BCAP Is an Interferon-Stimulated Gene That Enhances Type I Interferon Activity in Response to Lipopolysaccharide
Abstract
1. Introduction
2. Results
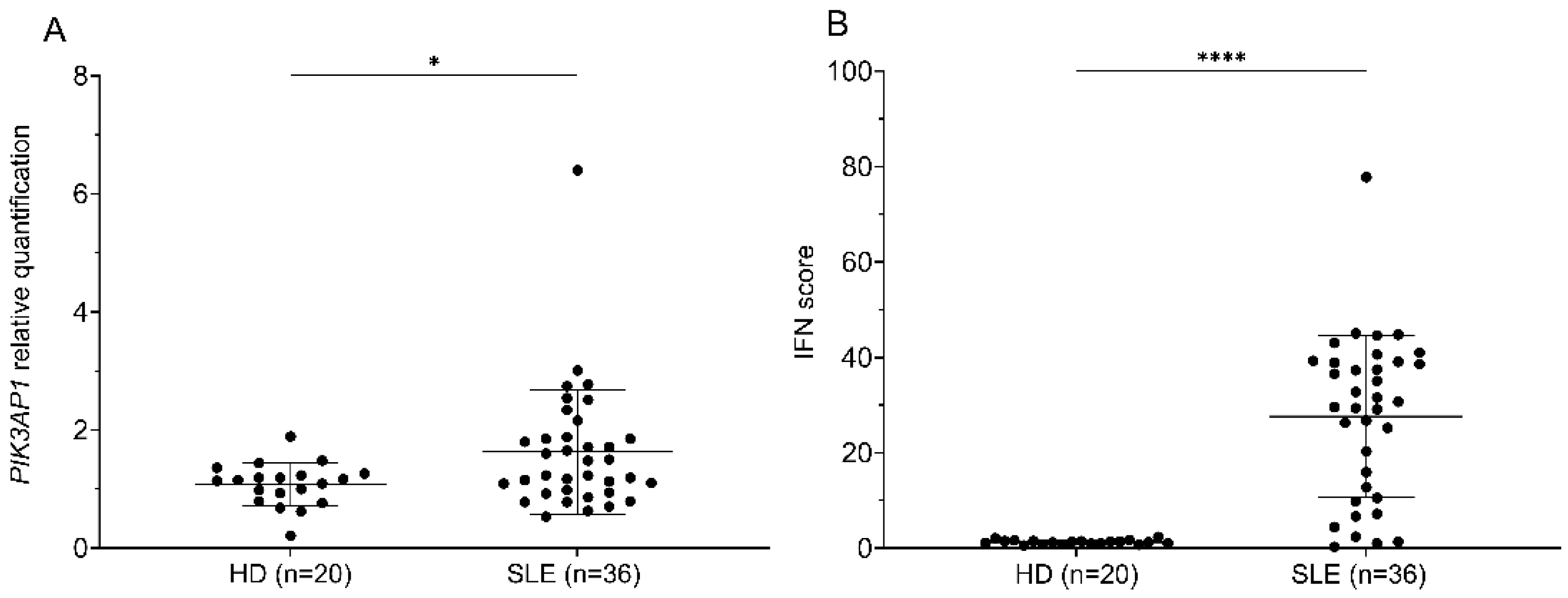
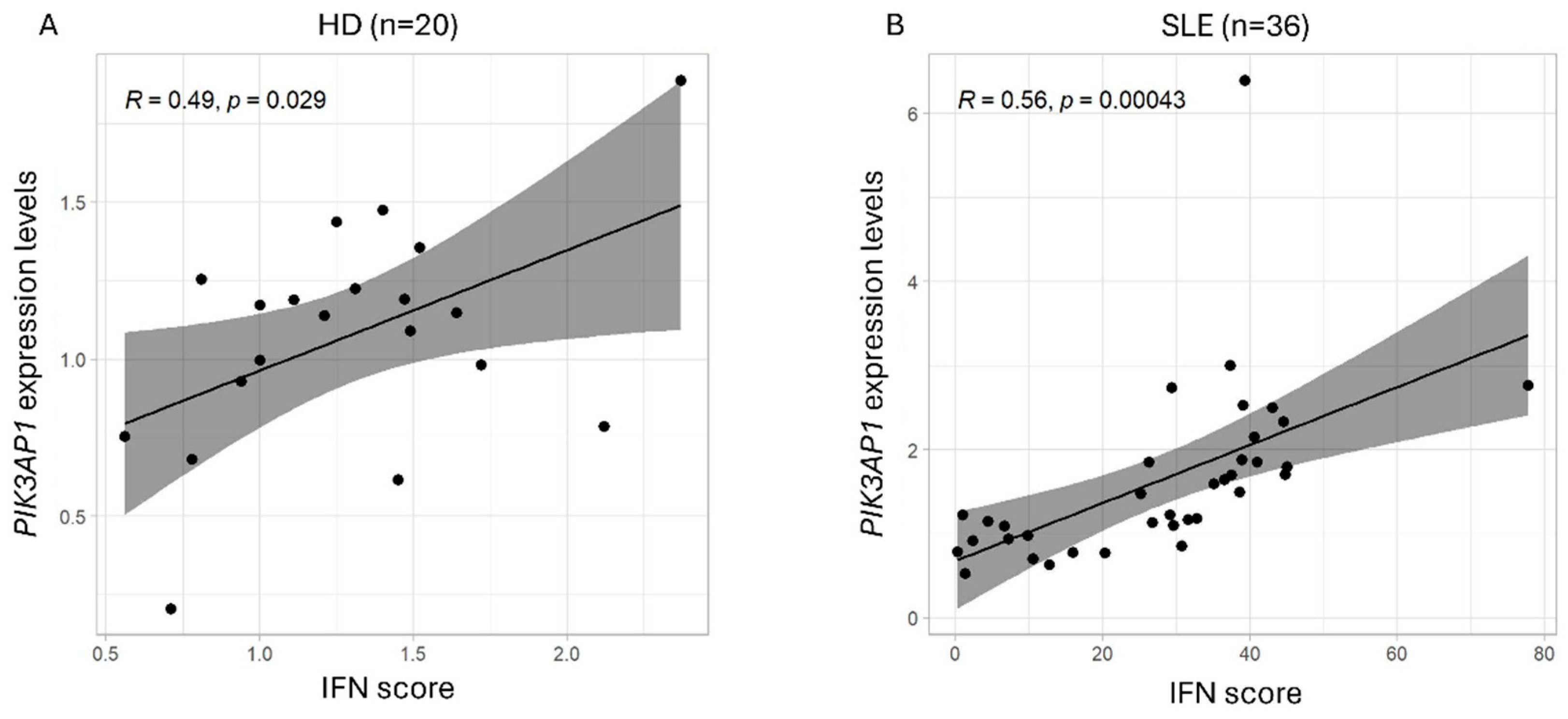
2.1. PIK3AP1 Expression and IFN Score Values Are Higher in Patients with SLE than in Controls
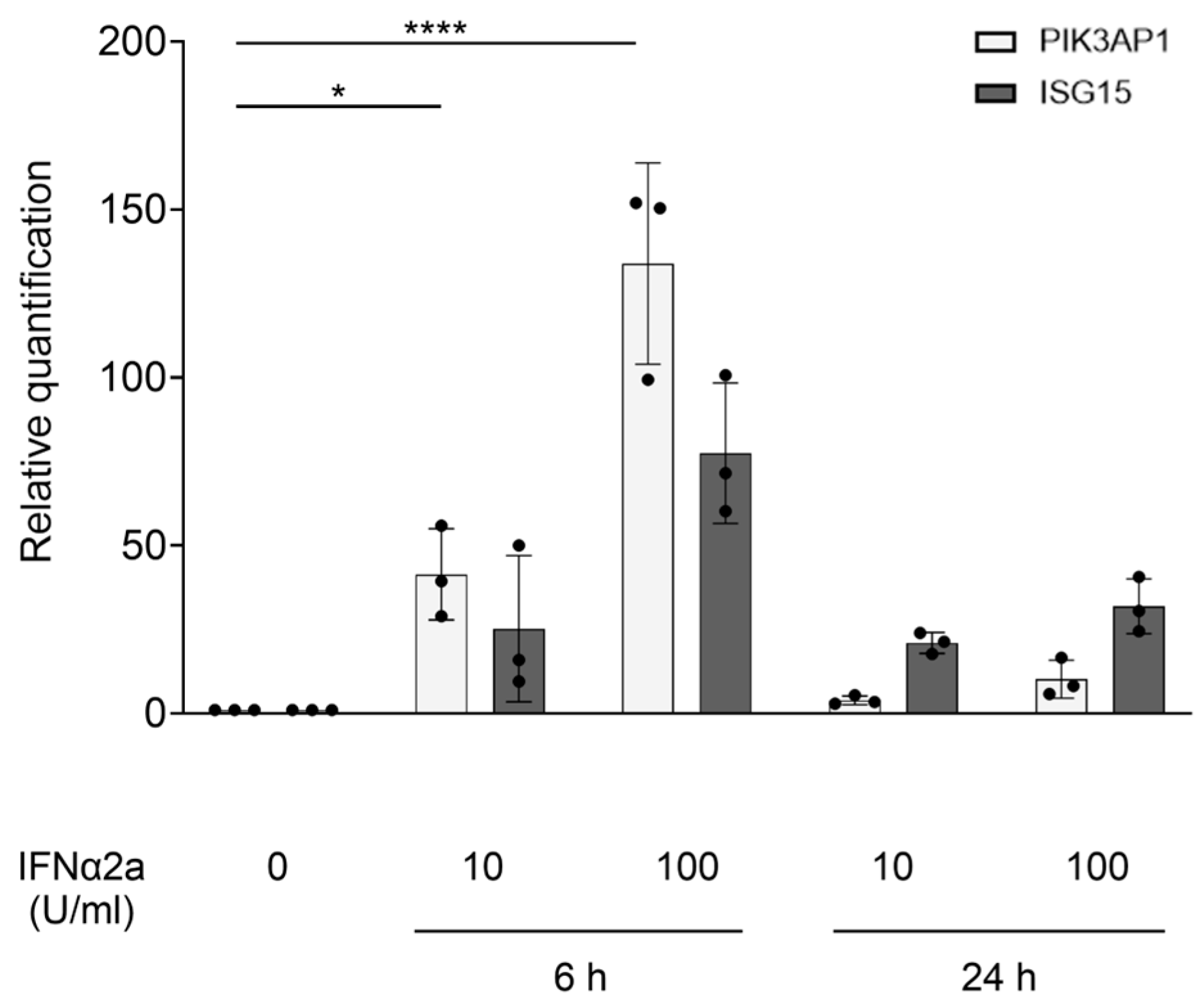
2.2. PIK3AP1 Is an ISG, and Its Hyperexpression Following cGAS Priming Is Inhibited by IFN Signaling Blockade via Tofacitinib or Anifrolumab
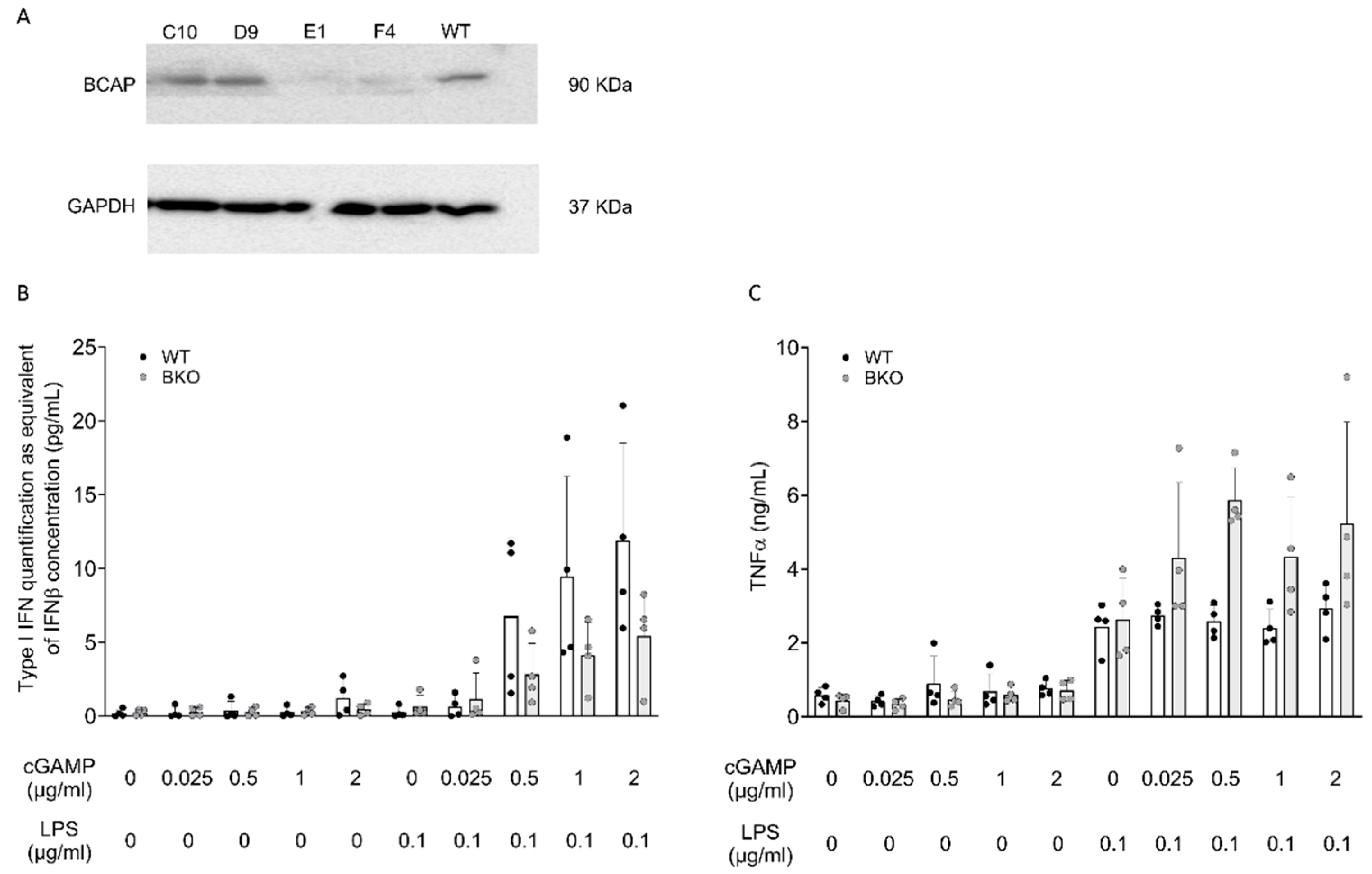
2.3. BCAP Skews LPS-Stimulated Cells Toward IFN-I Production
3. Discussion
4. Materials and Methods
4.1. Cell Cultures, Stimuli, and Inhibitors
4.2. Generation on HoxB8 BCAP Knockout Cells
- For caccgTTCCCAGGCGATATCTCCGG Rev aaacCCGGAGATATCGCCTGGGAAc Exon 3
- For caccgAGAACAACACCATCCCTATG Rev aaacCATAGGGATGGTGTTGTTCTc Exon 7
4.3. Hoxb8 Differentiation in GM-CSF
4.4. Western Blot Analysis
4.5. Functional Assays of Activation in Hoxb8-Derived Macrophages
4.6. Measure of PIK3AP1 Expression by Real-Time PCR
4.7. Calculation of the IFN Score by Real-Time PCR
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCAP | B-Cell Adapter for PI3K |
| cGAMP | Cyclic GMP-AMP |
| cGAS | Cyclic GMP-AMP Synthase |
| CI | Confidence Interval |
| IFN-I | Type I Interferon |
| IL | Interleukin |
| ISGs | Interferon-Stimulated Genes |
| LPS | Lipopolysaccharide |
| mTOR | Mammalian Target of Rapamycin |
| PI3K | Phosphatidylinositol 3-Kinase |
| PIK3AP1 | PI3K Adapter Protein 1 |
| TBK1 | TANK-Binding Kinase 1 |
| TIR | Toll-IL1-receptor |
| TIRAP | TIR adaptor protein |
| TLRs | Toll-Like Receptors |
| TNFα | Tumor Necrosis Factor α |
| SLE | Systemic Lupus Erythematosus |
| STING | Stimulator of Interferon Genes |
References
- Inabe, K.; Kurosaki, T. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood 2002, 99, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.D.; Hu, W.; Fulenchek, S.; Yamazaki, T.; Kurosaki, T.; Bazan, J.F.; Pasare, C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl. Acad. Sci. USA 2012, 109, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; MacFarlane, A.W.; Toft, M.; Lowell, C.A.; Campbell, K.S.; Hamerman, J.A. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proc. Natl. Acad. Sci. USA 2012, 109, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Ni, M.; Chen, C.; Akilesh, S.; Hamerman, J.A. Cutting Edge: BCAP Promotes Lupus-like Disease and TLR-Mediated Type I IFN Induction in Plasmacytoid Dendritic Cells. J. Immunol. 2019, 202, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Tesser, A.; Piperno, G.M.; Pin, A.; Piscianz, E.; Boz, V.; Benvenuti, F.; Tommasini, A. Priming of the cGAS-STING-TBK1 Pathway Enhances LPS-Induced Release of Type I Interferons. Cells 2021, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Redecke, V.; Wu, R.; Zhou, J.; Finkelstein, D.; Chaturvedi, V.; High, A.A.; Häcker, H. Hematopoietic progenitor cell lines with myeloid and lymphoid potential. Nat. Methods 2013, 10, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Piperno, G.M.; Naseem, A.; Silvestrelli, G.; Amadio, R.; Caronni, N.; Cervantes Luevano, K.E.; Liv, N.; Klumperman, J.; Colliva, A.; Ali, H.; et al. Wiskott-Aldrich syndrome protein restricts cGAS-STING activation by dsDNA immune complexes. JCI Insight 2020, 5, e132857. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.D.; Bazan, J.F.; Pasare, C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 2012, 11, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Deason, K.; Troutman, T.D.; Jain, A.; Challa, D.K.; Mandraju, R.; Brewer, T.; Ward, E.S.; Pasare, C. BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation. J. Exp. Med. 2018, 215, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Wang, H.; Suttles, J.; Graves, D.T.; Martin, M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J. Biol. Chem. 2011, 286, 44295–44305. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007, 76, 447–480. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Shen, W.W.; Zhong, J.; Huang, C.; Zhang, L.; Li, J. Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int. J. Mol. Med. 2014, 34, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Negishi, H.; Asagiri, M.; Nakajima, C.; Mizutani, T.; Takaoka, A.; Honda, K.; Taniguchi, T. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 2003, 306, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Murayama, G.; Chiba, A.; Kuga, T.; Makiyama, A.; Yamaji, K.; Tamura, N.; Miyake, S. Inhibition of mTOR suppresses IFNα production and the STING pathway in monocytes from systemic lupus erythematosus patients. Rheumatology 2020, 59, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, D.; Garantziotis, P.; Sentis, G.; Fanouriakis, A.; Bertsias, G.; Frangou, E.; Nikolopoulos, D.; Banos, A.; Boumpas, D.T. Restoration of aberrant gene expression of monocytes in systemic lupus erythematosus via a combined transcriptome-reversal and network-based drug repurposing strategy. BMC Genomics 2023, 24, 207. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wu, C.; Hong, R.; Sun, Y.; Qian, J.; Zhao, J.; Wang, Q.; Tian, X.; Wang, Y.; Li, M.; et al. Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: A real-world study and meta-analysis. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20953336. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pin, A.; Monasta, L.; Taddio, A.; Piscianz, E.; Tommasini, A.; Tesser, A. An Easy and Reliable Strategy for Making Type I Interferon Signature Analysis Comparable among Research Centers. Diagnostics 2019, 9, 113. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rosa, M.; Piperno, G.M.; Tesser, A.; Pin, A.; Sospiro, G.; Valencic, E.; Boz, V.; Pastore, S.; Tommasini, A.; Benvenuti, F. BCAP Is an Interferon-Stimulated Gene That Enhances Type I Interferon Activity in Response to Lipopolysaccharide. Int. J. Mol. Sci. 2025, 26, 7034. https://doi.org/10.3390/ijms26157034
Di Rosa M, Piperno GM, Tesser A, Pin A, Sospiro G, Valencic E, Boz V, Pastore S, Tommasini A, Benvenuti F. BCAP Is an Interferon-Stimulated Gene That Enhances Type I Interferon Activity in Response to Lipopolysaccharide. International Journal of Molecular Sciences. 2025; 26(15):7034. https://doi.org/10.3390/ijms26157034
Chicago/Turabian StyleDi Rosa, Marianna, Giulia Maria Piperno, Alessandra Tesser, Alessia Pin, Giada Sospiro, Erica Valencic, Valentina Boz, Serena Pastore, Alberto Tommasini, and Federica Benvenuti. 2025. "BCAP Is an Interferon-Stimulated Gene That Enhances Type I Interferon Activity in Response to Lipopolysaccharide" International Journal of Molecular Sciences 26, no. 15: 7034. https://doi.org/10.3390/ijms26157034
APA StyleDi Rosa, M., Piperno, G. M., Tesser, A., Pin, A., Sospiro, G., Valencic, E., Boz, V., Pastore, S., Tommasini, A., & Benvenuti, F. (2025). BCAP Is an Interferon-Stimulated Gene That Enhances Type I Interferon Activity in Response to Lipopolysaccharide. International Journal of Molecular Sciences, 26(15), 7034. https://doi.org/10.3390/ijms26157034






