Advanced Research on Biological Properties—A Study on the Activity of the Apis mellifera Antioxidant System and the Crystallographic and Spectroscopic Properties of 7-Diethylamino-4-hydroxycoumarin
Abstract
1. Introduction
2. Results and Discussion
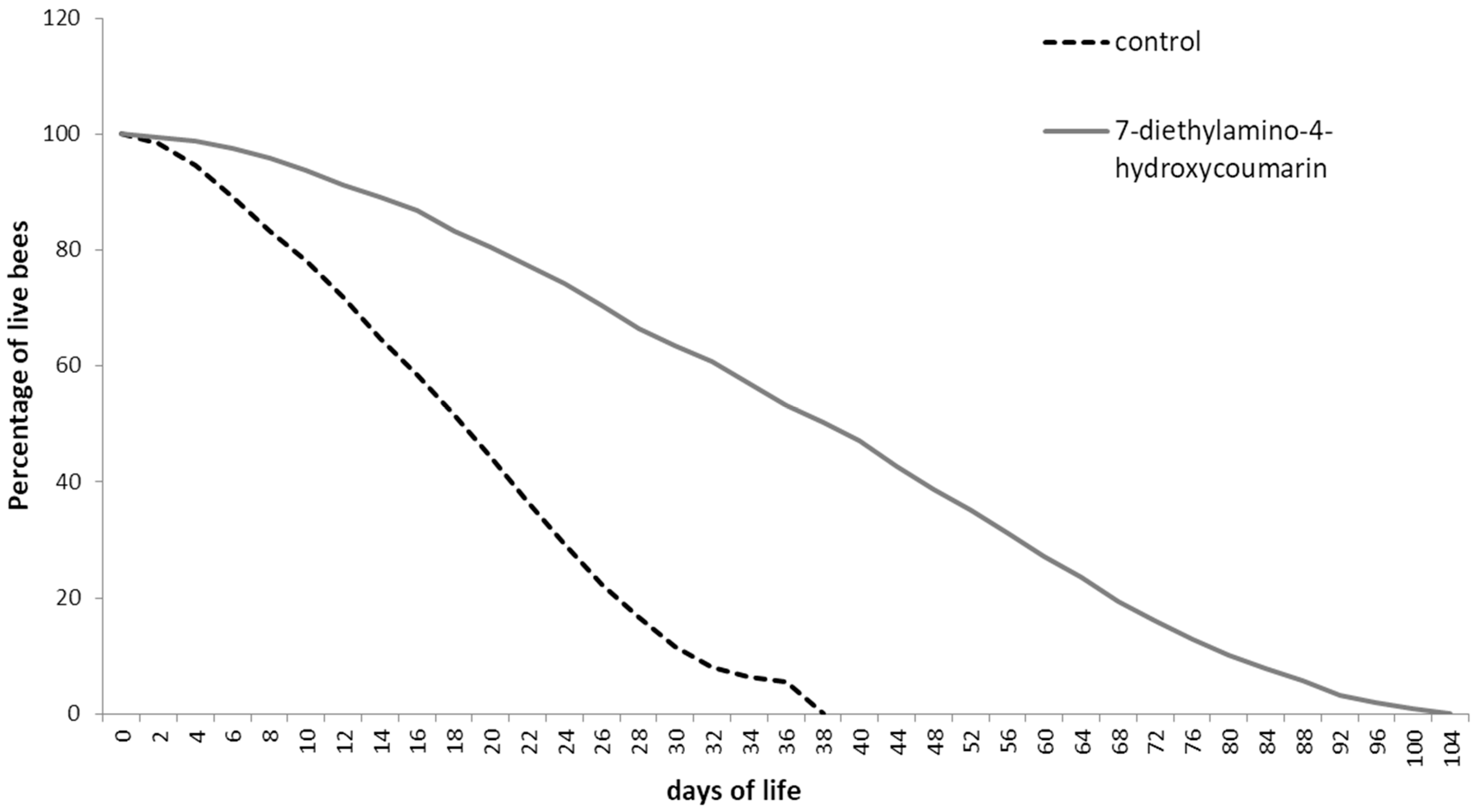
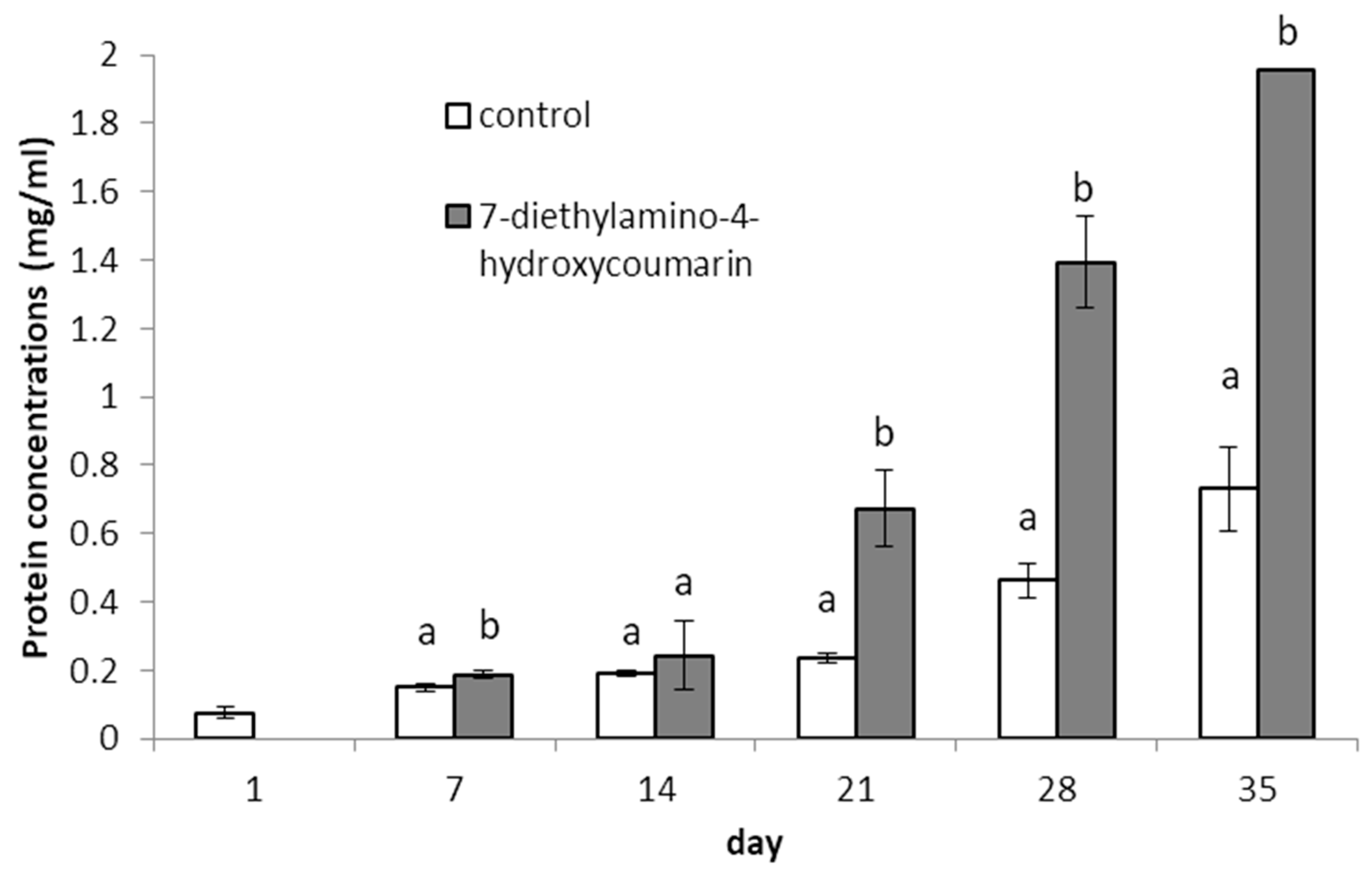
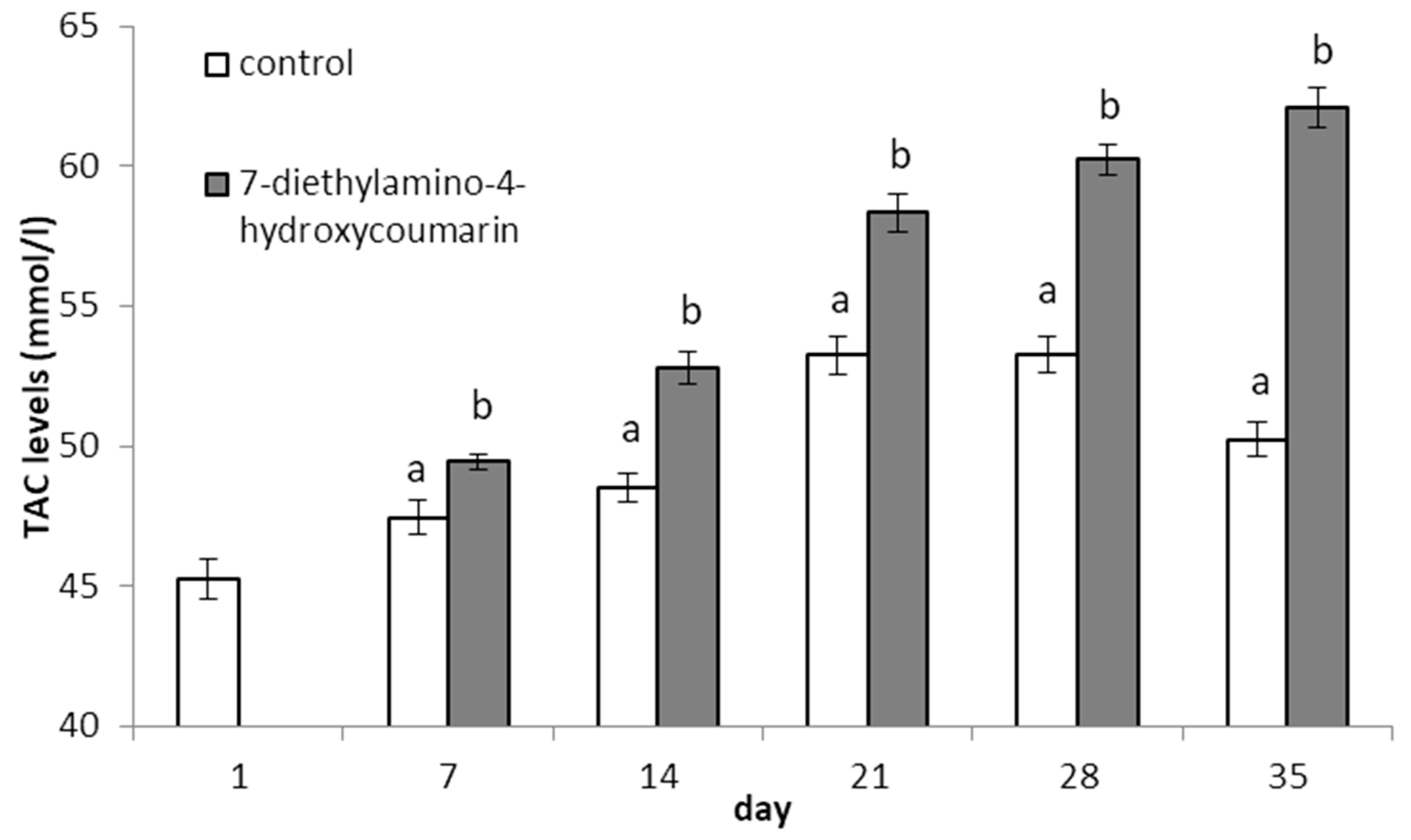
2.1. Determination of Biological Properties
- SOD—superoxide dismutase (two-way ANOVA; group: F(1,268) = 756.20, p = 0.0000; days of supplementation: F(4,264) = 5729.4, p = 0.0000, se ± 0.033727 (1st day), se ± 0.023849 (7–35 days); group × days of supplementation: F(4,264) = 340.50; p = 0.0000); se ± 0.115141 (control), se ± 0.178103 (7DOC);
- CAT—catalase (two-way ANOVA; group: F(1,268) = 987.15, p = 0.0000; se ± 0.129998 (control), se ± 0.280503 (7DOC); days of supplementation: F(4,264) = 2336.2, p = 0.0000, se ± 0.069046 (1st day), se ± 0.048823 (7–35 days); group × days of supplementation: F(4,264) = 548.70, p = 0.0000, se ± 0.069046);
- GST—glutathione S-transferase (two-way ANOVA; group: F(1,268) = 639.91, p = 0.0000; se ± 0.187744 (control), se ± 0.288596 (7DOC); days of supplementation: F(4,264) = 1948.2, p = 0.0000, se ± 0.088550 (1st day), se ± 0.062614 (7–35 days); group × days of supplementation: F(4,264) = 208.68, p = 0.0000, se ± 0.088550);
- GPx—Glutathione peroxidase (two-way ANOVA; group: F(1,268) = 718.09, p = 0.0000, se ± 0.168185 (control), se ± 0.331584 (7DOC); days of supplementation: F(4,264) = 1078.7, p = 0.0000, se ± 0.105886 (1st day), se ± 0.074873 (7–35 days); group × days of supplementation: F(4,264) = 634.88, p = 0.0000, se ± 0.105886).
2.2. Determination of Crystallographic Properties
2.2.1. Crystal Structure of 7-Diethylamino-4-hydroxycoumarin (7DOC)
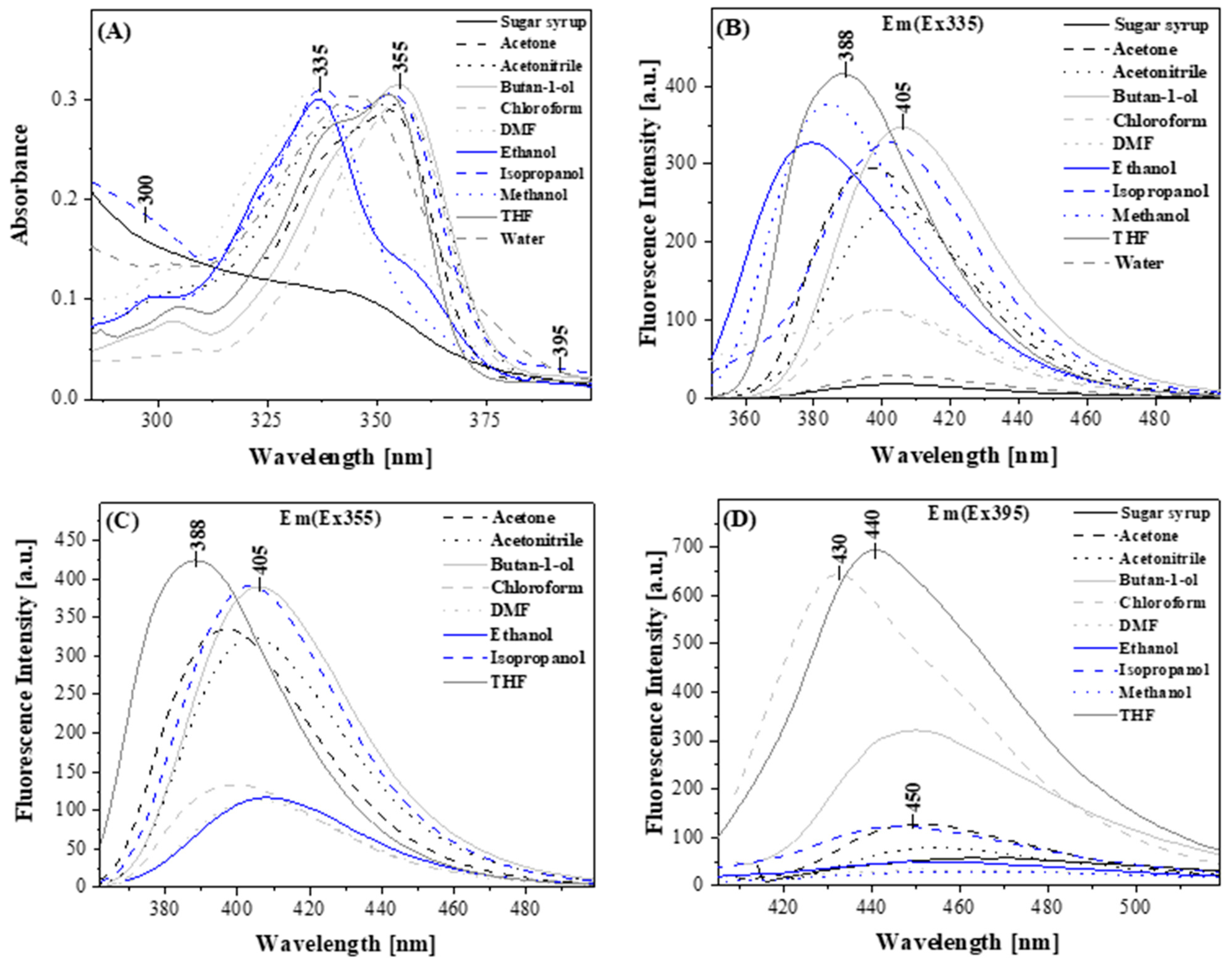
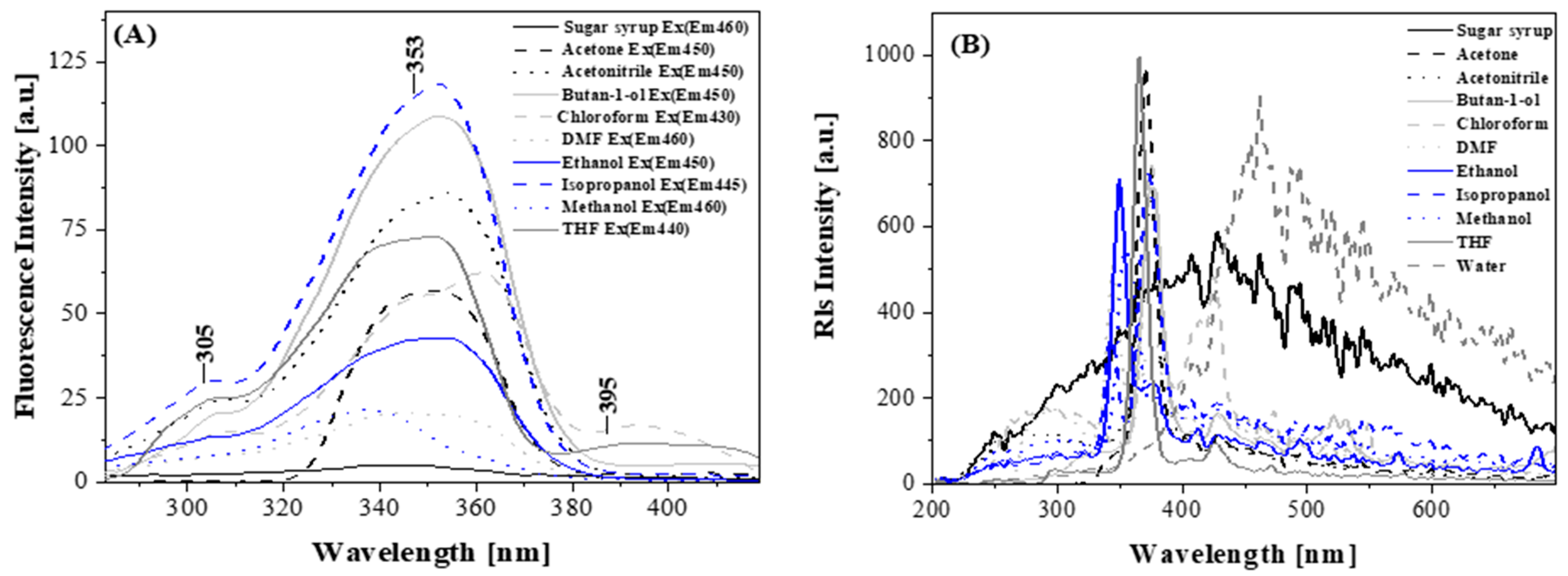
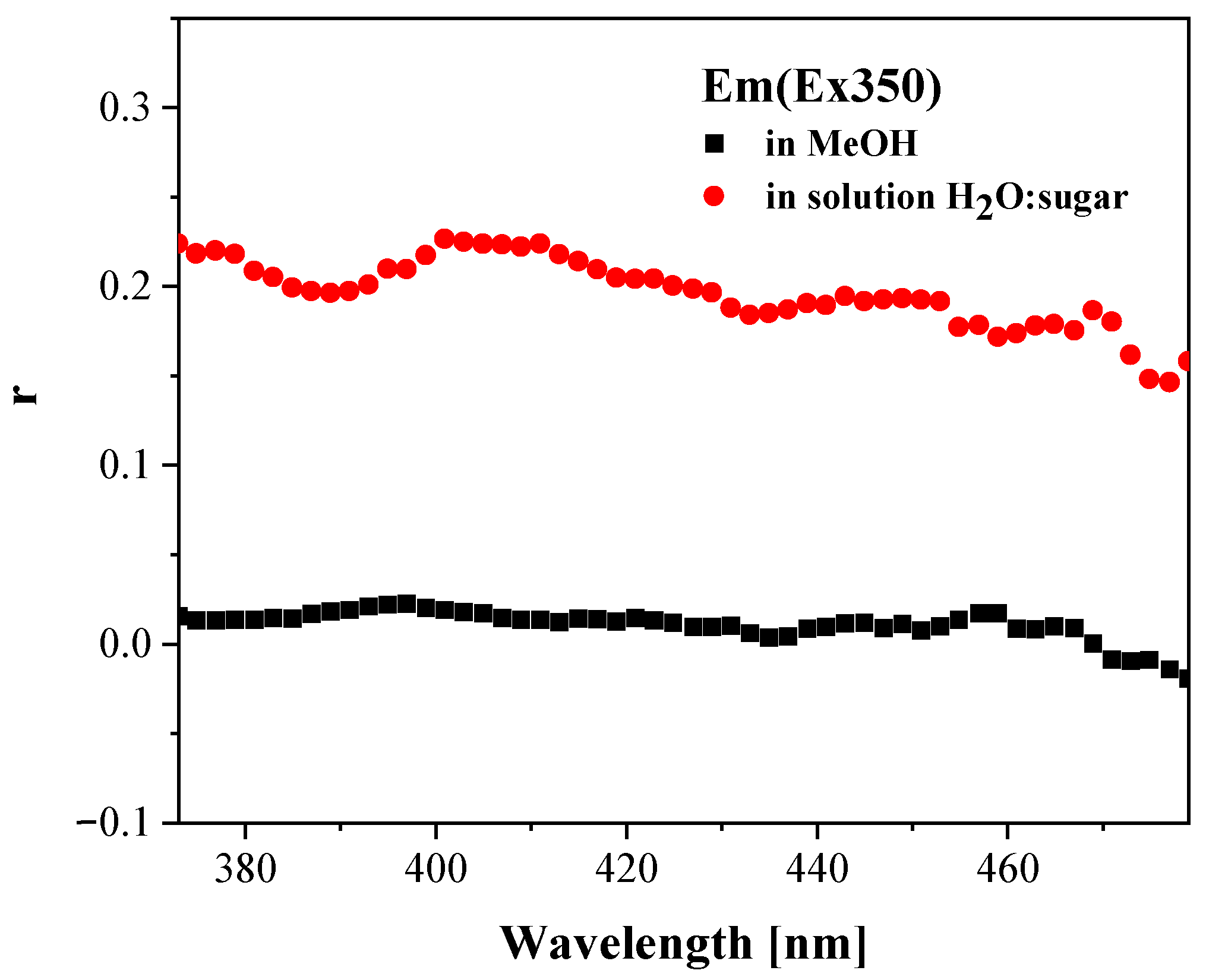
2.2.2. Results of Measurements Made by Means of Absorption Spectroscopy and Stationary Fluorescence with the RLS Technique
3. Materials and Methods
3.1. Determination of the Biological Properties
3.1.1. Experimental Protocols/Sampling
3.1.2. Longevity Test
3.1.3. Hemolymph Collection
3.1.4. Biochemical Analyses
- Superoxide dismutase (SOD) activity was determined using a commercial Sigma-Aldrich (19,160) SOD Determination Kit (Poznań, Poland);
- Catalase (CAT) activity was determined using a Catalase Assay Kit (219265-1KIT) from Sigma-Aldrich (Poznań, Poland);
- Glutathione peroxidase (GPx) activity was determined using a Glutathione Peroxidase Assay Kit (Sigma Aldrich, Schnelldorf, Germany, no. MAK437);
- Glutathione S-Transferase (GST) activity was determined using a Glutathione-S-Transferase (GST) Assay Kit (Sigma Aldrich, Saint Louis, MO, USA, no. CS0410-1KT);
- Total antioxidant capacity (TAC) was determined using a Total Antioxidant Capacity Assay Kit (MAK187-1KT) from Sigma-Aldrich (Poznań, Poland).
3.1.5. Statistical Analysis
3.1.6. Absorption Spectroscopy (UV-Vis), Electronic Fluorescence Spectroscopy and Resonance Light Scattering (RLS)
3.1.7. Anisotropy Measurements
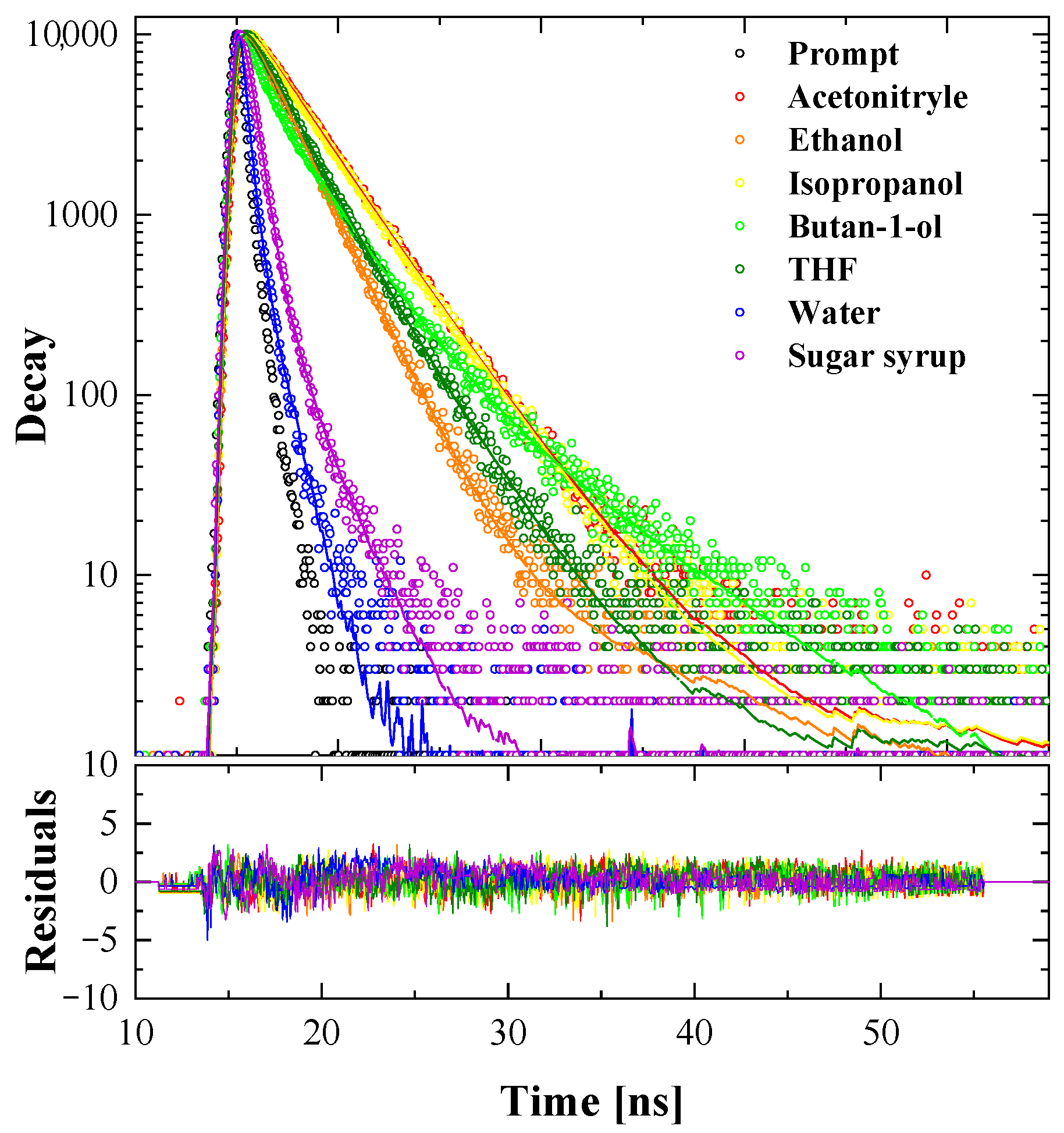
3.1.8. Fluorescence Lifetime Measurements—Time-Correlated Single Photon Counting (TCSPC)
3.1.9. Single-Crystal X-Ray Diffraction Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brutscher, L.M.; McMenamin, A.J.; Flenniken, M.L. The Buzz about Honey Bee Viruses. PLoS Pathog. 2016, 12, e1005757. [Google Scholar] [CrossRef]
- Brosi, B.J.; Delaplane, K.S.; Boots, M.; de Roode, J.C. Ecological and Evolutionary Approaches to Managing Honeybee Disease. Nat. Ecol. Evol. 2017, 1, 1250–1262. [Google Scholar] [CrossRef]
- Geldmann, J.; González-Varo, J.P. Conserving Honey Bees Does Not Help Wildlife. Science 2018, 359, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- De Paula, J.C.; Doello, K.; Mesas, C.; Kapravelou, G.; Cornet-Gómez, A.; Orantes, F.J.; Martínez, R.; Linares, F.; Prados, J.C.; Porres, J.M.; et al. Exploring Honeybee Abdominal Anatomy through Micro-CT and Novel Multi-Staining Approaches. Insects 2022, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkærne, S.; et al. Agricultural Policies Exacerbate Honeybee Pollination Service Supply-Demand Mismatches Across Europe. PLoS ONE 2014, 9, e82996. [Google Scholar] [CrossRef]
- Ullah, A.; Tlak Gajger, I.; Majoros, A.; Dar, S.A.; Khan, S.; Kalimullah; Haleem Shah, A.; Nasir Khabir, M.; Hussain, R.; Khan, H.U.; et al. Viral Impacts on Honey Bee Populations: A Review. Saudi J. Biol. Sci. 2021, 28, 523–530. [Google Scholar] [CrossRef]
- Meixner, M.D.; Francis, R.M.; Gajda, A.; Kryger, P.; Andonov, S.; Uzunov, A.; Topolska, G.; Costa, C.; Amiri, E.; Berg, S.; et al. Occurrence of Parasites and Pathogens in Honey Bee Colonies Used in a European Genotype-Environment Interactions Experiment. J. Apic. Res. 2014, 53, 215–229. [Google Scholar] [CrossRef]
- Alburaki, M.; Chen, D.; Skinner, J.A.; Meikle, W.G.; Tarpy, D.R.; Adamczyk, J.; Stewart, S.D. Honey Bee Survival and Pathogen Prevalence: From the Perspective of Landscape and Exposure to Pesticides. Insects 2018, 9, 65. [Google Scholar] [CrossRef]
- Piot, N.; Schweiger, O.; Meeus, I.; Yañez, O.; Straub, L.; Villamar-Bouza, L.; De la Rúa, P.; Jara, L.; Ruiz, C.; Malmstrøm, M.; et al. Honey Bees and Climate Explain Viral Prevalence in Wild Bee Communities on a Continental Scale. Sci. Rep. 2022, 12, 1904. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Flenniken, M.L. Recently Identified Bee Viruses and Their Impact on Bee Pollinators. Curr. Opin. Insect Sci. 2018, 26, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid Markedly Affects Hemolymph Proteolysis, Biomarkers, DNA Global Methylation, and the Cuticle Proteolytic Layer in Western Honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Gancarz, M.; Wiącek, D.; Nawrocka, A.; Strachecka, A. Imidacloprid Pesticide Causes Unexpectedly Severe Bioelement Deficiencies and Imbalance in Honey Bees Even at Sublethal Doses. Animals 2023, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological Stoichiometry of the Honeybee: Pollen Diversity and Adequate Species Composition Are Needed to Mitigate Limitations Imposed on the Growth and Development of Bees by Pollen Quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]
- Antoń, S.; Denisow, B. Pollination Biology and Breeding System in Five Nocturnal Species of Oenothera (Onagraceae): Reproductive Assurance and Opportunities for Outcrossing. Plant Syst. Evol. 2018, 304, 1231–1243. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M.; Ziółkowska, E. The Need for Weeds: Man-Made, Non-Cropped Habitats Complement Crops and Natural Habitats in Providing Honey Bees and Bumble Bees with Pollen Resources. Sci. Total Environ. 2022, 840, 156551. [Google Scholar] [CrossRef]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why Bees Are So Vulnerable to Environmental Stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef]
- Barroso-Arévalo, S.; Vicente-Rubiano, M.; Puerta, F.; Molero, F.; Sánchez-Vizcaíno, J.M. Immune Related Genes as Markers for Monitoring Health Status of Honey Bee Colonies. BMC Vet. Res. 2019, 15, 72. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Ellis, A.M.; Myers, S.S.; Ricketts, T.H. Do Pollinators Contribute to Nutritional Health? PLoS ONE 2015, 10, e114805. [Google Scholar] [CrossRef]
- Smith, M.R.; Singh, G.M.; Mozaffarian, D.; Myers, S.S. Effects of Decreases of Animal Pollinators on Human Nutrition and Global Health: A Modelling Analysis. Lancet Lond. Engl. 2015, 386, 1964–1972. [Google Scholar] [CrossRef]
- Dong, Z.-X.; Tang, Q.-H.; Li, W.-L.; Wang, Z.-W.; Li, X.-J.; Fu, C.-M.; Li, D.; Qian, K.; Tian, W.-L.; Guo, J. Honeybee (Apis Mellifera) Resistance to Deltamethrin Exposure by Modulating the Gut Microbiota and Improving Immunity. Environ. Pollut. 2022, 314, 120340. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a New Natural Supplement with Beneficial Effects on the Life-Span and Defence System of Honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. CBD Supplementation Has a Positive Effect on the Activity of the Proteolytic System and Biochemical Markers of Honey Bees (Apis mellifera) in the Apiary. Animals 2022, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants 2022, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Buczek, K.; Deryło, K.; Kutyła, M.; Rybicka-Jasińska, K.; Gryko, D.; Borsuk, G.; Rodzik, B.; Trytek, M. Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema Ceranae Spores to Infect Honeybees. Insects 2020, 11, 504. [Google Scholar] [CrossRef]
- Trytek, M.; Buczek, K.; Zdybicka-Barabas, A.; Wojda, I.; Borsuk, G.; Cytryńska, M.; Lipke, A.; Gryko, D. Effect of Amide Protoporphyrin Derivatives on Immune Response in Apis Mellifera. Sci. Rep. 2022, 12, 14406. [Google Scholar] [CrossRef]
- Farjan, M.; Łopieńska-Biernat, E.; Lipiński, Z.; Dmitryjuk, M.; Żółtowska, K. Supplementing with Vitamin C the Diet of Honeybees (Apis mellifera carnica) Parasitized with Varroa Destructor: Effects on Antioxidative Status. Parasitology 2014, 141, 770–776. [Google Scholar] [CrossRef]
- Pachla, A.; Ptaszyńska, A.A.; Wicha, M.; Kunat, M.; Wydrych, J.; Oleńska, E.; Małek, W. Insight into Probiotic Properties of Lactic Acid Bacterial Endosymbionts of Apis Mellifera L. Derived from the Polish Apiary. Saudi J. Biol. Sci. 2021, 28, 1890–1899. [Google Scholar] [CrossRef]
- Rascón, B.; Hubbard, B.P.; Sinclair, D.A.; Amdam, G.V. The Lifespan Extension Effects of Resveratrol Are Conserved in the Honey Bee and May Be Driven by a Mechanism Related to Caloric Restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Simone-Finstrom, M. Nutritional and Prebiotic Efficacy of the Microalga Arthrospira platensis (Spirulina) in Honey Bees. Apidologie 2020, 51, 898–910. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The Terpenes of Leaves, Pollen, and Nectar of Thyme (Thymus vulgaris) Inhibit Growth of Bee Disease-Associated Microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal Activity of the Essential Oil Obtained from Cryptocarya Alba against Infection in Honey Bees by Nosema Ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; Arathi, A. Dietary Phytochemicals, Honey Bee Longevity and Pathogen Tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising Antimicrobial Activity against the Honey Bee Parasite Nosema Ceranae by Methanolic Extracts from Chilean Native Plants and Propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Pașca, C.; Matei, I.A.; Diaconeasa, Z.; Rotaru, A.; Erler, S.; Dezmirean, D.S. Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens. Antibiotics 2021, 10, 960. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-H.; Zhang, R.-K.; Qiao, B.; Li, B.-Z.; Yuan, Y.-J. Engineering Budding Yeast for the Production of Coumarins from Lignin. Biochem. Eng. J. 2020, 160, 107634. [Google Scholar] [CrossRef]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Miłoś, A.; Ślusarczyk, L.; et al. Novel Coumarin-Thiadiazole Hybrids and Their Cu(II) and Zn(II) Complexes as Potential Antimicrobial Agents and Acetylcholinesterase Inhibitors. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Miłoś, A.; Jenkins, H.; Ślusarczyk, L.; Matwijczuk, A. Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin–Thiadiazole Hybrids. Int. J. Mol. Sci. 2022, 23, 6314. [Google Scholar] [CrossRef]
- Chamlagai, D.; Bora, P.; Bhatta, A.; Upadhyaya, J.; Phanrang, P.T.; Bora, U.; Mitra, S. Donor-Acceptor Functionalized Coumarin Derivatives: Synthesis, Fluorescence Modulation, Interaction with Human Serum Albumin and Acetylcholinesterase Inhibition Activity. J. Photochem. Photobiol. Chem. 2024, 447, 115273. [Google Scholar] [CrossRef]
- Raunio, H.; Pentikäinen, O.; Juvonen, R.O. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 4708. [Google Scholar] [CrossRef]
- Sarih, N.M.; Ciupa, A.; Moss, S.; Myers, P.; Slater, A.G.; Abdullah, Z.; Tajuddin, H.A.; Maher, S. Furo [3,2-c]Coumarin-Derived Fe3+ Selective Fluorescence Sensor: Synthesis, Fluorescence Study and Application to Water Analysis. Sci. Rep. 2020, 10, 7421. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Alexander, E.; Pham, D.; Gagoś, M.; Matwijczuk, A.; Gryczynski, Z.; Gryczynski, I. Spectral Properties of 4-Methylumbelliferone in PVA Films; Long-Lived Room Temperature Phosphorescence. Methods Appl. Fluoresc. 2024, 13, 015003. [Google Scholar] [CrossRef] [PubMed]
- Paleolog, J.; Wilde, J.; Miszczak, A.; Gancarz, M.; Strachecka, A. Antioxidation Defenses of Apis Mellifera Queens and Workers Respond to Imidacloprid in Different Age-Dependent Ways: Old Queens Are Resistant, Foragers Are Not. Animals 2021, 11, 1246. [Google Scholar] [CrossRef]
- Strachecka, A.; Migdał, P.; Kuszewska, K.; Skowronek, P.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Humoral and Cellular Defense Mechanisms in Rebel Workers of Apis Mellifera. Biology 2021, 10, 1146. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in Insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Farjan, M.; Dmitryjuk, M.; Lipiński, Z.; Biernat-Łopieńska, E.; Żółtowska, K. Supplementation of the Honey Bee Diet with Vitamin C: The Effect on the Antioxidative System of Apis mellifera Carnica Brood at Different Stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef]
- Li, Z.; Duan, J.; Chen, L.; Wang, Y.; Qin, Q.; Dang, X.; Zhou, Z. Melatonin Enhances the Antioxidant Capacity to Rescue the Honey Bee Apis Mellifera from the Ecotoxicological Effects Caused by Environmental Imidacloprid. Ecotoxicol. Environ. Saf. 2022, 239, 113622. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.-L.; Le Conte, Y. Pathological Effects of the Microsporidium Nosema Ceranae on Honey Bee Queen Physiology (Apis mellifera). J. Invertebr. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef]
- Nicewicz, Ł.; Nicewicz, A.W.; Kafel, A.; Nakonieczny, M. Set of Stress Biomarkers as a Practical Tool in the Assessment of Multistress Effect Using Honeybees from Urban and Rural Areas as a Model Organism: A Pilot Study. Environ. Sci. Pollut. Res. 2021, 28, 9084–9096. [Google Scholar] [CrossRef] [PubMed]
- Dziechciarz, P.; Strachecka, A.; Borsuk, G.; Olszewski, K. Effect of Rearing in Small-Cell Combs on Activities of Catalase and Superoxide Dismutase and Total Antioxidant Capacity in the Hemolymph of Apis Mellifera Workers. Antioxidants 2023, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total Antioxidant Capacity as a Tool to Assess Redox Status: Critical View and Experimental Data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules 2013, 18, 1394–1404. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; IntechOpen: London, UK, 2015; ISBN 978-953-51-2170-1. [Google Scholar]
- Xu, Z.; Han, S.J.; Lee, C.; Yoon, J.; Spring, D.R. Development of off–on Fluorescent Probes for Heavy and Transition Metal Ions. Chem. Commun. 2010, 46, 1679–1681. [Google Scholar] [CrossRef]
- Mathivanan, M.; Tharmalingam, B.; Devaraj, T.; Murugan, A.; Lin, C.-H.; Jothi, M.; Murugesapandian, B. A New 7-Diethylamino-4-Hydroxycoumarin Based Reversible Colorimetric/Fluorometric Probe for Sequential Detection of Al3+/PPi and Its Potential Use in Biodetection and Bioimaging Applications. New J. Chem. 2021, 45, 6067–6079. [Google Scholar] [CrossRef]
- Calovi, M.; Grozinger, C.M.; Miller, D.A.; Goslee, S.C. Summer Weather Conditions Influence Winter Survival of Honey Bees (Apis Mellifera) in the Northeastern United States. Sci. Rep. 2021, 11, 1553. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Y.; Han, M.; Miao, X. Longevity Extension of Worker Honey Bees (Apis mellifera) by Royal Jelly: Optimal Dose and Active Ingredient. PeerJ 2017, 5, e3118. [Google Scholar] [CrossRef]
- Okińczyc, P.; Paluch, E.; Franiczek, R.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżanowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial Activity of Apis mellifera L. and Trigona Sp. Propolis from Nepal and Its Phytochemical Analysis. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 129, 110435. [Google Scholar] [CrossRef]
- Johnson, R.M.; Mao, W.; Pollock, H.S.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Ecologically Appropriate Xenobiotics Induce Cytochrome P450s in Apis Mellifera. PLoS ONE 2012, 7, e31051. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. A Dietary Phytochemical Alters Caste-Associated Gene Expression in Honey Bees. Sci. Adv. 2015, 1, e1500795. [Google Scholar] [CrossRef]
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef]
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Bicak, B.; Gok, B.; Belmen, B.; Aydogan, F.; Yolacan, C. New Coumarin Derivative with Potential Antioxidant Activity: Synthesis, DNA Binding and in Silico Studies (Docking, MD, ADMET). Arab. J. Chem. 2023, 16, 104440. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The Antimicrobial Agents. Syst. Rev. Pharm. 2017, 8, 62–70. [Google Scholar] [CrossRef]
- Bubols, G.B.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The Antioxidant Activity of Coumarins and Flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Brandner, L.; Müller, T.J.J. Multicomponent Synthesis of Chromophores—The One-Pot Approach to Functional π-Systems. Front. Chem. 2023, 11, 1124209. [Google Scholar] [CrossRef]
- Recent Trends in Molecular Aggregates: An Exploration of Biomedicine—Heo—2022—Aggregate—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/agt2.159 (accessed on 27 March 2025).
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55–70. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Chen, L.; Qiu, Y.; Qian, L.; Zhang, K.; Yang, C. Crystal Structure and Photoluminescence of 7-(N,N′-Diethylamino)-Coumarin-3-Carboxylic Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 1136–1139. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Collings, P.J. Resonance Light Scattering: A New Technique for Studying Chromophore Aggregation. Science 1995, 269, 935–939. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Barberis, M.; Calabrese, D.; Galloni, M.; Nepi, M. Secondary metabolites in nectar-mediated plant-pollinator relationships. Plants 2023, 12, 550. [Google Scholar] [CrossRef]
- Dekebo, A.; Kim, M.J.; Son, M.; Jung, C. Comparative analysis of volatile organic compounds from flowers attractive to honey bees and bumblebees. J. Ecol. Environ. 2022, 46, 62–75. [Google Scholar] [CrossRef]
- Available online: https://patents.google.com/patent/CN111297851B/en (accessed on 20 May 2025).
- Łoś, A.; Strachecka, A. Fast and Cost-Effective Biochemical Spectrophotometric Analysis of Solution of Insect “Blood” and Body Surface Elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Schacterle, G.R.; Pollack, R.L. A Simplified Method for the Quantitative Assay of Small Amounts of Protein in Biologic Material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
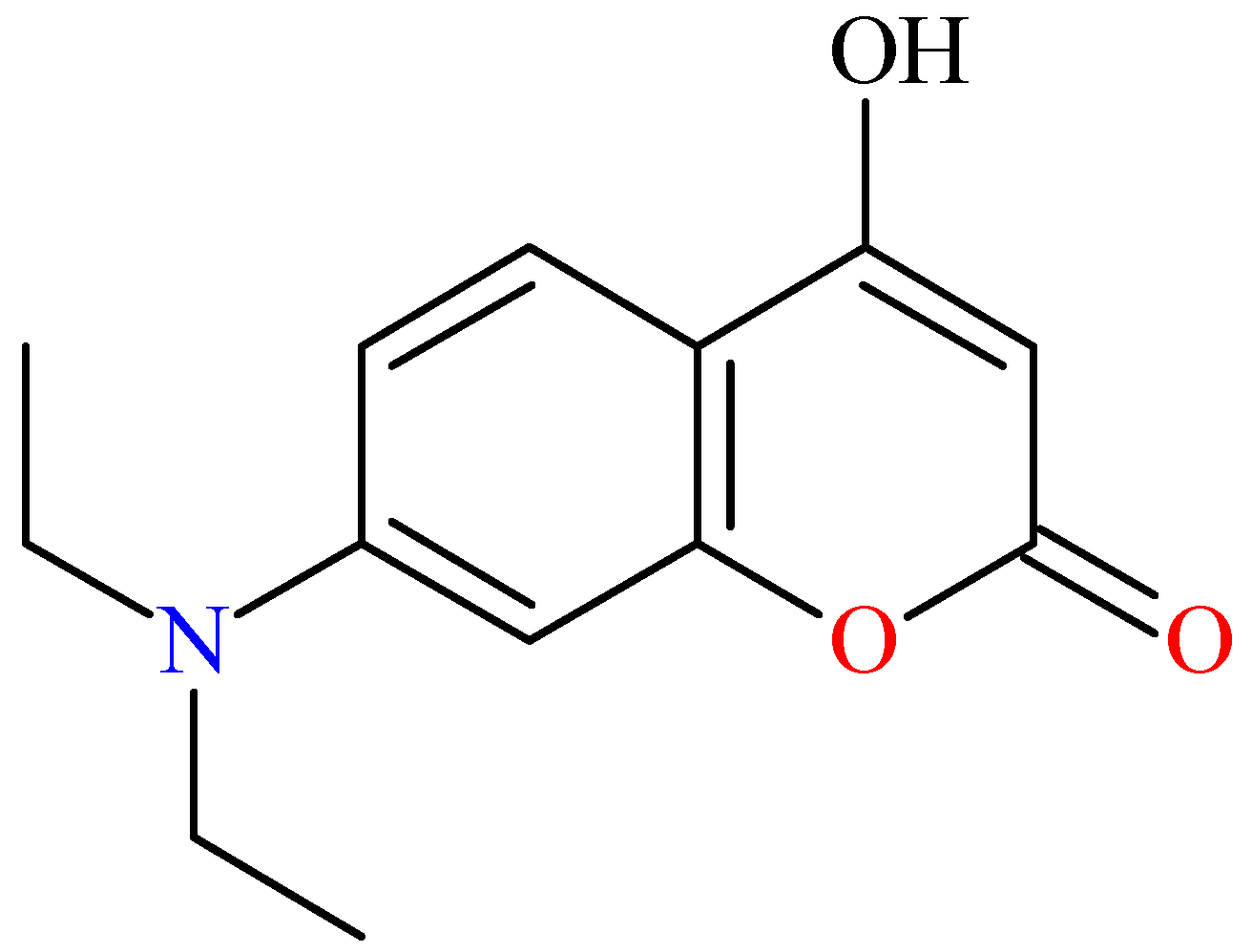

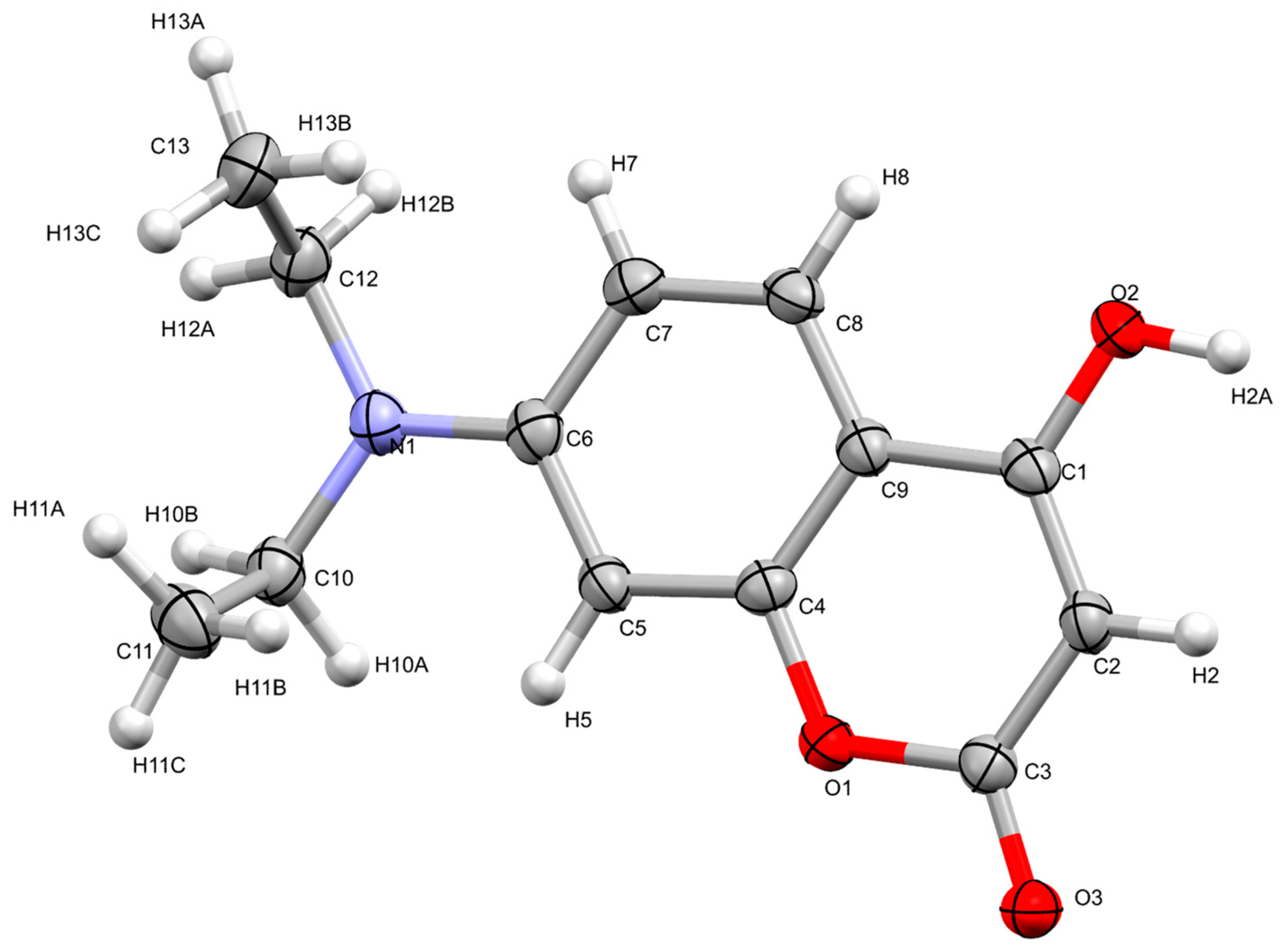
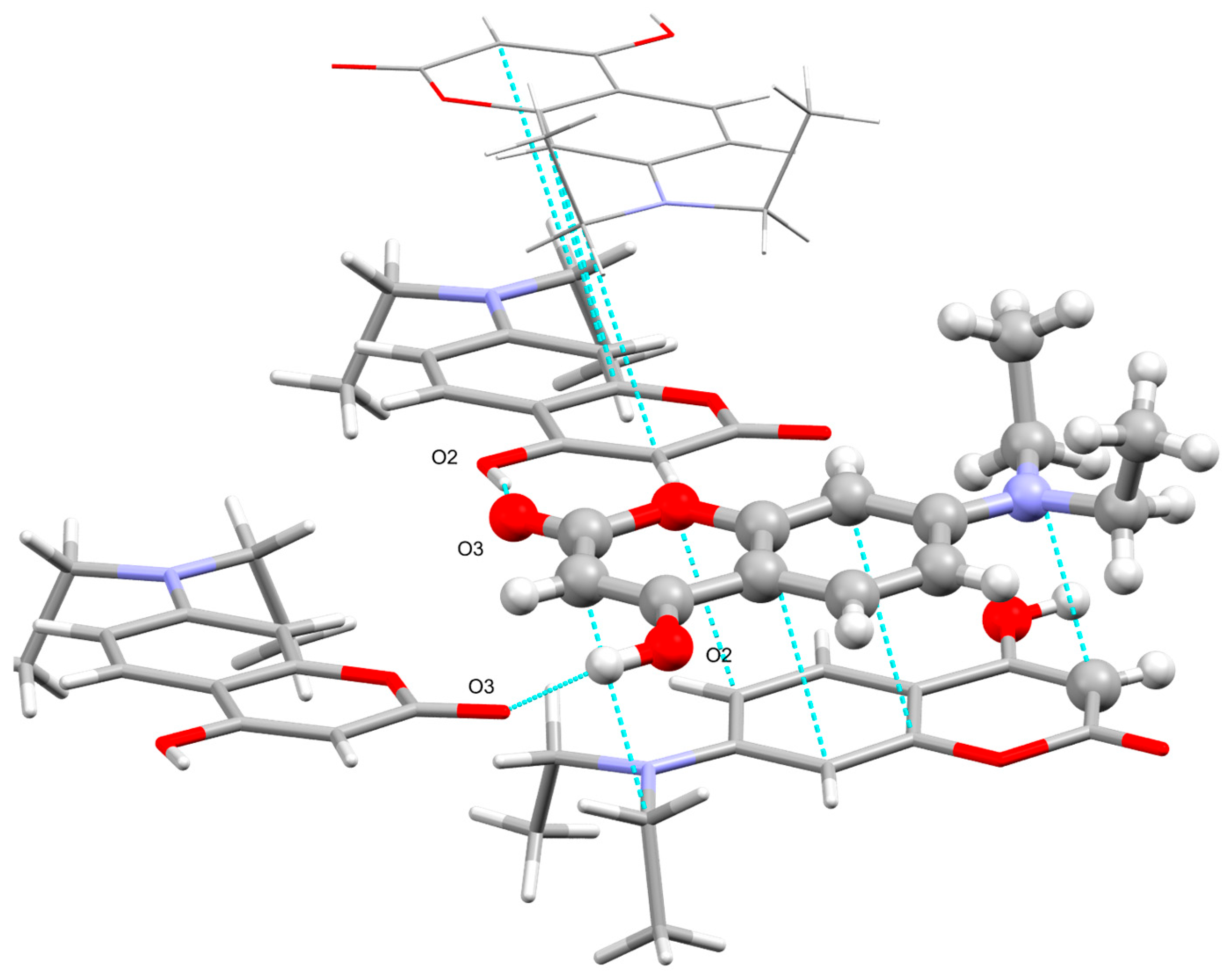
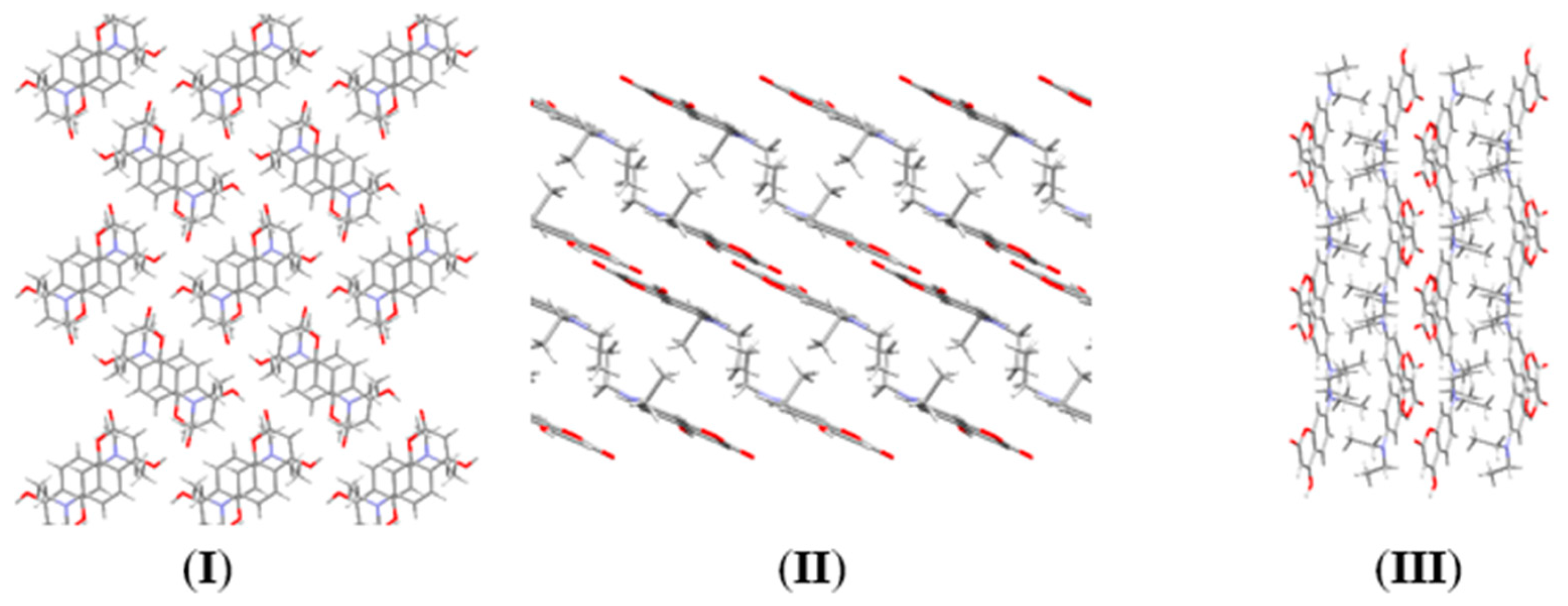
| 7-Diethylamino-4-hydroxycoumarin | |
|---|---|
| Chemical formula | C13H15NO3 |
| Mr | 233.26 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 8.5069 (4), 10.2185 (4), 13.5117 (5) |
| β (°) | 99.017 (4) |
| V (Å3) | 1160.03 (8) |
| Z,Z’ | 4,1 |
| Crystal size (mm) | 0.12 × 0.03 × 0.02 |
| Rint | 0.049 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.052, 0.127, 1.13 |
| Δρmax, Δρmin (e Å−3) | 0.21, −0.17 |
| CCDC Number | 2,434,788 |
| Nr | Solvent | Absorbance [nm] | Fluorescence Em (Ex335) [nm] | Fluorescence Em (Ex355) [nm] | Fluorescence Em (Ex395) [nm] |
|---|---|---|---|---|---|
| 1 | MeOH | 338 | 384 | - | 450 |
| 2 | EtOH | 337 | 379 | 408 | 448 |
| 3 | Isopropanol | 337 | 402 | 402 | 447 |
| 4 | Butan-1-ol | 355 | 405 | 405 | 450 |
| 5 | Acetonitrile | 354 | 403 | 406 | 452 |
| 6 | DMF | 335 | 400 | 406 | 450 |
| 7 | Acetone | 352 | 396 | 396 | 450 |
| 8 | THF | 353 | 388 | 388 | 440 |
| 9 | Chloroform | 355 | 400 | 400 | 430 |
| 10 | Water | 346 | 402 | - | - |
| 11 | Sugar syrup | 342 | 400 | - | 460 |
| Solvent | <τ> | τ1 | τ2 | τ3 | f1 | f2 | f3 |
|---|---|---|---|---|---|---|---|
| Acetonitrile | 2.61 ± 0.04 | 2.31 ± 0.05 | 3.24 ± 0.05 | 0.675 ± 0.001 | 0.325 ± 0.003 | ||
| Ethanol | 1.82 ± 0.02 | 1.76 ± 0.02 | 8.1 ± 0.6 | 0.991 ± 0.001 | 0.091 ± 0.001 | ||
| Propanol | 2.58 ± 0.07 | 1.8 ± 0.3 | 2.84 ± 0.01 | 0.242 ± 0.003 | 0.758 ± 0.003 | ||
| Butanol | 2.26 ± 0.02 | 0.45 ± 0.02 | 2.28 ± 0.02 | 5.51 ± 0.08 | 0.221 ± 0.001 | 0.660 ± 0.002 | 0.120 ± 0.002 |
| Tetrahydrofuran | 2.03 ± 0.02 | 1.71 ± 0.02 | 2.78 ± 0.03 | 0.698 ± 0.001 | 0.302 ± 0.002 | ||
| Water | 0.147 ± 0.004 | 0.089 ± 0.004 | 0.97 ± 0.02 | 0.934 ± 0.001 | 0.066 ± 0.001 | ||
| Sucrose syrup | 0.512 ± 0.005 | 0.395 ± 0.004 | 1.56 ± 0.03 | 0.899 ± 0.001 | 0.101 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rząd, K.; Budziak-Wieczorek, I.; Strachecka, A.; Staniszewska, P.; Staniszewski, A.; Gryboś, A.; Matwijczuk, A.; Gładyszewska, B.; Starzak, K.; Hoser, A.A.; et al. Advanced Research on Biological Properties—A Study on the Activity of the Apis mellifera Antioxidant System and the Crystallographic and Spectroscopic Properties of 7-Diethylamino-4-hydroxycoumarin. Int. J. Mol. Sci. 2025, 26, 7015. https://doi.org/10.3390/ijms26147015
Rząd K, Budziak-Wieczorek I, Strachecka A, Staniszewska P, Staniszewski A, Gryboś A, Matwijczuk A, Gładyszewska B, Starzak K, Hoser AA, et al. Advanced Research on Biological Properties—A Study on the Activity of the Apis mellifera Antioxidant System and the Crystallographic and Spectroscopic Properties of 7-Diethylamino-4-hydroxycoumarin. International Journal of Molecular Sciences. 2025; 26(14):7015. https://doi.org/10.3390/ijms26147015
Chicago/Turabian StyleRząd, Klaudia, Iwona Budziak-Wieczorek, Aneta Strachecka, Patrycja Staniszewska, Adam Staniszewski, Anna Gryboś, Alicja Matwijczuk, Bożena Gładyszewska, Karolina Starzak, Anna A. Hoser, and et al. 2025. "Advanced Research on Biological Properties—A Study on the Activity of the Apis mellifera Antioxidant System and the Crystallographic and Spectroscopic Properties of 7-Diethylamino-4-hydroxycoumarin" International Journal of Molecular Sciences 26, no. 14: 7015. https://doi.org/10.3390/ijms26147015
APA StyleRząd, K., Budziak-Wieczorek, I., Strachecka, A., Staniszewska, P., Staniszewski, A., Gryboś, A., Matwijczuk, A., Gładyszewska, B., Starzak, K., Hoser, A. A., Nowak, M. E., Figiel, M., Okoń, S., & Matwijczuk, A. P. (2025). Advanced Research on Biological Properties—A Study on the Activity of the Apis mellifera Antioxidant System and the Crystallographic and Spectroscopic Properties of 7-Diethylamino-4-hydroxycoumarin. International Journal of Molecular Sciences, 26(14), 7015. https://doi.org/10.3390/ijms26147015











