Neural Progenitor Cell- and Developing Neuron-Derived Extracellular Vesicles Differentially Modulate Microglial Activation
Abstract
1. Introduction
2. Results
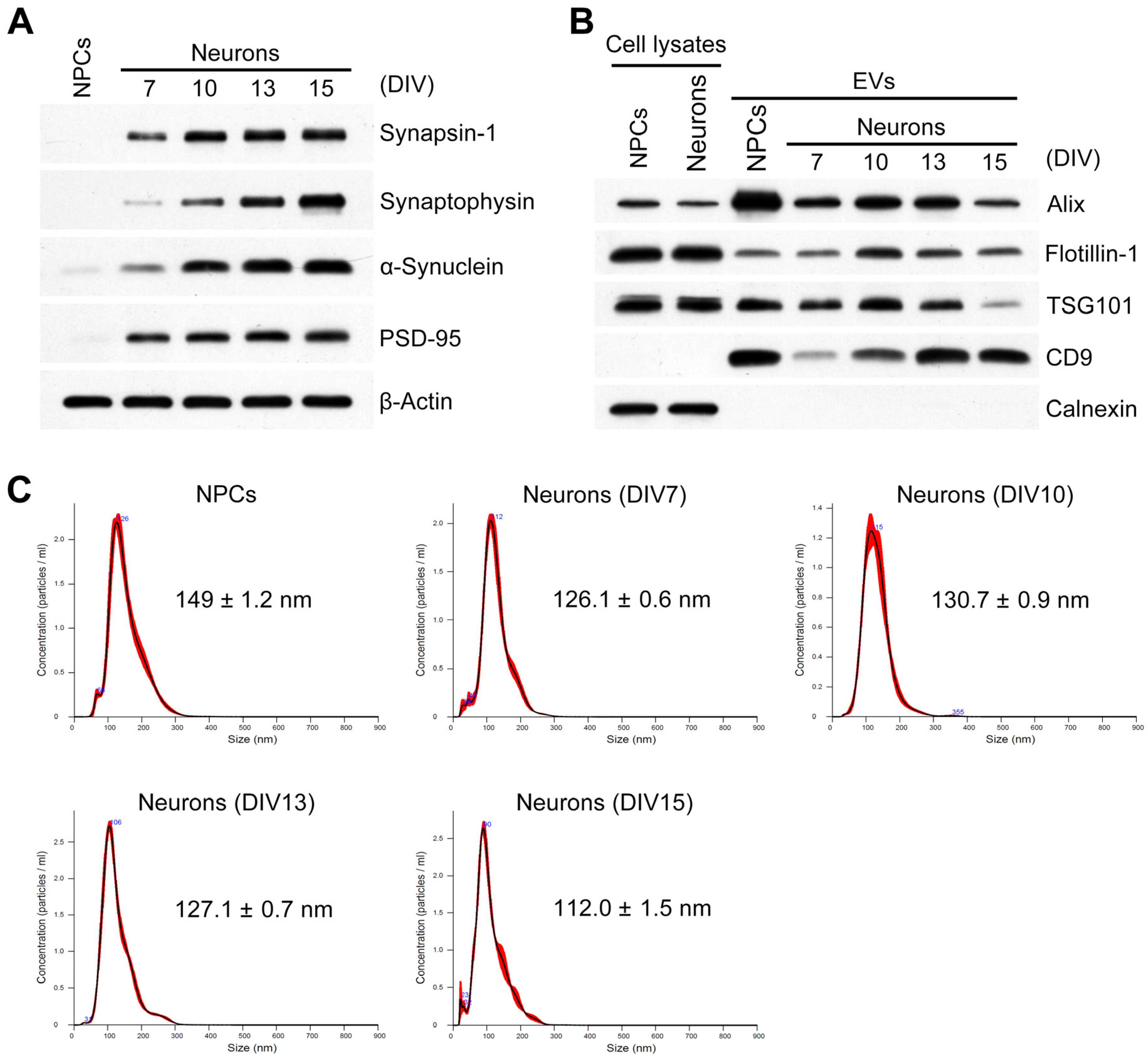
2.1. Characterization of Developing Neurons and Isolated EVs
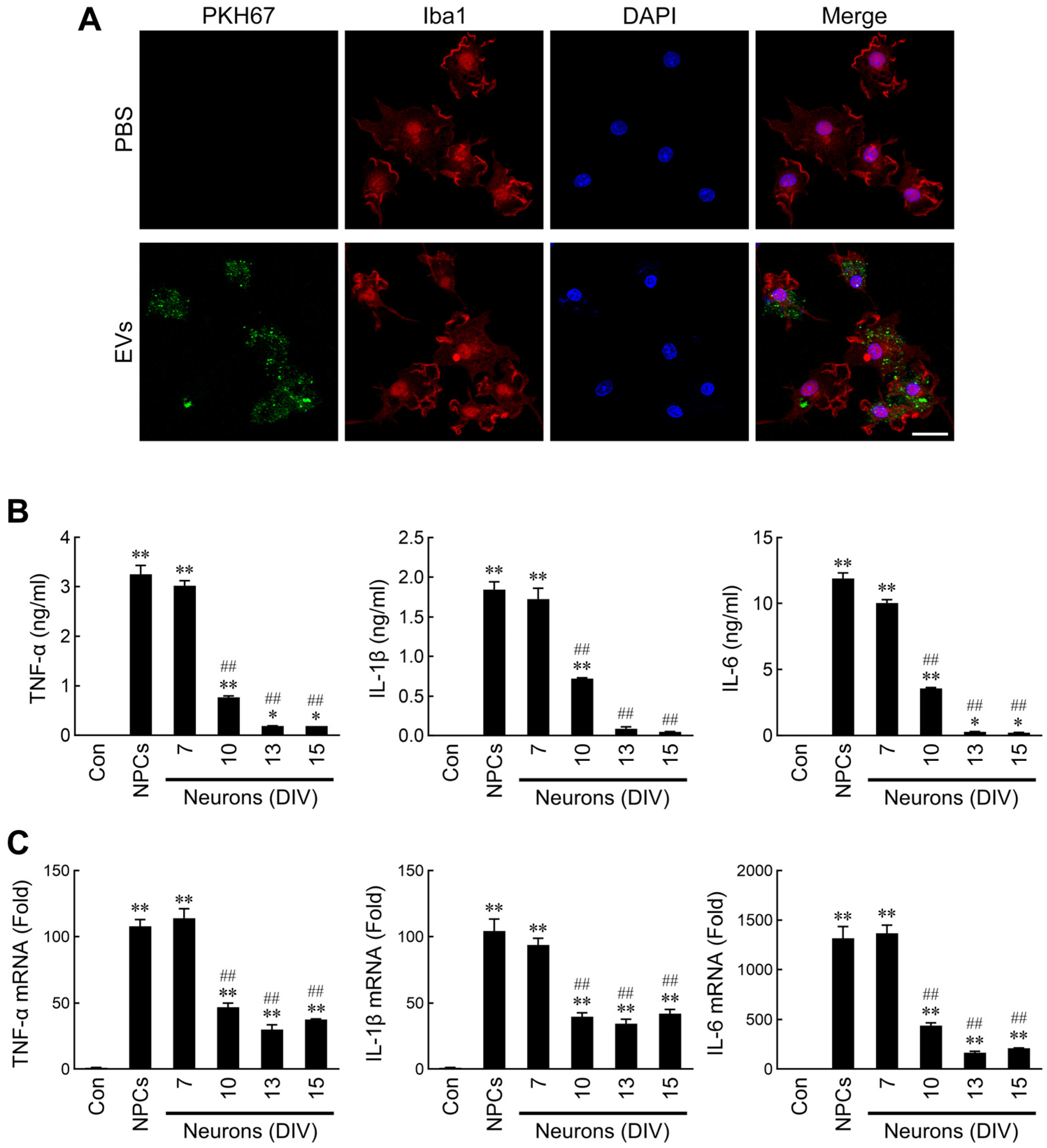
2.2. Differential Regulation of Microglial Activation by NPC- and Neuron-Derived EVs
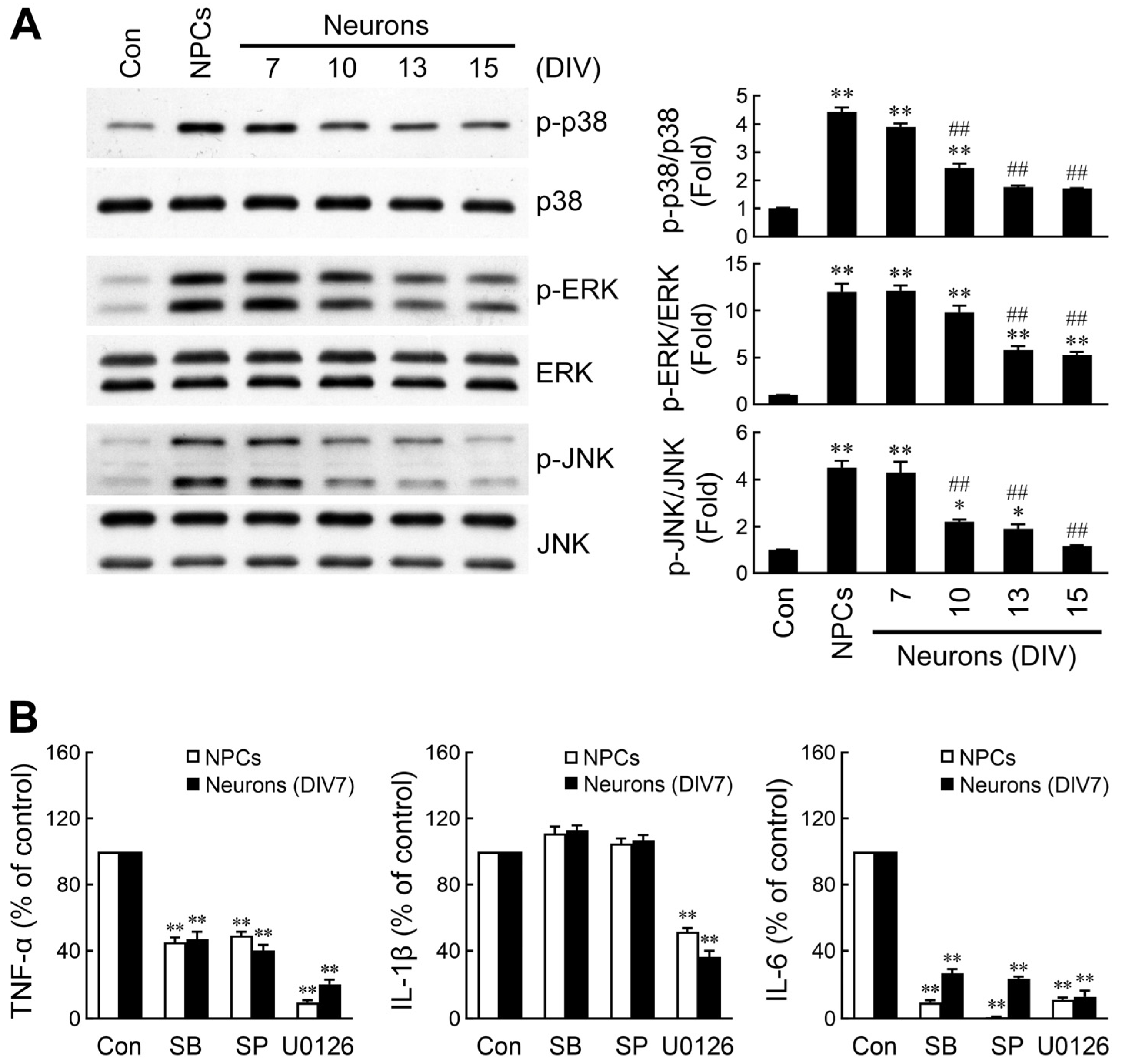
2.3. Divergent Activation of MAPK Signaling by NPC- and Neuron-Derived EVs
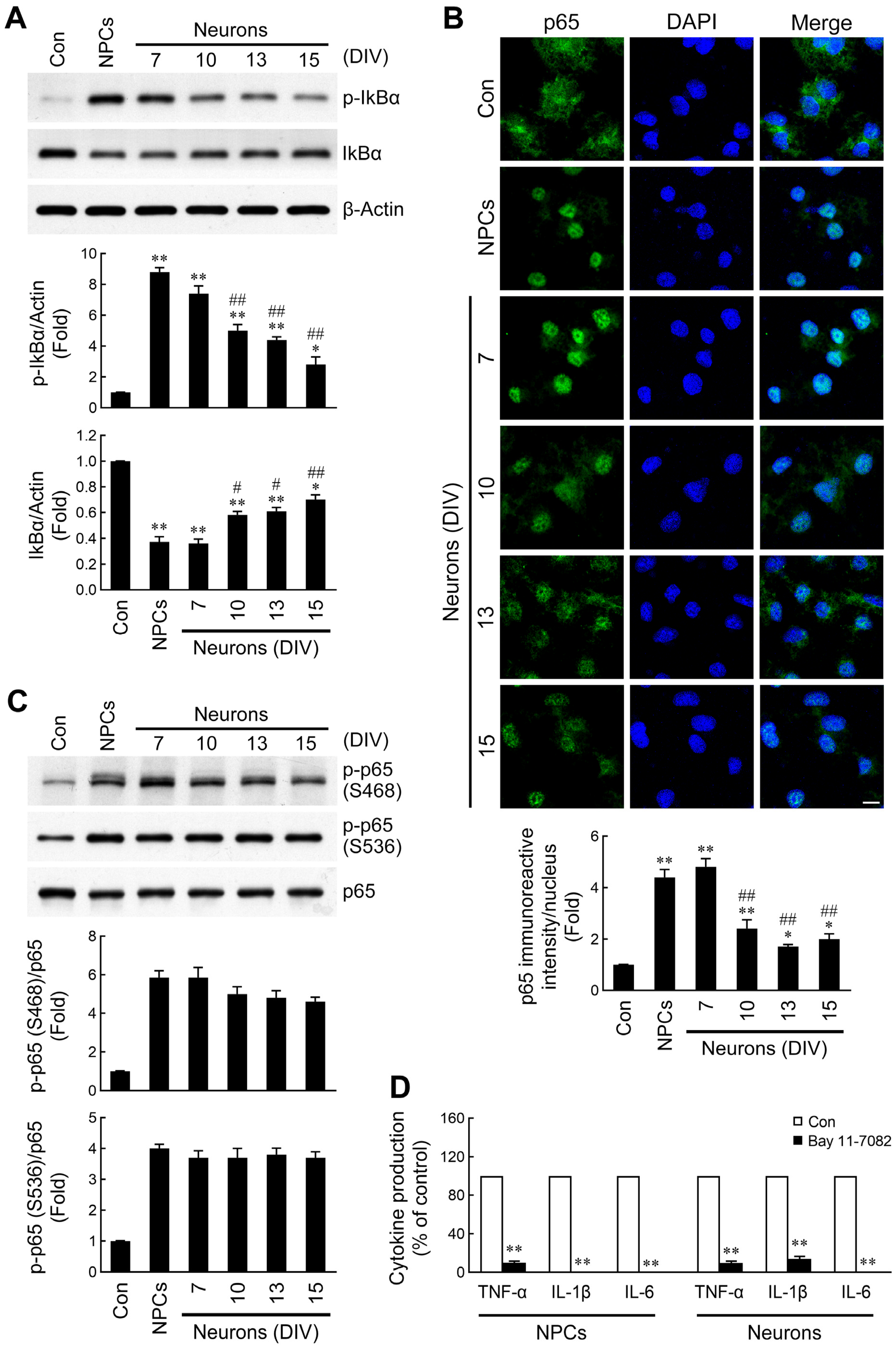
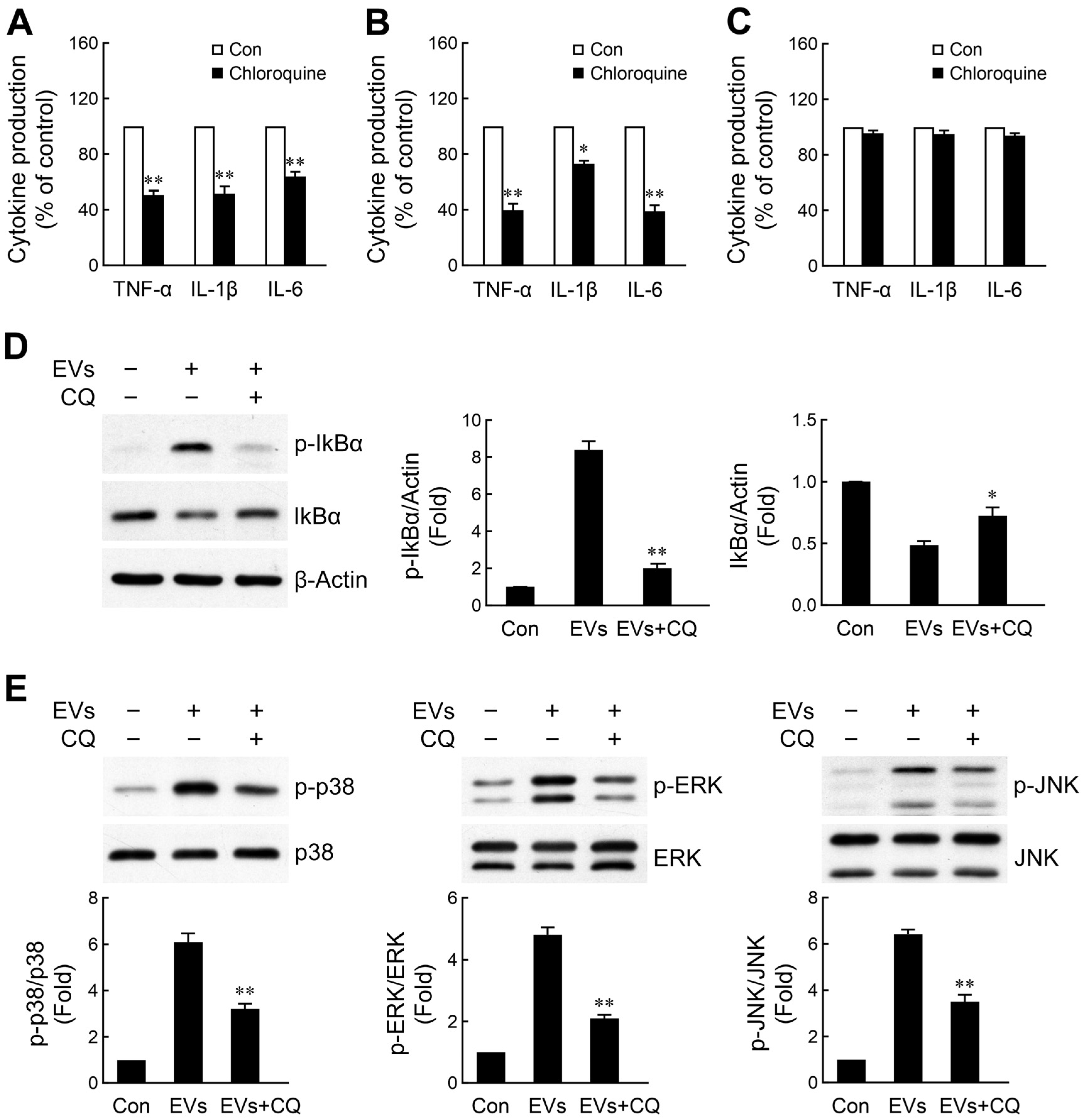
2.4. Discrepant Activation of NF-κB Signaling by NPC- and Neuron-Derived EVs
2.5. NPC- and Immature Neuron-Derived EVs Activate Microglia via Toll-like Receptor 7 (TLR7) Signaling Axis
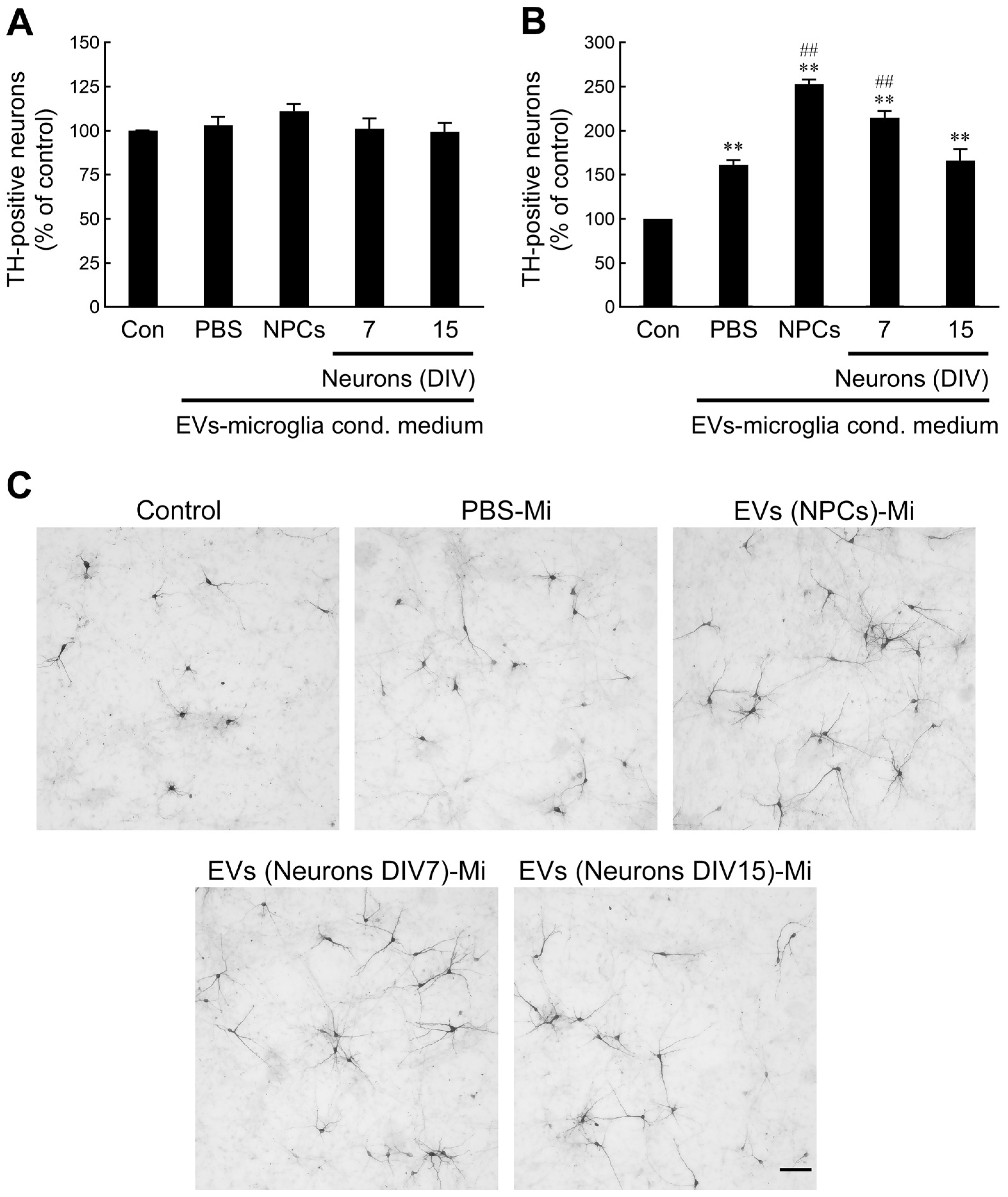
2.6. NPC- and Immature Neuron-Derived EV-Activated Microglia Promote Developing Dopaminergic (DA) Neuron Survival
3. Discussion
4. Materials and Methods
4.1. Neural Progenitor Cell Cultures
4.2. Mesencephalic Neuron-Enriched Cultures
4.3. Microglial Cultures
4.4. Dopaminergic Neuron Differentiation Assay
4.5. Isolation of EVs
4.6. Quantification and Size Evaluation of EVs
4.7. Labeling and Uptake of EVs
4.8. Real-Time RT-PCR Analysis
4.9. Western Blotting
4.10. Immunocytochemistry
4.11. Cytokines Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kierdorf, K.; Prinz, M. Microglia in steady state. J. Clin. Investig. 2017, 127, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Mosser, C.A.; Baptista, S.; Arnoux, I.; Audinat, E. Microglia in CNS development: Shaping the brain for the future. Prog. Neurobiol. 2017, 149–150, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 1999, 117, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Kohsaka, S.; Rezaie, P. The origin and cell lineage of microglia: New concepts. Brain Res. Rev. 2007, 53, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Garel, S. The mysterious origins of microglia. Nat. Neurosci. 2018, 21, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Navascués, J.; Calvente, R.; Marín-Teva, J.L.; Cuadros, M.A. Entry, dispersion and differentiation of microglia in the developing central nervous system. An. Acad. Bras. Cienc. 2000, 72, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Rigato, C.; Buckinx, R.; Le-Corronc, H.; Rigo, J.M.; Legendre, P. Pattern of invasion of the embryonic mouse spinal cord by microglial cells at the time of the onset of functional neuronal networks. Glia 2011, 59, 675–695. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, N.; Smolders, S.; Avila, A.; Notelaers, K.; Paesen, R.; Ameloot, M.; Brône, B.; Legendre, P.; Rigo, J.M. Complex invasion pattern of the cerebral cortex by microglial cells during development of the mouse embryo. Glia 2013, 61, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Bruttger, J.; Karram, K.; Wörtge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y. The behavior and functions of embryonic microglia. Anat. Sci. Int. 2022, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y. The multifaceted roles of embryonic microglia in the developing brain. Front. Cell. Neurosci. 2023, 17, 988952. [Google Scholar] [CrossRef] [PubMed]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada González, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Itoh, H.; Tsugawa, Y.; Nishida, Y.; Kurata, K.; Uemura, A.; Miyata, T. Embryonic pericytes promote microglial homeostasis and their effects on neural progenitors in the developing cerebral cortex. J. Neurosci. 2022, 42, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.P.; Breyak, E.; Nishinakamura, R.; Nakagawa, Y. The zinc finger transcription factor Sall1 is required for the early developmental transition of microglia in mouse embryos. Glia 2022, 70, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Hoek, R.M.; Ruuls, S.R.; Murphy, C.A.; Wright, G.J.; Goddard, R.; Zurawski, S.M.; Blom, B.; Homola, M.E.; Streit, W.J.; Brown, M.H.; et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 2000, 290, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Bonetto, V. Extracellular vesicles and a novel form of communication in the brain. Front. Neurosci. 2016, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Breakefield, X.O. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 2012, 3, 228. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. Lond. Ser. B. Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Pistono, C.; Bister, N.; Stanová, I.; Malm, T. Glia-derived extracellular vesicles: Role in central nervous system communication in health and disease. Front. Cell Dev. Biol. 2021, 8, 623771. [Google Scholar] [CrossRef] [PubMed]
- Schnatz, A.; Müller, C.; Brahmer, A.; Krämer-Albers, E.M. Extracellular vesicles in neural cell interaction and CNS homeostasis. FASEB Bioadv. 2021, 3, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Bahrini, I.; Song, J.H.; Diez, D.; Hanayama, R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci. Rep. 2015, 5, 7989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Harvey, B.T.; Richards, C.I.; Nixon, K. Neuron-derived extracellular vesicles modulate microglia activation and function. Biology 2021, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Cai, L.L.; Li, Y.; Wang, R.C.; Xu, Y.H.; Chen, Y.J.; Xie, Y.H.; Zhu, X.L.; Li, Y.F. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J. Nanobiotechnol. 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Garel, S. On place and time: Microglia in embryonic and perinatal brain development. Curr. Opin. Neurobiol. 2017, 47, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, D.M.; Zhang, S.; Nasrallah, C.M.; Lisgo, S.N.; Bordey, A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS ONE 2014, 9, e88810. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.C.; Feliciano, D.M. Neurovesicles in brain development. Cell. Mol. Neurobiol. 2016, 36, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal subventricular zone neural stem cells release extracellular vesicles that act as a microglial morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Stronati, E.; Conti, R.; Cacci, E.; Cardarelli, S.; Biagioni, S.; Poiana, G. Extracellular vesicle-induced differentiation of neural stem progenitor cells. Int. J. Mol. Sci. 2019, 20, 3691. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, N.; Heldt, J.; Leclere, N.; Gross, J. Differential response of immature and mature neurons to hypoxia in rat mesencephalic cultures. Dev. Neurosci. 2001, 23, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lesuisse, C.; Martin, L.J. Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J. Neurobiol. 2002, 51, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglial morphometric parameters correlate with the expression level of IL-1beta, and allow identifying different activated morphotypes. Front. Cell. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Forsbach, A.; Nemorin, J.G.; Montino, C.; Müller, C.; Samulowitz, U.; Vicari, A.P.; Jurk, M.; Mutwiri, G.K.; Krieg, A.M.; Lipford, G.B.; et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 2008, 180, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liao, K.; Niu, F.; Yang, L.; Dallon, B.W.; Callen, S.; Tian, C.; Shu, J.; Cui, J.; Sun, Z.; et al. Astrocyte EV-induced lincRNA-Cox2 regulates microglial phagocytosis: Implications for morphine-mediated neurodegeneration. Mol. Ther. Nucleic Acids 2018, 13, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, Y.; Jeyaram, A.; Jay, S.M.; Zou, L.; Chao, W. Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA- and TLR7-dependent mechanisms. J. Immunol. 2018, 201, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Niu, F.; Hu, G.; Yang, L.; Dallon, B.; Villarreal, D.; Buch, S. Morphine-mediated release of miR-138 in astrocyte-derived extracellular vesicles promotes microglial activation. J. Extracell. Vesicles 2020, 10, e12027. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, A.; Bencina, M.; Svajger, U.; Jeras, M.; Rozman, B.; Jerala, R. Mechanism of endosomal TLR inhibition by antimalarial drugs: Implications for drug design. J. Immunol. 2011, 186, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 8127774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, P.; Bonnet, P.A. Recent advances on small-molecule antagonists targeting TLR7. Molecules 2023, 28, 634. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Chiu, T.L.; Chang, H.F.; Hsu, H.R.; Pang, C.Y.; Liew, H.K.; Wang, M.J. Epigenetic regulation contributes to urocortin-enhanced midbrain dopaminergic neuron differentiation. Stem Cells 2015, 33, 1601–1617. [Google Scholar] [CrossRef] [PubMed]
- Zusso, M.; Methot, L.; Lo, R.; Greenhalgh, A.D.; David, S.; Stifani, S. Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1. J. Neurosci. 2012, 32, 11285–11298. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Nitsch, R.; Heinemann, U.; Haas, D.; Eder, C. Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signaling pathways. Eur. J. Neurosci. 2001, 14, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wollmer, M.A.; Lucius, R.; Wilms, H.; Held-Feindt, J.; Sievers, J.; Mentlein, R. ATP and adenosine induce ramification of microglia in vitro. J. Neuroimmunol. 2001, 115, 19–27. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, G.L.; Santander, N.; Zhang, X.; Kleemann, K.L.; Tran, L.; Katewa, A.; Conant, K.; Barraza, M.; Waddell, K.; Lizama, C.O.; et al. Radial glia integrin avb8 regulates cell autonomous microglial TGFbeta1 signaling that is necessary for microglial identity. Nat. Commun. 2025, 16, 2840. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Andres, R.H.; Fukuhara, T.; Bieri, G.; Hasegawa-Moriyama, M.; He, Y.; Guzman, R.; Wyss-Coray, T. Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci. 2012, 15, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Arnò, B.; Grassivaro, F.; Rossi, C.; Bergamaschi, A.; Castiglioni, V.; Furlan, R.; Greter, M.; Favaro, R.; Comi, G.; Becher, B.; et al. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat. Commun. 2014, 5, 5611. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Zhang, L.F.; Ding, P.S.; Liu, Y.J.; Wu, X.; Zhou, J.N. Microglial activation mediates host neuronal survival induced by neural stem cells. J. Cell Mol. Med. 2014, 18, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.M.A.; Goodkey, K.; Voronova, A. Regulation of microglia function by neural stem cells. Front. Cell. Neurosci. 2023, 17, 1130205. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2020, 68, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- VanRyzin, J.W.; Marquardt, A.E.; Pickett, L.A.; McCarthy, M.M. Microglia and sexual differentiation of the developing brain: A focus on extrinsic factors. Glia 2020, 68, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zou, L.; Yan, D.; Chen, H.; Xu, G.; Jian, W.; Cui, P.; Chao, W. Extracellular microRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. J. Immunol. 2017, 199, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglioli, A.; Efe, I.E.; Guneykaya, D.; Ivanov, A.; Huang, Y.; Orlowski, E.; Krüger, C.; Deisz, R.A.; Markovic, D.; Flüh, C.; et al. let-7 microRNAs regulate microglial function and suppress glioma growth through Toll-like receptor 7. Cell Rep. 2019, 29, 3460–3471. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Morsey, B.M.; Emanuel, K.M.; Fox, H.S. Sequence-specific extracellular microRNAs activate TLR7 and induce cytokine secretion and leukocyte migration. Mol. Cell. Biochem. 2021, 476, 4139–4151. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, J.; Proestler, E.; Riedel, A.; Nevermann, S.; Hertel, B.; Guenther, A.; Gattenlöhner, S.; Savai, R.; Larsson, K.; Saul, M.J. Small extracellular vesicle-derived miR-574-5p regulates PGE2-biosynthesis via TLR7/8 in lung cancer. J. Extracell. Vesicles 2021, 10, e12143. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Goldman, J.E.; Sekino, Y.; Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014, 34, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, S.Z.; Kuo, J.S.; Chen, W.F.; Wang, M.J. G-CSF protects dopaminergic neurons from 6-OHDA-induced toxicity via the ERK pathway. Neurobiol. Aging 2007, 28, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Lin, S.Z.; Kuo, J.S.; Huang, H.Y.; Tzeng, S.F.; Liao, C.H.; Chen, D.C.; Chen, W.F. Urocortin modulates inflammatory response and neurotoxicity induced by microglial activation. J. Immunol. 2007, 179, 6204–6214. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, A.; Gitler, A.D. Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PLoS ONE 2017, 12, e0171418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, T.-L.; Huang, H.-Y.; Liew, H.-K.; Chang, H.-F.; Wu, H.-R.; Wang, M.-J. Neural Progenitor Cell- and Developing Neuron-Derived Extracellular Vesicles Differentially Modulate Microglial Activation. Int. J. Mol. Sci. 2025, 26, 7099. https://doi.org/10.3390/ijms26157099
Chiu T-L, Huang H-Y, Liew H-K, Chang H-F, Wu H-R, Wang M-J. Neural Progenitor Cell- and Developing Neuron-Derived Extracellular Vesicles Differentially Modulate Microglial Activation. International Journal of Molecular Sciences. 2025; 26(15):7099. https://doi.org/10.3390/ijms26157099
Chicago/Turabian StyleChiu, Tsung-Lang, Hsin-Yi Huang, Hock-Kean Liew, Hui-Fen Chang, Hsin-Rong Wu, and Mei-Jen Wang. 2025. "Neural Progenitor Cell- and Developing Neuron-Derived Extracellular Vesicles Differentially Modulate Microglial Activation" International Journal of Molecular Sciences 26, no. 15: 7099. https://doi.org/10.3390/ijms26157099
APA StyleChiu, T.-L., Huang, H.-Y., Liew, H.-K., Chang, H.-F., Wu, H.-R., & Wang, M.-J. (2025). Neural Progenitor Cell- and Developing Neuron-Derived Extracellular Vesicles Differentially Modulate Microglial Activation. International Journal of Molecular Sciences, 26(15), 7099. https://doi.org/10.3390/ijms26157099




