Impact of Food Exposome on Atherosclerotic Plaque Stability: Metabolomic Insights from Human Carotid Endarterectomy Specimen
Abstract
1. Introduction
2. Results
2.1. Patient and Plaque Characteristics Associated with Plaque Stability
2.2. Molecular Characteristics of Stability in Carotid Atherosclerotic Plaque
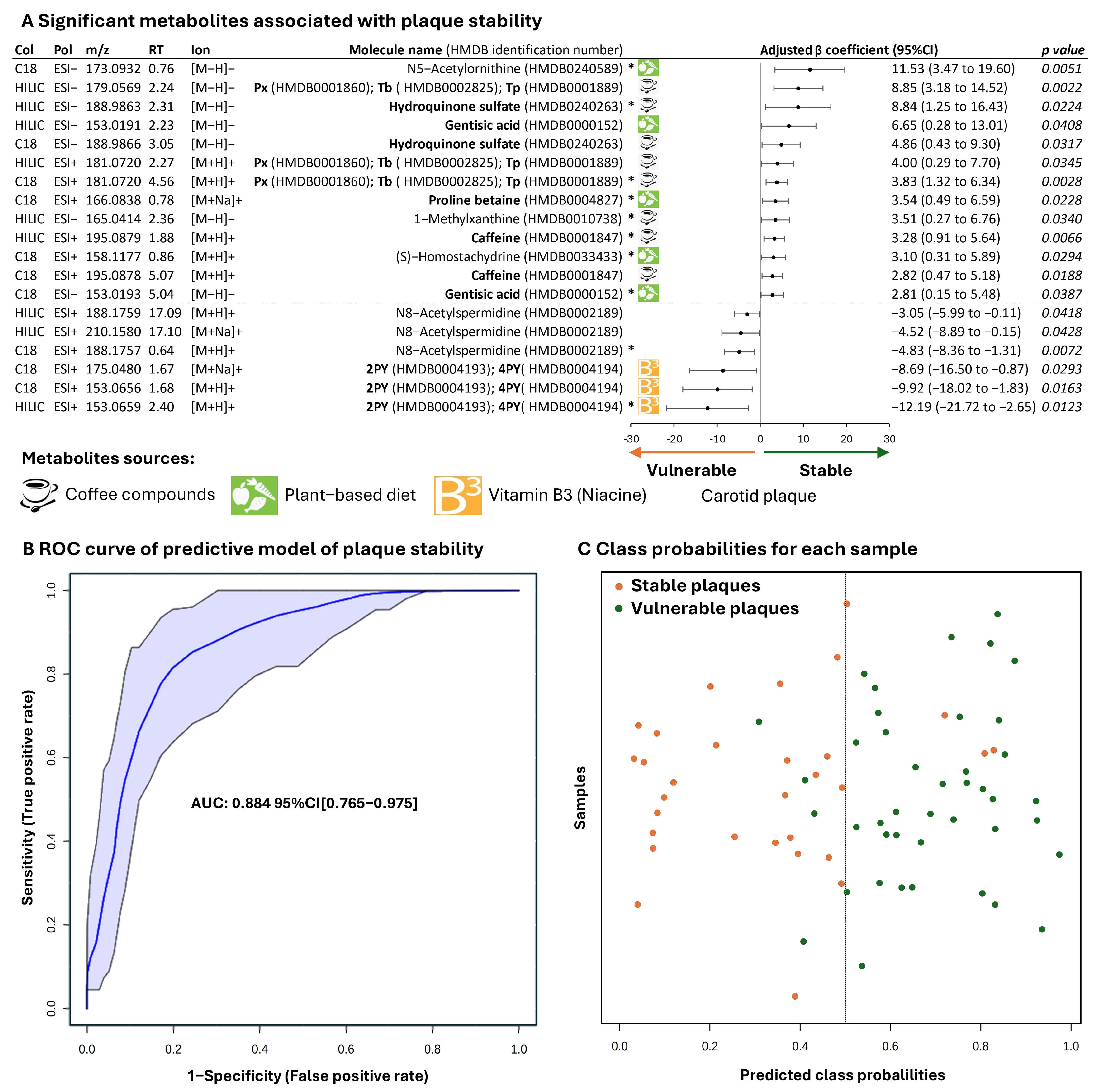
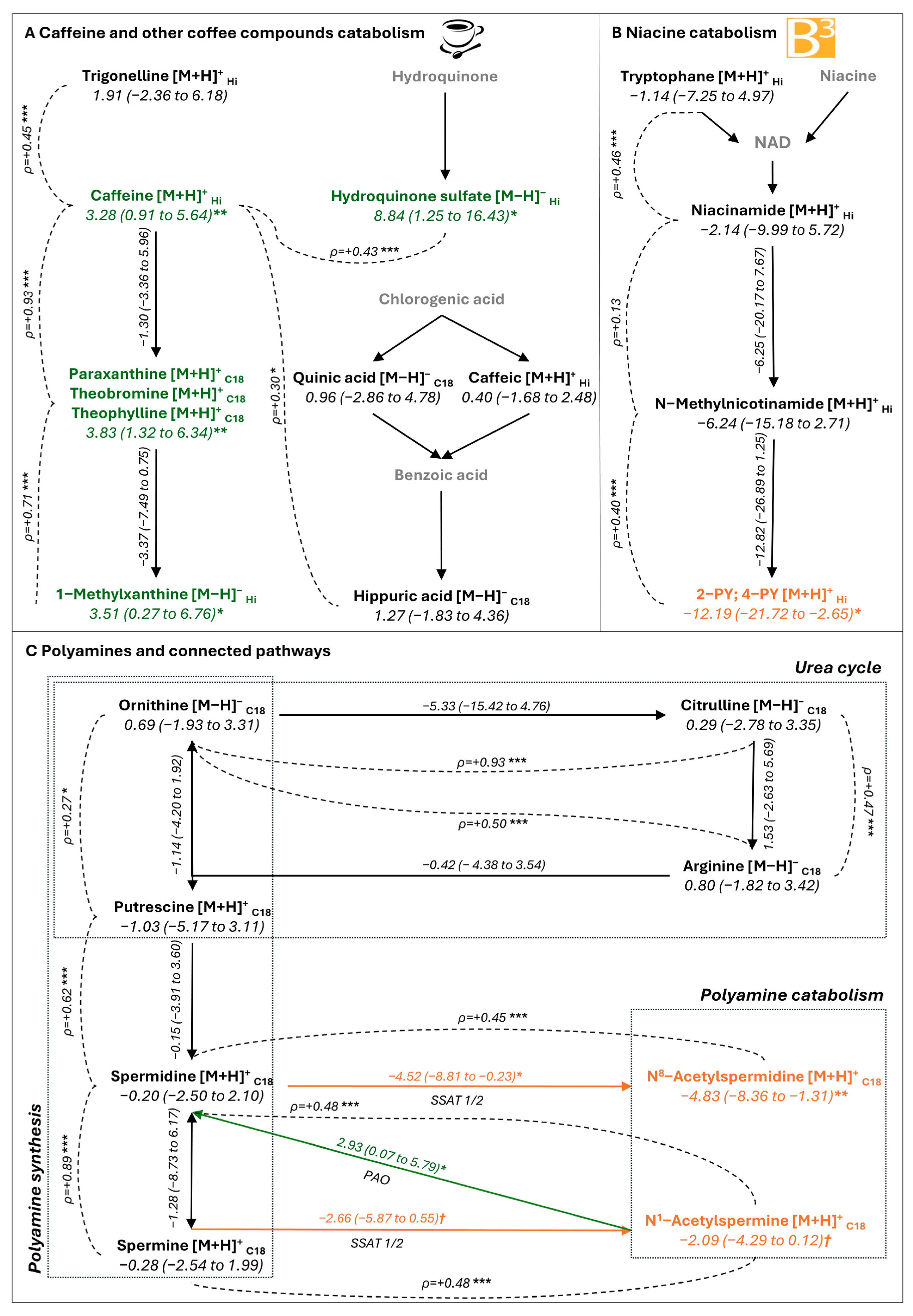
2.3. Metabolites Associated with Plaque Stability
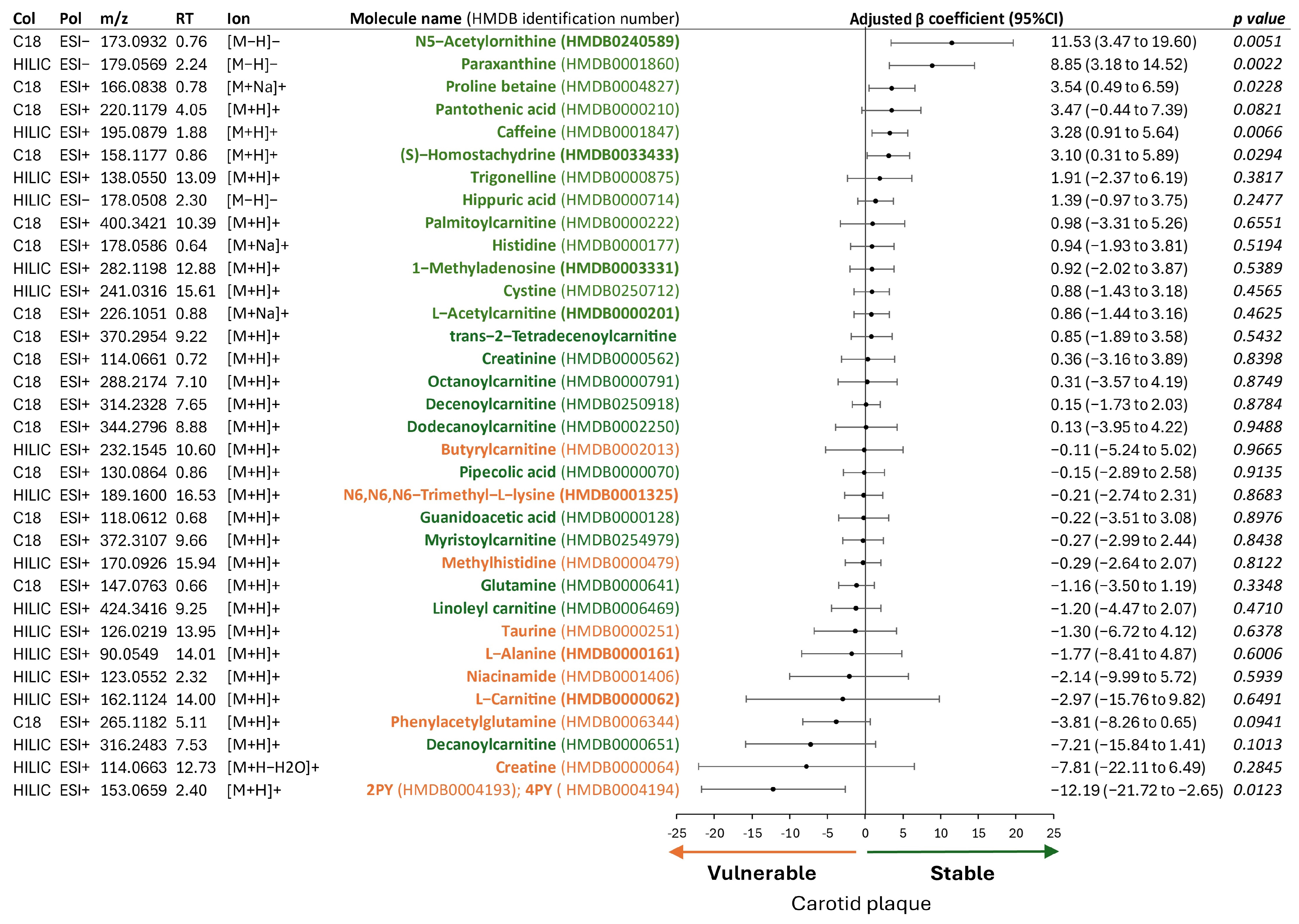
2.4. Metabolites Associated with Plaque Vulnerability
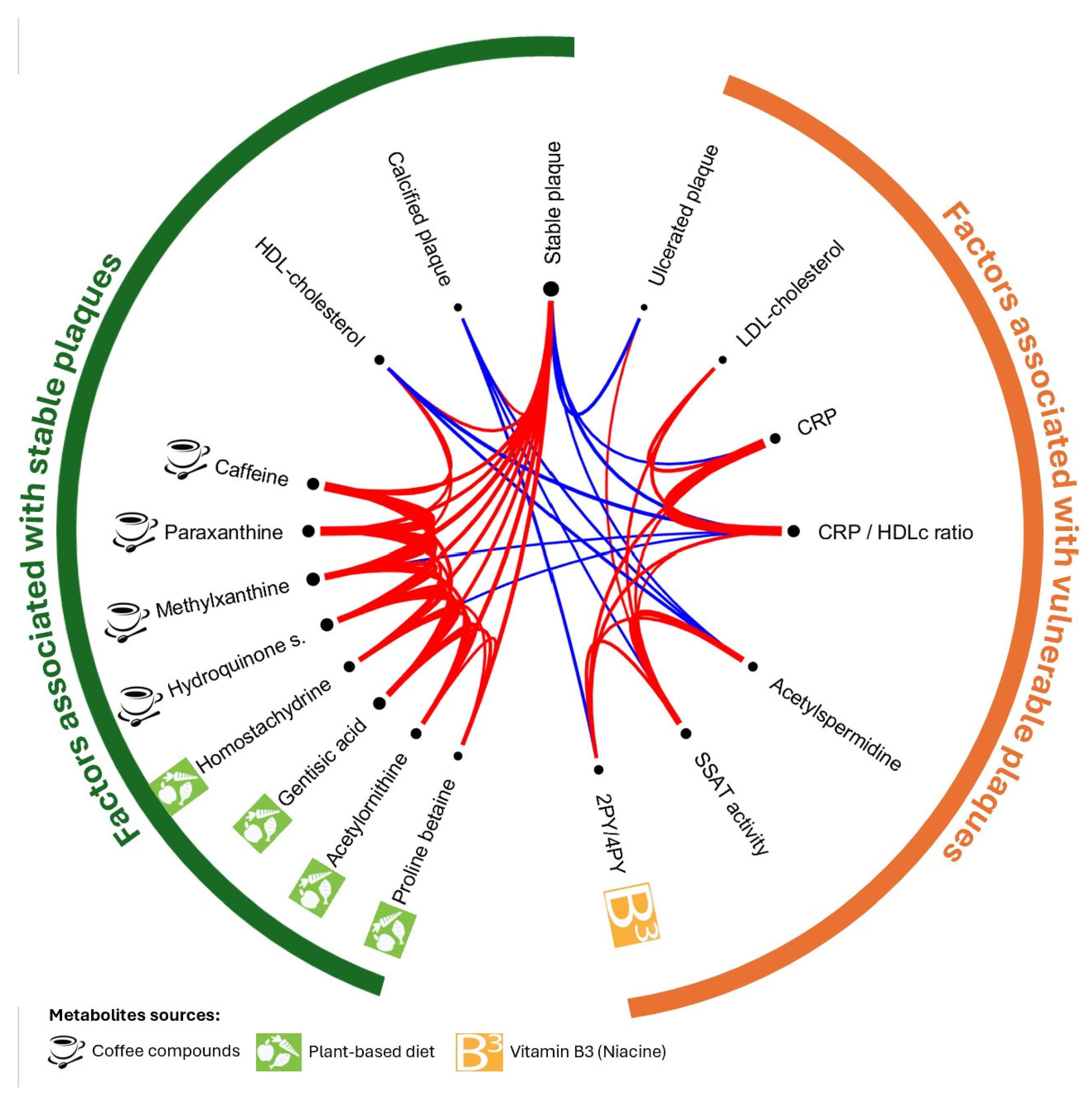
2.5. Associations Between Metabolites Inside Plaque, Systemic Inflammation, and Lipid Profile
2.6. Metabolites of Mediterranean Diet and Plaque Stability
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Population and Clinico-Biological Data Collection
4.2. Collection and Preparation of Carotid Plaque for Metabolomic Analysis
4.3. Untargeted Metabolomic Analysis of Carotid Plaque
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the ROC curve |
| BMI | Body Mass Index |
| CRP | C-Reactive Protein |
| CAS | Carotid Atherosclerotic Stenosis |
| CEA | Carotid Endarterectomy |
| DASH | Dietary Approaches to Stop Hypertension (DASH) |
| ESI | Electrospray Ionization |
| eGFR | estimated Glomerular Filtration rate by MDRD (Modification in Diet in Renal Disease) |
| Col | high performance-liquid chromatography column used |
| HDL-cholesterol | High-Density Lipoprotein cholesterol |
| HMDB | Human Metabolome Database |
| HILIC | Hydrophilic Interaction Liquid Chromatography |
| IS | Ischemic stroke |
| LC-MS | Liquid Chromatography coupled with tandem Mass Spectrometry |
| LDL-cholesterol | Low-Density Lipoprotein cholesterol |
| 2PY | N-methyl-2-pyridone-5-carboxamide |
| 4PY | N1-methyl-4-pyridone-3-carboxamide |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| Px | Paraxanthine |
| Pol | Polarity |
| PAO | Polyamine Oxydase |
| ROC | Receiver Operating Characteristic |
| RT | Retention Time |
| SSAT | Spermidine/Spermine n-Acetyltransferase |
| SVM | Support Vector Machine |
| Tb | Theobromine |
| Tp | Theophylline |
| TIA | Transient Ischemic Attack |
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Wang, X.; Yang, N.; He, L.; Wang, J.; Ping, F.; Xu, L.; Zhang, H.; Li, W.; et al. Comparative Analysis of Atherosclerotic Cardiovascular Disease Burden between Ages 20–54 and over 55 Years: Insights from the Global Burden of Disease Study 2019. BMC Med. 2024, 22, 303. [Google Scholar] [CrossRef] [PubMed]
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.D.; Münzel, T.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2021, 41, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; Grotta, R.L.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.; Goonetilleke, N.; Patel, S.; Keech, A. Colchicine in Atherosclerotic Cardiovascular Disease. Heart 2024, 110, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events with Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation Guideline on Endarterectomy and Stenting for Carotid Artery Stenosis. Eur. Stroke J. 2021, 6, I-XLVII. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Navi, B.B.; Merkler, A.E.; Baradaran, H.; Díaz, I.; Parikh, N.S.; Kasner, S.E.; Gladstone, D.J.; Iadecola, C.; Gupta, A. Reclassification of Ischemic Stroke Etiological Subtypes on the Basis of High-Risk Nonstenosing Carotid Plaque. Stroke 2020, 51, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Underhill, H.; Cai, J.; Tran, N.; Polissar, N.L.; Isaac, C.; Ferguson, M.S.; et al. Association between Carotid Plaque Characteristics and Subsequent Ischemic Cerebrovascular Events: A Prospective Assessment with MRI--Initial Results. Stroke 2006, 37, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Altaf, N.; MacSweeney, S.T.; Gladman, J.; Auer, D.P. Carotid Intraplaque Hemorrhage Predicts Recurrent Symptoms in Patients with High-Grade Carotid Stenosis. Stroke 2007, 38, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, C.; Drozdov, I.; Shalhoub, J.; Humphries, J.; Ladroue, C.; Didangelos, A.; Baumert, M.; Allen, M.; Davies, A.H.; Monaco, C.; et al. Comparative Lipidomics Profiling of Human Atherosclerotic Plaques. Circ. Cardiovasc. Genet. 2011, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, L.G.; Yeung, K.; Eldrup, N.; Eiberg, J.P.; Sillesen, H.H.; Davies, M.J. Proteomic Analysis of the Extracellular Matrix of Human Atherosclerotic Plaques Shows Marked Changes between Plaque Types. Matrix Biol. Plus 2024, 21, 100141. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Quercioli, L.; Pucci, A.; Rocchiccioli, S.; Ferrari, M.; Recchia, F.A.; McDonnell, L.A. Mass Spectrometry Imaging as a Tool to Investigate Region Specific Lipid Alterations in Symptomatic Human Carotid Atherosclerotic Plaques. Metabolites 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, H. Associations of the Hs-CRP/HDL-C Ratio with Cardiovascular Disease among US Adults: Evidence from NHANES 2015-2018. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103814. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Fillâtre, Y.; Martin, J.-F.; Lyan, B.; Pujos-Guillot, E.; Fezeu, L.; Hercberg, S.; Comte, B.; Galan, P.; Touvier, M.; et al. New Biomarkers of Coffee Consumption Identified by the Non-Targeted Metabolomic Profiling of Cohort Study Subjects. PLoS ONE 2014, 9, e93474. [Google Scholar] [CrossRef] [PubMed]
- Makiso, M.U.; Tola, Y.B.; Ogah, O.; Endale, F.L. Bioactive Compounds in Coffee and Their Role in Lowering the Risk of Major Public Health Consequences: A Review. Food Sci. Nutr. 2024, 12, 734–764. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chang, B.-E.; Pan, Y.-H.; Lin, B.-R.; Lian, Y.-C.; Lee, M.-S.; Yeung, S.-Y.; Lin, L.-D.; Jeng, J.-H. Antiplatelet, Antioxidative, and Anti-Inflammatory Effects of Hydroquinone. J. Cell. Physiol. 2019, 234, 18123–18130. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, A.P. The Toxicology of Hydroquinone—Relevance to Occupational and Environmental Exposure. Crit. Rev. Toxicol. 1999, 29, 283–330. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Ericson, U.; Hellstrand, S.; Orho-Melander, M.; Nilsson, P.M.; Fernandez, C.; Melander, O.; Ottosson, F. A Healthy Dietary Metabolic Signature Is Associated with a Lower Risk for Type 2 Diabetes and Coronary Artery Disease. BMC Med. 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Ottosson, F.; Ericson, U.; Hellstrand, S.; Rizzo, M.; Sukruang, K.; Pizza, V.; Orho-Melander, M.; Nilsson, P.M.; Kennbäck, C.; et al. Impact of a Short-Term Mediterranean Diet Intervention on Plasma Metabolites: A Pilot Study. Metabolomics 2024, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, T.; Azab, S.M.; Teo, K.K.; Thabane, L.; Anand, S.S.; Morrison, K.M.; de Souza, R.J.; Britz-McKibbin, P. Nutritional Metabolomics and the Classification of Dietary Biomarker Candidates: A Critical Review. Adv. Nutr. 2021, 12, 2333–2357. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, M.; Wang, Z.; Anderson, J.T.; Li, X.S.; Witkowski, M.; DiDonato, J.A.; Hilser, J.R.; Hartiala, J.A.; Haghikia, A.; Cajka, T.; et al. A Terminal Metabolite of Niacin Promotes Vascular Inflammation and Contributes to Cardiovascular Disease Risk. Nat. Med. 2024, 30, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-Term Coffee Consumption and Risk of Cardiovascular Disease: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Liu, Z.; Lin, S.; Macniven, R.; Akombi-Inyang, B.; Hall, J.; Feng, X.; Schutte, A.E.; Xu, X. Long-Term Consumption of 10 Food Groups and Cardiovascular Mortality: A Systematic Review and Dose Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2023, 14, 55–63. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, H.; Sun, Q.; Li, J.; Heianza, Y.; Van Dam, R.M.; Hu, F.B.; Rimm, E.; Manson, J.E.; Qi, L. Coffee Drinking Timing and Mortality in US Adults. Eur. Heart J. 2025, 46, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Zeng, L.; Grafenauer, S.; Schutte, A.E.; Xu, X. Long-Term Consumption of 6 Different Beverages and Cardiovascular Disease-Related Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Curr. Dev. Nutr. 2024, 8, 102095. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Wee, C.C.; Kovell, L.C.; Plante, T.B.; Miller, E.R.; Appel, L.J.; Mukamal, K.J.; Juraschek, S.P. Effects of Diet on 10-Year Atherosclerotic Cardiovascular Disease Risk (from the DASH Trial). Am. J. Cardiol. 2023, 187, 10–17. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Salen, P.; Martin, J.-L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors, and the Rate of Cardiovascular Complications After Myocardial Infarction. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Cirilli, I.; Marcheggiani, F.; Silvestri, S.; Orlando, P.; Muvhulawa, N.; Moetlediwa, M.T.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hlengwa, N.; et al. Potential Benefits of Coffee Consumption on Improving Biomarkers of Oxidative Stress and Inflammation in Healthy Individuals and Those at Increased Risk of Cardiovascular Disease. Molecules 2023, 28, 6440. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Martin, S. Health Effects of Coffee: Mechanism Unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine Effects on Systemic Metabolism, Oxidative-Inflammatory Pathways, and Exercise Performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Sahebkar, A.; Banach, M. Coffee or Tea: Anti-Inflammatory Properties in the Context of Atherosclerotic Cardiovascular Disease Prevention. Pharmacol. Res. 2023, 187, 106596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hyppönen, E. Habitual Coffee Intake and Plasma Lipid Profile: Evidence from UK Biobank. Clin. Nutr. 2021, 40, 4404–4413. [Google Scholar] [CrossRef] [PubMed]
- Uto-Kondo, H.; Ayaori, M.; Ogura, M.; Nakaya, K.; Ito, M.; Suzuki, A.; Takiguchi, S.; Yakushiji, E.; Terao, Y.; Ozasa, H.; et al. Coffee Consumption Enhances High-Density Lipoprotein-Mediated Cholesterol Efflux in Macrophages. Circ. Res. 2010, 106, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Noerman, S.; Kårlund, A.; Raita, J.; Meuronen, T.; Koistinen, V.; Landberg, R.; Hanhineva, K. Nutritional Metabolomics: Recent Developments and Future Needs. Curr. Opin. Chem. Biol. 2023, 77, 102400. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Yang, J.-L.; Ni, J.; You, K.; Li, X.; Song, Y.; Wang, X.; Li, J.; Shen, X.; et al. Gentisic Acid Prevents the Development of Atherosclerotic Lesions by Inhibiting SNX10-Mediated Stabilization of LRP6. Pharmacol. Res. 2024, 210, 107516. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A Review on Gentisic Acid as a Plant Derived Phenolic Acid and Metabolite of Aspirin: Comprehensive Pharmacology, Toxicology, and Some Pharmaceutical Aspects. Phytother. Res. 2019, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Ashidate, K.; Kawamura, M.; Mimura, D.; Tohda, H.; Miyazaki, S.; Teramoto, T.; Yamamoto, Y.; Hirata, Y. Gentisic Acid, an Aspirin Metabolite, Inhibits Oxidation of Low-Density Lipoprotein and the Formation of Cholesterol Ester Hydroperoxides in Human Plasma. Eur. J. Pharmacol. 2005, 513, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, M.; Wang, R.; Jiang, J.; Hu, Y.; Wang, W.; Wang, Y.; Li, H. The Predictive Value of the Hs-CRP/HDL-C Ratio, an Inflammation-Lipid Composite Marker, for Cardiovascular Disease in Middle-Aged and Elderly People: Evidence from a Large National Cohort Study. Lipids Health Dis. 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/Spermine-N(1)-Acetyltransferase: A Key Metabolic Regulator. Am. J. Physiol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.-M.; Zheng, W.; Shaalan, W.; Tang, J.; Bassiouny, H.S. Human Carotid Plaque Calcification and Vulnerability. Relationship between Degree of Plaque Calcification, Fibrous Cap Inflammatory Gene Expression and Symptomatology. Cerebrovasc. Dis. 2009, 27, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-León, A.M.; Camafort, M.; Sala-Vila, A.; Gilabert, R.; Núñez, I.; Castro-Barquero, S.; Fitó, M.; Lamuela-Raventós, R.M.; Pintó, X.; García-Arellano, A.; et al. The Mediterranean Diet Displays an Immunomodulatory Effect That Correlates with Beneficial Changes in Carotid Atherosclerosis. Cardiovasc. Res. 2025, 121, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; Lorenzo, A.D. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-Processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for Keeping the Food System within Environmental Limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Asymptomatic Patients | Symptomatic Patients | p Value | |
|---|---|---|---|---|
| n = 72 | n = 42 | n = 30 | ||
| Medical history before hospitalization | ||||
| Age, y | 69.00 [63.75–77.00] | 70.00 [64.00–77.50] | 69.00 [63.25–76.75] | 0.833 |
| Men | 56/72 (77.78%) | 31/42 (73.81%) | 25/30 (83.33%) | 0.338 |
| High blood pressure | 53/72 (73.61%) | 32/42 (76.19%) | 21/30 (70.00%) | 0.557 |
| Active smoker | 43/72 (59.72%) | 25/42 (59.52%) | 18/30 (60.00%) | 0.968 |
| Diabetes mellitus | 26/72 (37.50%) | 19/42 (45.24%) | 8/30 (26.67%) | 0.109 |
| Coronary disease | 22/72 (30.56%) | 15/42 (35.71%) | 7/30 (23.33%) | 0.261 |
| BMI, kg/m2 | 25.61 [24.09–27.51] (3) | 25.48 [24.53–27.72] | 26.17 [23.78–27.22] (3) | 0.980 |
| Obesity (BMI ≥ 30 kg/m2) | 12/69 (17.39%) | 9/42 (21.43%) | 3/27 (11.11%) | 0.270 |
| Ischemic stroke or TIA | 12/72 (16.67%) | 5/42 (11.90%) | 7/30 (23.33%) | 0.201 |
| Medical treatment before hospitalization | ||||
| Antiplatelet therapy | 53/72 (73.61%) | 36/42 (85.71%) | 17/30 (56.67%) | 0.006 |
| Lipid-lowering therapy | 43/72 (59.72%) | 32/42 (76.19%) | 11/30 (36.67%) | <0.001 |
| Antidiabetes therapy | 23/72 (31.94%) | 16/42 (38.10%) | 7/30 (23.33%) | 0.185 |
| Antihypertensive therapy | 55/72 (76.39%) | 33/42 (78.57%) | 22/30 (73.33%) | 0.606 |
| CAS and CEA characteristics | ||||
| NASCET measure of CAS, % | 77.50 [70.00–90.00] | 77.50 [70.00–90.00] | 77.50 [70.00–90.00] | 0.939 |
| CEA under antiplatelet therapy | 67/72 (93.06%) | 39/42 (92.90%) | 28/30 (93.30%) | 0.938 |
| Laboratory results at admission | ||||
| Hemoglobin, g/dL | 13.55 [12.90–14.90] | 13.70 [12.93–14.98] | 13.35 [12.90–14.60] | 0.451 |
| Hematocrit | 0.40 [0.38–0.44] | 0.40 [0.38–0.45] | 0.40 [0.37–0.43] | 0.457 |
| Platelet count, G/L | 233.00 [209.00–291.75] | 231.50 [203.75–290.00] | 240.50 [214.25–288.25] | 0.421 |
| Leukocyte count, G/L | 8.10 [6.59–9.30] (1) | 8.30 [6.95–9.45] | 7.92 [6.28–9.10] (1) | 0.261 |
| C-reactive protein, mg/L | 2.40 [1.18–6.50] | 1.75 [0.73–4.65] | 3.90 [2.03–7.65] | 0.022 |
| Fasting glycemia, g/L | 1.00 [0.86–1.19] (2) | 1.05 [0.87–1.35] (1) | 0.97 [0.86–1.12] (1) | 0.331 |
| Total cholesterol, g/L | 1.58 [1.30–1.87] (6) | 1.60 [1.31–1.78] (4) | 1.55 [1.24–1.92] (2) | 0.979 |
| LDL-cholesterol, g/L | 0.79 [0.56–1.04] (5) | 0.72 [0.50–0.91] (3) | 0.86 [0.66–1.16] (2) | 0.090 |
| HDL-cholesterol, g/L | 0.43 [0.36–0.49] (5) | 0.46 [0.38–0.57] (3) | 0.39 [0.35–0.43] (2) | 0.010 |
| Triglycerides, g/L | 1.23 [0.96–1.71] (5) | 1.25 [0.93–1.87] (3) | 1.20 [0.98–1.61] (2) | 0.809 |
| Ratio of CRP to HDL-cholesterol | 5.68 [2.77–14.92] (5) | 4.00 [1.43–10.06] (3) | 10.46 [5.03–19.49] (2) | 0.004 |
| eGRF, mL/min/1.73 m2 | 75.82 [58.25–93.43] | 63.72 [51.84–79.27] | 91.14 [76.07–98.93] | <0.001 |
| Macroscopic description of the plaque after CEA | ||||
| Calcified plaque | 17/72 (23.61%) | 14/42 (33.33%) | 3/30 (10.00%) | 0.022 |
| Ulcerated plaque | 11/72 (15.28%) | 2/42 (4.76%) | 9/30 (30.00%) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doche, E.; Leclercq, B.; Sulowski, C.; Magoncia, E.; Tardivel, C.; Svilar, L.; Sarlon-Bartoli, G.; Martin, J.-C.; Bartoli, M.; Rossillon, A.; et al. Impact of Food Exposome on Atherosclerotic Plaque Stability: Metabolomic Insights from Human Carotid Endarterectomy Specimen. Int. J. Mol. Sci. 2025, 26, 7018. https://doi.org/10.3390/ijms26147018
Doche E, Leclercq B, Sulowski C, Magoncia E, Tardivel C, Svilar L, Sarlon-Bartoli G, Martin J-C, Bartoli M, Rossillon A, et al. Impact of Food Exposome on Atherosclerotic Plaque Stability: Metabolomic Insights from Human Carotid Endarterectomy Specimen. International Journal of Molecular Sciences. 2025; 26(14):7018. https://doi.org/10.3390/ijms26147018
Chicago/Turabian StyleDoche, Emilie, Barbara Leclercq, Constance Sulowski, Ellen Magoncia, Catherine Tardivel, Ljubica Svilar, Gabrielle Sarlon-Bartoli, Jean-Charles Martin, Michel Bartoli, Alexandre Rossillon, and et al. 2025. "Impact of Food Exposome on Atherosclerotic Plaque Stability: Metabolomic Insights from Human Carotid Endarterectomy Specimen" International Journal of Molecular Sciences 26, no. 14: 7018. https://doi.org/10.3390/ijms26147018
APA StyleDoche, E., Leclercq, B., Sulowski, C., Magoncia, E., Tardivel, C., Svilar, L., Sarlon-Bartoli, G., Martin, J.-C., Bartoli, M., Rossillon, A., & Suissa, L. (2025). Impact of Food Exposome on Atherosclerotic Plaque Stability: Metabolomic Insights from Human Carotid Endarterectomy Specimen. International Journal of Molecular Sciences, 26(14), 7018. https://doi.org/10.3390/ijms26147018








