Limited Proteolysis as a Regulator of Lymphatic Vessel Function and Architecture
Abstract
1. Introduction
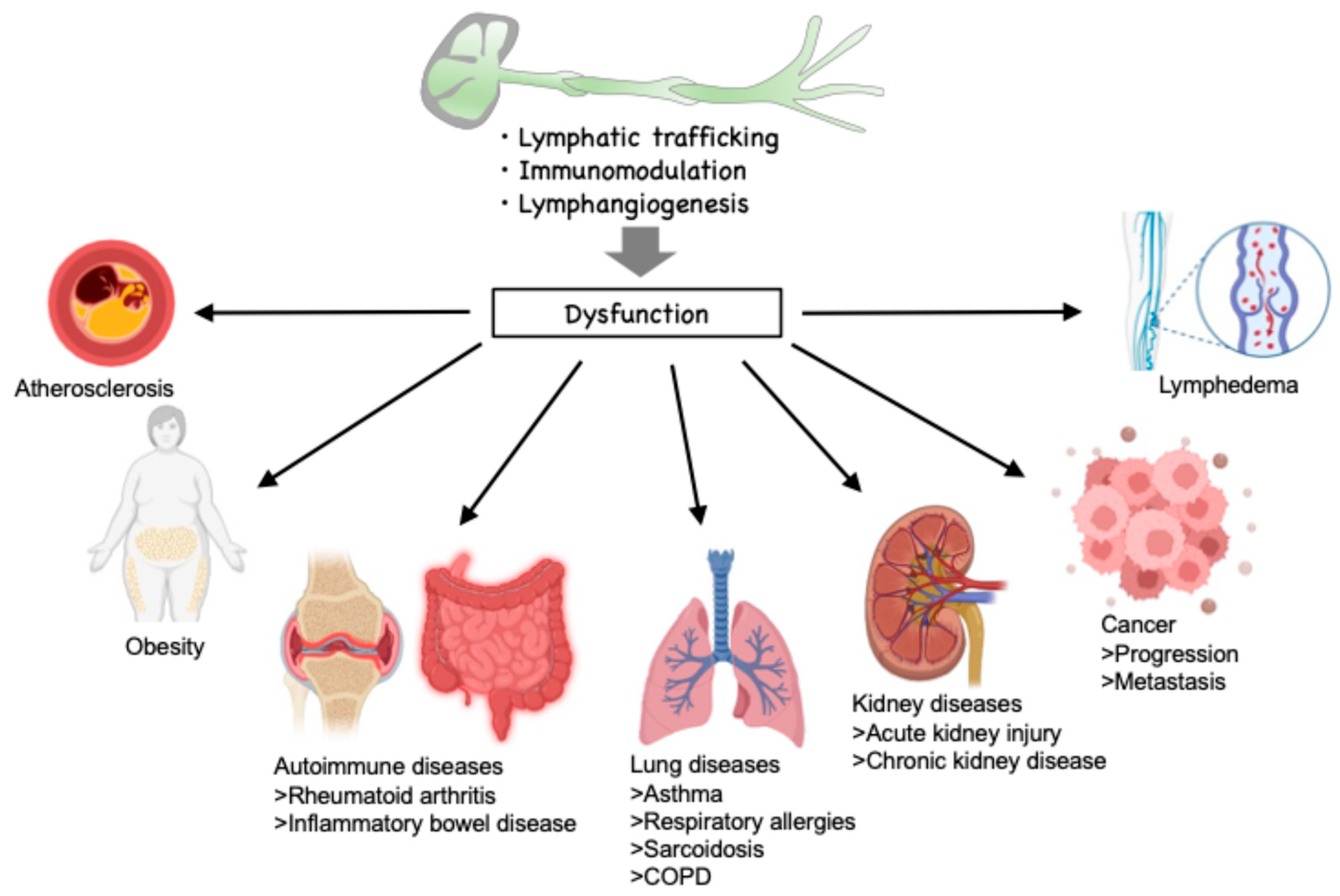
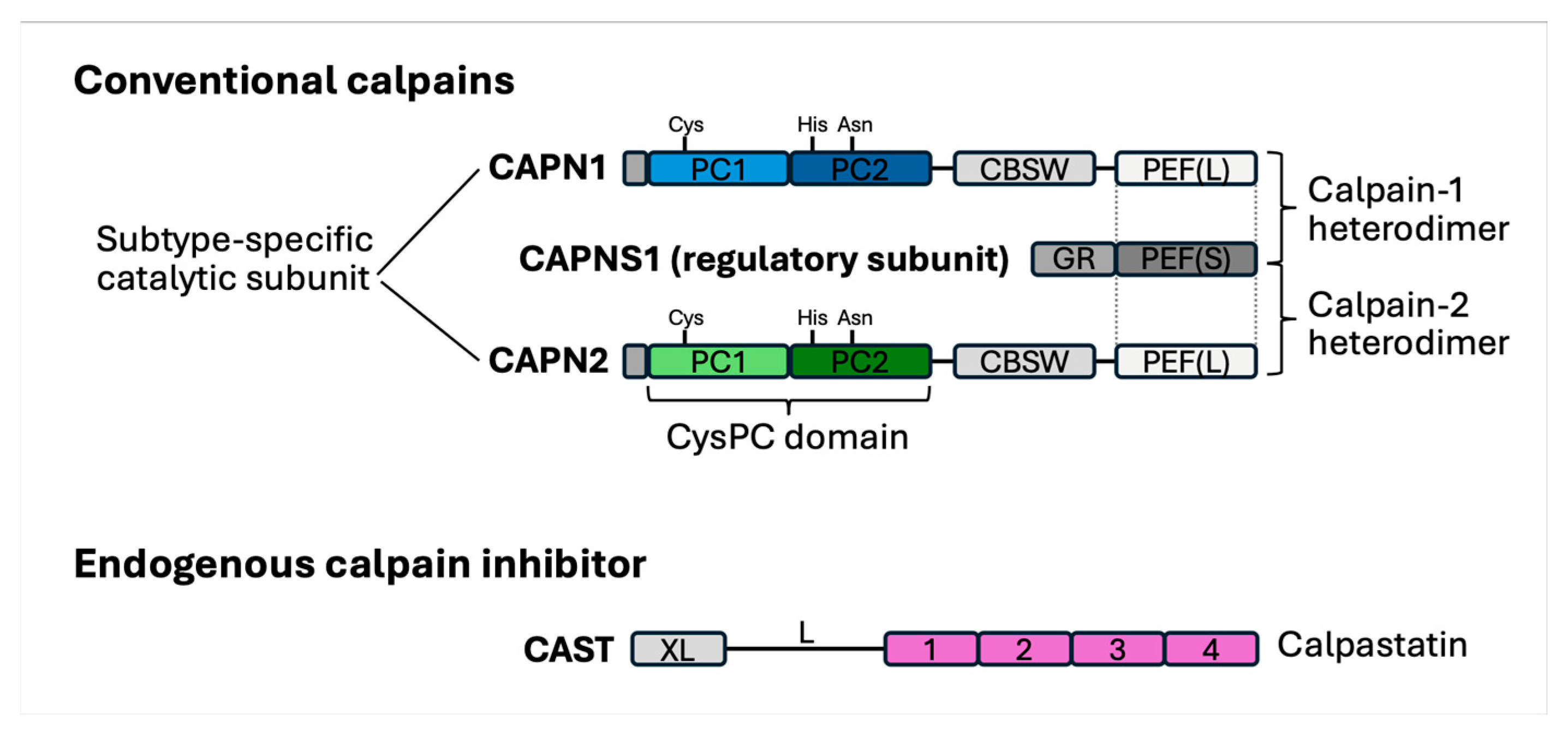
2. Limited Proteases in the Lymphatic Environment
3. Regulation of Lymphatic Inflammation by Limited Proteolysis
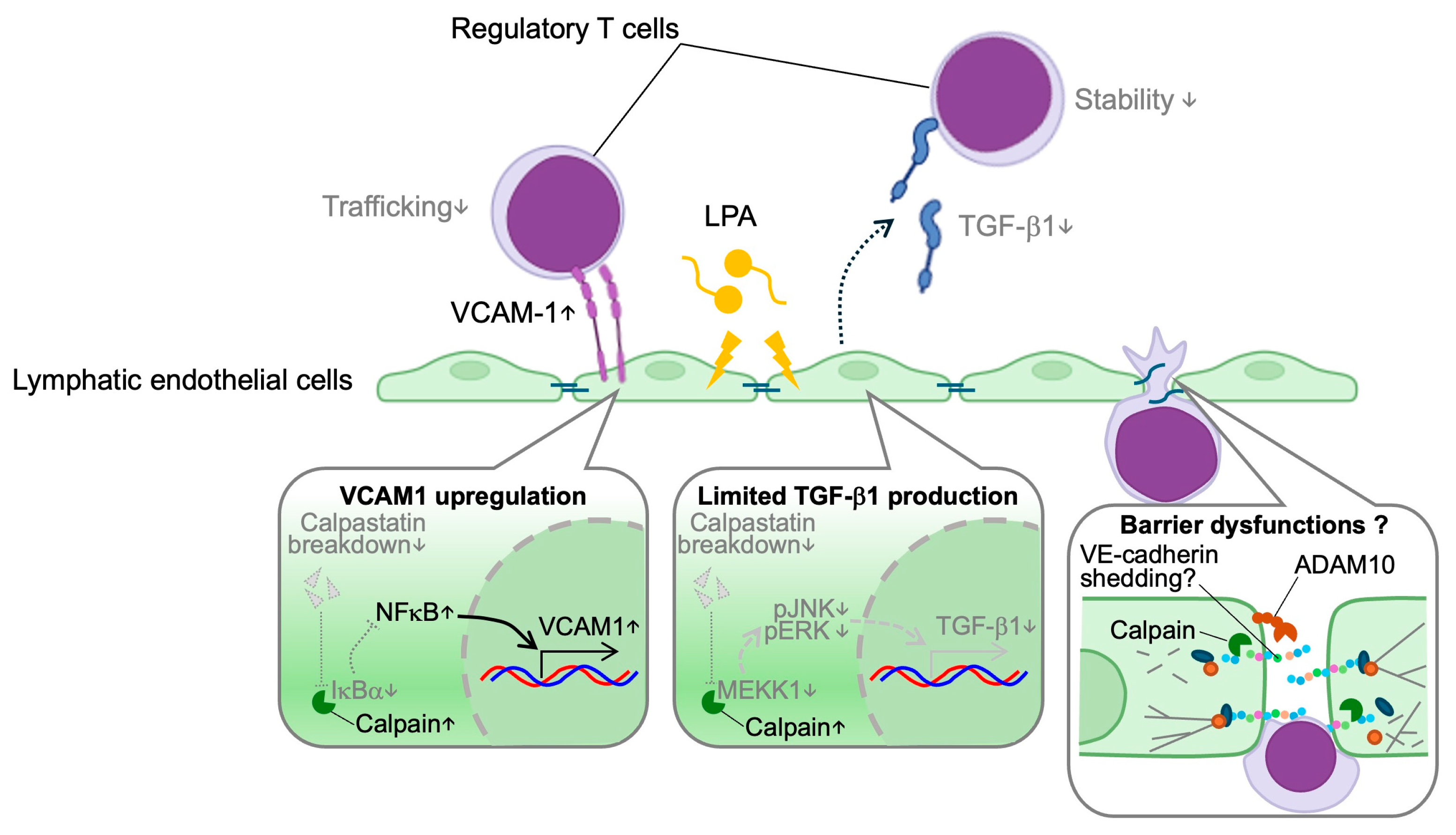
3.1. Lymphatic Trafficking
3.2. Immunomodulation
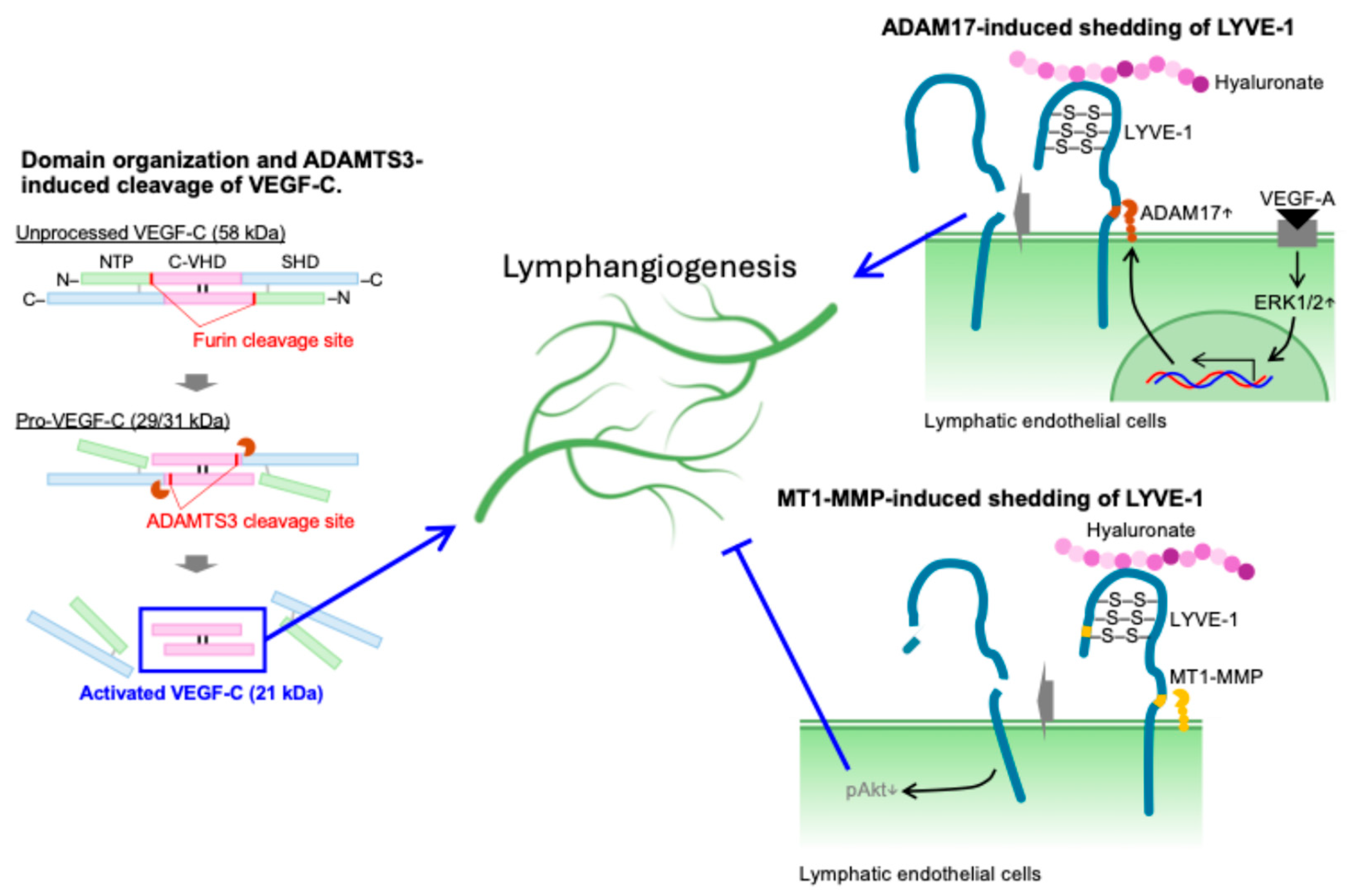
4. Regulation of Lymphangiogenesis by Limited Proteolysis
5. Therapeutic Potential and Future Perspective
| Name (Modality) | Company and Institute | Target Disease | Phase | References |
|---|---|---|---|---|
| ABT-957 (inhibitor) | AbbVie | Alzheimer’s disease | Phase I, terminated | [109,110] |
| NA-184 (inhibitor) | Abliva AB | Traumatic brain injury | Phase I is being planned | [111] |
| AMX0114 (antisense) | Amylyx Pharmaceuticals | Amyotrophic lateral sclerosis | Phase I | [112] |
| SJP-0008 (inhibitor) | Senju Pharmaceutical Co., Ltd. | Central retinal artery occlusion | Phase III | [113] |
6. Conclusive Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM10 | A disintegrin and metalloprotease 10 |
| ADAM17 | A disintegrin and metalloprotease 17 |
| ADAMTS3 | A disintegrin and metalloprotease with thrombospondin motifs-3 |
| ADAMTS14 | A disintegrin and metalloprotease with thrombospondin motifs-14 |
| BET | Bromodomain and extraterminal domain |
| CCBE1 | Collagen- and calcium-binding epidermal growth factor domains 1 |
| CHK1 | Checkpoint kinase 1 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FGF2 | Fibroblast growth factor-2 |
| IκBα | Inhibitor of κBα |
| JAM | Junctional adhesion molecules |
| LAG-3 | Lymphocyte activation gene-3 |
| LDLR | Low-density lipoprotein receptor |
| LPA | Lysophosphatidic acid |
| LYVE-1 | Lymphatic vessel endothelial hyaluronan receptor-1 |
| MEKK1 | Mitogen-activated kinase kinase kinase 1 |
| MFN2 | Mitofusin 2 |
| MMP2 | Matrix metalloproteinase2 |
| MMP9 | Matrix metalloproteinase9 |
| MT1-MMP | Membrane-type 1 matrix metalloproteinase |
| NF-κB | Nuclear factor-κB |
| siRNA | Small interfering RNA |
| SphK1 | Sphingosine kinases 1 |
| SphK2 | Sphingosine kinases 2 |
| SPL | Sphingosine-1-phosphate lyase |
| Spns2 | Spinster homolog 2 |
| SPP1 | Sphingosine-1-phosphate phosphatases 1 |
| SPP2 | Sphingosine-1-phosphate phosphatases 2 |
| TED4 | TEA domain family member 4 |
| TGF-β1 | Transforming growth factor-β1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VE-cadherin | Vascular endothelial-cadherin |
| VEGF-A | Vascular endothelial growth factor-A |
| VEGF-C | Vascular endothelial growth factor-C |
| VEGF-D | Vascular endothelial growth factor-D |
| VEGFR-3 | Vascular endothelial growth factor receptor-3 |
References
- Jalkanen, S.; Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 2020, 20, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Harris, N.R.; Caron, K.M. Lymphatic Vasculature: An Emerging Therapeutic Target and Drug Delivery Route. Annu. Rev. Med. 2021, 72, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Norrmén, C.; Petrova, T.V. Molecular mechanisms of lymphatic vascular development. Cell. Mol. Life Sci. 2007, 64, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Salmi, M.; Jalkanen, S. Lymph node lymphatic endothelial cells as multifaceted gatekeepers in the immune system. Trends Immunol. 2023, 44, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Card, C.M.; Yu, S.S.; Swartz, M.A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Investig. 2014, 124, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Guidi, C.J.; Tewalt, E.F.; Qiao, H.; Rouhani, S.J.; Ruddell, A.; Farr, A.G.; Tung, K.S.; Engelhard, V.H. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 2010, 207, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kedl, R.M.; Tamburini, B.A. Antigen archiving by lymph node stroma: A novel function for the lymphatic endothelium. Eur. J. Immunol. 2015, 45, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.D.; Tamburini, B.A.J. Lymph Node Lymphatic Endothelial Cell Expansion and Contraction and the Programming of the Immune Response. Front. Immunol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Angelillo, J.; Hugues, S. Lymphatic transport in anti-tumor immunity and metastasis. J. Exp. Med. 2025, 222, e20231954. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, B.J.; Radtke, A.J.; Randolph, G.J.; Wachter, B.T.; Greenwel, P.; Rovira, I.I.; Galis, Z.S.; Muratoglu, S.C. The emerging importance of lymphatics in health and disease: An NIH workshop report. J. Clin. Investig. 2023, 133, e171582. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyazaki, A. Hypercholesterolemia and Lymphatic Defects: The Chicken or the Egg? Front. Cardiovasc. Med. 2021, 8, 701229. [Google Scholar] [CrossRef] [PubMed]
- Karakousi, T.; Mudianto, T.; Lund, A.W. Lymphatic vessels in the age of cancer immunotherapy. Nat. Rev. Cancer 2024, 24, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 2015, 16, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Wang, W. The ubiquitin-proteasome system in the tumor immune microenvironment: A key force in combination therapy. Front. Immunol. 2024, 15, 1436174. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Zafar, M.A.; Singh, S.; Nanda, S.; Bashir, H.; Das, D.K.; Lamba, T.; Khan, M.A.; Kaur, G.; Agrewala, J.N. From defense to dysfunction: Autophagy’s dual role in disease pathophysiology. Eur. J. Pharmacol. 2024, 981, 176856. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyazaki, A. Emerging roles of calpain proteolytic systems in macrophage cholesterol handling. Cell. Mol. Life Sci. 2017, 74, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, B.E.; Vogel, T.P.; Bouchier-Hayes, L. Inflammatory caspase regulation: Maintaining balance between inflammation and cell death in health and disease. FEBS J. 2019, 286, 2628–2644. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Mullooly, M.; O’Donovan, N.; Sukor, S.; Crown, J.; Pierce, A.; McGowan, P.M. The ADAMs family of proteases: New biomarkers and therapeutic targets for cancer? Clin. Proteom. 2011, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Chen, S.; Tang, X.X. Role of ADAM and ADAMTS proteases in pathological tissue remodeling. Cell Death Discov. 2023, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.I.; Jenkins, B.J. The protease ADAM17 at the crossroads of disease: Revisiting its significance in inflammation, cancer, and beyond. FEBS J. 2024, 291, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Serra, R. Matrix Metalloproteinases in Health and Disease 3.0. Biomolecules 2024, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Valasciuc, E.; Anton, I.B.; Gosav, E.M.; Dima, N.; Cucu, A.I.; Costea, C.F.; Floria, D.E.; Hurjui, L.L.; Tarniceriu, C.C.; et al. Matrix Metalloproteinases: Pathophysiologic Implications and Potential Therapeutic Targets in Cardiovascular Disease. Biomolecules 2025, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.P.; Lim, H.Y.; Angeli, V. Leukocyte Trafficking via Lymphatic Vessels in Atherosclerosis. Cells 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Scogin, C.K.; Rizwan, K.; Morley, T.S.; Rutkowski, J.M. Characterizing Lymphangiogenesis and Concurrent Inflammation in Adipose Tissue in Response to VEGF-D. Front. Physiol. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Burman, A.; Haworth, O.; Hardie, D.L.; Amft, E.N.; Siewert, C.; Jackson, D.G.; Salmon, M.; Buckley, C.D. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J. Immunol. 2005, 174, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, D.; de Voogd, F.A.E.; Pruijt, M.J.; Grootjans, J.; van de Sande, M.G.; D’Haens, G.R. The Role of the Lymphatic System in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 1854. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G. Lymphatic trafficking of immune cells and insights for cancer metastasis. Clin. Exp. Metastasis 2024, 41, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, K.; Ren, J.; Peng, H.; Wang, J.; Wang, X.; Nasser, M.I.; Liu, C. The immune regulatory role of lymphangiogenesis in kidney disease. J. Transl. Med. 2024, 22, 1053. [Google Scholar] [CrossRef] [PubMed]
- Maisel, K.; Outtz Reed, H. The Lymphatic Vasculature in Lung Homeostasis and Disease. Annu. Rev. Physiol. 2024, 87, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Taketomi, Y.; Higashi, T.; Ohtaki, H.; Takaki, T.; Ohnishi, K.; Hosonuma, M.; Kono, N.; Akasu, R.; Haraguchi, S.; et al. Hypercholesterolemic Dysregulation of Calpain in Lymphatic Endothelial Cells Interferes With Regulatory T-Cell Stability and Trafficking. Arter. Thromb. Vasc. Biol. 2023, 43, e66–e82. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Akasu, R.; Miyazaki, A. Calpain proteolytic systems counteract endothelial cell adaptation to inflammatory environments. Inflamm. Regen. 2020, 40, 5. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyazaki, A. Defective Protein Catabolism in Atherosclerotic Vascular Inflammation. Front. Cardiovasc. Med. 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Akasu, R.; Miyazaki, A. Calpain-Associated Proteolytic Regulation of the Stromal Microenvironment in Cancer. Curr. Pharm. Des. 2021, 27, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T. Calpain and Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 16782. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Huibregtse, J.M.; Matouschek, A. A masked initiation region in retinoblastoma protein regulates its proteasomal degradation. Nat. Commun. 2020, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Akasu, R.; Miyazaki, T.; Elhussiny, M.Z.; Sugiura, Y.; Tomitsuka, Y.; Haraguchi, S.; Otsu, K.; Chowdhury, V.S.; Miyazaki, A. Calpain-mediated proteolytic production of free amino acids in vascular endothelial cells augments obesity-induced hepatic steatosis. J. Biol. Chem. 2022, 298, 101953. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Bréart, B.; Ramos-Perez, W.D.; Pitt, L.A.; Gobert, M.; Sunkara, M.; Lafaille, J.J.; Morris, A.J.; Schwab, S.R. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012, 2, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Cyster, J.G. Finding a way out: Lymphocyte egress from lymphoid organs. Nat. Immunol. 2007, 8, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Simmons, S.; Kawamura, S.; Inoue, A.; Orba, Y.; Tokudome, T.; Sunden, Y.; Arai, Y.; Moriwaki, K.; Ishida, J.; et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Investig. 2012, 122, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase in immunity and cancer: Silencing the siren. Trends Mol. Med. 2007, 13, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Le Stunff, H.; Peterson, C.; Thornton, R.; Milstien, S.; Mandala, S.M.; Spiegel, S. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J. Biol. Chem. 2002, 277, 8920–8927. [Google Scholar] [CrossRef] [PubMed]
- Pulli, I.; Löf, C.; Blom, T.; Asghar, M.Y.; Lassila, T.; Bäck, N.; Lin, K.L.; Nyström, J.H.; Kemppainen, K.; Toivola, D.M.; et al. Sphingosine kinase 1 overexpression induces MFN2 fragmentation and alters mitochondrial matrix Ca2+ handling in HeLa cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Riboni, L.; Abdel Hadi, L.; Navone, S.E.; Guarnaccia, L.; Campanella, R.; Marfia, G. Sphingosine-1-Phosphate in the Tumor Microenvironment: A Signaling Hub Regulating Cancer Hallmarks. Cells 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Tian, W.; Wu, T.T.; Xiang, M.; Vinh, R.; Chang, J.L.; Gu, S.; Lee, S.; Zhu, Y.; Guan, T.; et al. Abnormal Lymphatic Sphingosine-1-Phosphate Signaling Aggravates Lymphatic Dysfunction and Tissue Inflammation. Circulation 2023, 148, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, Y.; Milstien, S.; Spiegel, S. Development of small-molecule inhibitors of sphingosine-1-phosphate signaling. Pharmacol. Ther. 2011, 132, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Harlé, G.; Kowalski, C.; Dubrot, J.; Brighouse, D.; Clavel, G.; Pick, R.; Bessis, N.; Niven, J.; Scheiermann, C.; Gannagé, M.; et al. Macroautophagy in lymphatic endothelial cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2021, 218, e20201776. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Taketomi, Y.; Takimoto, M.; Lei, X.F.; Arita, S.; Kim-Kaneyama, J.R.; Arata, S.; Ohata, H.; Ota, H.; Murakami, M.; et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation 2011, 124, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. USA 2019, 116, 12980–12985. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; Zhang, Y.; Miao, X.; Li, S.; Yang, H.; Ling, Q.; Hoffmann, P.R.; Huang, Z. Use of a Mouse Model and Human Umbilical Vein Endothelial Cells to Investigate the Effect of Arsenic Exposure on Vascular Endothelial Function and the Associated Role of Calpains. Environ. Health Perspect. 2019, 127, 77003. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Kowalczyk, A.P. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Mol. Biol. Cell 2017, 28, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Flemming, S.; Burkard, N.; Renschler, M.; Vielmuth, F.; Meir, M.; Schick, M.A.; Wunder, C.; Germer, C.T.; Spindler, V.; Waschke, J.; et al. Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc. Res. 2015, 107, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.S.; Lai, S.C. Matrix metalloproteinase-9 leads to claudin-5 degradation via the NF-κB pathway in BALB/c mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis. PLoS ONE 2013, 8, e53370. [Google Scholar] [CrossRef] [PubMed]
- Casas, E.; Barron, C.; Francis, S.A.; McCormack, J.M.; McCarthy, K.M.; Schneeberger, E.E.; Lynch, R.D. Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments. Exp. Cell Res. 2010, 316, 353–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tokarz, V.L.; Pereira, R.V.S.; Jaldin-Fincati, J.R.; Mylvaganam, S.; Klip, A. Junctional integrity and directional mobility of lymphatic endothelial cell monolayers are disrupted by saturated fatty acids. Mol. Biol. Cell 2023, 34, ar28. [Google Scholar] [CrossRef] [PubMed]
- Kakei, Y.; Akashi, M.; Shigeta, T.; Hasegawa, T.; Komori, T. Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphat. Res. Biol. 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.L.; Bonica, J.; Shamseddine, A.A.; Hannun, Y.A.; Obeid, L.M. A role for caspase-2 in sphingosine kinase 1 proteolysis in response to doxorubicin in breast cancer cells—Implications for the CHK1-suppressed pathway. FEBS Open Bio. 2017, 8, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.J.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.R.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Gitenay, D.; Lallet-Daher, H.; Bernard, D. Caspase-2 regulates oncogene-induced senescence. Oncotarget 2014, 5, 5845–5847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muppani, N.; Nyman, U.; Joseph, B. TAp73alpha protects small cell lung carcinoma cells from caspase-2 induced mitochondrial mediated apoptotic cell death. Oncotarget 2011, 2, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Cianci, R.; Pagliari, D.; Landolfi, R.; Cammarota, G. Cellular mediators of inflammation: Tregs and TH17 cells in gastrointestinal diseases. Mediat. Inflamm. 2009, 2009, 132028. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Lee, Z.L.; Zapas, G.; Wu, L.; Jewell, C.M.; Abdi, R.; Bromberg, J.S. Regulatory T cell and endothelial cell crosstalk. Nat. Rev. Immunol. 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Brown, M.L. Harnessing proteases for T regulatory cell immunotherapy. Eur. J. Immunol. 2020, 50, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K. Furin: A mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 1997, 327, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Pesu, M.; Watford, W.T.; Wei, L.; Xu, L.; Fuss, I.; Strober, W.; Andersson, J.; Shevach, E.M.; Quezado, M.; Bouladoux, N.; et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 2008, 455, 246–250. [Google Scholar] [CrossRef] [PubMed]
- de Zoeten, E.F.; Lee, I.; Wang, L.; Chen, C.; Ge, G.; Wells, A.D.; Hancock, W.W.; Ozkaynak, E. Foxp3 processing by proprotein convertases and control of regulatory T cell function. J. Biol. Chem. 2009, 284, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Bauché, D.; Joyce-Shaikh, B.; Jain, R.; Grein, J.; Ku, K.S.; Blumenschein, W.M.; Ganal-Vonarburg, S.C.; Wilson, D.C.; McClanahan, T.K.; Malefyt, R.W.; et al. LAG3+ Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1+ Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis. Immunity 2018, 49, 342–352.e5. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, W.; Greer, P.A.; Tuder, R.M.; Toque, H.A.; Wang, K.K.; Caldwell, R.W.; Su, Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J. Clin. Investig. 2011, 121, 4548–4566. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Che, Y.; Murohara, T. Therapeutic Lymphangiogenesis Is a Promising Strategy for Secondary Lymphedema. Int. J. Mol. Sci. 2023, 24, 7774. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.M.; Fang, G.-H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef] [PubMed]
- Kukk, E.; Lymboussaki, A.; Taira, S.; Kaipainen, A.; Jeltsch, M.; Joukov, V.; Alitalo, K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggest a role in lymphatic vascular development. Development 1996, 122, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Saaristo, A.; Jussila, L.; Karila, K.A.; Lawrence, E.C.; Pajusola, K.; Bueler, H.; Eichmann, A.; Kauppinen, R.; Kettunen, M.I.; et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 2001, 98, 12677–12682. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Wirzenius, M.; Makinen, T.; Veikkola, T.; Haisma, H.J.; Achen, M.G.; Stacker, S.A.; Pytowski, B.; Yla-Herttuala, S.; Alitalo, K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am. J. Pathol. 2006, 169, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, M.; Jha, S.K.; Tvorogov, D.; Anisimov, A.; Leppänen, V.M.; Holopainen, T.; Kivelä, R.; Ortega, S.; Kärpanen, T.; Alitalo, K. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 2014, 129, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.C.Y.; White, M.J.; Eckalbar, W.L.; Szpiech, Z.A.; Oh, S.S.; Pino-Yanes, M.; Hu, D.; Goddard, P.; Huntsman, S.; Galanter, J.; et al. NHLBI Trans-Omics for Precision Medicine [TOPMed] Consortium. Whole-Genome Sequencing of Pharmacogenetic Drug Response in Racially Diverse Children with Asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Huyghe, J.R.; Locke, A.E.; Jackson, A.U.; Sim, X.; Stringham, H.M.; Teslovich, T.M.; Welch, R.P.; Fuchsberger, C.; Narisu, N.; et al. Common, low-frequency, and rare genetic variants associated with lipoprotein subclasses and triglyceride measures in Finnish men from the METSIM study. PLoS Genet. 2017, 13, e1007079. [Google Scholar] [CrossRef] [PubMed]
- Marouli, E.; Graff, M.; Medina-Gomez, C.; Lo, K.S.; Wood, A.R.; Kjaer, T.R.; Fine, R.S.; Lu, Y.; Schurmann, C.; Highland, H.M.; et al. Rare and low-frequency coding variants alter human adult height. Nature 2017, 542, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, P.; Dupont, L.; Helaers, R.; Coulie, R.; Tiller, G.E.; Peeden, J.; Colige, A.; Vikkula, M. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum. Mol. Genet. 2017, 26, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Muhl, L.; Padberg, Y.; Dupont, L.; Peterson-Maduro, J.; Stehling, M.; le Noble, F.; Colige, A.; Betsholtz, C.; Schulte-Merker, S.; et al. Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis. Nat. Commun. 2020, 11, 2724. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, L.; Karpanen, T.; Schulte, D.; Harris, N.C.; Koltowska, K.; Roukens, G.; Bower, N.I.; van Impel, A.; Stacker, S.A.; Achen, M.G.; et al. Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 2014, 141, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Villefranc, J.A.; Nicoli, S.; Bentley, K.; Jeltsch, M.; Zarkada, G.; Moore, J.C.; Gerhardt, H.; Alitalo, K.; Lawson, N.D. A truncation allele in vascular endothelial growth factor c reveals distinct modes of signaling during lymphatic and vascular development. Development 2013, 140, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Dang, X.; Shen, X.; Liu, Y.; Gu, J.; Peng, X.; Huang, Z.; Hong, W.; Cui, L.; Liu, C.Y. The YAP-TEAD4 complex promotes tumor lymphangiogenesis by transcriptionally upregulating CCBE1 in colorectal cancer. J. Biol. Chem. 2023, 299, 103012. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Tang, W.; Zhi, J. The Lymphangiogenic Factor CCBE1 Promotes Angiogenesis and Tumor Growth in Colorectal Cancer. Curr. Mol. Med. 2022, 22, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Veikkola, T.; Jussila, L.; Makinen, T.; Karpanen, T.; Jeltsch, M.; Petrova, T.V.; Kubo, H.; Thurston, G.; McDonald, D.M.; Achen, M.G.; et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001, 20, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, M.; Kaipainen, A.; Joukov, V.; Meng, X.; Lakso, M.; Rauvala, H.; Swartz, M.; Fukumura, D.; Jain, R.K.; Alitalo, K. Hyperplasia of lymphatic vessels in VEGFC transgenic mice. Science 1997, 276, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.M.; Enis, D.; Robciuc, M.R.; Nurmi, H.J.; Cohen, J.; Chen, M.; Yang, Y.; Dhillon, V.; Johnson, K.; Zhang, H.; et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Investig. 2016, 126, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Fukuda, H.; Araki, R.; Shudou, M.; Okazaki, H.; Tomono, Y.; Nakayama, H.; Fukuda, S.; Sakaue, T.; Shirakata, Y.; Sayama, K.; et al. Ectodomain Shedding of Lymphatic Vessel Endothelial Hyaluronan Receptor 1 [LYVE-1] Is Induced by Vascular Endothelial Growth Factor A [VEGF-A]. J. Biol. Chem. 2016, 291, 10490–10500. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 2016, 7, 10824. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Cao, R.; Jin, G.; Chan, K.M.; Cao, Y.; Zhou, Z. When MT1-MMP meets ADAMs. Cell Cycle 2012, 11, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Du, Y.; Liu, Y.; He, Y.; Yang, C.; Wang, W.; Gao, F. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS ONE 2014, 9, e92857. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsen, S.; Porse, A.; Erpicum, C.; Maertens, L.; Jürgensen, H.J.; Madsen, D.H.; Melander, M.C.; Gårdsvoll, H.; Høyer-Hansen, G.; Noel, A.; et al. Targeting a single function of the multifunctional matrix metalloprotease MT1-MMP: Impact on lymphangiogenesis. J. Biol. Chem. 2013, 288, 10195–10204. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Taketomi, Y.; Saito, Y.; Hosono, T.; Lei, X.F.; Kim-Kaneyama, J.R.; Arata, S.; Takahashi, H.; Murakami, M.; Miyazaki, A. Calpastatin counteracts pathological angiogenesis by inhibiting suppressor of cytokine signaling 3 degradation in vascular endothelial cells. Circ. Res. 2015, 116, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. ClinicalTrials.gov. 2016. Available online: http://clinicaltrials.gov/ct2/show/NCT02220738 (accessed on 10 July 2025).
- US National Library of Medicine. ClinicalTrials.gov. 2016. Available online: http://clinicaltrials.gov/ct2/show/NCT02573740 (accessed on 10 July 2025).
- Baudry, M.; Luo, Y.L.; Bi, X. Calpain-2 Inhibitors as Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. ClinicalTrials.gov. 2025. Available online: http://clinicaltrials.gov/ct2/show/NCT06665165 (accessed on 10 July 2025).
- Japan Registry of Clinical Trials. jrct.mhlw.go.jp. 2025. Available online: https://jrct.mhlw.go.jp/en-latest-detail/jRCT2011230042 (accessed on 10 July 2025).
- D’Alessio, S.; Correale, C.; Tacconi, C.; Gandelli, A.; Pietrogrande, G.; Vetrano, S.; Genua, M.; Arena, V.; Spinelli, A.; Peyrin-Biroulet, L.; et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Investig. 2014, 124, 3863–3878. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, T. Limited Proteolysis as a Regulator of Lymphatic Vessel Function and Architecture. Int. J. Mol. Sci. 2025, 26, 7144. https://doi.org/10.3390/ijms26157144
Miyazaki T. Limited Proteolysis as a Regulator of Lymphatic Vessel Function and Architecture. International Journal of Molecular Sciences. 2025; 26(15):7144. https://doi.org/10.3390/ijms26157144
Chicago/Turabian StyleMiyazaki, Takuro. 2025. "Limited Proteolysis as a Regulator of Lymphatic Vessel Function and Architecture" International Journal of Molecular Sciences 26, no. 15: 7144. https://doi.org/10.3390/ijms26157144
APA StyleMiyazaki, T. (2025). Limited Proteolysis as a Regulator of Lymphatic Vessel Function and Architecture. International Journal of Molecular Sciences, 26(15), 7144. https://doi.org/10.3390/ijms26157144





