Menopause as a Critical Turning Point in Lipedema: The Estrogen Receptor Imbalance, Intracrine Estrogen, and Adipose Tissue Dysfunction Model
Abstract
1. Introduction
2. Results
3. Discussion
3.1. The Role of Estradiol and Its Receptors
3.2. Intracrine Production of Estradiol in Adipose Tissue
3.3. Progesterone Resistance
3.4. How Menopause Affects Lipedema
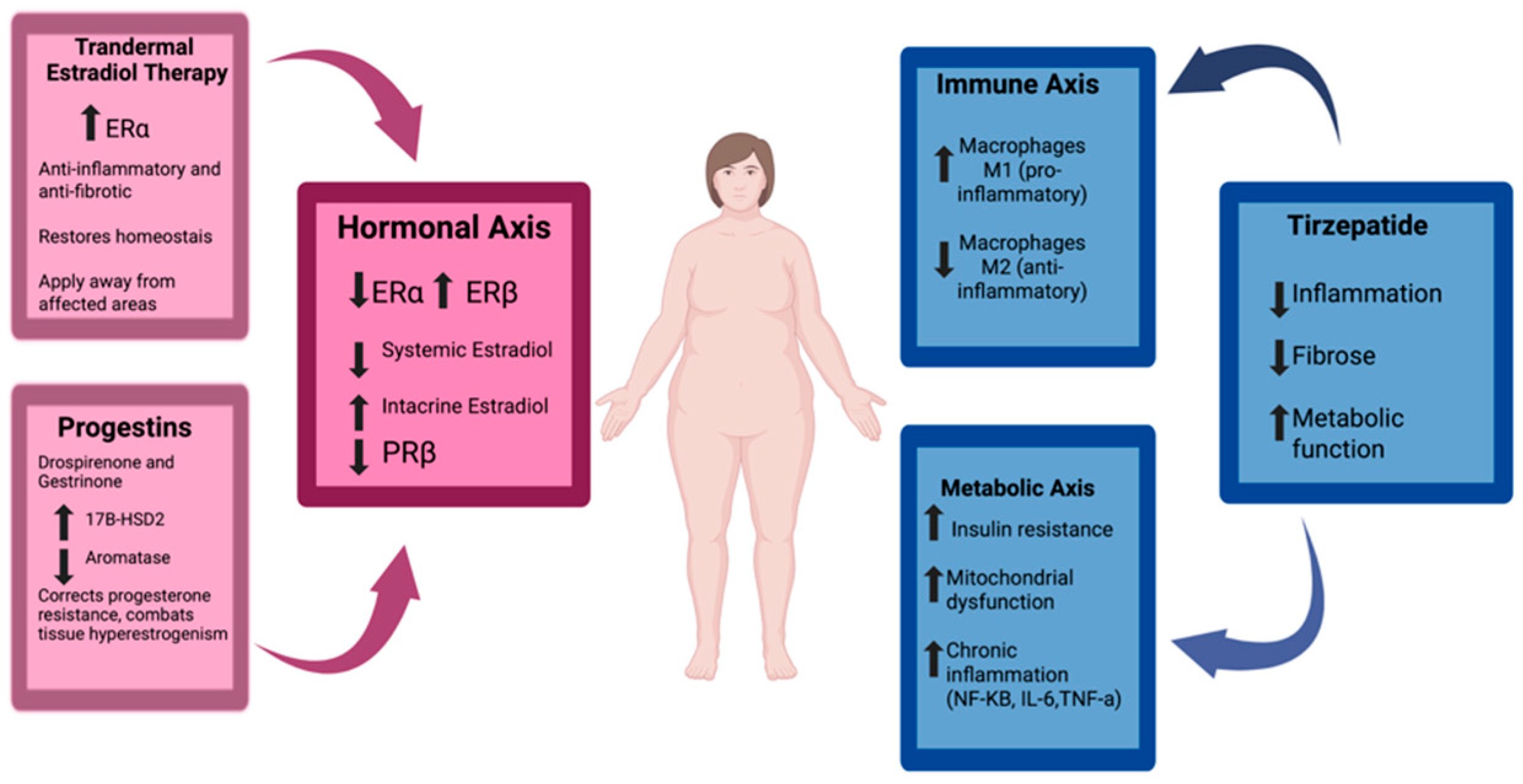
3.5. Therapeutic Implications
4. Material and Methods
- Estrogen receptor signaling (ERα, ERβ) in adipose tissue;
- Intracrine estrogen metabolism via aromatase, 17β-HSD1, and 17β-HSD2 enzymes;
- The role of menopause-induced estrogen deficiency in adipose tissue dysfunction;
- The immunometabolic consequences of receptor imbalance, including inflammation and fibrosis;
- Parallels between lipedema and other estrogen-driven gynecological disorders.
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ERα | Estrogen Receptor Alpha |
| ERβ | Estrogen Receptor Beta |
| PRβ | Progesterone Receptor Beta |
| 17β-HSD1 | 17β-Hydroxysteroid Dehydrogenase Type 1 |
| 17β-HSD2 | 17β-Hydroxysteroid Dehydrogenase Type 2 |
| HRT | Hormone Replacement Therapy |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| GLP-1 | Glucagon-Like Peptide-1 |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| LPL | Lipoprotein Lipase |
| GLUT4 | Glucose Transporter Type 4 |
| VEGF | Vascular Endothelial Growth Factor |
| IL-6/IL-1β/IL-18 | Interleukin-6/Interleukin-1 beta/Interleukin-18 |
| TNF-α | Tumor Necrosis Factor Alpha |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| NF-κB | Nuclear Factor Kappa B |
| JNK | c-Jun N-terminal Kinase |
| UCP1 | Uncoupling Protein 1 |
| RAAS | Renin–Angiotensin–Aldosterone System |
| STRAW | Stages of Reproductive Aging Workshop |
| SWAN | Study of Women’s Health Across the Nation |
| MASH | Metabolic Associated Steatohepatitis |
| CD163+ | Cluster of Differentiation 163 Positive (M2 macrophage marker) |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| ERK | Extracellular Signal-Regulated Kinase |
| DNA | Deoxyribonucleic Acid |
| E2 | Estradiol |
References
- Wold, L.E.; Hines, E.A., Jr.; Allen, E.V. Lipedema of the legs; A syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, P.; Georgiou, I.; Biermann, N.; Prantl, L.; Klein-Weigel, P.; Ghods, M. Lipedema-Pathogenesis, Diagnosis, and Treatment Options. Dtsch Arztebl Int. 2020, 117, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.C.M.; Amato, F.C.M.; Amato, J.L.S.; Benitti, D.A. Lipedema prevalence and risk factors in Brazil. J. Vasc. Bras. 2022, 21, e20210198. [Google Scholar] [CrossRef] [PubMed]
- Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the potential role of estro-gen in excessive adipose tissue accumulation. Int. J. Mol. Sci. 2021, 22, 11720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forner-Cordero, I.; Szolnoky, G.; Forner-Cordero, A.; Kemény, L. Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome—Systematic review. Clin. Obes. 2012, 2, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Torre, Y.S.; Wadeea, R.; Rosas, V.; Herbst, K.L. Lipedema: Friend and foe. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20170076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, N.; Sutton-Tyrrell, K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet. Gynecol. Clin. N. Am. 2011, 38, 417–423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simpson, E.R.; Merrill, J.C.; Hollub, A.J.; Graham-Lorence, S.; Mendelson, C.R. Regula-tion of estrogen biosynthesis by human adipose cells. Endocr. Rev. 1989, 10, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Michelini, S.; Abrego-Guandique, D.M.; Vaia, N.; Michelini, S.; Puleo, V.; Bertelli, M.; Caroleo, M.C.; Cannataro, R. Identification of Specific microRNAs in Adipose Tissue Affected by Lipedema. Curr. Issues Mol. Biol. 2024, 46, 11957–11974. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. American Heart Associa-tion Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardio-vascular disease risk: Implications for timing of early prevention: A Scientific State-ment From the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.; Ricolfi, L.; Bortolon, M.; Gabriele, G.; Zolesio, P.; Cione, E.; Cannataro, R. Observational Study on a Large Italian Population with Lipedema: Biochemical and Hormonal Profile, Anatomical and Clinical Evaluation, Self-Reported History. Int. J. Mol. Sci. 2024, 25, 1599. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Kamble, P.G.; Hetty, S.; Fanni, G.; Vranic, M.; Sarsenbayeva, A.; Kristófi, R.; Almby, K.; Svensson, M.K.; Pereira, M.J.; et al. Role of estrogen and its receptors in ad-ipose tissue glucose metabolism in pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 2022, 107, e1879–e1889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signalling through ERal-pha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, D.; Petrosino, J.; Aldoori, A.; Melgar-Bermudez, E.; Wells, A.; Ziouzenkova, O. Enzymatic intracrine regulation of white adipose tissue. Horm. Mol. Biol. Clin. Investig. 2014, 19, 39–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viana, D.P.C.; Câmara, L.C. Hormonal links between lipedema and gynecological dis-orders: Therapeutic roles of gestrinone and drospirenone. J. Adv. Med. Med. Res. 2025, 37, 175–188. [Google Scholar] [CrossRef]
- Szél, E.; Kemény, L.; Groma, G.; Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 2014, 83, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Poojari, A.; Dev, K.; Rabiee, A. Lipedema: Insights into morphology, pathophysiology, and challenges. Biomedicines 2022, 10, 3081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, S.N.; Welter, B.H.; Mantzke, K.A.; Price, T.M. Identification of progesterone re-ceptor in human subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 1998, 83, 509–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeitoun, K.; Takayama, K.; Sasano, H.; Suzuki, T.; Moghrabi, N.; Andersson, S.; Johns, A.; Meng, L.; Putman, M.; Carr, B.; et al. Deficient 17beta-hydroxysteroid dehydro-genase type 2 expression in endometriosis: Failure to metabolize 17beta-estradiol. J. Clin. Endocrinol. Metab. 1998, 83, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Bardhi, O.; Dubey, P.; Palmer, B.F.; Clegg, D.J. Oestrogens, adipose tissues and environmental exposures influence obesity and diabetes across the lifecycle. Proc. Nutr. Soc. 2024, 83, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Isern, S.U.; Herbst, K.L.; Bunnell, B.A. The Expression of Adipogenic Marker Is Significantly Increased in Estrogen-Treated Lipedema Adipocytes Differentiated from Adipose Stem Cells In Vitro. Biomedicines 2024, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Viana, D.P.D.C.; Câmara, L.C. Metabolic Therapy for Lipedema: Can Tirzepatide Overcome the Treatment Gap? J. Pharm. Res. Int. 2025, 37, 21–28. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and men-opause: The role of estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renke, G.; Kemen, E.; Scalabrin, P.; Braz, C.; Baesso, T.; Pereira, M.B. Cardio-metabolic health and HRT in menopause: Novel insights in mitochondrial biogenesis and RAAS. Curr. Cardiol. Rev. 2023, 19, e060223213459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. J. Clin. Endocrinol. Metab. 1997, 82, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A. Estrogens in adipose tissue physiology and obesity-related dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during meno-pause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, W.; Jiang, W.; Liao, W.; Yan, H.; Ai, W.; Pan, Q.; Brashear, W.A.; Xu, Y.; He, L.; Guo, S. An estrogen receptor α-derived peptide improves glucose homeostasis during obe-sity. Nat. Commun. 2024, 15, 3410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clegg, D.J.; Brown, L.M.; Woods, S.C.; Benoit, S.C. Gonadal hormones determine sensi-tivity to central leptin and insulin. Diabetes 2006, 55, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Tomada, I. Lipedema: From women’s hormonal changes to nutritional intervention. Endocrines 2025, 6, 24. [Google Scholar] [CrossRef]
- Pernoud, L.E.; Gardiner, P.A.; Fraser, S.D.; Dillon-Rossiter, K.; Dean, M.M.; Schaumberg, M.A. A systematic review and meta-analysis investigating differences in chronic inflammation and adiposity before and after menopause. Maturitas 2024, 190, 108119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The role of estrogen in adipose tissue metabolism: Insights into glucose homeostasis regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Renke, G.; Antunes, M.; Sakata, R.; Tostes, F. Effects, doses, and applicability of gestrinone in estrogen-dependent conditions and post-menopausal women. Pharmaceuticals 2024, 17, 1248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makabe, T.; Koga, K.; Miyashita, M.; Takeuchi, A.; Sue, F.; Taguchi, A.; Urata, Y.; Izumi, G.; Takamura, M.; Harada, M.; et al. Drospirenone reduces inflammatory cytokines, vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) expression in human endometriotic stromal cells. J. Reprod. Immunol. 2017, 119, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Antelmi, A.; Chetrite, G.; Muscat, A.; Mammi, C.; Marzolla, V.; Fabbri, A.; Zennaro, M.C.; Fève, B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: Potential implications for the treatment of metabolic syndrome. Endocrinology 2011, 152, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Tankó, L.B.; Christiansen, C. Effects of 17beta-oestradiol plus different doses of dro-spirenone on adipose tissue, adiponectin and atherogenic metabolites in postmeno-pausal women. J. Intern. Med. 2005, 258, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Kives, S.; Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Gynaecology and Fertility Group, organizador. Cochrane Database Syst. Rev. 2012, 2012, CD002122. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Farquhar, C. Endometriosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2014, 2014, CD009590. [Google Scholar] [CrossRef] [PubMed]
- European Society of Human Reproduction and Embryology. Information on Endometriosis: Patient Leaflet Based on the ESHRE Guideline on Endometriosis; ESHRE: Grimbergen, Belgium, 2025. [Google Scholar]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of symptoms of the menopause: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Park, H. Metabolic disorders in menopause. Metabolites 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, Y.; Jin, J.; Sun, Y.; Kong, X.; Shen, Z.; Yan, R.; Huang, R.; Liu, X.; Xia, W.; Ma, J.; et al. Tirzepatide’s role in targeting adipose tissue macrophages to reduce obesity- related inflammation and improve insulin resistance. Int. Immunopharmacol. 2024, 143 Pt 2, 113499. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samms, R.J.; Zhang, G.; He, W.; Ilkayeva, O.; Droz, B.A.; Bauer, S.M.; Stutsman, C.; Pirro, V.; Collins, K.A.; Furber, E.C.; et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 2022, 64, 101550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regmi, A.; Aihara, E.; Christe, M.E.; Varga, G.; Beyer, T.P.; Ruan, X.; Beebe, E.; O’Farrell, L.S.; Bellinger, M.A.; Austin, A.K.; et al. Tirzepatide modulates the regulation of adipocyte nutrient metabolism through long-acting activation of the GIP receptor. Cell Metab. 2024, 36, 1534–1549.e7. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V. Lipedema: Progress, challenges, and the road ahead. Obes. Rev. 2025, 27, e13953. [Google Scholar] [CrossRef] [PubMed]
| Pathophysiological Change | Author and Year | Summary of Findings |
|---|---|---|
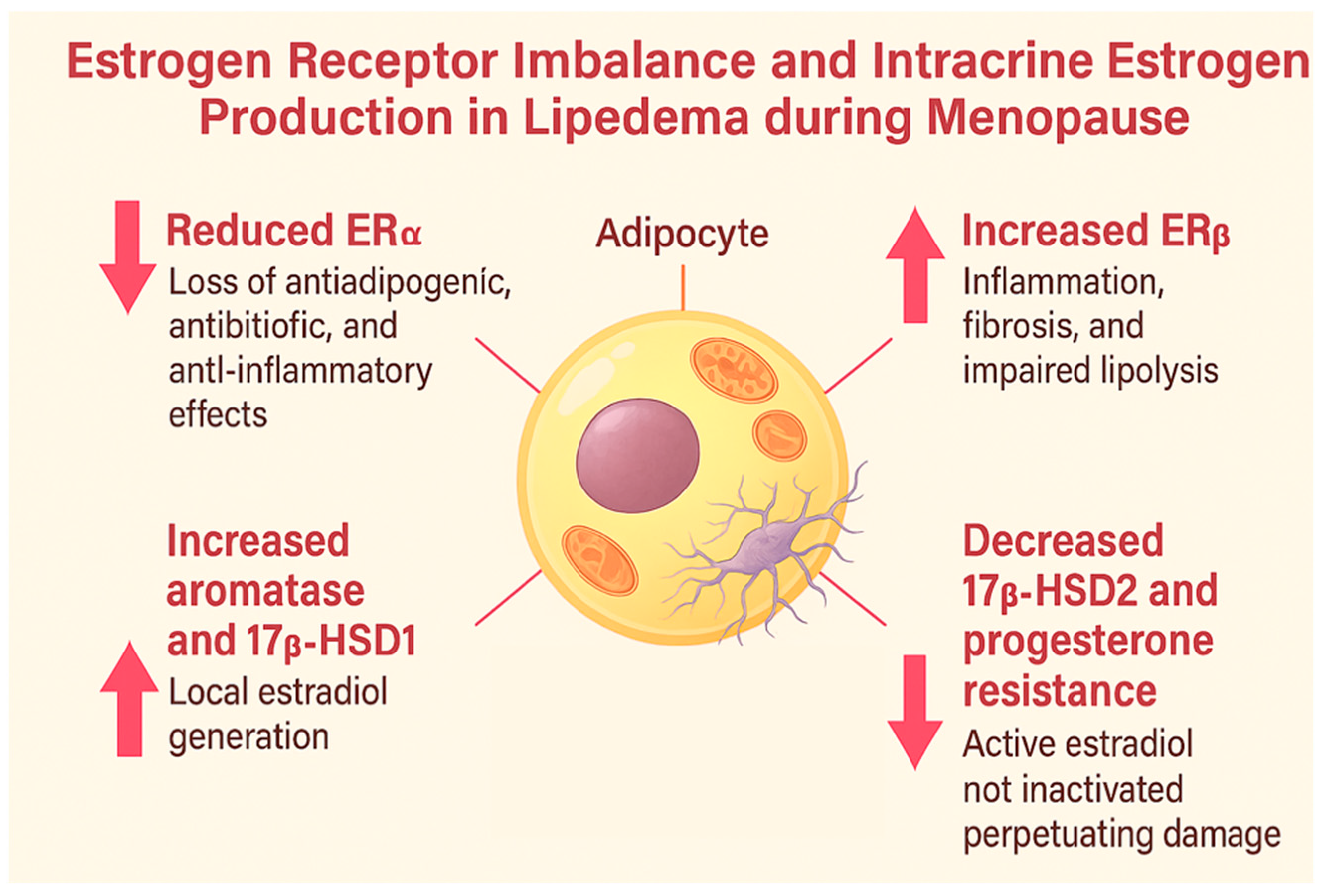
| Estrogen Receptors (ERα and ERβ) | Simpson et al., 1989 [8] | The climacteric period decreases ERα expression and compensatorily increases ERβ in adipose tissue. |
| Foryst-Ludwig and Kintscher, 2010 [13] | Estrogen deficiency in menopause and lipedema reduces ERα and increases ERβ, promoting inflammation, fibrosis, and insulin resistance. | |
| Katzer K et al., 2021 [4] | Dysregulation of estrogen receptors (ERα/ERβ) and local estrogen production in adipose tissue may lead to excessive fat accumulation, particularly in the lower body, a hallmark of lipedema. | |
| Aromatase (CYP19A1) | Simpson et al., 1989 [8] | Subcutaneous adipose tissue synthesizes estrogens via aromatase and 17β-HSD. |
| Szél et al., 2014 [16] | Lipedema exhibits increased aromatase activity and enzymatic dysregulation. | |
| 17β-HSD 1 and 2 | Zeitoun et al., 1998 [19] | In conditions like endometriosis, 17β-HSD2 deficiency prevents the conversion of estradiol into estrone. |
| Szél et al., 2014 [16] | There is an increase in 17β-HSD1, which converts estrone into active estradiol, intensifying local estrogenic activation. | |
| Bardhi et al., 2024 [20] | 17β-HSDs in adipose tissue convert weak steroids like estrone into potent forms such as estradiol, underscoring the role of local hormone metabolism in adipose function. | |
| Al-Ghadban et al., 2024 [21] | Estrogen enhances HSD17B7 and LIPE expression in lipedema cells, supporting a direct role of estrogen metabolism in disease pathogenesis. | |
| Viana and Câmara, 2025 [22] | Progesterone resistance reduces 17β-HSD2 activity, impairing estradiol inactivation. | |
| Progesterone Resistance | O’Brien et al., 1998 [18] | Subcutaneous adipose tissue expresses progesterone receptors, suggesting an active local hormonal role. |
| Viana and Câmara, 2025 [15] | The failure of progesterone to modulate adipose tissue allows intracrine estradiol to sustain the inflammatory state. | |
| Mitochondrial Dysfunction | Geraci et al., 2021 [23] Renke et al., 2023 [24] | Mitochondrial dysfunction induced by estrogen deficiency reduces basal metabolism, contributing to sarcopenia and insulin resistance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto da Costa Viana, D.; Caseri Câmara, L.; Borges Palau, R. Menopause as a Critical Turning Point in Lipedema: The Estrogen Receptor Imbalance, Intracrine Estrogen, and Adipose Tissue Dysfunction Model. Int. J. Mol. Sci. 2025, 26, 7074. https://doi.org/10.3390/ijms26157074
Pinto da Costa Viana D, Caseri Câmara L, Borges Palau R. Menopause as a Critical Turning Point in Lipedema: The Estrogen Receptor Imbalance, Intracrine Estrogen, and Adipose Tissue Dysfunction Model. International Journal of Molecular Sciences. 2025; 26(15):7074. https://doi.org/10.3390/ijms26157074
Chicago/Turabian StylePinto da Costa Viana, Diogo, Lucas Caseri Câmara, and Robinson Borges Palau. 2025. "Menopause as a Critical Turning Point in Lipedema: The Estrogen Receptor Imbalance, Intracrine Estrogen, and Adipose Tissue Dysfunction Model" International Journal of Molecular Sciences 26, no. 15: 7074. https://doi.org/10.3390/ijms26157074
APA StylePinto da Costa Viana, D., Caseri Câmara, L., & Borges Palau, R. (2025). Menopause as a Critical Turning Point in Lipedema: The Estrogen Receptor Imbalance, Intracrine Estrogen, and Adipose Tissue Dysfunction Model. International Journal of Molecular Sciences, 26(15), 7074. https://doi.org/10.3390/ijms26157074







