, H.; Peng, Z. Candida albicans survival and biofilm formation under starvation conditions. Int. Endod. J. 2013, 46, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Traore, O.; Springthorpe, V.; Sattar, S. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J. Appl. Microbiol. 2002, 92, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.; Sultan, O.; Mitchell, B.G.; Jenney, A.; Kiernan, M.; Brewster, D.J.; Russo, P.L. How long do nosocomial pathogens persist on inanimate surfaces? A scoping review. J. Hosp. Infect. 2024, 147, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Enrico, A.; Pettersson, T.; Ek, M.; Herland, A.; Niklaus, F.; Stemme, G.; Wågberg, L. Bactericidal surfaces prepared by femtosecond laser patterning and layer-by-layer polyelectrolyte coating. J. Colloid Interface Sci. 2020, 575, 286–297. [Google Scholar] [CrossRef]
- Prudnikov, E.; Polishchuk, I.; Sand, A.; Hamad, H.A.; Massad-Ivanir, N.; Segal, E.; Pokroy, B. Self-assembled fatty acid crystalline coatings display superhydrophobic antimicrobial properties. Mater. Today Bio 2023, 18, 100516. [Google Scholar] [CrossRef]
- Prudnikov, E.; Abu Hamad, H.; Polishchuk, I.; Katsman, A.; Segal, E.; Pokroy, B. Superhydrophobic Fatty Acid-Based Spray Coatings with Dual-Mode Antifungal Activity. ACS Appl. Bio Mater. 2025, 8, 5970–5983. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Kang, E.T.; Chan-Park, M.B. Chitosan-based peptidopolysaccharides as cationic antimicrobial agents and antibacterial coatings. Biomacromolecules 2018, 19, 2156–2165. [Google Scholar] [CrossRef]
- Marzullo, P.; Presentato, A.; D’Anna, F.; Campisciano, V.; Alduina, R.; Tornatore, E.; Giacalone, F.; Liotta, L.F.; Gruttadauria, M. A new synthetic approach for high surface area mesoporous silica and its use towards sustainable antifouling materials. RSC Sustain. 2025, 3, 2352–2365. [Google Scholar] [CrossRef]
- Anandapillai, P.T.; Kamali, S.M.; Malathy, V.; Dhairiyasamy, R. Evaluating silver nanoparticles, copper coatings, and zinc oxide nanostructures for antimicrobial medical device applications. Matéria 2024, 29, e20240742. [Google Scholar] [CrossRef]
- Ciacotich, N.; Kvich, L.; Sanford, N.; Wolcott, J.; Bjarnsholt, T.; Gram, L. Copper-silver alloy coated door handles as a potential antibacterial strategy in clinical settings. Coatings 2020, 10, 790. [Google Scholar] [CrossRef]
- Raja, F.N.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Rosenberg, M.; Ilić, K.; Juganson, K.; Kahru, A. Potential Ecotoxicological Effects of Antimicrobial Surface Coatings: A Literature Survey Backed Up by Analysis of Market Reports. Int. J. Environ. Res. Public Health 2019, 16, 3494. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.N.; Salimijazi, H.; Labbaf, S.; Amya, M.; Ehsani, P.; Mehrbod, P. Antibacterial and virucidal evaluation of ultrafine wire arc sprayed German silver coatings. J. Therm. Spray Technol. 2023, 32, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Ortí-Lucas, R.M.; Muñoz-Miguel, J. Effectiveness of surface coatings containing silver ions in bacterial decontamination in a recovery unit. Antimicrob. Resist. Infect. Control 2017, 6, 61. [Google Scholar] [CrossRef]
- Varghese, S.; ElFakhri, S.O.; Sheel, D.W.; Sheel, P.; Bolton, F.J.E.; Foster, H.A. Antimicrobial activity of novel nanostructured Cu-SiO2 coatings prepared by chemical vapour deposition against hospital related pathogens. Amb Express 2013, 3, 53. [Google Scholar] [CrossRef]
- Olivieri, F.; Orlo, E.; Spinelli, E.; Castaldo, R.; Gentile, G.; Licoccia, S.; Lavorgna, M.; Lavorgna, M. Dual Biocide Behaviour of Quaternary Ammonium Functionalized Mesoporous Silica Nanoparticles Loaded with Thymus Essential Oil for Stone Conservation. Nanomaterials 2025, 15, 866. [Google Scholar] [CrossRef]
- Moreno, V.V.; Yohai, L.; Procaccini, R.; Pellice, S. Silver-functionalized mesoporous silica nanoparticle coatings: Optimal thermal stability and ionic activity for antimicrobial applications. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136387. [Google Scholar] [CrossRef]
- Tao, L.; Vojnits, K.; Lam, M.I.; Lu, X.; Pakpour, S.; Liu, J. Antibacterial Activity of Zinc Oxide Thin Films by Atomic Layer Deposition for Personal Protective Equipment Applications. arXiv 2023, arXiv:2302.02039. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Canta, M.; Cauda, V. The investigation of the parameters affecting the ZnO nanoparticle cytotoxicity behaviour: A tutorial review. Nieznany (Eur. PMC Tutor. Rev.) 2020, 8, 6157–6174. [Google Scholar] [CrossRef]
- Alp, E.; Olivieri, F.; Aulitto, M.; Castaldo, R.; Contursi, P.; Cocca, M.; Gentile, G. The effect of ZnO nanoparticles morphology on the barrier and antibacterial properties of hybrid ZnO/graphene oxide/montmorillonite coatings for flexible packaging. Surf. Interfaces 2024, 55, 105307. [Google Scholar] [CrossRef]
- Matijaković Mlinarić, N.; Wawrzaszek, B.; Kowalska, K.; Selmani, A.; Učakar, A.; Vidmar, J.; Kušter, M.; Van de Velde, N.W.; Trebše, P.; Sever Škapin, A.; et al. Poly(Allylamine Hydrochloride) and ZnO Nanohybrid Coating for the Development of Hydrophobic, Antibacterial, and Biocompatible Textiles. Nanomaterials 2024, 14, 570. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, C.; Singh, P.; Mukhopadhyay, S.; Gupta, A.; Walvekar, B. High-performance multifunctional cotton fabric with integrated green-synthesized ZnO nanoparticle coating: UV protection and antibacterial performance. Surf. Coat. Technol. 2024, 491, 131171. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15806–15810. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Zhang, Y.; Yin, Y.; Chen, B.; Liang, Y.; Jiang, G.; Zare, R.N. Contact between water vapor and silicate surface causes abiotic formation of reactive oxygen species in an anoxic atmosphere. Proc. Natl. Acad. Sci. USA 2023, 120, e2302014120. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Liu, J. Introduction: Heterogeneous Single-Atom Catalysis. Chem. Rev. 2020, 120, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-Atom Catalysts: From Design to Application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Colilla, M.; Manzano, M.; Vallet-Regí, M. In vitro stability of SBA-15 under physiological conditions. Microporous Mesoporous Mater. 2010, 132, 442–452. [Google Scholar] [CrossRef]
- Hartmann, M.; Vinu, A. Mechanical stability and porosity analysis of large-pore SBA-15 mesoporous molecular sieves by mercury porosimetry and organics adsorption. Langmuir 2002, 18, 8010–8016. [Google Scholar] [CrossRef]
- Galarneau, A.; Nader, M.; Guenneau, F.; Di Renzo, F.; Gedeon, A. Understanding the stability in water of mesoporous SBA-15 and MCM-41. J. Phys. Chem. C 2007, 111, 8268–8277. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Newalkar, B.L.; Choudary, N.V.; Turaga, U.T.; Vijayalakshmi, R.; Kumar, P.; Komarneni, S.; Bhat, T.S. Potential adsorbent for light hydrocarbon separation: Role of SBA-15 framework porosity. Chem. Mater. 2003, 15, 1474–1479. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Laha, D.; Bhattacharya, D.; Pramanik, A.; Santra, C.R.; Pramanik, P.; Karmakar, P. Evaluation of copper iodide and copper phosphate nanoparticles for their potential cytotoxic effect. Toxicol. Res. 2012, 1, 131–136. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zhang, J.G.; Wang, X.C.; Chen, J. Excessive copper induces the production of reactive oxygen species, which is mediated by phospholipase D, nicotinamide adenine dinucleotide phosphate oxidase and antioxidant systems. J. Integr. Plant Biol. 2008, 50, 157–167. [Google Scholar] [CrossRef]
- El Houbbadi, S.; Laskowska, M.; Walcarius, A.; Doskocz, M.; Maximenko, A.; Olejniczak, Z.; Laskowski, Ł. Revealing the molecular structure of copper phosphonate groups anchored inside SBA-15 silica channels: Theoretical and experimental study. Appl. Surf. Sci. 2024, 669, 160425. [Google Scholar] [CrossRef]
- Laskowska, M.; Laskowski, L.; Jelonkiewicz, J. SBA-15 mesoporous silica activated by metal ions–Verification of molecular structure on the basis of Raman spectroscopy supported by numerical simulations. J. Mol. Struct. 2015, 1100, 21–26. [Google Scholar] [CrossRef]
- Laskowski, L.; Laskowska, M. Functionalization of SBA-15 mesoporous silica by Cu-phosphonate units: Probing of synthesis route. J. Solid State Chem. 2014, 220, 221–226. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Dulski, M.; Zubko, M.; Jelonkiewicz, J.; Perzanowski, M.; Vila, N.; Walcarius, A. Multi-step functionalization procedure for fabrication of vertically aligned mesoporous silica thin films with metal-containing molecules localized at the pores bottom. Microporous Mesoporous Mater. 2019, 274, 356–362. [Google Scholar] [CrossRef]
- Laskowski, L.; Laskowska, M.; Jelonkiewicz, J.; Dulski, M.; Wojtyniak, M.; Fitta, M.; Balanda, M. SBA-15 mesoporous silica free-standing thin films containing copper ions bounded via propyl phosphonate units-preparation and characterization. J. Solid State Chem. 2016, 241, 143–151. [Google Scholar] [CrossRef]
- Pastukh, S.; Laskowska, M.; Dulski, M.; Krzykawski, T.; Parlinski, K.; Piekarz, P. Ab initio studies for characterization and identification of nanocrystalline copper pyrophosphate confined in mesoporous silica. Nanotechnology 2021, 32, 415701. [Google Scholar] [CrossRef]
- Borodko, Y.; Ager, J.W.; Marti, G.E.; Song, H.; Niesz, K.; Somorjai, G.A. Structure sensitivity of vibrational spectra of mesoporous silica SBA-15 and Pt/SBA-15. J. Phys. Chem. B 2005, 109, 17386–17390. [Google Scholar] [CrossRef]
- McMillan, P.F.; Piriou, B. Raman spectroscopic studies of silicate and related glass structure: A review. Bull. Mineral. 1983, 106, 57–75. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts—Applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Tang, N.; Liu, X.; Jia, M.R.; Shi, X.Y.; Fu, J.W.; Guan, D.X.; Ma, L.Q. Amine- and thiol-bifunctionalized mesoporous silica material for immobilization of Pb and Cd: Characterization, efficiency, and mechanism. Chemosphere 2022, 291, 132771. [Google Scholar] [CrossRef]
- Munaweera, I.; Pathiraja, A.S.; Kottegoda, N. Surface Functionalized Mesoporous Silica Nanoparticles for Enhanced Removal of Heavy Metals: A Review. Vidyodaya J. Sci. 2023, 1. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Innovative Silver-Based Capping System for Mesoporous Silica Nanocarriers Able to Exploit a Twofold Anticorrosive Mechanism in Composite Polymer Coatings: Tailoring Benzotriazole Release and Capturing Chloride Ions. ACS Appl. Mater. Interfaces 2021, 13, 48141–48152. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, M.; Rabe, A.; Budiyanto, E.; Friedel Ortega, K.; Najafishirtari, S.; Tüysüz, H.; Behrens, M. Dynamics of Reactive Oxygen Species on Cobalt-Containing Spinel Oxides in Cyclic CO Oxidation. Catalysts 2021, 11, 1312. [Google Scholar] [CrossRef]
- Lu, X.; Kuai, L.; Huang, F.; Jiang, J.; Song, J.; Liang, Y.; Peng, W.; Luo, Y.; Li, Y.; Dong, H.; et al. Single-atom catalysts-based catalytic ROS clearance for efficient psoriasis treatment and relapse prevention via restoring ESR1. Nat. Commun. 2023, 14, 6767. [Google Scholar] [CrossRef]
- Chen, S.; Huang, F.; Mao, L.; Zhang, Z.; Lin, H.; Yan, Q.; Lu, X.; Shi, J. High Fe-Loading Single-Atom Catalyst Boosts ROS Production by Density Effect for Efficient Antibacterial Therapy. Nano-Micro Lett. 2025, 17, 32. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Alexandris, S.; Papadopoulos, P.; Sakellariou, G.; Steinhart, M.; Butt, H.J.; Floudas, G. Interfacial energy and glass temperature of polymers confined to nanoporous alumina. Macromolecules 2016, 49, 7400–7414. [Google Scholar] [CrossRef]
- ISO 11133; Microbiology of Food, Animal Feed and Water-Preparation, Production, Storage and Performance Testing of Culture Media. ISO: Geneve, Switzerland, 2014.
- Sahrawat, P.; Kowalczyk, P.; Koszelewski, D.; Szymczak, M.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. Influence of Open Chain and Cyclic Structure of Peptidomimetics on Antibacterial Activity in E. coli Strains. Molecules 2022, 27, 3633. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef]
- Kadeřábková, N.; Mahmood, A.J.; Mavridou, D.A. Antibiotic susceptibility testing using minimum inhibitory concentration (MIC) assays. NPJ Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef]
- Milovanovic, V.; Minic, R.; Vakic, J.; Ivanovic, S.; Cupic, V.; Borozan, S.; Nesic, A.; Zivkovic, I. MTT based L-aminoacid oxidase activity test for determination of antivenom potency against Vipera ammodytes envenomation. Toxicon 2021, 192, 57–65. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Modanloo, M. An overview of the most common methods for assessing cell viability. J. Res. Med. Dent. Sci. 2017, 5, 33–41. [Google Scholar] [CrossRef]




| Element Content (At. %) | ||||
|---|---|---|---|---|
| Si:P | P:Cu | |||
| Sample | Si | P | P | Cu |
| MSTF-Cu1.25% | 98.9 | 1.1 | 48.0 | 52.0 |
| MSTF-Cu2.5% | 97.6 | 2.4 | 48.9 | 51.1 |
| MSTF-Cu5% | 95.0 | 5.0 | 50.8 | 49.2 |
| MSTF-Cu10% | 91.2 | 8.8 | 56.7 | 43.3 |
| Material | H2O CA [°] | Glycerin CA [°] | [mJ/m2] |
|---|---|---|---|
| MSTF-Cu1.25% | 63.22 | 58.92 | 53.74612 |
| MSTF-Cu2.5% | 66.15 | 58.57 | 46.92306 |
| MSTF-Cu5% | 68.05 | 58.07 | 57.47949 |
| MSTF-Cu10% | 68.55 | 62.97 | 35.84863 |
| Sample | Molar Ratio TEOS:PPTES |
|---|---|
| MSTF-Cu1.25% | 79:1 |
| MSTF-Cu2.5% | 39:1 |
| MSTF-Cu5% | 19:1 |
| MSTF-Cu10% | 9:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, M.; Kowalczyk, P.; Karczmarska, A.; Pogoda, K.; Zubko, M.; Laskowski, Ł. Functionalised Mesoporous Silica Thin Films as ROS-Generating Antimicrobial Coatings. Int. J. Mol. Sci. 2025, 26, 7154. https://doi.org/10.3390/ijms26157154
Laskowska M, Kowalczyk P, Karczmarska A, Pogoda K, Zubko M, Laskowski Ł. Functionalised Mesoporous Silica Thin Films as ROS-Generating Antimicrobial Coatings. International Journal of Molecular Sciences. 2025; 26(15):7154. https://doi.org/10.3390/ijms26157154
Chicago/Turabian StyleLaskowska, Magdalena, Paweł Kowalczyk, Agnieszka Karczmarska, Katarzyna Pogoda, Maciej Zubko, and Łukasz Laskowski. 2025. "Functionalised Mesoporous Silica Thin Films as ROS-Generating Antimicrobial Coatings" International Journal of Molecular Sciences 26, no. 15: 7154. https://doi.org/10.3390/ijms26157154
APA StyleLaskowska, M., Kowalczyk, P., Karczmarska, A., Pogoda, K., Zubko, M., & Laskowski, Ł. (2025). Functionalised Mesoporous Silica Thin Films as ROS-Generating Antimicrobial Coatings. International Journal of Molecular Sciences, 26(15), 7154. https://doi.org/10.3390/ijms26157154







