Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications
Abstract
1. Introduction
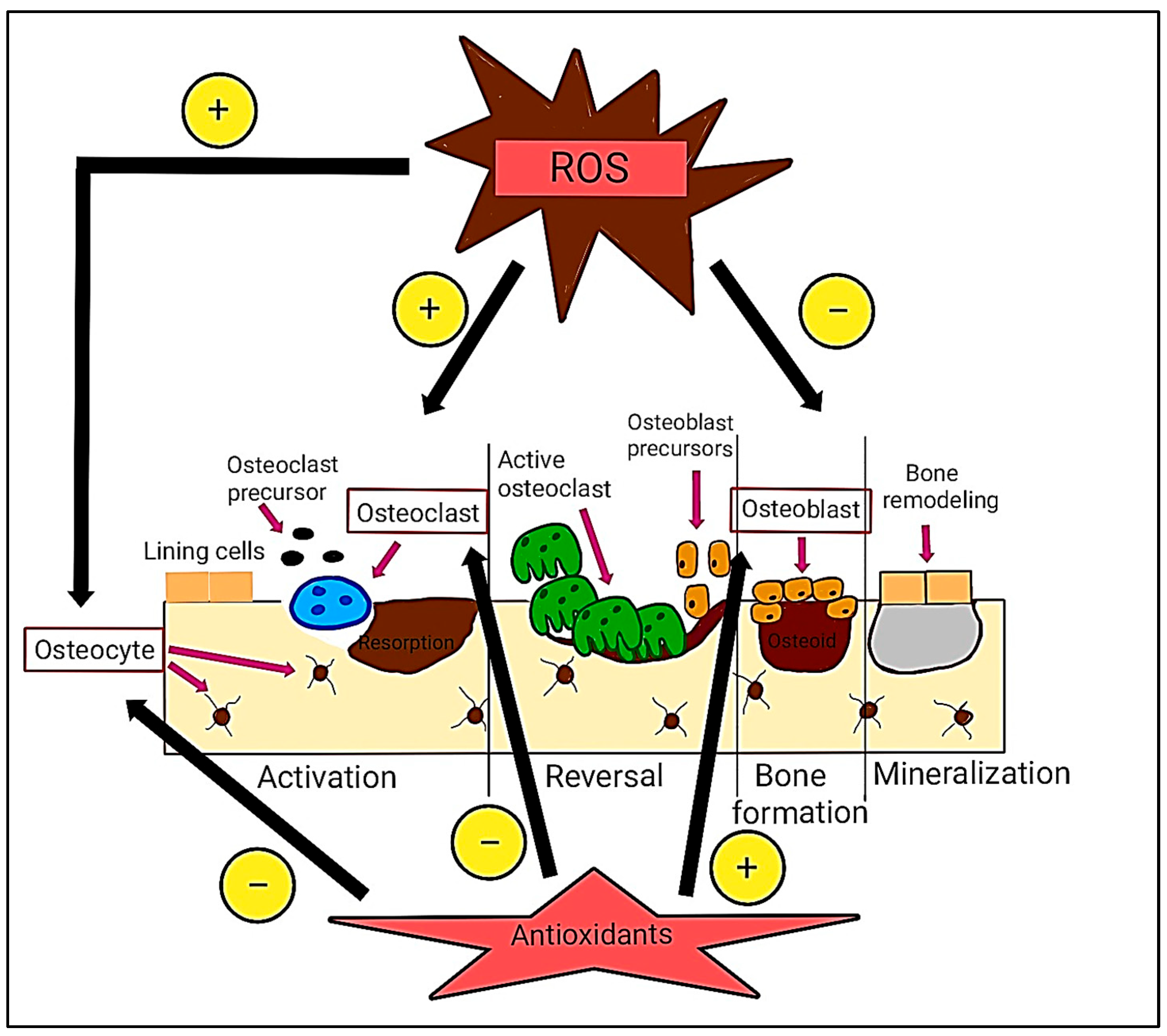
1.1. Oxidative Stress in Bone
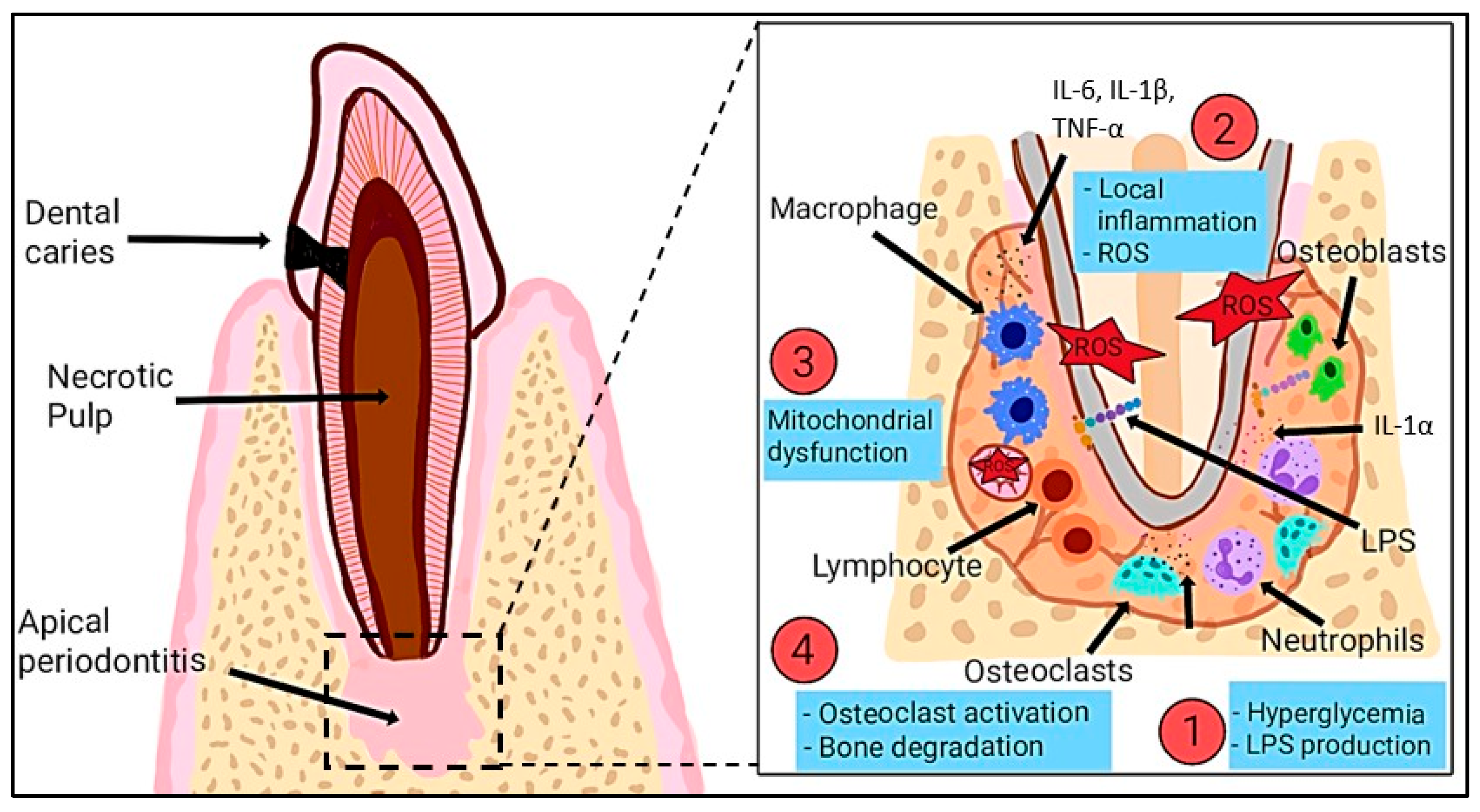
1.2. Oxidative Stress in Dental
2. Synthesis and Extraction of Palm Raw Materials
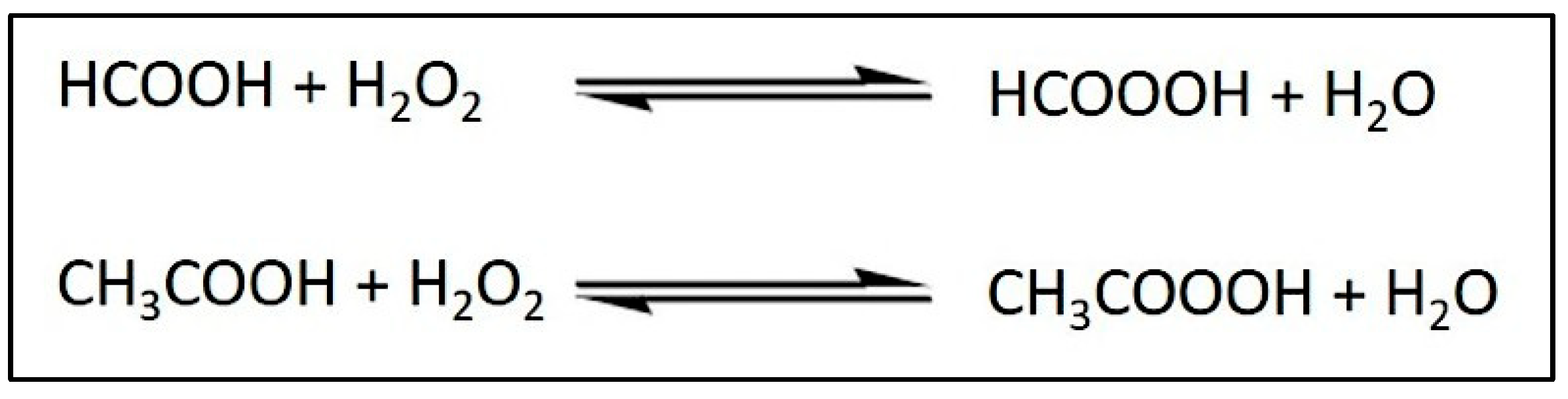
2.1. Epoxidized Palm Olein
2.2. Oil Palm Leaves
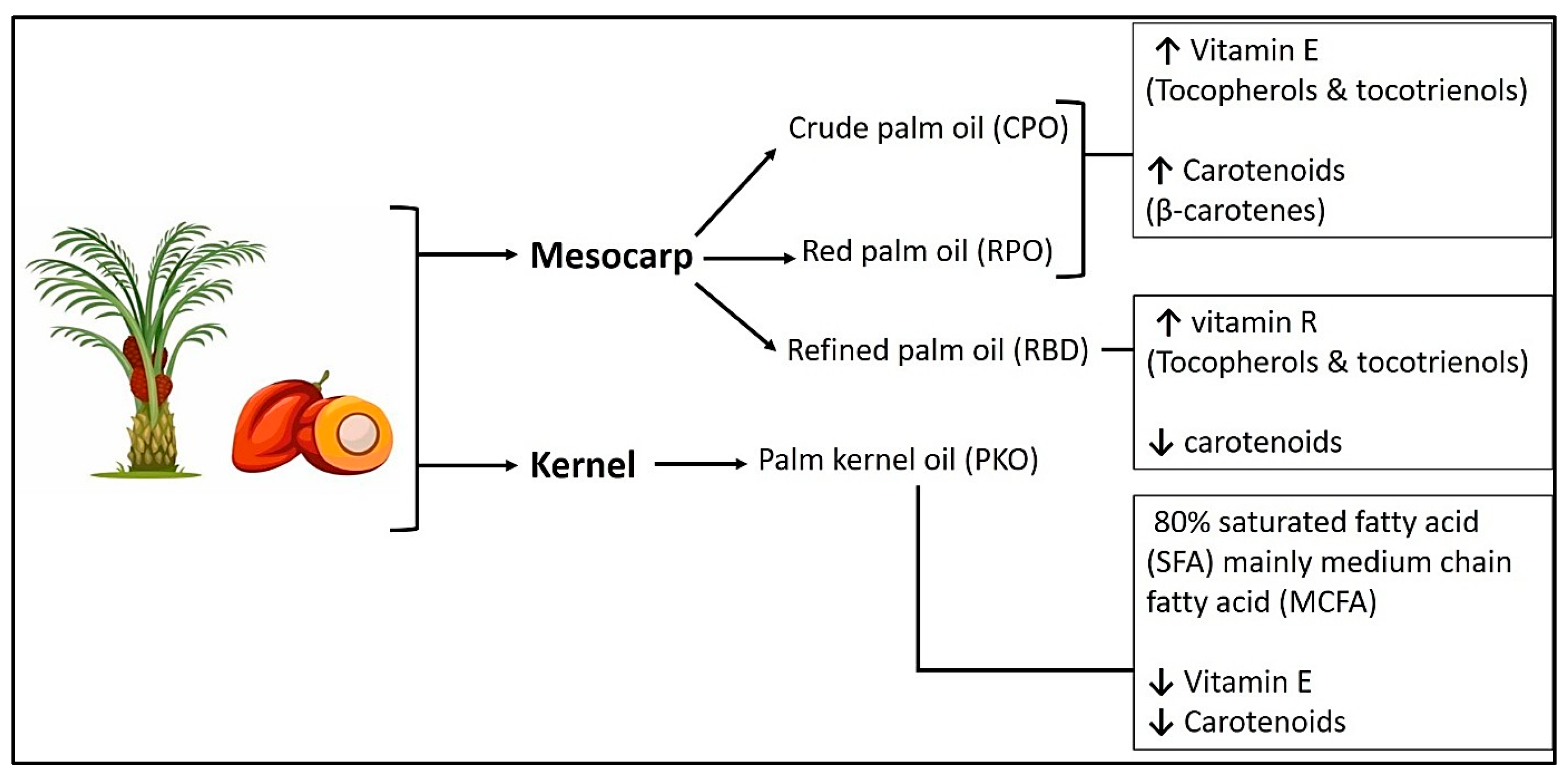
2.3. Palm Fruit
3. Bioactive Compound of Palm Oil
3.1. Phenolic Compounds
3.2. Carotenoids
3.3. Tocopherols and Tocotrienols
4. Protective Roles of Palm Oil and Its Bioactive Compounds
4.1. Mechanism of Action: Antioxidant Properties of Palm Oil
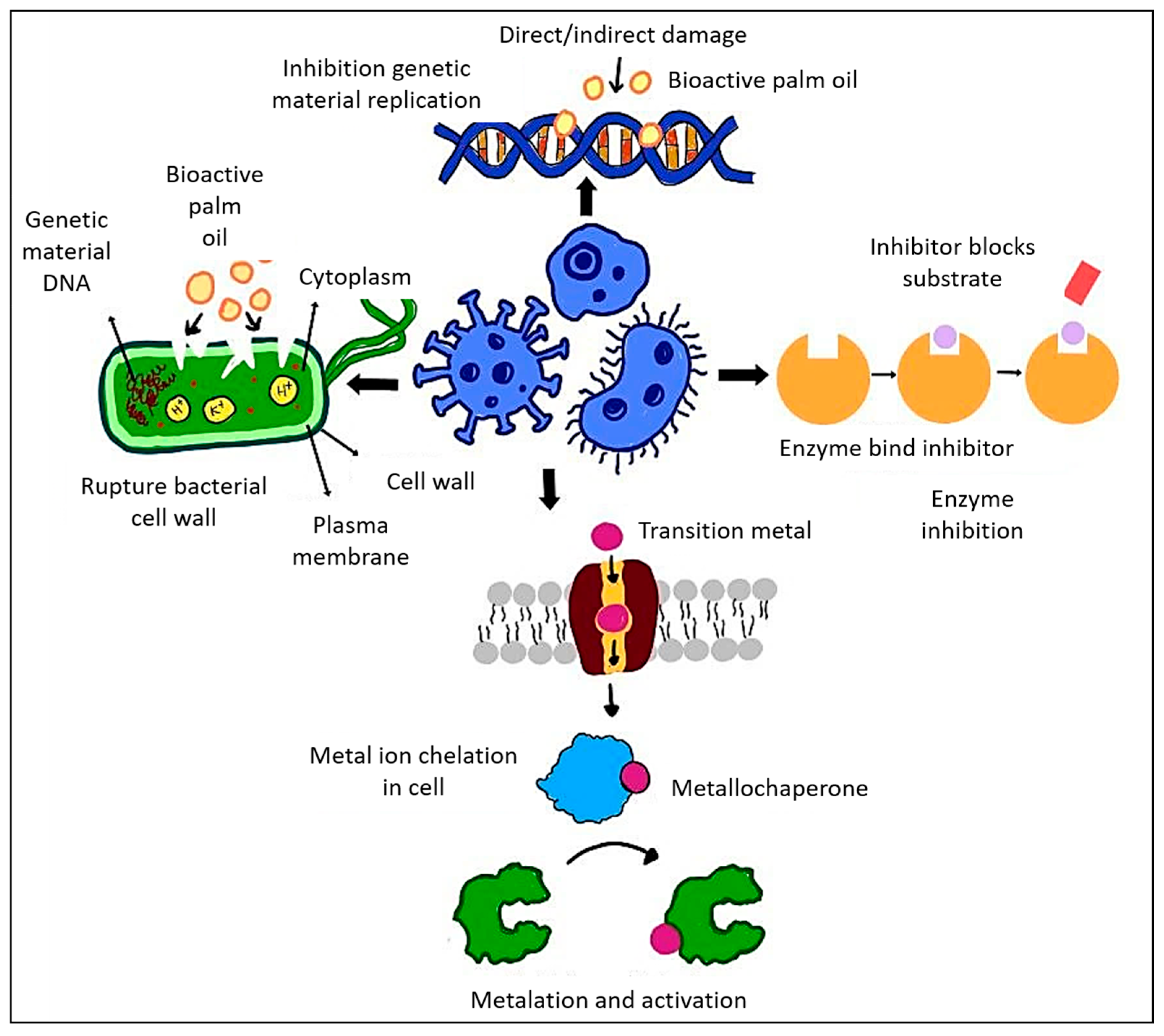
4.2. Mechanism of Action: Antimicrobial Properties of Palm Oil
| Type of Palm Oil | Bacteria | Type of Bacteria | Mechanism of Action (Antimicrobial) | Findings | References |
|---|---|---|---|---|---|
| Red palm oil (RPO) and palm kernel oil (PKO) | P. acnes, S. epidermidis | Gram-positive | Lauric acid in PKO disrupts bacterial membranes. | RPO alone had no effect. PKO showed strong activity (up to 23 mm zone); combo (80:20 PKO:RPO) also effective. | [129] |
| Palm oil from the mesocarp of the oil palm fruit | Clinical and ATCC strains (e.g., E. coli, Pseudomonas, Streptococcus) | Gram-negative | Antimicrobial due to low pH, hydrogen peroxide, and fatty acid content. Heat reduces activity. | Delta palm oil was most effective, especially against E. coli (29 mm), better than some antibiotics. | [123] |
| Palm oil (Elaeis guineensis) | Staphylococcus aureus, MRSA | Gram-positive | Enhances antibiotic uptake (oxacillin) via membrane disruption. | Palm oil enhanced oxacillin efficacy (FICI < 0.5), showing synergy against multidrug-resistant S. aureus. | [134] |
| Palm oil-derived sophorolipids | C. albicans, S. bombicola, S. riodocensis | Yeast, gram-positive | Amphipathic surfactants disrupt fungal membranes and inhibit adhesion and biofilm formation. | Active against C. albicans; surfactants showed emulsification and antifungal potential. | [136] |
| Oil palm empty fruit bunch | S. aureus, E. coli | Gram-positive Gram-negative | Lignin disrupts gram-positive cell walls more easily; gram-negatives resist via the outer membrane. | Strong reduction in S. aureus; minimal effect on E. coli. | [140] |
| Crude palm oil (CPO) | S. aureus, E. coli, P. acnes, S. epidermidis | Gram-positive Gram-negative | Glycerol esters are ineffective; sucrose esters selectively inhibit Gram-positive bacteria. | Sucrose-fatty acid esters inhibited S. aureus; others showed minimal antibacterial effect. | [146] |
| Oil Palm Rhizosphere-Associated Actinomycete, Streptomyces palmae CMU-AB204T | Multiple pathogens (e.g., B. subtilis, E. coli, K. pneumoniae, S. aureus, P. aeruginosa) | Gram-positive Gram-negative | Active compounds (AB204-A–F, anguinomycin, leptomycin) inhibit protein/DNA synthesis. | AB204-E/F is active against gram-positive bacteria; anguinomycin and leptomycin showed broad antimicrobial effects. | [148] |
| Palm Oil Mill Effluent | S. aureus, E. coli, C. albicans | Gram-positive Gram-negative Yeast | Disrupts cell walls or metabolic pathways (selective antimicrobial effect). | BDE-MA/PVA and BCX-MA/PVA hydrogels inhibited S. aureus (~31 mm); no effect on E. coli or C. albicans. | [149] |
| Elaeis guineensis Jacq leaves | Staphylococcus aureus | Gram-positive | Promotes wound healing via antimicrobial, epithelial, and collagen-enhancing properties. | Leaf extract eliminated S. aureus in rat wounds by day 16, similar to BETADINE®. | [150] |
5. Advantages and Limitations of Palm Oil for Dental and Bone Tissue Engineering
5.1. Potential Incorporation of Palm Oil into 3D Bioprinted Hydrogels for Oxidative Stress Modulation
5.2. Impact of Palm Oil on Biocompatibility and Cellular Responses in Regenerative Medicine
5.3. Limitations
6. Conclusions and Future Perspective
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | Activator Protein 1 |
| CPO | Crude Palm Oil |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| EPO | Epoxidized Palm Olein |
| ERK | Extracellular Signal-Regulated Kinase |
| FGF | Fibroblast Growth Factor |
| JNK | c-Jun N-Terminal Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| Nf-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cell |
| NLC | Nanostructured Lipid Carrier |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| OPG | Osteoprotegerin |
| OPL | Oil Palm Leaf |
| PKO | Palm Kernel Oil |
| PME | Palm Methyl Ester |
| PPP | Palm-Based Polyester Polyol |
| RANKL | Receptor Activator of Nuclear Factor Kappa-Β Ligand |
| ROS | Reactive Oxygen Species |
| RPO | Red Palm Oil |
| SL | Sophorolipid |
References
- Mohd, N.; Razali, M.; Ghazali, M.J.; Kasim, N.H.A. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Kasim, N.H.A. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Science. J. Sci. 1993, 15, 119–120. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Causa, F.; Ambrosio, L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med. Devices 2007, 4, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.E.; Bita, B.; Negut, I. From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics 2024, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Tajau, R.; Rohani, R.; Salleh, M.Z. Physicochemical and Thermal Properties of Acrylated Palm Olein as a Promising Biopolymer. J. Polym. Environ. 2020, 28, 2734–2748. [Google Scholar] [CrossRef]
- Kadandale, S.; Marten, R.; Smith, R. The palm oil industry and noncommunicable diseases. Bull. World Health Organ. 2019, 97, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Parveez, G.K.A.; Tarmizi, A.H.A.; Sundram, S.; Loh, S.K.; Ong-Abdullah, M.; Palam, K.D.P.; Salleh, K.M.; Ishak, S.M.; Idris, Z. Oil palm economic performance in Malaysia and R & D progress in 2020. J. Oil Palm Res. 2021, 33, 181–214. [Google Scholar] [CrossRef]
- Hoe, B.C.; Chan, E.S.; Ramanan, R.N.; Ooi, C.W. Recent development and challenges in extraction of phytonutrients from palm oil. Compr. Rev. Food Sci. Food Saf. 2020, 19, 4031–4061. [Google Scholar] [CrossRef] [PubMed]
- Yarra, R.; Jin, L.; Zhao, Z.; Cao, H. Progress in tissue culture and genetic transformation of oil palm: An overview. Int. J. Mol. Sci. 2019, 20, 5353. [Google Scholar] [CrossRef] [PubMed]
- Tajau, R.; Rohani, R.; Alias, M.S.; Mudri, N.H.; Halim, K.A.A.; Harun, M.H.; Isa, N.M.; Ismail, R.C.; Faisal, S.M.; Talib, M.; et al. Emergence of polymeric material utilising sustainable radiation curable palm oil-based products for advanced technology applications. Polymers 2021, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Nahidi, M.; Cheraghali, F. Physico-chemical properties, fatty acid profile and nutrition in palm oil. Arch. Adv. Biosci. 2015, 6, 117–134. [Google Scholar]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 9812, 355–362. [Google Scholar] [CrossRef]
- Sazuan, N.S.A.; Zubairi, S.I.; Mohd, N.H.; Daik, R. Synthesising injectable molecular self-curing polymer from monomer derived from lignocellulosic oil palm empty fruit bunch biomass: A review on treating Osteoarthritis. Arab. J. Chem. 2023, 16, 104500. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.U. Insight into hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Deepa, G.; Thulasidasan, A.K.T.; Anto, R.J.; Pillai, J.J.; Kumar, G.S.V. Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 2012, 7, 4077–4088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stavila, E.; Yuliati, F.; Adharis, A.; Laksmono, J.A.; Iqbal, M. Recent advances in synthesis of polymers based on palm oil and its fatty acids. RSC Adv. 2023, 13, 14747–14775. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, H.; Prociak, A. Influence of Palm Oil-Based Polyol on the Properties of Flexible Polyurethane Foams. J. Polym. Environ. 2012, 20, 438–445. [Google Scholar] [CrossRef]
- Saad, N.M.; Zubir, S.A. Palm kernel oil polyol-based polyurethane as shape memory material: Effect of polyol molar ratio. J. Phys. Sci. 2019, 30, 77–89. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Rink, C.; Roy, S. Tocotrienols: The Emerging Face of Natural Vitamin E Chandan. NIH Public Access 2007, 23, 203–261. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Dell’Anna, M.L. Oxidative stress. Vitiligo 2010, 231–237. [Google Scholar] [CrossRef]
- Da Silva Martins, D.; Boteon, A.P.; Ferreira, A.M.; Debortolli, A.L.B.; Grizzo, I.C.; Ionta, F.Q.; Carvalho, T.S.; Buzalaf, M.A.R.; Rios, D.; Honório, H.M. Can the combination of proanthocyanidin and vitamin e or palm oil effectively protect enamel against in vitro erosive and abrasive challenges? J. Appl. Oral Sci. 2024, 32, e20240100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.C.; Wang, C.Y.; Lee, A.K.X.; Yeh, C.L.; Lin, C.P. Assessment of the release of vascular endothelial growth factor from 3D-printed poly-ε-caprolactone/hydroxyapatite/calcium sulfate scaffold with enhanced osteogenic capacity. Polymers 2020, 12, 1455. [Google Scholar] [CrossRef] [PubMed]
- Bădilă, A.E.; Rădulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Rădulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Zimel, M.; Heath, E.; Hogan, V.; Raz, A. Galectin-3 in bone tumor microenvironment: A beacon for individual skeletal metastasis management. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-acetylcysteine and Lipoic Acid Down-Regulate Starvation-Induced Apoptosis, RANKL/OPG Ratio and Sclerostin in Osteocytes: Involvement of JNK and ERK1/2 Signalling. Calcif. Tissue Int. 2015, 96, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local communication on and within bone controls bone remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Neiers, F.; Feron, G.; Canon, F. The Relationship Between Salivary Redox, Diet, and Food Flavor Perception. Front. Nutr. 2021, 7, 612735. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Né, Y.G.; Lima, W.F.; Mendes, P.F.S.; Baia-da-Silva, D.C.; Bittencourt, L.O.; Nascimento, P.C.; de Souza-Rodrigues, R.D.; Paranhos, L.R.; Martins-Júnior, P.A.; Lima, R.R. Dental Caries and Salivary Oxidative Stress: Global Scientific Research Landscape. Antioxidants 2023, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Beikler, T.; Kinney, J.S.; Ramseier, C.A.; Wong, D.T. Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontology 2000 2009, 50, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Hans, V.M. Epithelial antimicrobial peptides: Guardian of the oral cavity. Int. J. Pept. 2014, 2014, 370297. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.F.; Herzberg, M.C. Autonomous immunity in mucosal epithelial cells: Fortifying the barrier against infection. Microbes Infect. 2016, 18, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative stress and antioxidant system in periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Ionta, F.Q.; de Alencar, C.R.B.; Val, P.P.; Boteon, A.P.; Jordão, M.C.; Honório, H.M.; Buzalaf, M.A.R.; Rios, D. Effect of vegetable oils applied over acquired enamel pellicle on initial erosion. J. Appl. Oral Sci. 2017, 25, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ionta, F.Q.; de Alencar, C.R.B.; Santos, N.M.D.; Bergantin, B.T.P.; Val, P.P.; Honório, H.M.; de Oliveira, T.M.; Rios, D. Effect of palm oil alone or associated to stannous solution on enamel erosive-abrasive wear: A randomized in situ/ex vivo study. Arch. Oral Biol. 2018, 95, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, G.; Ramakrishnan, T.; Parthasarathy, H.; Raja, M.; Raj, S. Fenugreek, diabetes, and periodontal disease: A cross-link of sorts! J. Indian Soc. Periodontol. 2018, 22, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al-Juhaimi, F.Y.; Ahmed, I.A.M.; Osman, M.A.; Gassem, M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019, 278, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of biodegradable palm oil-based polyurethane as potential biomaterial for biomedical applications. Polymers 2020, 12, 1842. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Fontanini, C.; Baltanás, M.A. High yield epoxidation of fatty acid methyl esters with performic acid generated in situ. Chem. Eng. J. 2008, 144, 466–475. [Google Scholar] [CrossRef]
- Rangarajan, B.; Havey, A.; Grulke, E.A.; Culnan, P.D. Kinetic parameters of a two-phase model for in situ epoxidation of soybean oil. J. Am. Oil Chem. Soc. 1995, 72, 1161–1169. [Google Scholar] [CrossRef]
- Jalil, M.J.; Zaini, M.S.M.; Yamin, A.F.M.; Azmi, I.S.; Chang, S.H.; Morad, N.; Hadi, A. Synthesis and physicochemical properties of epoxidized oleic acid-based palm oil. IOP Conf. Ser. Earth Environ. Sci. 2019, 291, 012046. [Google Scholar] [CrossRef]
- Derawi, D.; Salimon, J. Optimization on epoxidation of palm olein by using performic acid. E-J. Chem. 2010, 7, 1440–1448. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.; Zakaria, Z.; Sasidharan, S.; Kalnisha, P.; Lachimanan, Y.; Ramanathan, S. Antimicrobial Activity of Elaeis guineensis Leaf. Pharmacologyonline 2007, 3, 379–386. [Google Scholar]
- Ibraheem, Z.; Sattar, M.; Abdullah, N.; Hassaan, R.; Johns, E. Toxicity, phytochemical content and antioxidant activity assessment studies for a standardized ethanolic fraction of palm oil leaf extract. Pharmacogn. Commun. 2012, 2, 21–30. [Google Scholar] [CrossRef]
- Romes, N.B.; Hamid, M.A.; Hashim, S.E.; Wahab, R.A. Statistical modelling of ultrasonic-aided extraction of Elaeis guineensis leaves for better-quality yield and total phenolic content. Indones. J. Chem. 2019, 19, 811–826. [Google Scholar] [CrossRef]
- Faramayuda, F.; Windyaswari, A.S.; Karlina, Y.; Maulana, M.R.; Guntina, R.K. Effect of Extraction Method on Antioxidant Activity of Palm Palm Leaves (Elaeis guineensis Jacq.). Med. Sains J. Ilm. Kefarmasian 2024, 9, 67–76. [Google Scholar] [CrossRef]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z.; Leong, S.W.; Ismail, I.S.; Khatib, A.; Shaari, K.; Abas, F. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Herrera, M.C.; De Castro, M.D.L. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J. Chromatogr. A 2005, 1100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Teixeira, C.B.; Macedo, G.A.; Macedo, J.A.; da Silva, L.H.M.; Rodrigues, A.M.D.C. Simultaneous extraction of oil and antioxidant compounds from oil palm fruit (Elaeis guineensis) by an aqueous enzymatic process. Bioresour. Technol. 2013, 129, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Neo, Y.P.; Ariffin, A.; Tan, C.P.; Tan, Y.A. Determination of oil palm fruit phenolic compounds and their antioxidant activities using spectrophotometric methods. Int. J. Food Sci. Technol. 2008, 43, 1832–1837. [Google Scholar] [CrossRef]
- Odion, E.E.; Ogboru, R.O.; Ighene, M.O. Identification of Compounds in Elaeis guineensis Fruits using GC-MS. Dhaka Univ. J. Pharm. Sci. 2020, 19, 153–159. [Google Scholar] [CrossRef]
- Emmanuel, S.S.; Adesibikan, A.A. Extraction and phytochemical screening of Elaeis guineensis shell waste. Int. J. Recent Res. Phys. Chem. Sci. (IJRRPCS) 2020, 7, 1–8. [Google Scholar]
- Odion, E.E.; Bafor, E.; Uchendu, A.; Geourge, I.; Omoruyi, O. Fruit Extract of Oil Palm (Elaeis guineensis (Jacq.)) Inhibits Uterine Contractility in Ex-Vivo Mouse Models. Nig. J. Pharm. Res. 2023, 17, 1929–1938. [Google Scholar] [CrossRef]
- Romes, N.B.; Wahab, R.A.; Hamid, M.A. Proximate Analysis and Bioactivity Study on Acoustically isolated Elaeis guineensis leaves extract. In Proceedings of the 2nd International Conference on Biosciences and Medical Engineering (ICBME2019): Towards Innovative Research and Cross-Disciplinary Collaborations, Bali, Indonesia, 11–12 April 2019. [Google Scholar] [CrossRef]
- Awolola, G.V.; Emmanuel, S.S.; Adesibikan, A.A. Evaluation of phytoconstituent and wound-healing potential of methanolic waste shell extract of Elaeis guineensis Jacquin in female rats. Phytomed. Plus 2021, 1, 100126. [Google Scholar] [CrossRef]
- Madièye, S.; Firmin, S.B.; Abdou, S.; Fatou, K.D.; Charlot, D.; Mamadou, N.; Awa, N.-S.; Guata, Y.S. Anti-inflammatory and analgesic activities of methanolic extract of Elaeis guineensis Jacq. leaves (Arecaceae) and its fractions. Afr. J. Pharm. Pharmacol. 2023, 17, 43–51. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ariffin, A.; Tan, C.P.; Tan, Y.A. Phenolic acid analysis and antioxidant activity assessment of oil palm (E. guineensis) fruit extracts. Food Chem. 2010, 122, 353–359. [Google Scholar] [CrossRef]
- Rodríguez, J.C.; Gómez, D.; Pacetti, D.; Núnnez, O.; Gagliardi, R.; Frega, N.G.; Ojeda, M.L.; Loizzo, M.R.; Tundis, R.; Lucci, P. Effects of the Fruit Ripening Stage on Antioxidant Capacity, Total Phenolics, and Polyphenolic Composition of Crude Palm Oil from Interspecific Hybrid Elaeis oleifera × Elaeis guineensis. J. Agric. Food Chem. 2016, 64, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Alexandri, M.; Fernandes, K.V.; Freire, D.M.G.; Mallouchos, A.; Koutinas, A.A. Extraction of phenolic compounds from palm oil processing residues and their application as antioxidants. Food Technol. Biotechnol. 2019, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic investigation of phytochemicals involved in green synthesis of gold nanoparticles using aqueous Elaeis guineensis leaves extract: Role of phenolic compounds and flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Fairus, S.; Mohamed, I.N. The effects and potential mechanism of oil palm phenolics in cardiovascular health: A review on current evidence. Nutrients 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bubols, G.B.; Da Rocha Vianna, D.; Medina-Remón, A.; Von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The Antioxidant Activity of Coumarins and Flavonoids. Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Tow, W.K.; Goh, A.P.T.; Sundralingam, U.; Palanisamy, U.D.; Sivasothy, Y. Flavonoid composition and pharmacological properties of Elaeis guineensis jacq. Leaf extracts: A systematic review. Pharmaceuticals 2021, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M. Protein adducts and protein oxidation as molecular mechanisms of flavonoid bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.M.F.M.; Hossain, M.S.; Khalil, H.P.S.A.; Zulkifli, M.; Al-Gheethi, A.; Asis, A.J.; Yahaya, A.N.A. Treatment of palm oil refinery effluent using tannin as a polymeric coagulant: Isotherm. kinetics, and thermodynamics analyses. Polymers 2020, 12, 2353. [Google Scholar] [CrossRef] [PubMed]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletak, L. Condensed and Hydrolysable Tannins as Antioxidants Influencing the Health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, F.; Vaissayre, V.; Serret, J.; Avallone, S.; Domonhédo, H.; Jacob, F.; Dussert, S. Natural diversity in the carotene, tocochromanol and fatty acid composition of crude palm oil. Food Chem. 2021, 365, 130638. [Google Scholar] [CrossRef] [PubMed]
- Kresnowati, M.T.A.P.; Lestari, D.; Anshori, M.; Jafar, R.M. Production of carotenoids from oil palm empty fruit bunches. IOP Conf. Ser. Earth Environ. Sci. 2020, 460, 012025. [Google Scholar] [CrossRef]
- Raj, T.; Hashim, F.H.; Huddin, A.B.; Hussain, A.; Ibrahim, M.F.; Abdul, P.M. Classification of oil palm fresh fruit maturity based on carotene content from Raman spectra. Sci. Rep. 2021, 11, 18315. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Navrotsky, A.; Leppert, V.J.; Paskowitz, M.J.; Risbud, S.H.; Ludwig, T.; Seifert, H.J.; Aldinger, F.; Mitomo, M. Thermochemistry of Si6-zAlzOzN8-z (z = 0 to 3.6) materials. J. Mater. Res. 1999, 14, 4630–4636. [Google Scholar] [CrossRef]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure. biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Barthelemy, V.; Schreiber, N.; Brun, P.; Lebouvier, N.; Jauffrais, T. Effects of nitrogen availability on the antioxidant activity and carotenoid content of the microalgae Nephroselmis sp. Mar. Drugs 2020, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Azman, N.H.E.N.; Goon, J.A.; Ghani, S.M.A.; Hamid, Z.; Ngah, W.Z.W. Comparing palm oil, tocotrienol-rich fraction and α-tocopherol supplementation on the antioxidant levels of older adults. Antioxidants 2018, 7, 74. [Google Scholar] [CrossRef]
- Ulatowski, L.M.; Manor, D. Vitamin E and neurodegeneration. Neurobiol. Dis. 2015, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.A.; Tsuchiya, M.; Goth, S.; Kaga, V.E.; Packer, L. Antioxidant Action of a-Tocopherol and ce-Tocotrienol in Membranes. Vitam. E Health Dis. 2023, 235–244. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of Vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Ambra, R.; Virgili, F. Tocotrienols: A family of molecules with specific biological activities. Antioxidants 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef] [PubMed]
- Szulczewska-Remi, A.; Nogala-Kałucka, M.; Nowak, K.W. Study on the influence of palm oil on blood and liver biochemical parameters. beta-carotene and tocochromanols content as well as antioxidant activity in rats. J. Food Biochem. 2019, 43, e12707. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.; Ismail, R.; Ghazali, R.; Idris, Z. Total phenolic contents and antioxidant activity of palm oils and palm kernel oils at various refining processes. J. Oil Palm Res. 2018, 30, 682–692. [Google Scholar] [CrossRef]
- Abdullah, F.; Ramu, N.A.S. Identification of hydrophilic phenolic compounds derived from palm oil products. J. Oil Palm Res. 2020, 32, 258–270. [Google Scholar] [CrossRef]
- Khemiri, M.; Khaldi, F.; Hamzaoui, A.; Chaouachi, B.; Hamzaoui, M.; Becher, S.B.; Bellagha, I.; Barsaoui, S. Cystic pulmonary malformations: Clinical and radiological polymorphism. A report on 30 cases. Rev. Pneumol. Clin. 2009, 65, 333–340. [Google Scholar] [CrossRef] [PubMed]
- de Menezes Nogueira, I.; Avelino, F.; de Oliveira, D.R.; Souza, N.F.; Rosa, M.F.; Mazzetto, S.E.; Lomonaco, D. Organic solvent fractionation of acetosolv palm oil lignin: The role of its structure on the antioxidant activity. Int. J. Biol. Macromol. 2019, 122, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J. Organosolv Ethanol Lignin from Hybrid Poplar as a Radical Scavenger: Relationship Between Lignin Structure, Extraction Conditions, and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Gosselink, R.J.A.; Van Der Putten, J.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 2013, 103, 78–85. [Google Scholar] [CrossRef]
- Sholahuddin, S.; Arinawati, D.Y.; Nathan, V.K.; Asada, C.; Nakamura, Y. Antioxidant and antimicrobial activities of lignin-derived products from all steam-exploded palm oil mill lignocellulosic biomass waste. Chem. Biol. Technol. Agric. 2024, 11, 5. [Google Scholar] [CrossRef]
- Izuddin, W.I.; Loh, T.C.; Nayan, N.; Akit, H.; Foo, H.L.; Noor, A.M. Antioxidant Enzyme System Modulation by Dietary Palm Oils. Palm Kernel Oil and Soybean Oil in Laying Hens. Animals 2023, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.N.A.A.; Malik, S.A.; Embi, K.; Ropi, N.A.M.; Yaakob, H.; Cheng, K.K.; Sarmidi, M.R.; Leong, H.Y. Carotenoids and antioxidant activity in virgin palm oil (VPO) produced from palm mesocarp with low heat aqueous-enzyme extraction techniques. Mater. Today Proc. 2019, 42, 148–152. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, A.G.S.; Siahaan, D. Antioxidant Activity of Bioactive Constituents from Crude Palm Oil and Palm Methyl Ester. Int. J. Oil Palm 2019, 2, 46–52. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Edo, G.I.; Makinde, M.G.; Nwosu, L.C.; Ozgor, E.; Akhayere, E. Physicochemical and Pharmacological Properties of Palm Oil: An Approach for Quality. Safety, and Nutrition Evaluation of Palm Oil. Food Anal. Methods 2022, 15, 2290–2305. [Google Scholar] [CrossRef]
- Rohmah, M.; Rahmadi, A.; Raharjo, S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein. Heliyon 2022, 8, e08913. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte, L.; Santagapita, P.R.; Díaz, L.E.; Quintanilla-Carvajal, M.X. Edible gelatin-based nanofibres loaded with oil encapsulating high-oleic palm oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124673. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Marnewick, J.L.; Oguntibeju, O.O.; Davids, L.M. Red Palm Oil Ameliorates Oxidative Challenge and Inflammatory Responses Associated with Lipopolysaccharide-Induced Hepatic Injury by Modulating NF-κβ and Nrf2/GCL/HO-1 Signaling Pathways in Rats. Antioxidants 2022, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Harmileni, H.; Hidayani, T.R.; Marfitania, T. Antimicrobial Activity of Palm Oil (Elaeis guineensis) Leaves Extract against Skin. In Proceedings of the Natural Medicine (ICOLIFEMED 2024), Medan, Indonesia, 5–6 December 2024; Atlantis Press International BV: Amsterdam, The Netherlands, 2024; Volume 84. [Google Scholar] [CrossRef]
- Nainggolan, M.; Sinaga, A.G.S. The modification of red palm oil and palm kernel oil as antibacterial liquid soap. Rasayan J. Chem. 2021, 14, 36–40. [Google Scholar] [CrossRef]
- Silva, N.d.S.e.; Farias, F.d.S.; Freitas, M.M.d.S.; Hernández, E.J.G.P.; Dantas, V.V.; Oliveira, M.E.C.; Joele, M.R.S.P.; Lourenço, L.d.F.H. Artificial intelligence application for classification and selection of fish gelatin packaging film produced with incorporation of palm oil and plant essential oils. Food Packag. Shelf Life 2021, 27, 100611. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties. mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Davidson, P.M.; Zhong, Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013, 61, 12720–12726. [Google Scholar] [CrossRef] [PubMed]
- Lalouckova, K.; Skrivanova, E.; Rondevaldova, J.; Frankova, A.; Soukup, J.; Kokoska, L. In vitro antagonistic inhibitory effects of palm seed crude oils and their main constituent. lauric acid, with oxacillin in Staphylococcus aureus. Sci. Rep. 2021, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Hovorková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef]
- Alfian, A.R.; Watchaputi, K.; Sooklim, C.; Soontorngun, N. Production of new antimicrobial palm oil-derived sophorolipids by the yeast Starmerella riodocensis sp. nov. against Candida Albicans Hyphal Biofilm formation. Microb. Cell Factories 2022, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Fu, S.; An, Z.; Feng, Y.; Wang, R.; Ji, P. Effects of sophorolipids on fungal and oomycete pathogens in relation to pH solubility. J. Appl. Microbiol. 2020, 128, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- De Rienzo, M.A.D.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Dengle-Pulate, V.; Chandorkar, P.; Bhagwat, S.; Prabhune, A.A. Antimicrobial and SEM studies of sophorolipids synthesized using lauryl alcohol. J. Surfactants Deterg. 2014, 17, 543–552. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Kingkaew, W.; Myint, N.N.; Sakulshah, N.; Khomlaem, C.; Saisriyoot, M.; Srinophakun, P. Quantification of antioxidant and antimicrobial properties of oil palm empty fruit bunch lignin based on organosolv extraction. Agric. Nat. Resour. 2024, 58, 303–312. [Google Scholar] [CrossRef]
- Lee, E.; Song, Y.; Lee, S. Antimicrobial Property and Biodegradability of Lignin Nanofibers. In Proceedings of the 2014 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM14), Daejeon, Republic of Korea, 24–28 August 2014; pp. 1–5. [Google Scholar]
- Hussin, M.H.; Rahim, A.A.; Ibrahim, M.N.M.; Brosse, N. The capability of ultrafiltrated alkaline and organosolv oil palm (Elaeis guineensis) fronds lignin as green corrosion inhibitor for mild steel in 0.5 M HCl solution. Measurement 2016, 78, 90–103. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Rocca, D.M.; Vanegas, J.P.; Fournier, K.; Becerra, M.C.; Scaiano, J.C.; Lanterna, A.E. Biocompatibility and photo-induced antibacterial activity of lignin-stabilized noble metal nanoparticles. RSC Adv. 2018, 8, 40454–40463. [Google Scholar] [CrossRef] [PubMed]
- Latif, N.H.A.; Rahim, A.A.; Brosse, N.; Hussin, M.H. The structural characterization and antioxidant properties of oil palm fronds lignin incorporated with p-hydroxyacetophenone. Int. J. Biol. Macromol. 2019, 130, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Naulidia, R.A.; Juliana, E.; Handayani, S.; Damayanti, F.; Setiasih, S. Enzymatic Synthesis of Glycerol and Sucrose-Palm Oil Fatty Acid Esters Produced and Their Potency as Antimicrobial Agents. In Proceedings of the 8th International Conference of the Indonesian Chemical Society (ICICS) 2019, Bogor, Indonesia, 6–7 August 2019. [Google Scholar]
- Valentin, R.; Alignan, M.; Giacinti, G.; Renaud, F.N.R.; Raymond, B.; Mouloungui, Z. Pure short-chain glycerol fatty acid esters and glycerylic cyclocarbonic fatty acid esters as surface active and antimicrobial coagels protecting surfaces by promoting superhydrophilicity. J. Colloid Interface Sci. 2012, 365, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sujarit, K.; Mori, M.; Dobashi, K.; Shiomi, K.; Pathom-aree, S.L.W. New Antimicrobial Phenyl Alkenoic Acids Isolated from an Oil Palm Rhizosphere-Associated. Microorganisms 2020, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Binma-ae, H.; Prasertsan, P.; Choorit, W. Preparation and Characterization of Biopolymers Recovered from Palm Oil Mill Effluent and Their Complex Hydrogels Compared to Commercial Xylan. Waste Biomass Valorization 2020, 11, 5109–5121. [Google Scholar] [CrossRef]
- Rajoo, A.; Ramanathan, S.; Mansor, S.M.; Sasidharan, S. Formulation and evaluation of wound healing activity of Elaeis guineensis Jacq leaves in a Staphylococcus aureus infected Sprague Dawley rat model. J. Ethnopharmacol. 2021, 266, 113414. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szłyk, E. Effect of refining processes on antioxidant capacity. total contents of phenolics and carotenoids in palm oils. Food Chem. 2011, 129, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.; Walvekar, R.; Khalid, M.; Vaka, M.; Mujawar, N.M.; Gupta, T.C.S.M. Recent progress and challenges in transformer oil nanofluid development: A review on thermal and electrical properties. IEEE Access 2019, 7, 151422–151438. [Google Scholar] [CrossRef]
- Mah, S.H.; Sundrasegaran, S.; Lau, H.L.N. Topical Nanoemulsion Hydrogel Formulated from Supercritical Fluid Extracted Palm-Pressed Fiber Oil and Virgin Coconut Oil with Antioxidant and Antibacterial Effects. J. Oleo Sci. 2024, 73, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.S.C.; Danish, M.; Shaari, K.; Fakurazi, S. One-pot synthesis of polysaccharide/gelatin amorphous hydrogels impregnated with a bioflavonoid derived from Elaeis guineensis leaf: Wound healing and drug release properties. DARU J. Pharm. Sci. 2024, 32, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Kasim, N.H.A. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Mohd, N.; Hayaty, N.; Kasim, A.; Razali, M. 3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration. Int. J. Mol. Sci. 2025, 26, 3510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C. Recent advances in 3D printing hydrogel for topical drug delivery. MedComm-Biomater. Appl. 2022, 1, 1. [Google Scholar] [CrossRef]
- Zulkifli, N.N.B.; Badri, K.B.H.; Nor, M.A.A.M.; Amin, K.A.M. Palm kernel oil-based polyurethane film: Biocompatibility and antibacterial activity studies. AIP Conf. Proc. 2017, 1817, 020005. [Google Scholar] [CrossRef]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids extraction and quantification: A review. Anal. Methods 2013, 5, 2916–2924. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals. applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Andrés, M.P.S.; Otero, J.; Vera, S. High performance liquid chromatography method for the simultaneous determination of α-, γ- And δ-tocopherol in vegetable oils in presence of hexadecyltrimethylammonium bromide/n-propanol in mobile phase. Food Chem. 2011, 126, 1470–1474. [Google Scholar] [CrossRef]
| Raw Materials | Synthesis Technique | Synthesis Process | Outcome | References |
|---|---|---|---|---|
| Epoxidized palm olein (EPO) | Polycondensation | EPO was reacted with glutaric acid (1:0.7 molar ratio) at 210 °C for 6 h to produce polyols. | The resulting palm oil-based polyol is a liquid with acid value 1.95 mg KOH/g, hydroxyl value 84.50 mg KOH/g, molecular weight 6698, and viscosity decreasing from 24.55 Pa·s (25 °C) to 8.68 Pa·s (40 °C). Pour and cloud points: 12 °C. | [56] |
| Fresh oil palm leaves (OPLs) | Ultrasonic-aided extraction (UAE) | OPLs were dried, powdered, and extracted using ethanol (0–100%) and different solvent-to-solid ratios. Sonication (20 kHz, 130 W) preserved active compounds. Extracts were centrifuged, filtered, evaporated, freeze-dried, and stored at 4 °C. | Optimized UAE resulted in high extraction yield and rich phenolic content. Solvent concentration, time, and sonication intensity significantly influenced efficiency. | [64] |
| Fresh oil palm leaves (OPLs) | Ethanolic extraction | Leaves were dried, ground, and mixed with 50% ethanol. The mixture was sonicated (30 min), centrifuged, and freeze-dried. Extract was stored at 4 °C. | Moisture: 18.8%, ash: 5.2%, protein: 11.2%, fat: 7.1%, carbohydrates: 57.7%, energy: 339.5 kcal/100 g. | [74] |
| Fresh oil palm leaves (OPLs) | Maceration and reflux | Leaves were extracted using 70% ethanol. Extracts were dissolved in methanol to prepare a 1000 ppm stock solution. | Maceration yielded 14.93%, while reflux gave a higher yield of 27.26% due to enhanced extraction efficiency. | [65] |
| Freshly dumped shell of Elaeis guineensis Jacquin | Methanolic extraction | Shells were washed, dried, ground, and soaked in methanol for 72 h. | Extract contains tannins, alkaloids, terpenoids, saponins, phenolics, and flavonoids. Highest contents: phenolics (10.4%), tannins (5.67%), flavonoids (4.67%). | [75] |
| Palm fruit | Boiling and triturating extraction | Fruits were boiled, mashed, and mixed with water to separate the mesocarp. The mixture was filtered, concentrated, and refrigerated. | Extracts from ethyl acetate, dichloromethane, and n-hexane fractions contained high levels of fatty acids, especially hexadecanoic acid and oleic acid. | [71] |
| Palm leaves | Methanolic extraction | Tannins are extracted by boiling the leaves in methanol and filtering. Terpenes and alkaloids are obtained using an alkaline solution with dichloromethane, then separated and dried. Flavonoids are extracted by boiling in water, followed by liquid-liquid extraction with ethyl acetate and butanol. | Methanolic extract (ME): flavonoids, alkaloids, tannins, sterols, triterpenes. Tannin-free extract (ME-TF): flavonoids, alkaloids, terpenes. Terpene fraction (TF): only terpenes. Alkaloid fraction (AF): only alkaloids. Flavonoid fraction (FF): only flavonoids. | [76] |
| Fresh palm shells | Methanolic extraction | Shells were washed, dried, ground, and soaked in methanol for 72 h. The extract was filtered and concentrated. | Highest content: phenolics (11.4%), tannins (6.67%), and flavonoids (5.67%). | [72] |
| Palm fruit | Aqueous extraction | A total of 500 g of fruits were cleaned, boiled for 45 min, mashed, and filtered to separate the mesocarp. The extract was then filtered again, concentrated, and stored at 4 °C, yielding 95 g (19%). | Extraction content not reported. The yield was 19% (95 g). | [73] |
| Study Focus | Method of Identification | Antioxidant Components | Mechanism of Action (Antioxidant) | Antioxidant Findings | References |
|---|---|---|---|---|---|
| Phenolic content in crude vs. refined palm oils | DPPH Assay | Phenolic compounds. | Neutralize radicals, chelate metals. | Extra virgin olive oil (EVOO) (70%) > crude palm oil (CPO) (45%) > Crude Palm Kernel Oil (CPKO) (30%); refined oils lower; lowest IC50 in EVOO and CPO. | [110] |
| Lignin extracted from palm biomass | DPPH Assay | Phenolics, methoxyl, conjugated bonds. | Radical stabilization via resonance. | Lignin and its fractions (MeOH-F, ACT-F, and EtOH-F). MeOH-F and ACT-F had IC50 ~42–43 µg/mL; better than BHT and Irganox. | [113] |
| Lignin from mesocarp fibers with enzymes | Total phenolic content (TPC) and ferric reducing antioxidant power assay (FRAP) | Phenolics, carotenoids, tocopherols, tocotrienols. | Cell wall breakdown releases antioxidants. | Enzyme-treated oils had higher antioxidant activity; carotenoid yield increased by 153%. | [118] |
| Crude palm oil (CPO) and palm oil methyl ester (PME) antioxidant properties | DPPH Assay | Carotenoids, tocotrienols. | Scavenging radicals, lipid protection. | PME showed higher activity (69.3%) than CPO (30.1%); PME IC50 = 5.9 µg/mL. | [120] |
| Palm oil waste: antioxidant screening | DPPH Assay | Multiple phenolics. | Hydrogen donation, metal ion chelation. | Palm kernel cake had the highest phenolics and antioxidant capacity. | [79] |
| Palm oil from regions and nitric oxide (NO) scavenging | Nitric oxide (NO) scavenging activity assay | Phenolic (flavone): 7-dihydroxyflavone (chrysin). | Radical scavenging, Maillard reaction products. | Delta oil lowest (501.7 µg/mL); Ascorbic acid strongest (65.5 µg/mL). | [123] |
| nanostructured lipid carriers (NLC) to enhance β-carotene antioxidant activity | DPPH Assay ABTS Assay | Carotenoids, phenolics. | Encapsulation reduces oxidation and enhances activity. | βC-NLC exhibited significant antioxidant activity, achieving 91.47% in ABTS and 24.72% in DPPH free radical scavenging assays. | [124] |
| high-oleic palm oil (HOPO) based nanofibers | ABTS Assay | Carotenoids, phenolics. | Absorb/react with free radicals, preserve antioxidants. | Nanofibers had high activity and were stable after processing. | [125] |
| The protective role of red palm oil (RPO) in mitigating liver damage induced by lipopolysaccharide (LPS). | FRAP, ORAC, TEAC | Tocopherols, tocotrienols, β-/α-carotene. | Nrf2 activation, NF-κB inhibition, reduces oxidative stress. | RPO restored FRAP; no change in ORAC/TEAC values. | [126] |
| ultra-high pressure (UHP)-treated palm fruits | DPPH, FRAP, ABTS | Phenolic acids, flavonoids. | Electron/hydrogen donation, ROS reduction, metal chelation. | UHP improved antioxidant levels; strong correlation with total phenolic content. | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, S.; Mohd, N.; Abu Kasim, N.H.; Razali, M. Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications. Int. J. Mol. Sci. 2025, 26, 6975. https://doi.org/10.3390/ijms26146975
Masri S, Mohd N, Abu Kasim NH, Razali M. Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications. International Journal of Molecular Sciences. 2025; 26(14):6975. https://doi.org/10.3390/ijms26146975
Chicago/Turabian StyleMasri, Syafira, Nurulhuda Mohd, Noor Hayaty Abu Kasim, and Masfueh Razali. 2025. "Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications" International Journal of Molecular Sciences 26, no. 14: 6975. https://doi.org/10.3390/ijms26146975
APA StyleMasri, S., Mohd, N., Abu Kasim, N. H., & Razali, M. (2025). Mechanistic Insight into the Antioxidant and Antimicrobial Activities of Palm Oil-Derived Biomaterials: Implications for Dental and Therapeutic Applications. International Journal of Molecular Sciences, 26(14), 6975. https://doi.org/10.3390/ijms26146975








