Fever in Children with Cancer: Pathophysiological Insights Using Blood Transcriptomics
Abstract
1. Introduction
2. Results
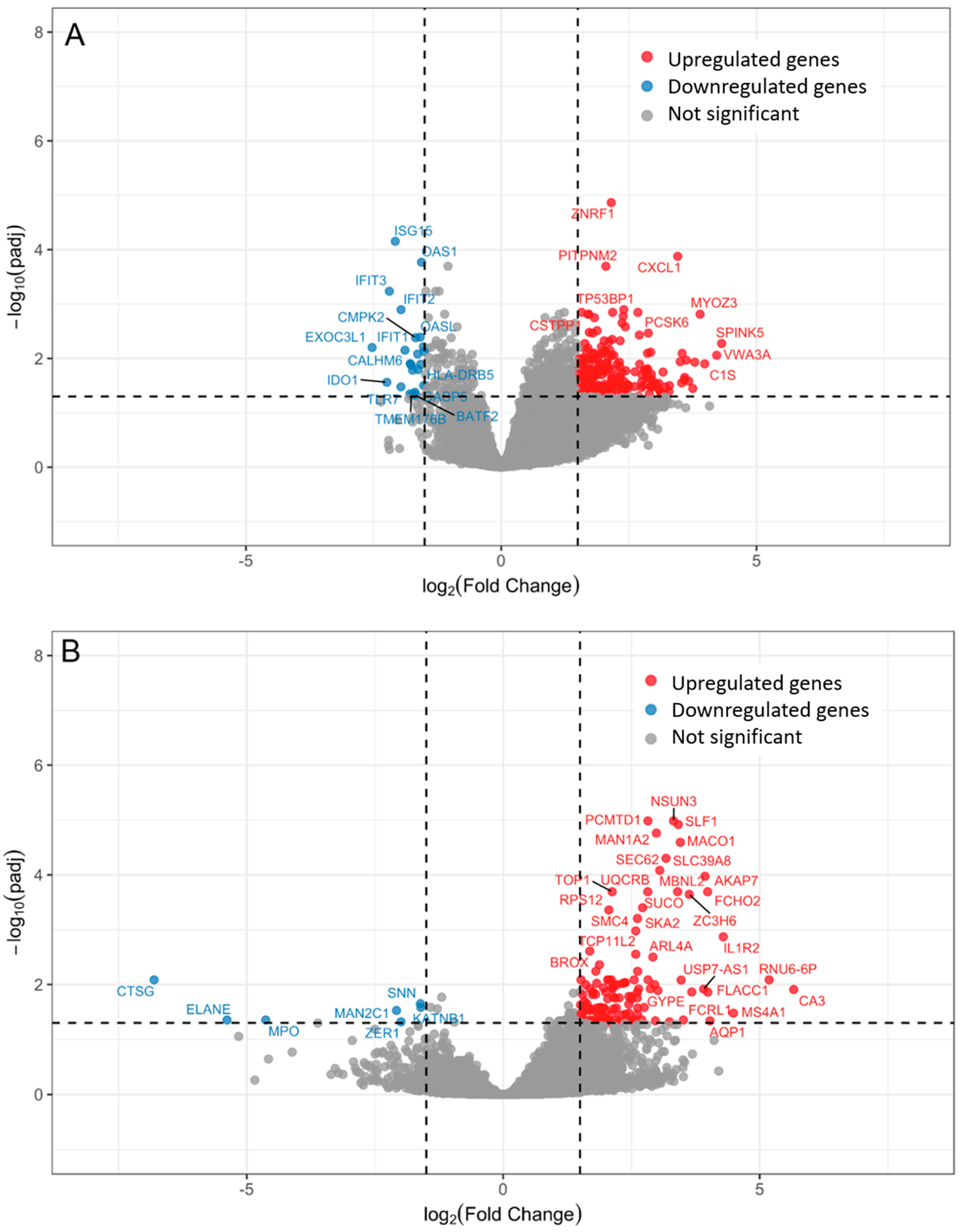
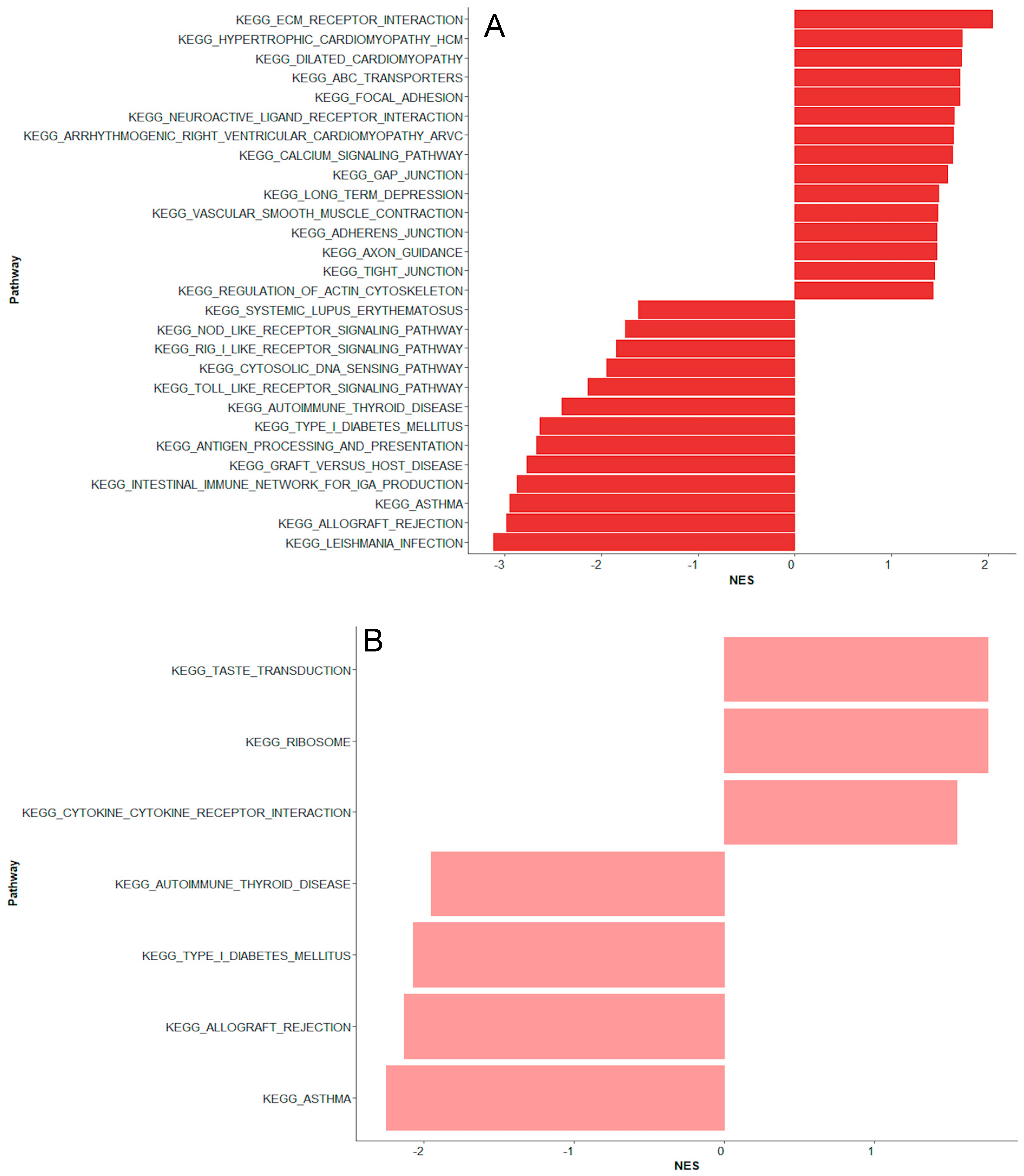
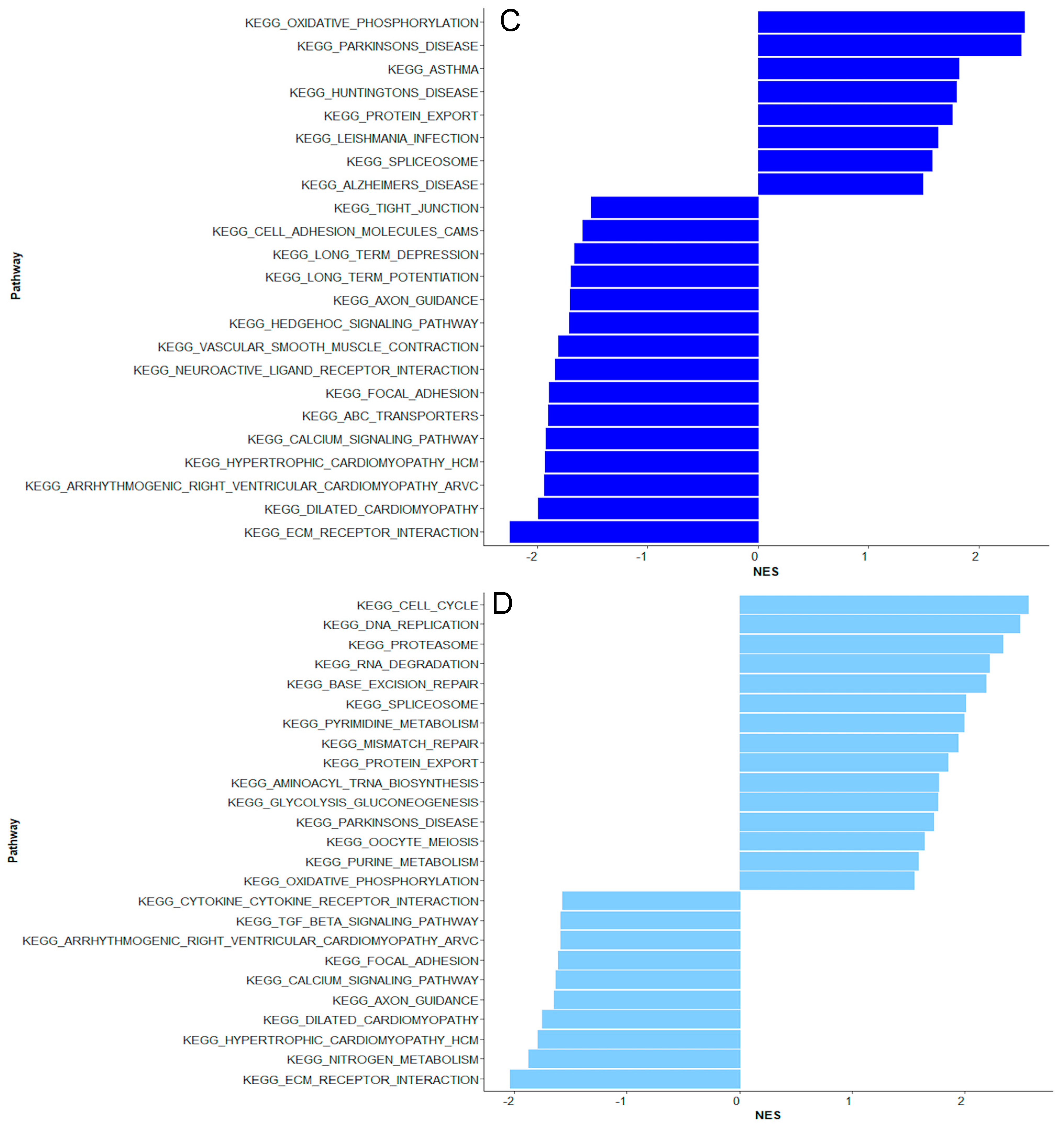
2.1. Bacteremia Versus Unexplained Fever with Lov C-Reactive Protein
2.2. Unexplained Fever with High C-Reactive Protein Versus Bacteremia
2.3. Coagulase-Negative Staphylococci vs. Bacteremia Caused by Gram-Negative and Other Gram-Positive Bacteria
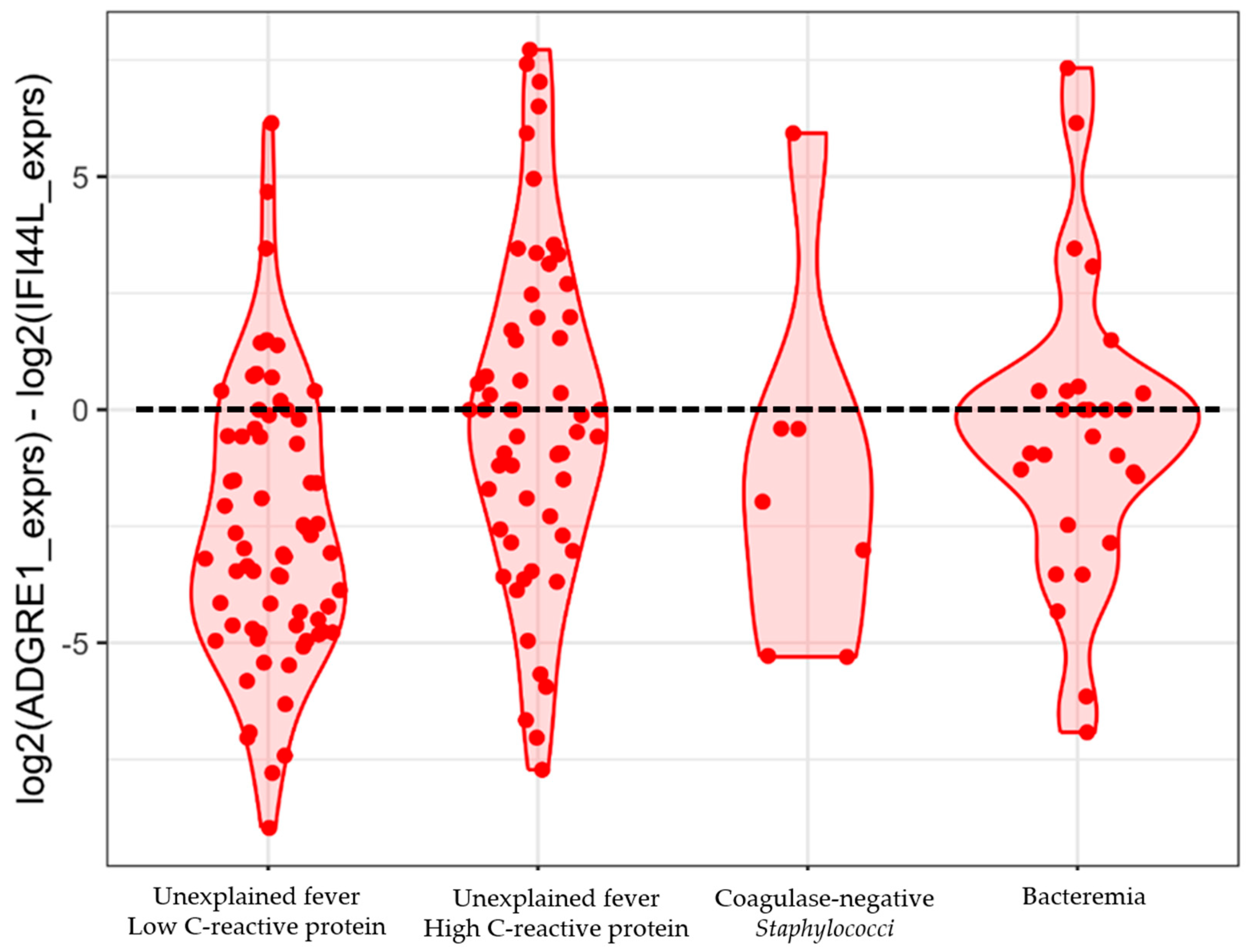
2.4. Testing the Performance of a 2-Gene Signature
3. Discussion
4. Materials and Methods
4.1. Definitions
- Bacteremia: Positive blood cultures, excluding coagulase-negative staphylococci
- Unexplained fever with low CRP: Negative blood culture and a maximum CRP below 35 mg/L
- Unexplained fever with high CRP: Negative blood culture and a maximum CRP of 35 mg/L or above
- Coagulase-negative staphylococci
4.2. RNA Sequencing and Quality Control
4.3. Differential Expression and Pathway Analysis
4.4. Study Approvals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castagnola, E.; Fontana, V.; Caviglia, I.; Caruso, S.; Faraci, M.; Fioredda, F.; Garrè, M.L.; Moroni, C.; Conte, M.; Losurdo, G.; et al. A Prospective Study on the Epidemiology of Febrile Episodes during Chemotherapy-Induced Neutropenia in Children with Cancer or after Hemopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2007, 45, 1296–1304. [Google Scholar] [CrossRef]
- van der Velden, F.J.S.; de Vries, G.; Martin, A.; Lim, E.; von Both, U.; Kolberg, L.; Carrol, E.D.; Khanijau, A.; Herberg, J.A.; De, T.; et al. Febrile illness in high-risk children: A prospective, international observational study. Eur. J. Pediatr. 2023, 182, 543–554. [Google Scholar] [CrossRef]
- Sørensen, M.C.L.; Andersen, M.M.; Rostgaard, K.; Schmiegelow, K.; Mikkelsen, T.S.; Wehner, P.S.; Olsen, M.; Søegaard, S.H.; Hjalgrim, L.L. Treatment-related mortality among children with cancer in Denmark during 2001–2021. Acta Oncol. 2024, 63, 294–302. [Google Scholar] [CrossRef]
- Inaba, H.; Pei, D.; Wolf, J.; Howard, S.C.; Hayden, R.T.; Go, M.; Varechtchouk, O.; Hahn, T.; Buaboonnam, J.; Metzger, M.L.; et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 2017, 28, 386–392. [Google Scholar] [CrossRef]
- Alp, S.; Akova, M. Management of febrile neutropenia in the era of bacterial resistance. Ther. Adv. Infect. Dis. 2013, 1, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Alali, M.; Mayampurath, A.; Dai, Y.; Bartlett, A.H. A prediction model for bacteremia and transfer to intensive care in pediatric and adolescent cancer patients with febrile neutropenia. Sci. Rep. 2022, 12, 7429. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.F.; de Sá Rodrigues, K.E.; Vanucci, M.F.; da Silva, P.L.L.; Baeta, T.; Oliveira, I.P.; Romanelli, R.M.d.C. Bloodstream infection in pediatric patients with febrile neutropenia induced by chemotherapy. Hematol. Transfus. Cell Ther. 2023, 45, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, N.D.; Gan, G.G.; Adeeba, K.; Sam, I.-C. Bacteremia in patients with febrile neutropenia after chemotherapy at a university medical center in Malaysia. Int. J. Infect. Dis. 2007, 11, 513–517. [Google Scholar] [CrossRef]
- Klein, K.; van Litsenburg, R.R.L.; de Haas, V.; Dors, N.; van den Heuvel-Eibrink, M.M.; Knops, R.R.G.; Tissing, W.J.E.; Versluys, B.A.; Zwaan, C.M.; Kaspers, G.J.L. Causes of early death and treatment-related death in newly diagnosed pediatric acute myeloid leukemia: Recent experiences of the Dutch Childhood Oncology Group. Pediatr. Blood Cancer 2020, 67, e28099. [Google Scholar] [CrossRef]
- Torres, J.P.; De la Maza, V.; Kors, L.; Villarroel, M.; Piemonte, P.; Izquierdo, G.; Salgado, C.; Tordecilla, J.; Contardo, V.; Farfán, M.J.; et al. Respiratory Viral Infections and Coinfections in Children with Cancer, Fever and Neutropenia: Clinical Outcome of Infections Caused by Different Respiratory Viruses. Pediatr. Infect. Dis. J. 2016, 35, 949–954. [Google Scholar] [CrossRef]
- Vissing, N.H.; Lausen, B.; Hutchings Hoffmann, M.; Als-Nielsen, B.; Schmiegelow, K.; Helweg-Larsen, J.; Arendrup, M.C.; Nygaard, U. Aspergillus flavus Infections in Children with Leukemia Despite Liposomal Amphotericin-B Prophylaxis. Pediatr. Infect. Dis. J. 2021, 40, 749–752. [Google Scholar] [CrossRef]
- Ishii, E.; Hara, T.; Mizuno, Y.; Ueda, K. Vincristine-induced fever in children with leukemia and lymphoma. Cancer 1988, 61, 660–662. [Google Scholar] [CrossRef]
- Chappell, T.L.; Pflaster, E.G.; Namata, R.; Bell, J.; Miller, L.H.; Pomputius, W.F.; Boutilier, J.J.; Messinger, Y.H. Bloodstream Infections in Childhood Acute Myeloid Leukemia and Machine Learning Models: A Single-institutional Analysis. J. Pediatr. Hematol. Oncol. 2025, 47, e26–e33. [Google Scholar] [CrossRef]
- Avilés-Robles, M.; Schnur, J.J.; Dorantes-Acosta, E.; Márquez-González, H.; Ocampo-Ramírez, L.A.; Chawla, N.V. Predictors of Septic Shock or Bacteremia in Children Experiencing Febrile Neutropenia Post-Chemotherapy. J. Pediatr. Infect. Dis. Soc. 2022, 11, 498–503. [Google Scholar] [CrossRef]
- van der Velden, F.J.S.; Gennery, A.R.; Emonts, M. Biomarkers for Diagnosing Febrile Illness in Immunocompromised Children: A Systematic Review of the Literature. Front. Pediatr. 2022, 10, 828569. [Google Scholar] [CrossRef]
- Haeusler, G.M.; Phillips, R.; Slavin, M.A.; Babl, F.E.; De Abreu Lourenco, R.; Mechinaud, F.; Thursky, K.A.; Australian PICNICC study group and the PREDICT network. Re-evaluating and recalibrating predictors of bacterial infection in children with cancer and febrile neutropenia. EClinicalMedicine 2020, 23, 100394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gan, M.; Ge, M.; Dong, X.; Yan, G.; Zhou, Q.; Yu, H.; Wang, X.; Cao, Y.; Lu, G.; et al. Diagnostic Performance and Clinical Impact of Metagenomic Next-Generation Sequencing for Pediatric Infectious Diseases. J. Clin. Microbiol. 2023, 61, e00115-23. [Google Scholar] [CrossRef] [PubMed]
- Arbefeville, S.S.; Timbrook, T.T.; Garner, C.D. Evolving strategies in microbe identification—A comprehensive review of biochemical, MALDI-TOF MS and molecular testing methods. J. Antimicrob. Chemother. 2024, 79, i2–i8. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.D.; Ammann, R.A.; Fisher, B.; Patel, P.; Phillips, R.; Beauchemin, M.P.; Carlesse, F.; Castagnola, E.; Davis, B.L.; et al. Guideline for the Management of Fever and Neutropenia in Pediatric Patients with Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J. Clin. Oncol. 2023, 41, 1774–1785. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Nygaard, U.; Nielsen, A.B.; Dungu, K.H.S.; Drici, L.; Holm, M.; Ottenheijm, M.E.; Nielsen, A.B.; Glenthøj, J.P.; Schmidt, L.S.; Cortes, D.; et al. Proteomic profiling reveals diagnostic signatures and pathogenic insights in multisystem inflammatory syndrome in children. Commun. Biol. 2024, 7, 688. [Google Scholar] [CrossRef]
- Buonsenso, D.; Sodero, G.; Valentini, P. Transcript host-RNA signatures to discriminate bacterial and viral infections in febrile children. Pediatr. Res. 2022, 91, 454–463. [Google Scholar] [CrossRef]
- Jenner, R.G.; Young, R.A. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 2005, 3, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Cohen, S.; Glowinski, R.; Ramilo, O. Host transcriptional signatures as predictive markers of infection in children. Curr. Opin. Infect. Dis. 2021, 34, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Kaforou, M.; Herberg, J.A.; Wright, V.J.; Coin, L.J.M.; Levin, M. Diagnosis of Bacterial Infection Using a 2-Transcript Host RNA Signature in Febrile Infants 60 Days or Younger. JAMA 2017, 317, 1577–1578. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, I.; Rodriguez-Manzano, J.; Moniri, A.; Kaforou, M.; Herberg, J.A.; Levin, M.; Georgiou, P. Translation of a Host Blood RNA Signature Distinguishing Bacterial From Viral Infection Into a Platform Suitable for Development as a Point-of-Care Test. JAMA Pediatr. 2021, 175, 417–419. [Google Scholar] [CrossRef]
- Mahajan, P.; Kuppermann, N.; Mejias, A.; Suarez, N.; Chaussabel, D.; Casper, T.C.; Smith, B.; Alpern, E.R.; Anders, J.; Atabaki, S.M.; et al. Association of RNA Biosignatures with Bacterial Infections in Febrile Infants Aged 60 Days or Younger. JAMA 2016, 316, 846–857. [Google Scholar] [CrossRef]
- Herberg, J.A.; Kaforou, M.; Wright, V.J.; Shailes, H.; Eleftherohorinou, H.; Hoggart, C.J.; Cebey-López, M.; Carter, M.J.; Janes, V.A.; Gormley, S.; et al. Diagnostic Test Accuracy of a 2-Transcript Host RNA Signature for Discriminating Bacterial vs Viral Infection in Febrile Children. JAMA 2016, 316, 835–845. [Google Scholar] [CrossRef]
- Anderson, S.T.; Kaforou, M.; Brent, A.J.; Wright, V.J.; Banwell, C.M.; Chagaluka, G.; Crampin, A.C.; Dockrell, H.M.; French, N.; Hamilton, M.S.; et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N. Engl. J. Med. 2014, 370, 1712–1723. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Ganesamoorthy, D.; Wilson, C.; Raman, S.; George, S.; Snelling, P.J.; Phillips, N.; Irwin, A.; Sharp, N.; Marsney, R.L.; et al. Host gene expression signatures to identify infection type and organ dysfunction in children evaluated for sepsis: A multicentre cohort study. Lancet Child Adolesc. Health 2024, 8, 325–338. [Google Scholar] [CrossRef]
- Wright, V.J.; Herberg, J.A.; Kaforou, M.; Shimizu, C.; Eleftherohorinou, H.; Shailes, H.; Barendregt, A.M.; Menikou, S.; Gormley, S.; Berk, M.; et al. Diagnosis of Kawasaki Disease Using a Minimal Whole-Blood Gene Expression Signature. JAMA Pediatr. 2018, 172, e182293. [Google Scholar] [CrossRef] [PubMed]
- Habgood-Coote, D.; Wilson, C.; Shimizu, C.; Barendregt, A.M.; Philipsen, R.; Galassini, R.; Calle, I.R.; Workman, L.; Agyeman, P.K.A.; Ferwerda, G.; et al. Diagnosis of childhood febrile illness using a multi-class blood RNA molecular signature. Med 2023, 4, 635–654.e5. [Google Scholar] [CrossRef] [PubMed]
- Viz-Lasheras, S.; Gómez-Carballa, A.; Bello, X.; Rivero-Calle, I.; Dacosta, A.I.; Kaforou, M.; Habgood-Coote, D.; Cunnington, A.J.; Emonts, M.; Herberg, J.A.; et al. A diagnostic host-specific transcriptome response for Mycoplasma pneumoniae pneumonia to guide pediatric patient treatment. Nat. Commun. 2025, 16, 673. [Google Scholar] [CrossRef]
- Viz-Lasheras, S.; Gómez-Carballa, A.; Pardo-Seco, J.; Bello, X.; Rivero-Calle, I.; Dacosta, A.I.; Kaforou, M.; Habgood-Coote, D.; Cunnington, A.J.; Emonts, M.; et al. A 5-transcript signature for discriminating viral and bacterial etiology in pediatric pneumonia. iScience 2025, 28, 111747. [Google Scholar] [CrossRef]
- Channon-Wells, S.; Habgood-Coote, D.; Vito, O.; Galassini, R.; Wright, V.J.; Brent, A.J.; Heyderman, R.S.; Anderson, S.T.; Eley, B.; Martinón-Torres, F.; et al. Integration and validation of host transcript signatures, including a novel 3-transcript tuberculosis signature, to enable one-step multiclass diagnosis of childhood febrile disease. J. Transl. Med. 2024, 22, 802. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Henao, R.; Montgomery, J.L.; Nawrocki, J.W.; Aydin, M.; Lydon, E.C.; Ko, E.R.; Petzold, E.; Nicholson, B.P.; Cairns, C.B.; et al. Discriminating Bacterial and Viral Infection Using a Rapid Host Gene Expression Test. Crit. Care Med. 2021, 49, 1651–1663. [Google Scholar] [CrossRef]
- Wahlund, M.; Sinha, I.; Broliden, K.; Saghafian-Hedengren, S.; Nilsson, A.; Berggren, A. The Feasibility of Host Transcriptome Profiling as a Diagnostic Tool for Microbial Etiology in Childhood Cancer Patients with Febrile Neutropenia. Int. J. Mol. Sci. 2020, 21, 5305. [Google Scholar] [CrossRef]
- Probst, V.; Smedegaard, L.M.; Simonyan, A.; Guo, Y.; Østrup, O.; Dungu, K.H.S.; Vissing, N.H.; Nygaard, U.; Bagger, F.O. A Protocol for Low-Input RNA-Sequencing of Patients with Febrile Neutropenia Captures Relevant Immunological Information. Int. J. Mol. Sci. 2023, 24, 10251. [Google Scholar] [CrossRef]
- Haeusler, G.M.; Garnham, A.L.; Li-Wai-Suen, C.S.; Clark, J.E.; Babl, F.E.; Allaway, Z.; Slavin, M.A.; Mechinaud, F.; Smyth, G.K.; Phillips, B.; et al. Blood transcriptomics identifies immune signatures indicative of infectious complications in childhood cancer patients with febrile neutropenia. Clin. Transl. Immunol. 2022, 11, e1383. [Google Scholar] [CrossRef]
- Weischendorff, S.; De Pietri, S.; Rathe, M.; Frandsen, T.L.; Hasle, H.; Nielsen, C.H.; Moser, C.; Müller, K. Markers of neutrophil chemotaxis for identification of blood stream infections in children with acute lymphoblastic leukemia undergoing induction treatment. Eur. J. Haematol. 2023, 110, 762–771. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lai, T.-Y.; Tsai, M.-K.; Chang, Y.-C.; Ho, Y.-H.; Yu, I.-S.; Yeh, T.-W.; Chou, C.-C.; Lin, Y.-S.; Lawrence, T.; et al. The ubiquitin ligase ZNRF1 promotes caveolin-1 ubiquitination and degradation to modulate inflammation. Nat. Commun. 2017, 8, 15502. [Google Scholar] [CrossRef]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Shidham, A.; Wong, H.R.; Khatri, P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci. Transl. Med. 2015, 7, 287ra71. [Google Scholar] [CrossRef]
- Cazalis, M.-A.; Lepape, A.; Venet, F.; Frager, F.; Mougin, B.; Vallin, H.; Paye, M.; Pachot, A.; Monneret, G. Early and dynamic changes in gene expression in septic shock patients: A genome-wide approach. Intensive Care Med. Exp. 2014, 2, 20. [Google Scholar] [CrossRef]
- Tang, B.M.; Huang, S.J.; McLean, A.S. Genome-wide transcription profiling of human sepsis: A systematic review. Crit. Care 2010, 14, R237. [Google Scholar] [CrossRef]
- Wong, H.R. Genome-wide expression profiling in pediatric septic shock. Pediatr. Res. 2013, 73, 564–569. [Google Scholar] [CrossRef]
- Tosi, M.; Coloretti, I.; Meschiari, M.; De Biasi, S.; Girardis, M.; Busani, S. The Interplay between Antibiotics and the Host Immune Response in Sepsis: From Basic Mechanisms to Clinical Considerations: A Comprehensive Narrative Review. Antibiotics 2024, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Dalskov, L.; Gad, H.H.; Hartmann, R. Viral recognition and the antiviral interferon response. EMBO J. 2023, 42, e112907. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, M.; Tabarani, C.M.; Bartholoma, N.; Rosenberg, H.F.; Domachowske, J.B. Nasopharyngeal detection of respiratory viruses in febrile neutropenic children. Clin. Pediatr. 2012, 51, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Santolaya, M.E. Respiratory viral infections in children with cancer and febrile neutropenia and children undergoing hematopoietic stem cell transplantation. Curr. Opin. Infect. Dis. 2024, 37, 407–412. [Google Scholar] [CrossRef]
- Barnbrock, A.; Berger, A.; Lauten, M.; Demmert, M.; Klusmann, J.-H.; Ciesek, S.; Bochennek, K.; Lehrnbecher, T. Frequency and clinical impact of viraemia in paediatric patients undergoing therapy for cancer. Sci. Rep. 2024, 14, 14867. [Google Scholar] [CrossRef]
- Handous, I.; Achour, B.; Marzouk, M.; Rouis, S.; Hazgui, O.; Brini, I.; Khelif, A.; Hannachi, N.; Boukadida, J. Co-infections of human herpesviruses (CMV, HHV-6, HHV-7 and EBV) in non-transplant acute leukemia patients undergoing chemotherapy. Virol. J. 2020, 17, 37. [Google Scholar] [CrossRef]
- Obrová, K.; Grumaz, S.; Remely, M.; Czurda, S.; Krickl, I.; Herndlhofer, S.; Gleixner, K.V.; Sperr, W.R.; Größlinger, L.; Frank, T.; et al. Presence of viremia during febrile neutropenic episodes in patients undergoing chemotherapy for malignant neoplasms. Am. J. Hematol. 2021, 96, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Phasuk, N.; Keatkla, J.; Rattanasiri, S.; Techasaensiri, C.; Anurathapan, U.; Apiwattanakul, N. Monitoring of cytomegalovirus infection in non-transplant pediatric acute lymphoblastic leukemia patients during chemotherapy. Medicine 2019, 98, e14256. [Google Scholar] [CrossRef] [PubMed]
- Cabler, S.S.; Storch, G.A.; Weinberg, J.B.; Walton, A.H.; Brengel-Pesce, K.; Aldewereld, Z.; Banks, R.K.; Cheynet, V.; Reeder, R.; Holubkov, R.; et al. Viral DNAemia and DNA Virus Seropositivity and Mortality in Pediatric Sepsis. JAMA Netw. Open 2024, 7, e240383. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, X.; Wu, M.; Jiang, L.; Wang, F.; He, S. IFI27 may predict and evaluate the severity of respiratory syncytial virus infection in preterm infants. Hereditas 2021, 158, 3. [Google Scholar] [CrossRef]
- Min, Z.; Ye, Z.; Gang, L.; Boyu, D.; Xueyan, X. IFI27 as a potential indicator for severe Enterovirus 71-infected hand foot and mouth disease. Virus Res. 2020, 289, 198149. [Google Scholar] [CrossRef]
- Tang, B.M.; Shojaei, M.; Parnell, G.P.; Huang, S.; Nalos, M.; Teoh, S.; O’Connor, K.; Schibeci, S.; Phu, A.L.; Kumar, A.; et al. A novel immune biomarker IFI27 discriminates between influenza and bacteria in patients with suspected respiratory infection. Eur. Respir. J. 2017, 49, 1602098. [Google Scholar] [CrossRef]
- Shojaei, M.; McLean, A.S. Interferon-stimulated gene IFI27 as a multifaceted candidate target in precision medicine. Trends Immunol. 2025, 46, 219–228. [Google Scholar] [CrossRef]
- Villamayor, L.; López-García, D.; Rivero, V.; Martínez-Sobrido, L.; Nogales, A.; DeDiego, M.L. The IFN-stimulated gene IFI27 counteracts innate immune responses after viral infections by interfering with RIG-I signaling. Front. Microbiol. 2023, 14, 1176177. [Google Scholar] [CrossRef]
- Schmiegelow, K. Early Termination of Empirical Antibiotics in High-Risk Febrile Neutropenia in Children with Cancer: An Open-Label, Randomised, Controlled Trial. Available online: https://clinicaltrials.gov/study/NCT04637464 (accessed on 11 April 2025).
- Murdoch Childrens Research Institute. Early Versus Late Stopping of Antibiotics in Children with Cancer and High-Risk Febrile Neutropenia. Available online: https://clinicaltrials.gov/study/NCT04948463 (accessed on 11 April 2025).
- Bodkin, N.; Ross, M.; McClain, M.T.; Ko, E.R.; Woods, C.W.; Ginsburg, G.S.; Henao, R.; Tsalik, E.L. Systematic comparison of published host gene expression signatures for bacterial/viral discrimination. Genome Med. 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Chawla, D.G.; Cappuccio, A.; Tamminga, A.; Sealfon, S.C.; Zaslavsky, E.; Kleinstein, S.H. Benchmarking transcriptional host response signatures for infection diagnosis. Cell Syst. 2022, 13, 974–988.e7. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- GeneCards. Available online: www.genecards.com (accessed on 1 March 2025).
- Hintze, J.L.; Nelson, R.D. Violin Plots: A Box Plot-Density Trace Synergism. Am. Stat. 1998, 52, 181–184. [Google Scholar] [CrossRef]
- Sidiropoulos, N.; Sohi, S.H.; Pedersen, T.L.; Porse, B.T.; Winther, O.; Rapin, N.; Bagger, F.O. SinaPlot: An Enhanced Chart for Simple and Truthful Representation of Single Observations Over Multiple Classes. J. Comput. Graph. Stat. 2018, 27, 673–676. [Google Scholar] [CrossRef]

| Febrile Episodes | WBC ×109 cells/L | ANC ×109 cells/L | CRP mg/L | PCT ng/mL |
|---|---|---|---|---|
| Positive blood culture | ||||
| Bacteremia | ||||
| 0.5 (0.1–2.4) | 0.0 (0.0–1.4) | 84 (40–130) | 5.9 (0.7–15.9) |
| 0.3 (0.1–0.5) | 0.0 (0.0–0.0) | 200 (150–287) | 4.3 (0.4–95.0) |
| Coagulase-negative staphylococci | 1.5 (0.5–2.3) | 0.8 (0.0–1.6) | 58 (17–115) | 0.5 (0.1–1.3) |
| Negative blood culture | ||||
| C-reactive protein < 35 mg/L | ||||
| 3.6 (1.6–5.9) | 2.5 (0.8–4.6) | 7 (4–20) | 0.2 (0.1–0.3) |
| 1.6 (1.1–3.2) | 0.3 (0.1–1.1) | 19 (8–25) | 0.2 (0.1–0.3) |
| C-reactive protein ≥ 35 mg/L | 0.7 (0.3–0.6) | 0.0 (0.0–0.3) | 88 (54–137) | 0.7 (0.3–1.5) |
| Gene Symbol | Gene Name | log2 FC | padj | Gene Function |
|---|---|---|---|---|
| Immune response and regulation | ||||
| CXCL1 | C-X-C Motif Chemokine Ligand 1 | 3.46 | 0.0001 | Chemotactic activity for neutrophils |
| ZNRF1 | Zinc and Ring Finger 1 | 2.16 | <0.0001 | Regulation of inflammation, TLR4-activated immune response, controlling TLR3 trafficking |
| BACH2 | BTB Domain and CNC Homolog 2 | 1.72 | 0.0015 | Regulates T-cell function, B-cell maturation, and inflammatory response |
| TP53BP1 | Tumor Protein 53 Binding Protein 1 | 2.40 | 0.0013 | DNA repair in lymphocytes; class switch recombination in B cells |
| ISG15 | ISG15 Ubiquitin-Like Modifier | −2.07 | <0.0001 | Interferon-induced; essential in innate antiviral immune response |
| OAS1 | 2′-5′-Oligoadenylate Synthetase 1 | −1.56 | 0.0002 | Interferon-induced; essential in innate antiviral immune response |
| IFIT3 | Interferon-Induced Protein with Tetratricopeptide Repeats 3 | −2.19 | 0.0006 | Interferon-induced antiviral protein; inhibitor of cellular and viral processes, cell migration, proliferation, signaling, and viral replication |
| IFIT2 | Interferon-Induced Protein with Tetratricopeptide Repeats 2 | −1.96 | 0.0013 | Interferon-induced antiviral protein; inhibits expression of viral messenger RNAs |
| OASL | 2′-5′-Oligoadenylate Synthetase Like | −1.59 | 0.0041 | Negative regulation of viral genome replication, IL-27-mediated signaling pathway |
| CMPK2 | Cytidine/Uridine Monophosphate Kinase 2 | −1.68 | 0.0042 | Immunomodulatory and antiviral activities |
| IFIT1 | Interferon-Induced Protein with Tetratricopeptide Repeats 1 | −1.89 | 0.0071 | Interferon-induced antiviral protein; inhibits viral replication |
| Other | ||||
| SS18L1 | SS18L1 Subunit of BAF Chromatin Remodeling Complex | 1.70 | 0.0015 | Calcium-responsive transactivator; an essential subunit of a neuron-specific chromatin-remodeling complex |
| CSTPP1 | Centriolar Satellite-Associated Tubulin Polyglutamylase Complex Regulator 1 | 1.58 | 0.0014 | Cytoskeletal organization, nuclear shape, and cilium disassembly |
| PITPNM2 | Phosphatidylinositol Transfer Protein Membrane Associated 2 | 2.05 | 0.0002 | Calcium ion binding and lipid binding |
| MYOZ3 | Myozenin 3 | 3.90 | 0.0015 | Skeletal and cardiac muscle; calcineurin-interacting, helps tether calcineurin to the sarcomere |
| ARHGEF9 | Cdc42 Guanine Nucleotide Exchange Factor 9 | 2.18 | 0.0014 | Brain-specific; receptor recruitment in GABAergic and glycinergic synapses |
| DUSP6 | Dual Specificity Phosphatase 6 | −1.51 | 0.0075 | Inactivates MAP kinases associated with cellular proliferation and differentiation |
| PCSK6 | Proprotein Convertase Subtilisin/Kexin Type 6 | 2.68 | 0.0014 | Vascular remodeling; processes protein and peptide precursors in the liver, gut, and brain |
| ODF3B | Outer Dense Fiber of Sperm Tails 3B | −1.52 | 0.0061 | Functional element |
| EXOC3L1 | Exocyst Complex Component 3 Like 1 | −2.53 | 0.0063 | Part of the exocyst; possible role in regulating exocytosis of insulin granules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smedegaard, L.M.; Dungu, K.H.S.; Guo, Y.; Hjalgrim, L.L.; Probst, V.; Mariani, L.; Grosen, D.; Kristensen, I.; Tuckuviene, R.; Schmiegelow, K.; et al. Fever in Children with Cancer: Pathophysiological Insights Using Blood Transcriptomics. Int. J. Mol. Sci. 2025, 26, 7126. https://doi.org/10.3390/ijms26157126
Smedegaard LM, Dungu KHS, Guo Y, Hjalgrim LL, Probst V, Mariani L, Grosen D, Kristensen I, Tuckuviene R, Schmiegelow K, et al. Fever in Children with Cancer: Pathophysiological Insights Using Blood Transcriptomics. International Journal of Molecular Sciences. 2025; 26(15):7126. https://doi.org/10.3390/ijms26157126
Chicago/Turabian StyleSmedegaard, Lotte Møller, Kia Hee Schultz Dungu, Yuliu Guo, Lisa Lyngsie Hjalgrim, Victoria Probst, Luca Mariani, Dorthe Grosen, Ines Kristensen, Ruta Tuckuviene, Kjeld Schmiegelow, and et al. 2025. "Fever in Children with Cancer: Pathophysiological Insights Using Blood Transcriptomics" International Journal of Molecular Sciences 26, no. 15: 7126. https://doi.org/10.3390/ijms26157126
APA StyleSmedegaard, L. M., Dungu, K. H. S., Guo, Y., Hjalgrim, L. L., Probst, V., Mariani, L., Grosen, D., Kristensen, I., Tuckuviene, R., Schmiegelow, K., Bagger, F. O., Vissing, N. H., & Nygaard, U. (2025). Fever in Children with Cancer: Pathophysiological Insights Using Blood Transcriptomics. International Journal of Molecular Sciences, 26(15), 7126. https://doi.org/10.3390/ijms26157126






