Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations
Abstract
1. Introduction
2. Mechanism of Action
3. Clinical Development
3.1. Phase I Studies
3.2. Phase II Studies
| Study ID (NCT Trial Number) | Treatment Arm | Number of Patients | Patient Population | ORR (%) | Most Common Grade ≥ 3 (%) | Any Grade Hypoxia (%) | Grade ≥ 3 Hypoxia (%) |
|---|---|---|---|---|---|---|---|
| LITESPARK001 (NCT02974738) [22,28] | Belzutifan for ccRCC | 55 | ccRCC previously treated with ≥1 therapy | 25 | Anemia (27) | 17 (31) | 9 (16) |
| LITESPARK-004 (NCT03401788) [8,29] | Belzutifan | 61 | ccRCC with VHL disease, not all pretreated | 49 | Anemia and hypertension (8) | 1 (2) | 1 (2) |
| LITESPARK-013 (NCT04489771) [26] | Belzutifan 120 mg 200 mg | 76 78 | ccRCC previously treated with one to three therapies | 23.7 23.1 | Hypoxia (21.1) Anemia (26.9) | 18 (23.7) 21 (26.9) | 16 (21.1) 17 (21.8) |
| LITESPARK-005 (NCT04195750) [9,30] | Belzutifan | 374 | ccRCC previously treated with immune and antiangiogenic therapies | 21.9 | Anemia (32.5) | 54 (14.5) | 39 (10.5) |
| LITESPARK-003 (NCT03634540) [27,31] | Belzutifan + Cabozantinib | 52 | ccRCC previously treated with immune therapy | 30.8 | Hypertension (27) | 2 (4) | 2 (4) |
3.3. Phase III Studies
3.4. Ongoing Clinical Trials
| Trial Number NCT Identifier | Phase | Intervention | Setting | Line of Therapy | Recruitment Status |
|---|---|---|---|---|---|
| Phase I Studies | |||||
| NCT06234605 [41] | I | HC-7366 Monotherapy versus HC-7366 + Belzutifan | Locally advanced (unresectable) or metastatic clear cell renal cell carcinoma (ccRCC) | 1st line | Recruiting |
| NCT05030506 [42] | I | Belzutifan + Lenvatinib Experimental Arm: Belzutifan + Lenvatinib + Pembrolizumab | Locally advanced (unresectable) or metastatic clear cell renal cell carcinoma (ccRCC) | 2nd line Experimental Arm: 1st line | Active, Not Recruiting |
| NCT04626479 [43] | I | Pembrolizumab/Quavonlimab + Lenvatinib versus Favezelimab/Pembrolizumab + Lenvatinib versus Pembrolizumab + Belzutifan + Lenvatinib versus Pembrolizumab + Lenvatinib versus Vibostolimab/Pembrolizumab + Belzutifan | Metastatic ccRCC | 1st line | Active, Not Recruiting |
| NCT02974738 [22,28] | I | Belzutifan Monotherapy with ccRCC and advanced solid tumors | Locally advanced ccRCC or metastatic solid tumors | 1 line+ | Active, Not Recruiting |
| NCT02293980 [44] | I | MK-3795, formerly called PT2385 versus MK-3795 + Nivolumab + Belzutifan versus MK-3795 + Cabozantinib | Advanced or metastatic clear-cell renal cell carcinoma (ccRCC) | 1 line+ | Active, Not Recruiting |
| NCT04846920 [45] | I | Belzutifan 160 mg BID Belzutifan 160 mg TID Belzutifan 200 mg TID Belzutifan 120 mg QD | Advanced clear-cell renal cell carcinoma (ccRCC) | 2 lines+ | Active, Not Recruiting |
| NCT04626518 [46] | I | Coformulation Pembrolizumab/Quavonlimab versus Coformulation Favezelimab/Pembrolizumab versus Pembrolizumab + MK-4830 versus Pembrolizumab + Belzutifan versus Belzutifan + Lenvatinib versus Pembrolizumab + Lenvatinib | Advanced clear-cell renal cell carcinoma (ccRCC) | 2 lines+ | Active, Not Recruiting |
| NCT05468697 [47] | I/II | Belzutifan Monotherapy versus Belzutifan + Palbociclib | Advanced clear-cell renal cell carcinoma (ccRCC) | 2 lines+ | Recruiting |
| Phase II Studies | |||||
| NCT03634540 (MK-6482-003) [27,31] | II | Belzutifan + Cabozantinib (Treatment Naïve) versus Belzutifan + Cabozantinib (Prior Immunotherapy) | Advanced or metastatic clear-cell renal cell carcinoma (ccRCC) | 1st/2nd line | Active, Not Recruiting |
| NCT03108066 [48] | II | MK-3795 (PT2385) | Von Hippel-Lindau (VHL) disease-associated clear cell renal cell carcinoma (ccRCC) | 1st line | Completed |
| NCT03401788 (MK-6482-004) [8,29] | II | Belzutifan Monotherapy | VHL disease + RCC tumor | 1st line | Active, Not Recruiting |
| NCT04489771 [26,49] | II | Belzutifan 200 mg versus Belzutifan 120 mg | Advanced or metastatic clear-cell renal cell carcinoma (ccRCC) | 2nd line | Active, Not Recruiting |
| Phase III Studies | |||||
| NCT05239728 [34] | III | Belzutifan + Pembrolizumab versus Placebo + Pembrolizumab | Adjuvant treatment of clear cell renal cell carcinoma (ccRCC) post-nephrectomy | 1st line | Active, Not Recruiting |
| NCT05899049 [50] | III | Pembrolizumab + Belzutifan + Lenvatinib versus Pembrolizumab/Quavonlimab + Lenvatinib | Advanced clear cell renal cell carcinoma (ccRCC) | 1st line | Active, Not Recruiting |
| NCT04736706 [37] | III | Pembrolizumab + Belzutifan + Lenvatinib or Pembrolizumab/Quavonlimab + Lenvatinib versus Pembrolizumab plus Lenvatinib | Advanced clear cell renal cell carcinoma (ccRCC) | 1st line | Active, Not Recruiting |
| NCT04586231 [39] | III | Belzutifan + Lenvatinib versus Cabozantinib | Locally advanced (inoperable) or metastatic clear cell renal cell carcinoma (RCC) | 1st/2nd line | Active, Not Recruiting |
| NCT04195750 [9,30] | III | Belzutifan versus Everolimus | Advanced or metastatic clear-cell renal cell carcinoma (ccRCC) | 2 lines+ | Active, Not Recruiting |
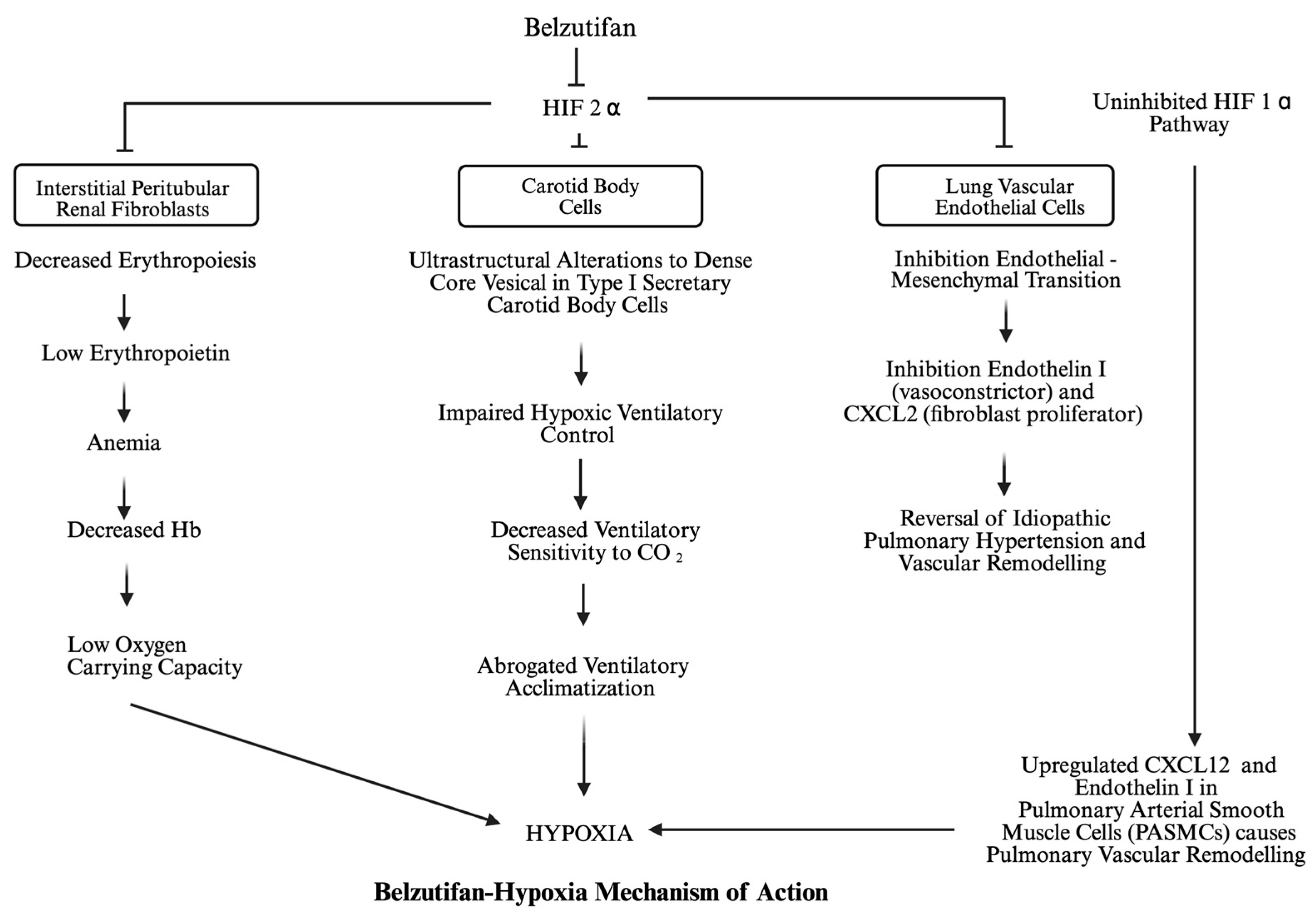
4. Mechanism of Belzutifan-Induced Hypoxia
5. Monitoring of Patients with Belzutifan
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| VHL | von Hippel-Lindau |
| ccRCC | Clear cell renal cell carcinoma |
| HIF-1α | Hypoxia-inducible factor-1α |
| HIF-2α | Hypoxia-inducible factor-2α |
| FDA | Food and Drug Administration |
| VEGF | Vascular endothelial growth factor |
| CNS | Central nervous system |
| PNET | Pancreatic neuroendocrine tumors |
| TKI | Tyrosine kinase inhibitor |
| ORR | Overall response rate |
| PFS | Progression-free survival |
| OS | Overall survival |
References
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: Findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Raval, R.R.; Lau, K.W.; Tran, M.G.B.; Sowter, H.M.; Mandriota, S.J.; Li, J.-L.; Pugh, C.W.; Maxwell, P.H.; Harris, A.L.; Ratcliffe, P.J. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 2005, 25, 5675–5686. [Google Scholar] [CrossRef] [PubMed]
- Carroll, V.A.; Ashcroft, M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: Implications for targeting the HIF pathway. Cancer Res. 2006, 66, 6264–6270. [Google Scholar] [CrossRef] [PubMed]
- Wiesener, M.S.; Jurgensen, J.S.; Rosenberger, C.; Scholze, C.; Hörstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Bratslavsky, G.; Sudarshan, S.; Neckers, L.; Linehan, W.M. Pseudohypoxic pathways in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 4667–4671. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Heiss, B.L.; Joeng, H.-K.; Weinstock, C.; Gao, X.; Pierce, W.F.; Chukwurah, B.; Bhatnagar, V.; Fiero, M.H.; Amiri-Kordestani, L.; et al. FDA Approval Summary: Belzutifan for Patients with Advanced Renal Cell Carcinoma. Clin. Cancer Res. 2024, 30, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Brave, M.H.; Weinstock, C.; Mehta, G.U.; Bradford, D.; Gittleman, H.; Bloomquist, E.W.; Charlab, R.; Hamed, S.S.; Miller, C.P.; et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clin. Cancer Res. 2022, 28, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Peltola, K.; de Velasco, G.; Burotto, M.; Suarez, C.; Ghatalia, P.; Iacovelli, R.; Lam, E.T.; Verzoni, E.; et al. Belzutifan versus Everolimus for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 391, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; Michaelson, M.D.; Gorbunova, V.A.; Gore, M.E.; Rusakov, I.G.; et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011, 378, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; Walsh, M.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 2018, 94, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, K.; Rizzi, J.P.; Huang, H.; Grina, J.A.; Schlachter, S.T.; Wang, B.; Wehn, P.M.; Yang, H.; Dixon, D.D.; et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a Hypoxia-Inducible Factor 2alpha (HIF-2alpha) Inhibitor for the Treatment of Clear Cell Renal Cell Carcinoma. J. Med. Chem. 2019, 62, 6876–6893. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr. HIF2 Inhibitor Joins the Kidney Cancer Armamentarium. J. Clin. Oncol. 2018, 36, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, T.H.; Tomchick, D.R.; Machius, M.; Guo, Y.; Bruick, R.K.; Gardner, K.H. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc. Natl. Acad. Sci. USA 2009, 106, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.M.; Rizzi, J.P.; Han, G.; Wehn, P.M.; Cao, Z.; Du, X.; Cheng, T.; Czerwinski, R.M.; Dixon, D.D.; Goggin, B.S.; et al. A Small-Molecule Antagonist of HIF2alpha Is Efficacious in Preclinical Models of Renal Cell Carcinoma. Cancer Res. 2016, 76, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Infante, J.R.; Lam, E.T.; Figlin, R.A.; Rini, B.I.; Brugarolas, J.; Zojwalla, N.J.; Lowe, A.M.; Wang, K.; Wallace, E.M.; et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2alpha Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2018, 36, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Plimack, E.; Bauer, T.; Merchan, J.; Papadopoulos, K.; McDermott, D.; Michaelson, M.; Appleman, L.; Thamake, S.; Zojwalla, N.; et al. 911PD—A first-in-human phase I/II trial of the oral HIF-2a inhibitor PT2977 in patients with advanced RCC. Ann. Oncol. 2019, 30, v361–v362. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.R.; Neumann, H.P.; Richard, S. von Hippel-Lindau disease: A clinical and scientific review. Eur. J. Hum. Genet. 2011, 19, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Sadowski, S.M.; Linehan, W.M.; Libutti, S.K.; Patel, D.; Nilubol, N.; Kebebew, E. Association of VHL Genotype With Pancreatic Neuroendocrine Tumor Phenotype in Patients With von Hippel-Lindau Disease. JAMA Oncol. 2018, 4, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Administration UFaD. WELIREG® (Belzutifan) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/215383s010s011lbl.pdf (accessed on 22 May 2025).
- Agarwal, N.; Brugarolas, J.; Ghatalia, P.; George, S.; Haanen, J.; Gurney, H.; Ravilla, R.; Van der Veldt, A.; Beuselinck, B.; Pokataev, I.; et al. Randomized phase II dose comparison LITESPARK-013 study of belzutifan in patients with advanced clear cell renal cell carcinoma. Ann. Oncol. 2024, 35, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; McDermott, D.F.; Merchan, J.; Bauer, T.M.; Figlin, R.; Heath, E.I.; Michaelson, M.D.; Arrowsmith, E.; D’SOuza, A.; Zhao, S.; et al. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: An open-label, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 553–562. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. NCT02974738. A Trial of Belzutifan (PT2977, MK-6482) Tablets in Patients with Advanced Solid Tumors (MK-6482-001). 2022. Available online: https://clinicaltrials.gov/study/NCT02974738 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT03401788. A Phase 2 Study of Belzutifan (PT2977, MK-6482) for the Treatment of Von Hippel Lindau (VHL) Disease-Associated Renal Cell Carcinoma (RCC) (MK-6482-004). 2024. Available online: https://clinicaltrials.gov/study/NCT03401788 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT04195750 (Litespark-005). A Study of Belzutifan (MK-6482) Versus Everolimus in Participants with Advanced Renal Cell Carcinoma (MK-6482-005). 2025. Available online: https://clinicaltrials.gov/study/NCT04195750 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT03634540. A Trial of Belzutifan (PT2977, MK-6482) in Combination with Cabozantinib in Patients with Clear Cell Renal Cell Carcinoma (ccRCC) (MK-6482-003). 2024. Available online: https://clinicaltrials.gov/study/NCT03634540 (accessed on 22 May 2025).
- Choueiri, T.K.; Ghatalia, P.; de Velasco, G.; Albiges, L.; Burotto, M.; Suarez, C.; Brugarolas, J.; Iacovelli, R.; Jalkanen, K.; Lam, E.T.; et al. 39 Safety profile of belzutifan monotherapy in patients with renal cell carcinoma: A pooled analysis of 4 clinical trials. Oncologist 2024, 29, S2–S3. [Google Scholar] [CrossRef]
- Wang, E.; Rupe, E.S.; Mukhida, S.S.; Johns, A.C.; Campbell, M.T.; Shah, A.Y.; Zurita, A.J.; Gao, J.; Goswami, S.; Jonasch, E.; et al. Belzutifan Efficacy and Tolerability in Patients with Sporadic Metastatic Clear Cell Renal Cell Carcinoma. Eur. Urol. Focus 2024, 11, 150–158. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. NCT05239728. A Study of Belzutifan (MK-6482) Plus Pembrolizumab (MK-3475) versus Placebo Plus Pembrolizumab in Participants with Clear Cell Renal Cell Carcinoma Post Nephrectomy (MK-6482-022). 2025. Available online: https://clinicaltrials.gov/study/NCT05239728 (accessed on 22 May 2025).
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. NCT04736706. A Study of Pembrolizumab (MK-3475) in Combination with Belzutifan (MK-6482) and Lenvatinib (MK-7902), or Pembrolizumab/Quavonlimab (MK-1308A) in Combination With Lenvatinib, Versus Pembrolizumab and Lenvatinib, for Treatment of Advanced Clear Cell Renal Cell Carcinoma (MK-6482-012). 2024. Available online: https://clinicaltrials.gov/study/NCT04736706 (accessed on 22 May 2025).
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. NCT04586231, A Study of Belzutifan (MK-6482) in Combination with Lenvatinib Versus Cabozantinib for Treatment of Renal Cell Carcinoma (MK-6482-011). 2024. Available online: https://clinicaltrials.gov/study/NCT04586231 (accessed on 22 May 2025).
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- clinicaltrials.gov. NCT06234605. A Study of HC-7366 in Combination with Belzutifan (WELIREG™) in Patients with Renal Cell Carcinoma. 2025. Available online: https://clinicaltrials.gov/study/NCT06234605?term=eif2ak4&viewType=Table&checkSpell=&rank=3 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT05030506. A Study of Belzutifan (MK-6482) as Monotherapy and in Combination with Lenvatinib (E7080/MK-7902) With or Without Pembrolizumab (MK-3475) in China Participants with Advanced Renal Cell Carcinoma (MK-6482-010). 2023. Available online: https://clinicaltrials.gov/study/NCT05030506 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT04626479. Substudy 03A: A Study of Immune and Targeted Combination Therapies in Participants with First Line (1L) Renal Cell Carcinoma (MK-3475-03A). 2025. Available online: https://clinicaltrials.gov/study/NCT04626479 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT02293980. A Phase 1, Dose-Escalation Trial of PT2385 Tablets in Patients with Advanced Clear Cell Renal Cell Carcinoma (MK-3795-001). 2024. Available online: https://clinicaltrials.gov/study/NCT02293980 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT04846920. A Study of Belzutifan (MK-6482) in Participants with Advanced Clear Cell Renal Cell Carcinoma (MK-6482-018). 2024. Available online: https://www.clinicaltrials.gov/study/NCT04846920 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT04626518. Substudy 03B: A Study of Immune and Targeted Combination Therapies in Participants with Second Line Plus (2L+) Renal Cell Carcinoma (MK-3475-03B). 2024. Available online: https://clinicaltrials.gov/study/NCT04626518?term=AREA%5BBasicSearch%5D(D-Tyrosine%20AND%20PDCD1)&rank=7 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT05468697. A Study of Belzutifan (MK-6482) in Combination with Palbociclib versus Belzutifan Monotherapy in Participants with Advanced Renal Cell Carcinoma (MK-6482-024/LITESPARK-024). 2024. Available online: https://clinicaltrials.gov/study/NCT05468697 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT03108066. MK-3795 (PT2385) for the Treatment of von Hippel-Lindau Disease-Associated Clear Cell Renal Cell Carcinoma (MK-3795-003). 2024. Available online: https://clinicaltrials.gov/study/NCT03108066 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT04489771. A Study of Belzutifan (MK-6482) in Participants with Advanced Renal Cell Carcinoma (MK-6482-013). 2024. Available online: https://clinicaltrials.gov/study/NCT04489771 (accessed on 22 May 2025).
- clinicaltrials.gov. NCT05899049. A Study of Pembrolizumab (MK-3475) in Combination With Belzutifan (MK-6482) and Lenvatinib (MK-7902), or Pembrolizumab/Quavonlimab (MK-1308A) in Combination With Lenvatinib, vs Pembrolizumab and Lenvatinib, for Treatment of Advanced Clear Cell Renal Cell Carcinoma (MK-6482-012)-China Extension Study. 2024. Available online: https://clinicaltrials.gov/study/NCT05899049 (accessed on 22 May 2025).
- Curry, L.; Soleimani, M. Belzutifan: A novel therapeutic for the management of von Hippel-Lindau disease and beyond. Future Oncol. 2024, 20, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Domínguez, A.; Ortega-Sáenz, P.; Gao, L.; Colinas, O.; García-Flores, P.; Bonilla-Henao, V.; Aragonés, J.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; et al. Acute O(2) sensing through HIF2alpha-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 2020, 13, eaay9452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Prange-Barczynska, M.; Fielding, J.W.; Zhang, M.; Burrell, A.L.; Lima, J.D.; Eckardt, L.; Argles, I.L.; Pugh, C.W.; Buckler, K.J.; et al. Marked and rapid effects of pharmacological HIF-2alpha antagonism on hypoxic ventilatory control. J. Clin. Investig. 2020, 130, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Gale, D.P.; Connor, T.; Adams, S.; de Boer, J.; Gascoyne, D.M.; Williams, O.; Maxwell, P.H.; Ancliff, P.J. Dysregulation of the HIF pathway due to VHL mutation causing severe erythrocytosis and pulmonary arterial hypertension. Blood 2011, 117, 3699–3701. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.P.; Harten, S.K.; Reid, C.D.L.; Tuddenham, E.G.D.; Maxwell, P.H. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood 2008, 112, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Babicheva, A.; McDermott, K.M.; Gu, Y.; Ayon, R.J.; Song, S.; Wang, Z.; Gupta, A.; Zhou, T.; Sun, X.; et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Cell Mol. Physiol. 2018, 314, L256–L275. [Google Scholar] [CrossRef]
- Ghosh, M.C.; Zhang, D.-L.; Ollivierre, W.H.; Noguchi, A.; Springer, D.A.; Linehan, W.M.; Rouault, T.A. Therapeutic inhibition of HIF-2alpha reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood 2021, 137, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Machireddy, N.; Evans, C.E.; Machado, R.; Zhang, X.; Zhao, Y.-Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2alpha Inhibitor. Am. J. Respir. Crit. Care Med. 2018, 198, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, M.; Moszyńska, A.; Króliczewski, J.; Cabaj, A.; Bartoszewska, S.; Charzyńska, A.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell. Mol. Biol. Lett. 2022, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Gassmann, M.; Wielockx, B. Erythrocytosis: The HIF pathway in control. Blood 2013, 122, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef] [PubMed]
- van Patot, M.C.T.; Gassmann, M. Hypoxia: Adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2alpha. High Alt. Med. Biol. 2011, 12, 157–167. [Google Scholar] [CrossRef]
- Branco-Price, C.; Zhang, N.; Schnelle, M.; Evans, C.; Katschinski, D.M.; Liao, D.; Ellies, L.; Johnson, R.S. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell 2012, 21, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, P.; Högel, H.; Seyednasrollah, F.; Rantanen, K.; Elo, L.L.; Jaakkola, P.M. Hypoxia-inducible factor (HIF)-prolyl hydroxylase 3 (PHD3) maintains high HIF2A mRNA levels in clear cell renal cell carcinoma. J. Biol. Chem. 2019, 294, 3760–3771. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’nEill, L.A. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016, 539, 112–117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharczyk, J.; Bhatt, A.; Bauer, L.; Economides, M. Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations. Int. J. Mol. Sci. 2025, 26, 7094. https://doi.org/10.3390/ijms26157094
Kucharczyk J, Bhatt A, Bauer L, Economides M. Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations. International Journal of Molecular Sciences. 2025; 26(15):7094. https://doi.org/10.3390/ijms26157094
Chicago/Turabian StyleKucharczyk, John, Anshini Bhatt, Laura Bauer, and Minas Economides. 2025. "Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations" International Journal of Molecular Sciences 26, no. 15: 7094. https://doi.org/10.3390/ijms26157094
APA StyleKucharczyk, J., Bhatt, A., Bauer, L., & Economides, M. (2025). Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations. International Journal of Molecular Sciences, 26(15), 7094. https://doi.org/10.3390/ijms26157094






