A Diet Rich in Essential Amino Acids Inhibits the Growth of HCT116 Human Colon Cancer Cell In Vitro and In Vivo
Abstract
1. Introduction
2. Results
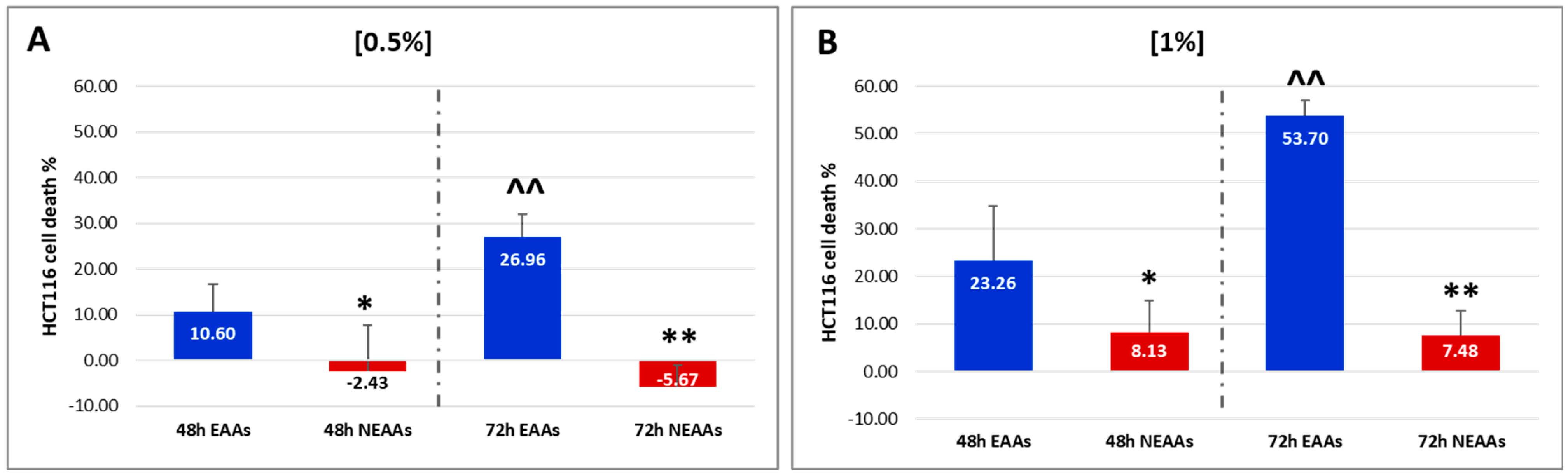
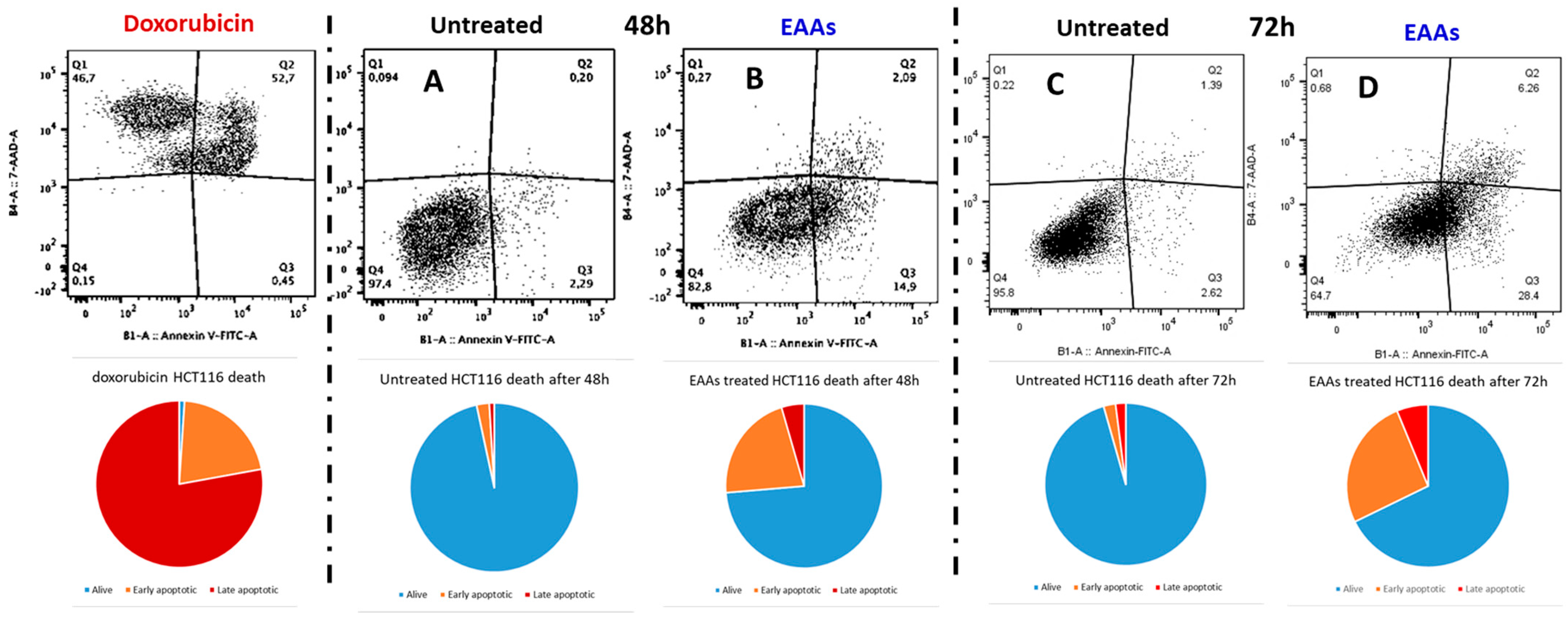
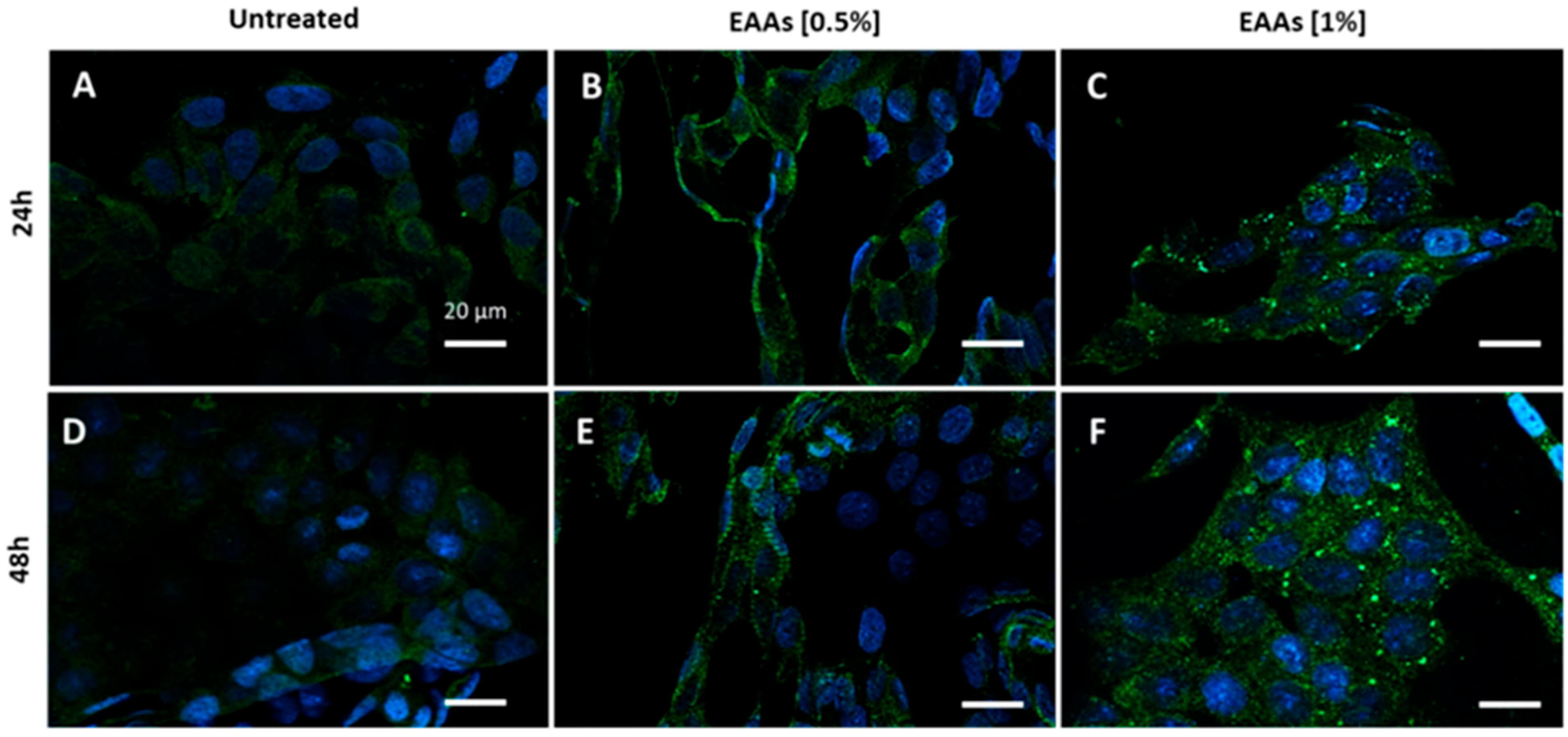
2.1. In Vitro Experiments
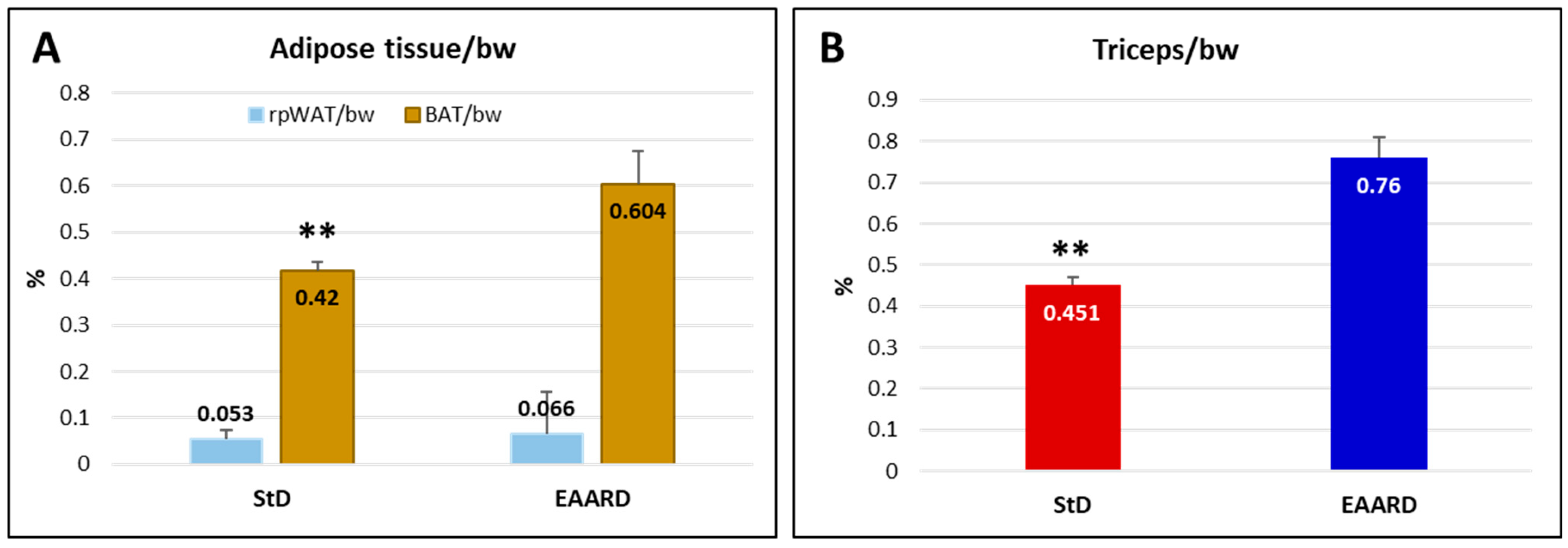
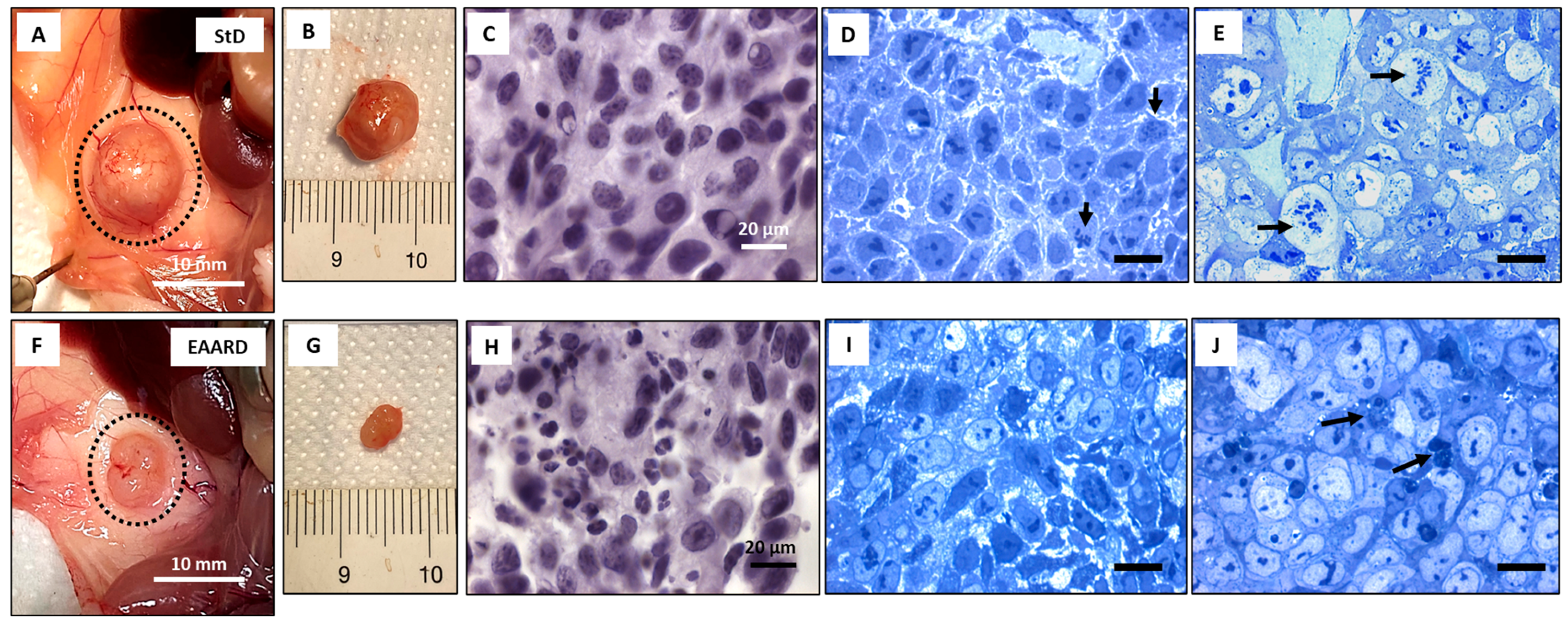
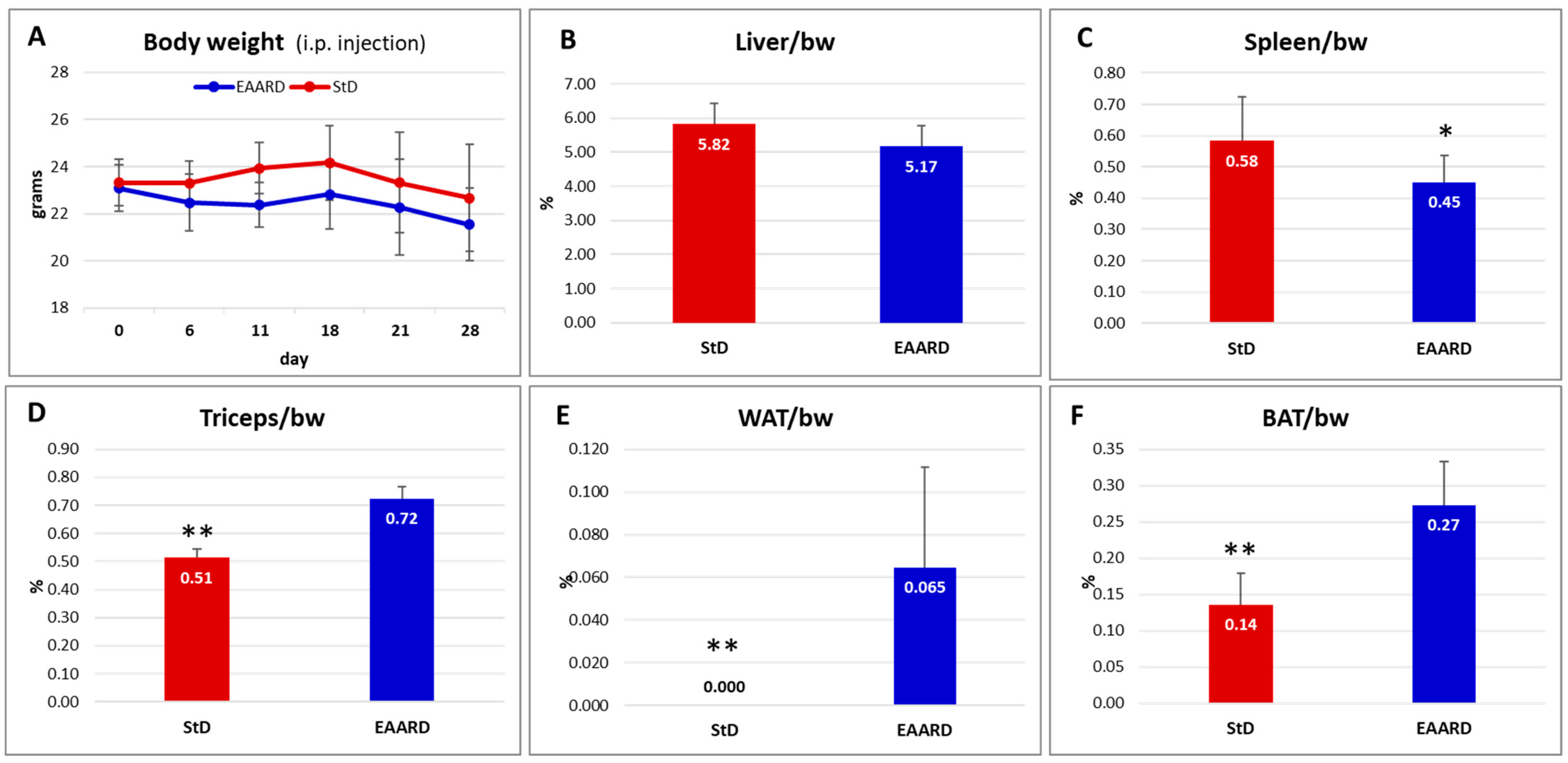
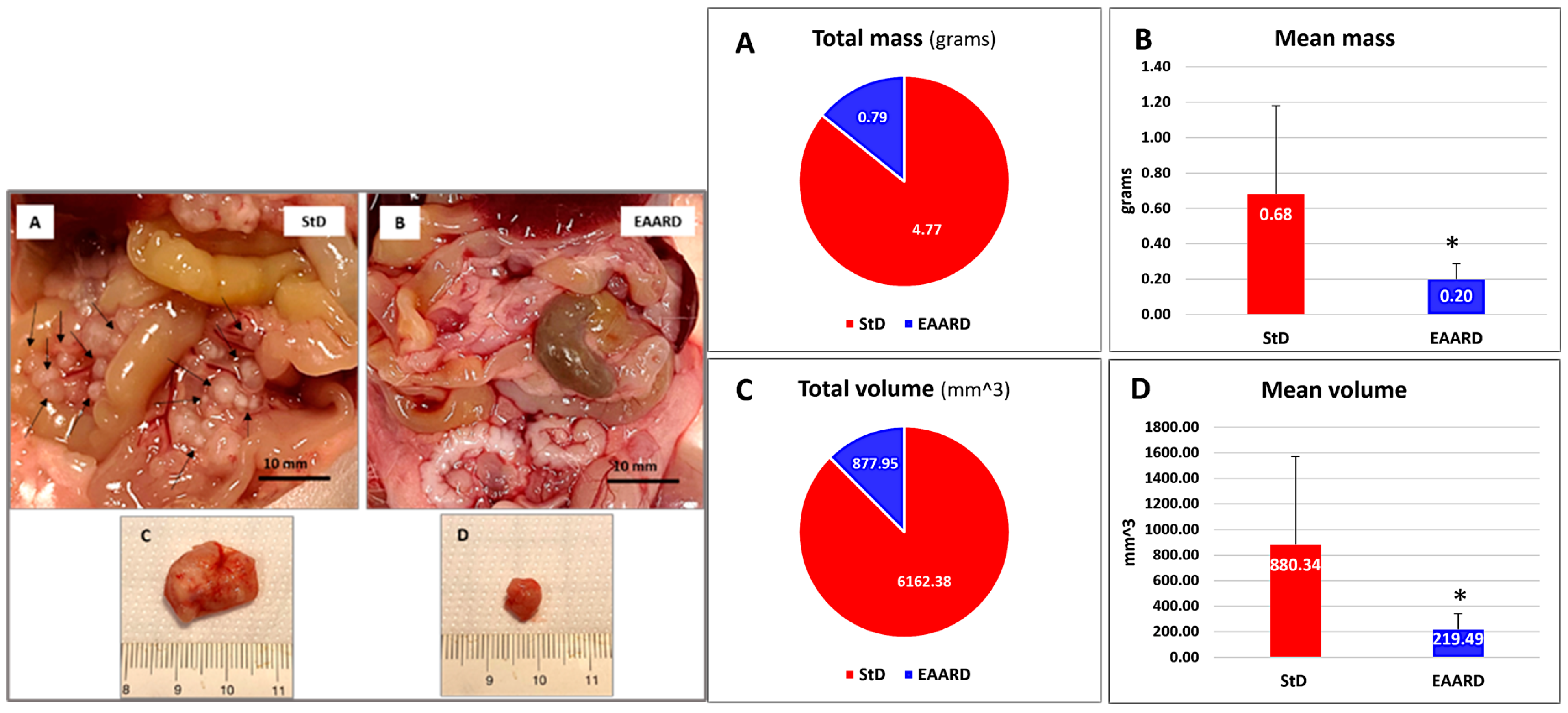
2.2. In Vivo Experiments
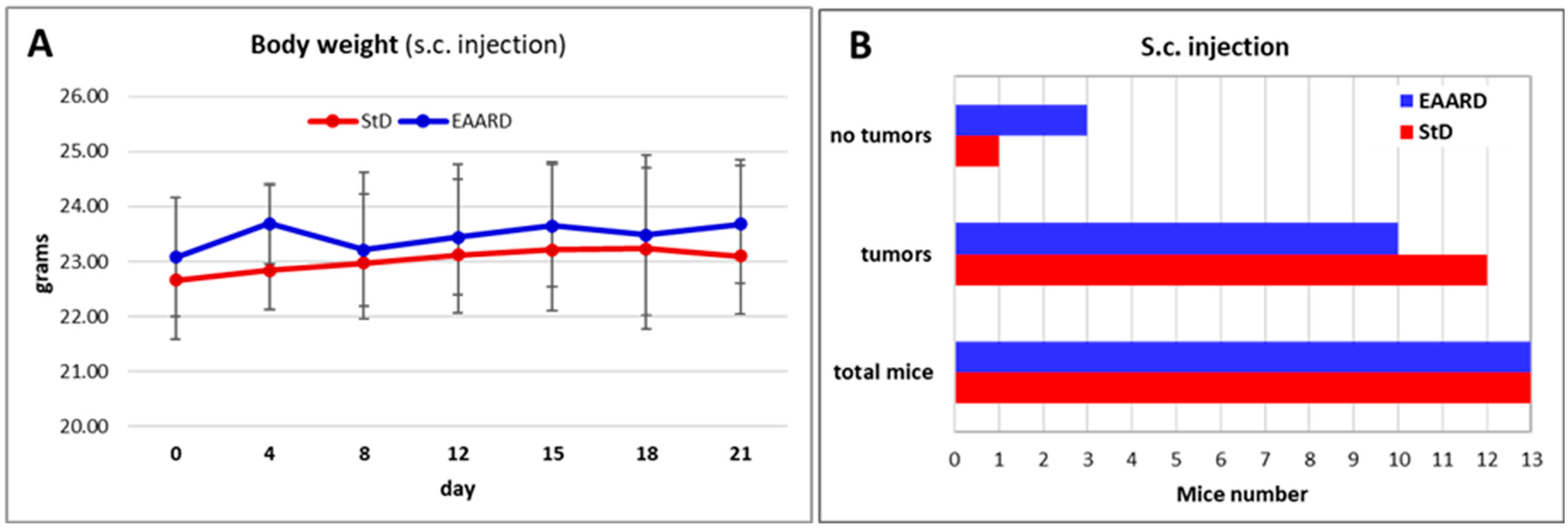
2.2.1. Sub-Cutaneous (s.c.) HCT116 Injection
2.2.2. Intraperitoneal (i.p.) HCT116 Injection
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
4.2. In Vivo Experiments
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUT | Autophagy |
| BAT | Brown adipose tissue |
| EAA | Essential amino acids |
| EAARD | Essential amino acids rich diet |
| ER | Endoplasmic reticulum |
| iNOS | Inducible nitric oxide synthase |
| NEAA | Non-Essential amino acids |
| StD | Standard diet |
| rpWAT | Retro peritoneal white adipose tissue |
| WAT | White adipose tissue |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Conti, L.; Del Cornò, M.; Gessani, S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit. Rev. Oncol. Hematol. 2020, 145, 102834. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Mayers, J.R.; Torrence, M.E.; Danai, L.V.; Papagiannakopoulos, T.; Davidson, S.M.; Bauer, M.R.; Lau, A.N.; Ji, B.W.; Dixit, P.D.; Hosios, A.M.; et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 2016, 353, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human colon cancer cell HCT 116 xenografts in nude mice: Evaluation of tumor growth and immunohistochemistry. Oncol. Rep. 2005, 13, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Vander Heiden, M.G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Deberardinis, R.J. Understanding the intersections between metabolism and cancer biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Dioguardi, F.S.; Flati, V.; Corsetti, G.; Pasini, E.; Romano, C. Is the response of tumors dependent on the dietary input of some amino acids or ratios among essential and non-essential amino acids? All that glitters is not gold. Int. J. Mol. Sci. 2018, 19, 3631. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, staging, and emerging treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; Fornelli, C.; Nisoli, E.; Penna, F. Amino acids in cancer and cachexia: An integrated view. Cancers 2022, 14, 5691. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284, 1726–1737. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Codenotti, S.; Giugno, L.; Pasini, E.; Fanzani, A.; Scarabelli, T.; Dioguardi, F.S. Intake of special amino acids mixture leads to blunted murine colon cancer growth in vitro and in vivo. Cells 2024, 13, 1210. [Google Scholar] [CrossRef]
- Nan, Y.; Su, H.; Zhou, B.; Liu, S. The function of natural compounds in important anticancer mechanisms. Front. Oncol. 2023, 12, 1049888. [Google Scholar] [CrossRef]
- Su, W.; Liao, M.; Tan, H.; Chen, Y.; Zhao, R.; Jin, W.; Zhu, S.; Zhang, Y.; He, L.; Liu, B. Identification of autophagic target RAB13 with small-molecule inhibitor in low-grade glioma via integrated multi-omics approaches coupled with virtual screening of traditional Chinese medicine databases. Cell Prolif. 2021, 54, e13135. [Google Scholar] [CrossRef]
- Fairlie, W.D.; Tran, S.; Lee, E.F. Crosstalk between apoptosis and autophagy signaling pathways. Int. Rev. Cell Mol. Biol. 2020, 352, 115–158. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yu, Y.; He, S.; Cheng, J.; Gong, Y.; Zhang, Z.; Yang, X.; Xu, B.; Liu, X.; Li, C.Y.; et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2017, 385, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.; Ruocco, C.; Tedesco, L.; Carruba, M.O.; Valerio, A.; Nisoli, E. An amino acid-defined diet impairs tumour growth in mice by promoting endoplasmic reticulum stress and mTOR inhibition. Mol. Metab. 2022, 60, 101478. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef]
- Ma, Y.; Hendershot, L.M. The role of the unfolded protein response in tumour development: Friend or foe? Nat. Rev. Cancer 2004, 4, 966–977. [Google Scholar] [CrossRef]
- Li, J.; Lee, B.; Lee, A.S. Endoplasmic reticulum stress-induced apoptosis: Multiple pathways and activation of p53–up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J. Biol. Chem. 2006, 281, 7260–7270. [Google Scholar] [CrossRef]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef]
- Warburg, O. The metabolism of carcinoma cells 1. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Codenotti, S.; Pasini, E.; Fanzani, A.; Dioguardi, F.S. Essential amino acids-rich diet decreased adipose tissue storage in adult mice: A preliminary histopathological study. Nutrients 2022, 14, 2915. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Ballarò, R.; Beltrà, M.; De Lucia, S.; García Castillo, L.; Costelli, P. The skeletal muscle as an active player against cancer cachexia. Front. Physiol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer cachexia: Beyond weight loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef]
- Solerte, S.B.; Gazzaruso, C.; Bonacasa, R.; Rondanelli, M.; Zamboni, M.; Basso, C.; Locatelli, E.; Schifino, N.; Giustina, A.; Fioravanti, M. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am. J. Cardiol. 2008, 101, 69E–77E. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef]
- Kruman, I.I.; Fowler, A.K. Impaired one carbon metabolism and DNA methylation in alcohol toxicity. J. Neurochem. 2014, 129, 770–780. [Google Scholar] [CrossRef]
- Keum, N.N.; Giovannucci, E.L. Folic Acid fortification and colorectal cancer risk. Am. J. Prev. Med. 2014, 46, S65–S72. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Gupta, V.; Gopinath, P.; Mazurek, S.; Bamezai, R.N. Pyruvate kinase M2 and cancer: An updated assessment. FEBS Lett. 2014, 588, 2685–2692. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of diets with varying essential/nonessential amino acid ratios on mouse lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef]


| EAAs Mix | NEAAs Mix | StD | EAARD | |
|---|---|---|---|---|
| KCal/Kg | -- | -- | 3952 | 3995 |
| Carbohydrates (%) | -- | -- | 54.61 | 61.76 |
| Lipids (%) | -- | -- | 7.5 | 6.12 |
| Nitrogen (%) | -- | -- | 21.8 ° | 20 * |
| Proteins: % of total N content | -- | -- | 95.93 | -- |
| Free AA: % of total N content | -- | -- | 4.07 | 100 |
| EAA/NEAA (% in grams) | -- | -- | < or <<0.9 | 6.14 (86/14) |
| Free AA composition | ||||
| L-Leucine (bcaa) | 13.53% | -- | -- | 13.53% |
| L-Isoleucine (bcaa) | 9.65% | -- | -- | 9.65% |
| L-Valine (bcaa) | 9.65% | -- | -- | 9.65% |
| L-Lysine | 11.6% | -- | 0.97% | 11.6% |
| L-Threonine | 8.7% | -- | -- | 8.7% |
| L-Histidine | 11.6% | -- | -- | 11.6% |
| L-Phenylalanine | 7.73% | -- | -- | 7.73% |
| L-Methionine | 4.35% | -- | 0.45% | 4.35% |
| L-Tyrosine | 5.80% | 1.0% | -- | 5.80% |
| L-Tryptophan | 3.38% | -- | 0.28% | 3.38% |
| L-Cystine/Cysteine | 8.20% | -- | 0.39% | 8.20% |
| L-Alanine | -- | 35.0% | -- | -- |
| L-Glycine | -- | 15.0% | 0.88% | -- |
| L- Arginine | -- | 14.0% | 1.1% | -- |
| L-Proline | -- | 12.0% | -- | -- |
| L-Glutamine | -- | 12.0% | -- | -- |
| L-Serine | 2.42% | 6.0% | -- | 2.42% |
| L-Glutamic Acid | -- | 2.0% | -- | -- |
| L-Asparagine | -- | 2.0% | -- | -- |
| L-Aspartic Acid | -- | 1.0% | -- | -- |
| Ornithine-αKG | 2.42% | -- | -- | 2.42% |
| N-acetylcysteine | 0.97% | -- | -- | 0.97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetti, G.; Romano, C.; Codenotti, S.; Pasini, E.; Fanzani, A.; Scarabelli, T.; Dioguardi, F.S. A Diet Rich in Essential Amino Acids Inhibits the Growth of HCT116 Human Colon Cancer Cell In Vitro and In Vivo. Int. J. Mol. Sci. 2025, 26, 7014. https://doi.org/10.3390/ijms26147014
Corsetti G, Romano C, Codenotti S, Pasini E, Fanzani A, Scarabelli T, Dioguardi FS. A Diet Rich in Essential Amino Acids Inhibits the Growth of HCT116 Human Colon Cancer Cell In Vitro and In Vivo. International Journal of Molecular Sciences. 2025; 26(14):7014. https://doi.org/10.3390/ijms26147014
Chicago/Turabian StyleCorsetti, Giovanni, Claudia Romano, Silvia Codenotti, Evasio Pasini, Alessandro Fanzani, Tiziano Scarabelli, and Francesco S. Dioguardi. 2025. "A Diet Rich in Essential Amino Acids Inhibits the Growth of HCT116 Human Colon Cancer Cell In Vitro and In Vivo" International Journal of Molecular Sciences 26, no. 14: 7014. https://doi.org/10.3390/ijms26147014
APA StyleCorsetti, G., Romano, C., Codenotti, S., Pasini, E., Fanzani, A., Scarabelli, T., & Dioguardi, F. S. (2025). A Diet Rich in Essential Amino Acids Inhibits the Growth of HCT116 Human Colon Cancer Cell In Vitro and In Vivo. International Journal of Molecular Sciences, 26(14), 7014. https://doi.org/10.3390/ijms26147014








