IFNγ Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells
Abstract
1. Introduction
2. Results
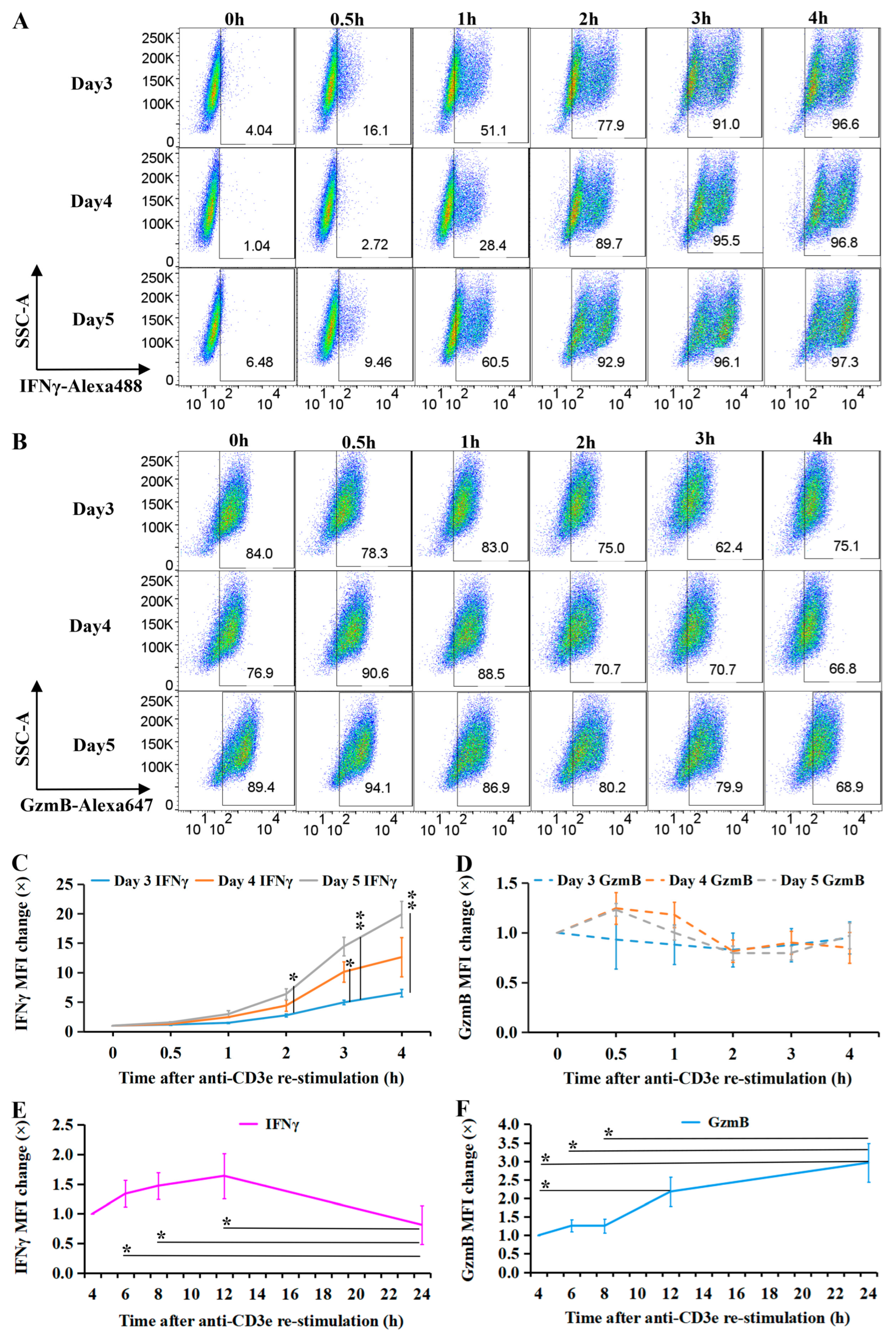
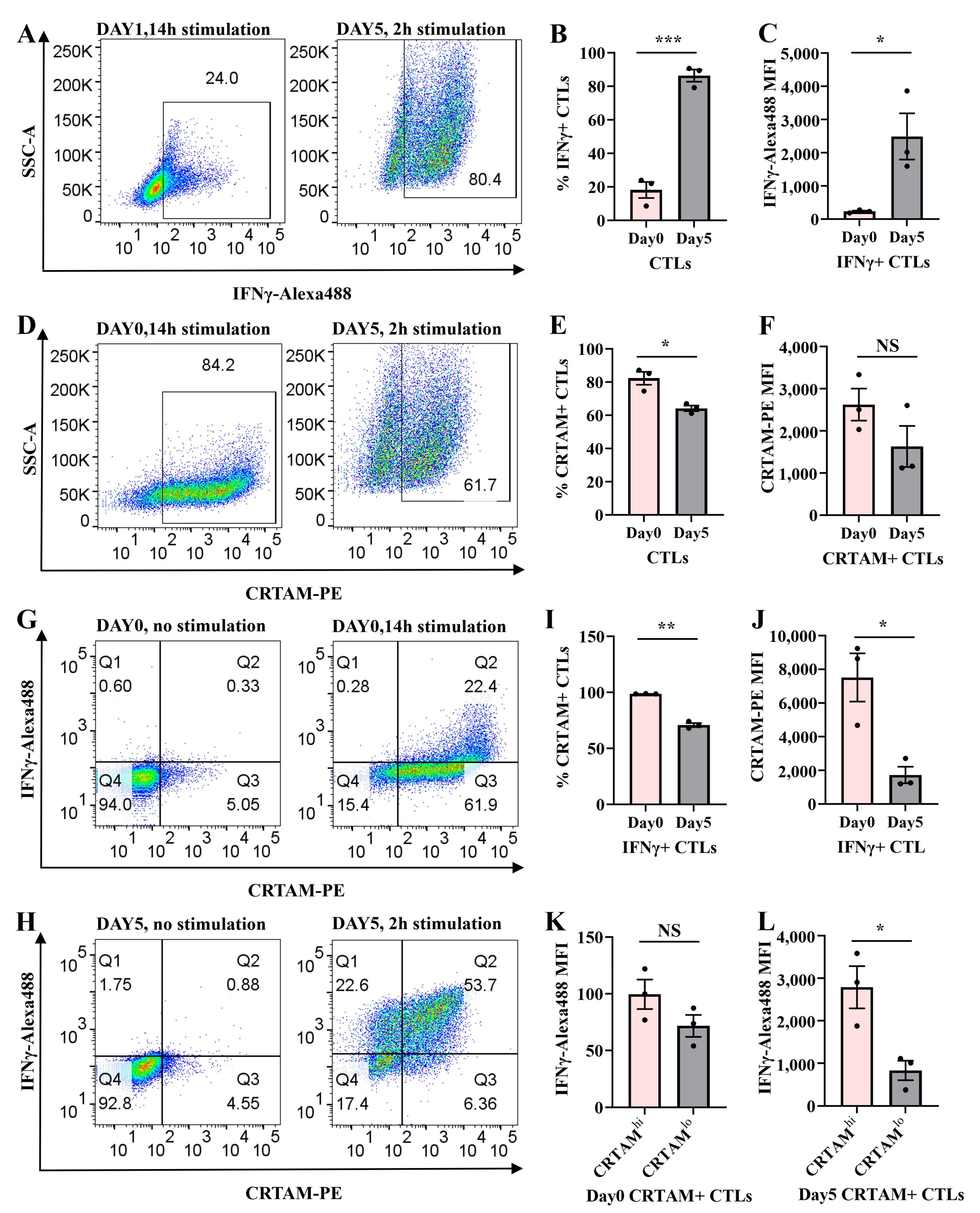
2.1. Transient IFNγ Expression Depends on TCR Reactivation in Resting CTLs
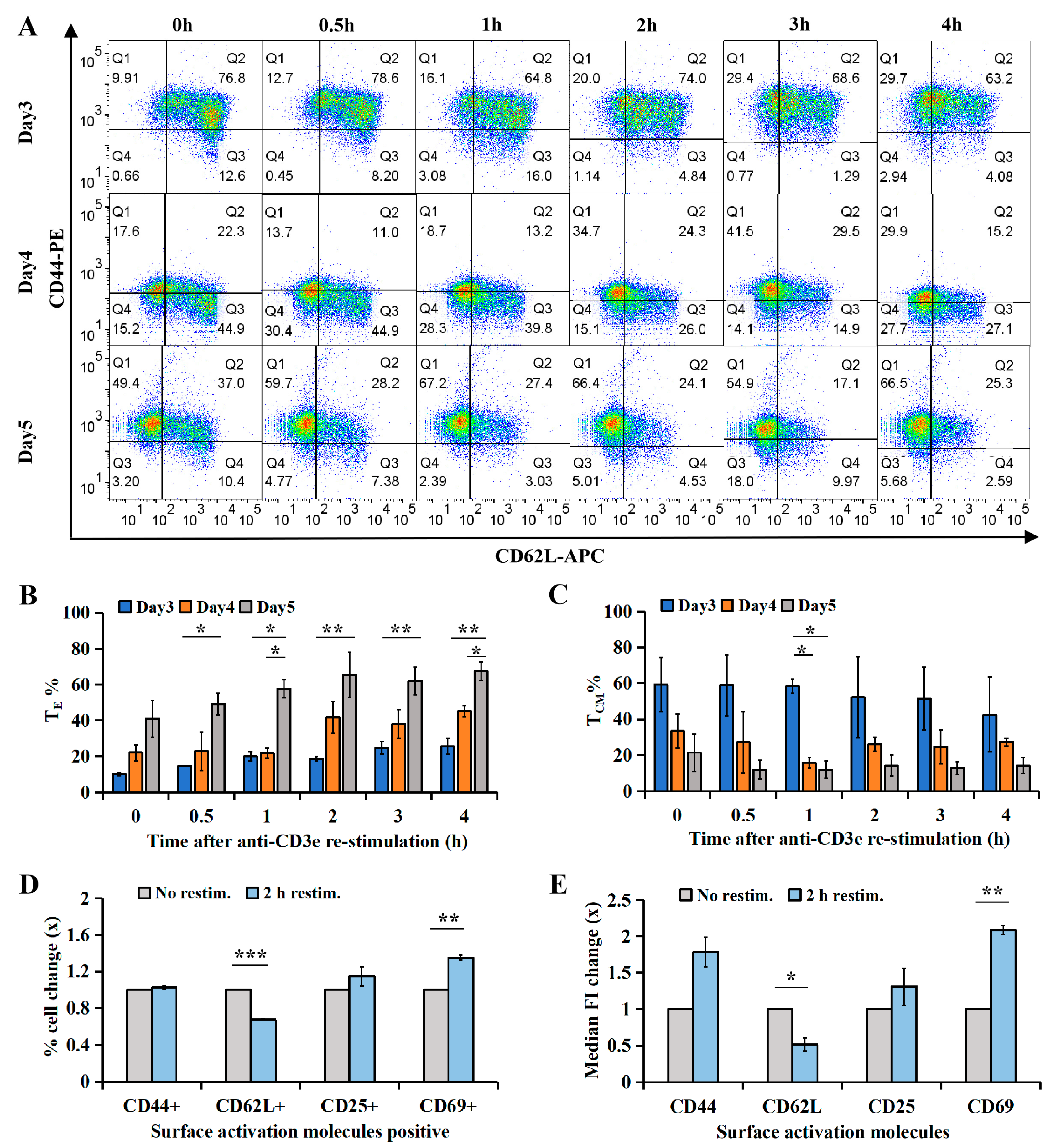
2.2. CTL Subtype Dynamics upon TCR Re-Stimulation
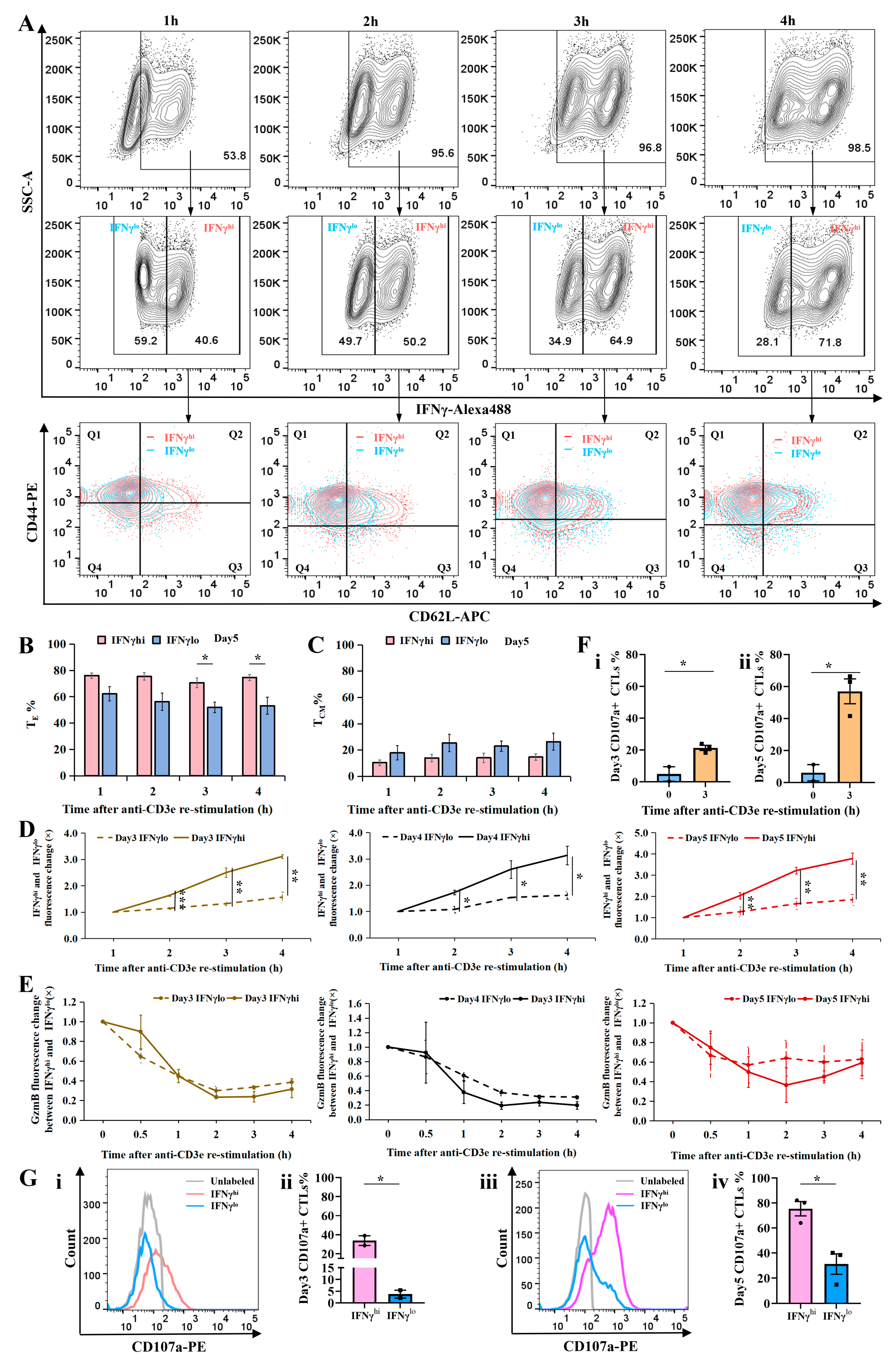
2.3. Cellular Subtype and Cytotoxicity Analysis of IFNγhi and IFNγlo CTLs
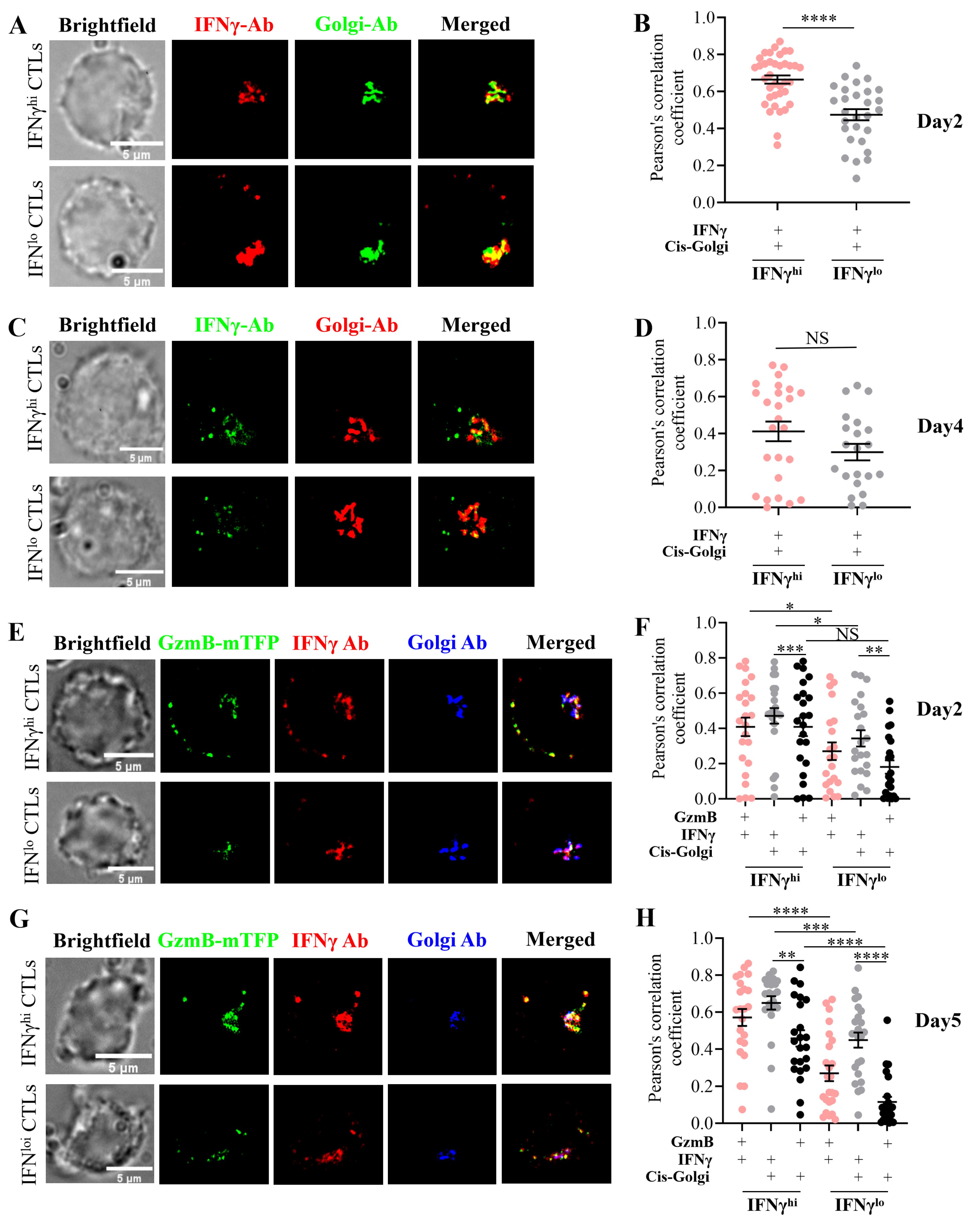
2.4. Subcellular Localization Analysis of IFNγ in IFNγhi and IFNγlo CTLs
2.5. Temporal Regulation of CRTAM and IFNγ in Naive and Activated CTLs
3. Discussion
4. Methods
4.1. Mice
4.2. Cell Isolation
4.3. Cell Culture
4.4. Immunocytochemistry
4.5. Structured Illumination Microscopy
Structured Illumination Microscopy Setup
4.6. Colocalization Analysis
4.7. Flow Cytometry
Intracellular IFNγ Measurement
4.8. Surface Marker Analysis
4.9. Degranulation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Weninger, W.; Manjunath, N.; von Andrian, U.H. Migration and differentiation of CD8+ T cells. Immunol. Rev. 2002, 186, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Stemberger, C.; Huster, K.M.; Koffler, M.; Anderl, F.; Schiemann, M.; Wagner, H.; Busch, D.H. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity 2007, 27, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.C.; Masopust, D. Diversity in T cell memory: An embarrassment of riches. Immunity 2009, 31, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Huster, K.M.; Busch, V.; Schiemann, M.; Linkemann, K.; Kerksiek, K.M.; Wagner, H.; Busch, D.H. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 2004, 101, 5610–5615. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.H.; Lee, S.; Kwak, M.; Kim, B.S.; Chung, Y. CD8 T-cell subsets: Heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weigelin, B.; den Boer, A.T.; Wagena, E.; Broen, K.; Dolstra, H.; de Boer, R.J.; Figdor, C.G.; Textor, J.; Friedl, P. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 2021, 12, 5217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perdomo-Celis, F.; Taborda, N.A.; Rugeles, M.T. CD8+ T-Cell Response to HIV Infection in the Era of Antiretroviral Therapy. Front. Immunol. 2019, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Itoh, Y.; Takumi, A.; Ishihara, C.; Arase, N.; Yokosuka, T.; Koseki, H.; Yamasaki, S.; Takai, Y.; Miyoshi, J.; et al. CRTAM confers late-stage activation of CD8+ T cells to regulate retention within lymph node. J. Immunol. 2009, 183, 4220–4228. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guilloty, F.; Pipkin, M.E.; Djuretic, I.M.; Levanon, D.; Lotem, J.; Lichtenheld, M.G.; Groner, Y.; Rao, A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009, 206, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.A.; Kroy, D.C.; Odorizzi, P.M.; Johnnidis, J.B.; Dolfi, D.V.; Barnett, B.E.; Bikoff, E.K.; Robertson, E.J.; Lauer, G.M.; Reiner, S.L.; et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012, 338, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.R.; Koup, R.A. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004, 75, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.J.; Borst, J.; Oorschot, V.; Fukuda, M.; Krähenbühl, O.; Tschopp, J.; Slot, J.W.; Geuze, H.J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991, 173, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 1991, 266, 21327–21330. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Griffiths, G.M. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 1999, 5, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Assarsson, E.; Lukacher, A.E.; Ljunggren, H.G.; Jensen, P.E. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol. 2003, 170, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Bold, T.D.; Ernst, J.D. CD4+ T cell-dependent IFN-γ production by CD8+ effector T cells in Mycobacterium tuberculosis infection. J. Immunol. 2012, 189, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, A.P.; Williams, T.M.; Strobl, B.; Watling, D.; Briscoe, J.; Kerr, I.M. The antiviral response to gamma interferon. J. Virol. 2002, 76, 9060–9068. [Google Scholar] [CrossRef] [PubMed]
- Rhein, B.A.; Powers, L.S.; Rogers, K.; Anantpadma, M.; Singh, B.K.; Sakurai, Y.; Bair, T.; Miller-Hunt, C.; Sinn, P.; Davey, R.A.; et al. Interferon-γ Inhibits Ebola Virus Infection. PLoS Pathog. 2015, 11, e1005263. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazet, J.M.; Mahale, J.N.; Tong, O.; Watson, R.A.; Lechuga-Vieco, A.V.; Pirgova, G.; Lau, V.W.C.; Attar, M.; Koneva, L.A.; Sansom, S.N.; et al. IFNγ signaling in cytotoxic T cells restricts anti-tumor responses by inhibiting the maintenance and diversity of intra-tumoral stem-like T cells. Nat. Commun. 2023, 14, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Q.; Du, Q.; Varley, P.R.; Goswami, J.; Liang, Z.; Wang, R.; Li, H.; Stolz, D.B.; Geller, D.A. Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response. Br. J. Cancer 2018, 118, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, R.B.; Strle, K.; Arvikar, S.L.; Weis, J.J.; Steere, A.C. Lyme arthritis: Linking infection, inflammation and autoimmunity. Nat. Rev. Rheumatol. 2021, 17, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016, 17, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chitirala, P.; Chang, H.F.; Martzloff, P.; Harenberg, C.; Ravichandran, K.; Abdulreda, M.H.; Berggren, P.O.; Krause, E.; Schirra, C.; Leinders-Zufall, T.; et al. Studying the biology of cytotoxic T lymphocytes in vivo with a fluorescent granzyme B-mTFP knock-in mouse. Elife 2020, 9, e58065. [Google Scholar] [CrossRef] [PubMed]
- Estl, M.; Blatt, P.; Li, X.; Becherer, U.; Chang, H.F.; Rettig, J.; Pattu, V. Various Stages of Immune Synapse Formation Are Differently Dependent on the Strength of the TCR Stimulus. Int. J. Mol. Sci. 2020, 21, 2475. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L.; Long, E.O. Cytotoxic immunological synapses. Immunol. Rev. 2010, 235, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schirra, C.; Wirkner, M.L.; Tu, S.M.; Lin, C.H.; Hohmann, M.; Alawar, N.; Chouaib, A.; Becherer, U.; Pattu, V.; et al. Lytic IFNγ is stored in granzyme B-containing cytotoxic granules and co-secreted by effector CD8+ T cells. BioRxiv 2025. [Google Scholar] [CrossRef]
- Bhat, P.; Leggatt, G.; Waterhouse, N.; Frazer, I.H. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017, 8, e2836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, J.H.; Sidhu, S.S.; Chan, A.C. Regulation of a late phase of T cell polarity and effector functions by Crtam. Cell 2008, 132, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Boles, K.S.; Barchet, W.; Diacovo, T.; Cella, M.; Colonna, M. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005, 106, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Badr Mel, S.; Miyauchi, K.; Ishihara, C.; Onishi, R.; Guo, Z.; Sasaki, Y.; Ike, H.; Takumi, A.; Tsuji, N.M.; et al. CRTAM determines the CD4+ cytotoxic T lymphocyte lineage. J. Exp. Med. 2016, 213, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Wieckowski, S.; Gupta, B.; Iancu, E.M.; Baumgaertner, P.; Baitsch, L.; Michielin, O.; Romero, P.; Rufer, N. Single cell analysis reveals similar functional competence of dominant and nondominant CD8 T-cell clonotypes. Proc. Natl. Acad. Sci. USA 2011, 108, 15318–15323. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.R.; Ngiow, S.F.; Manne, S.; Baxter, A.E.; Khan, O.; Wang, P.; Staupe, R.; Abdel-Hakeem, M.S.; Huang, H.; Mathew, D.; et al. Shared and distinct biological circuits in effector, memory and exhausted CD8+ T cells revealed by temporal single-cell transcriptomics and epigenetics. Nat. Immunol. 2022, 23, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wu, L.; Tian, R.; Gao, B.; Zhao, Y.; He, B.; Sun, D.; Zhao, B.; Li, Y.; Zhu, K.; et al. Integrative mapping of human CD8+ T cells in inflammation and cancer. Nat. Methods 2025, 22, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bzeih, H. The Role of Synaptobrevin2 in Exo-Endocytosis in Primary Mouse Cytotoxic lymphocytes. 2016. Available online: https://publikationen.sulb.uni-saarland.de/handle/20.500.11880/27034 (accessed on 15 February 2022).
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattu, V.; Krause, E.; Chang, H.-F.; Rettig, J.; Li, X. IFNγ Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells. Int. J. Mol. Sci. 2025, 26, 7024. https://doi.org/10.3390/ijms26147024
Pattu V, Krause E, Chang H-F, Rettig J, Li X. IFNγ Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells. International Journal of Molecular Sciences. 2025; 26(14):7024. https://doi.org/10.3390/ijms26147024
Chicago/Turabian StylePattu, Varsha, Elmar Krause, Hsin-Fang Chang, Jens Rettig, and Xuemei Li. 2025. "IFNγ Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells" International Journal of Molecular Sciences 26, no. 14: 7024. https://doi.org/10.3390/ijms26147024
APA StylePattu, V., Krause, E., Chang, H.-F., Rettig, J., & Li, X. (2025). IFNγ Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells. International Journal of Molecular Sciences, 26(14), 7024. https://doi.org/10.3390/ijms26147024





