Cryopreservation and Validation of Microfragmented Adipose Tissue for Autologous Use in Knee Osteoarthritis Treatment
Abstract
1. Introduction
2. Results
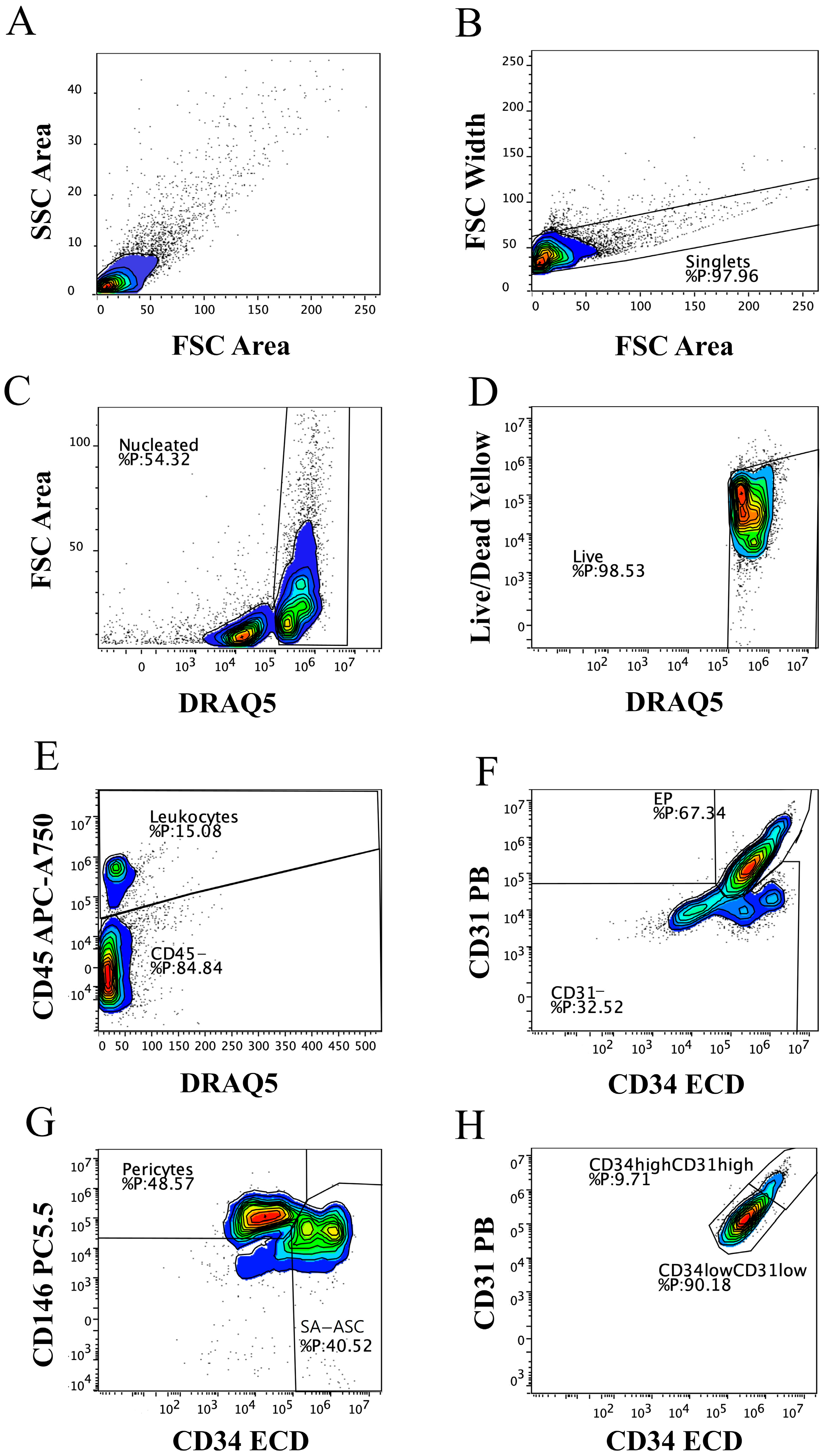
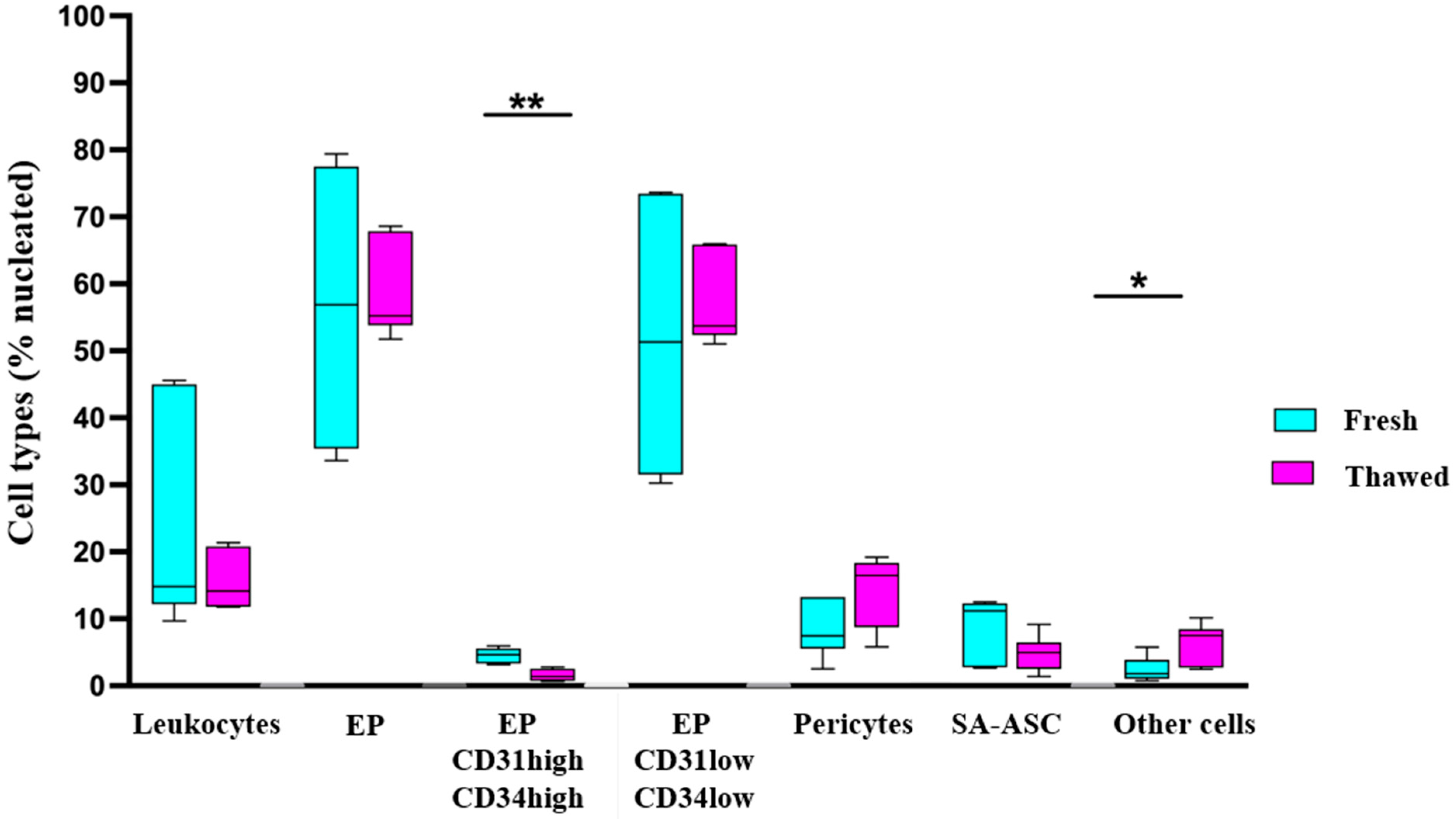
2.1. Immunophenotyping of MFAT Samples
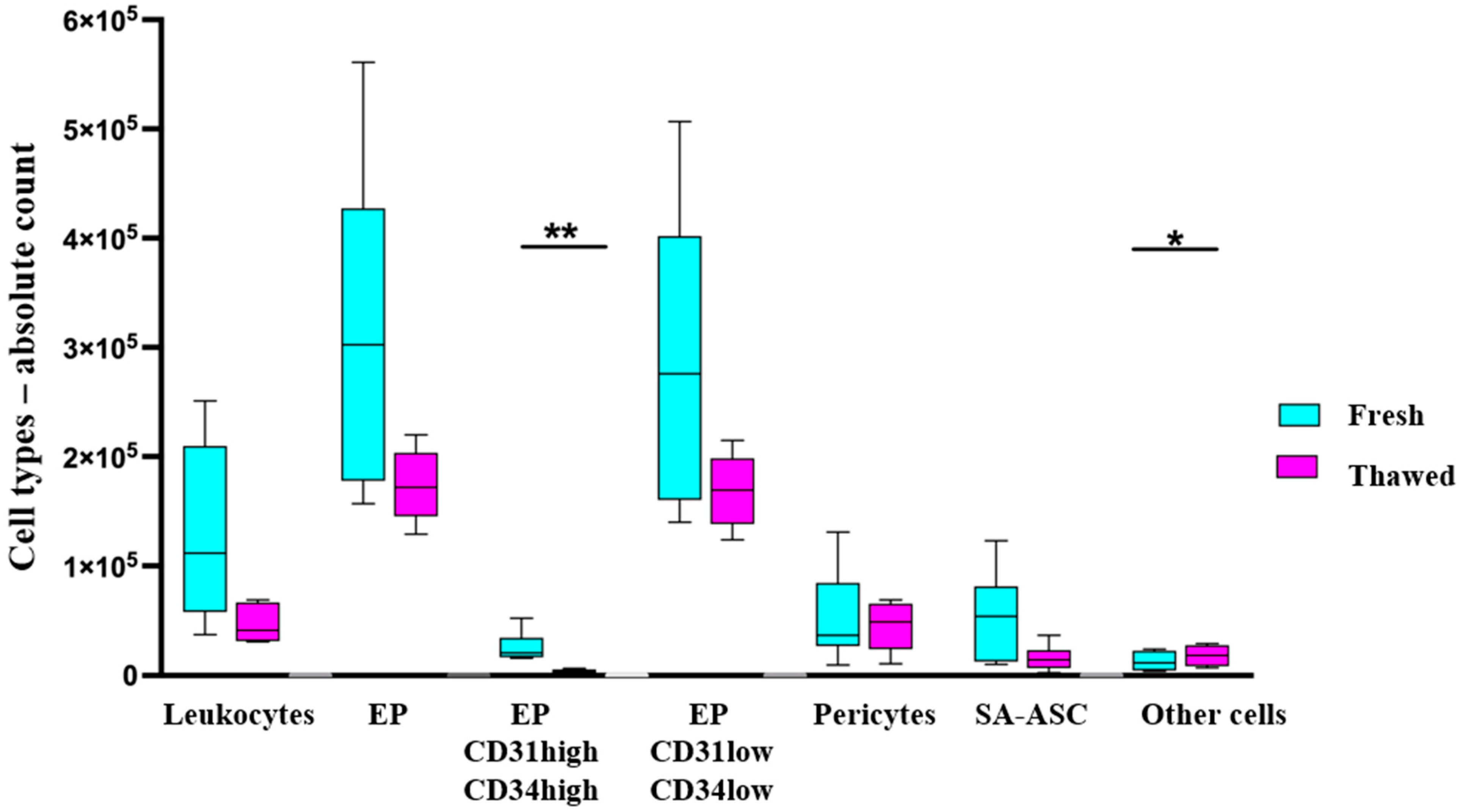
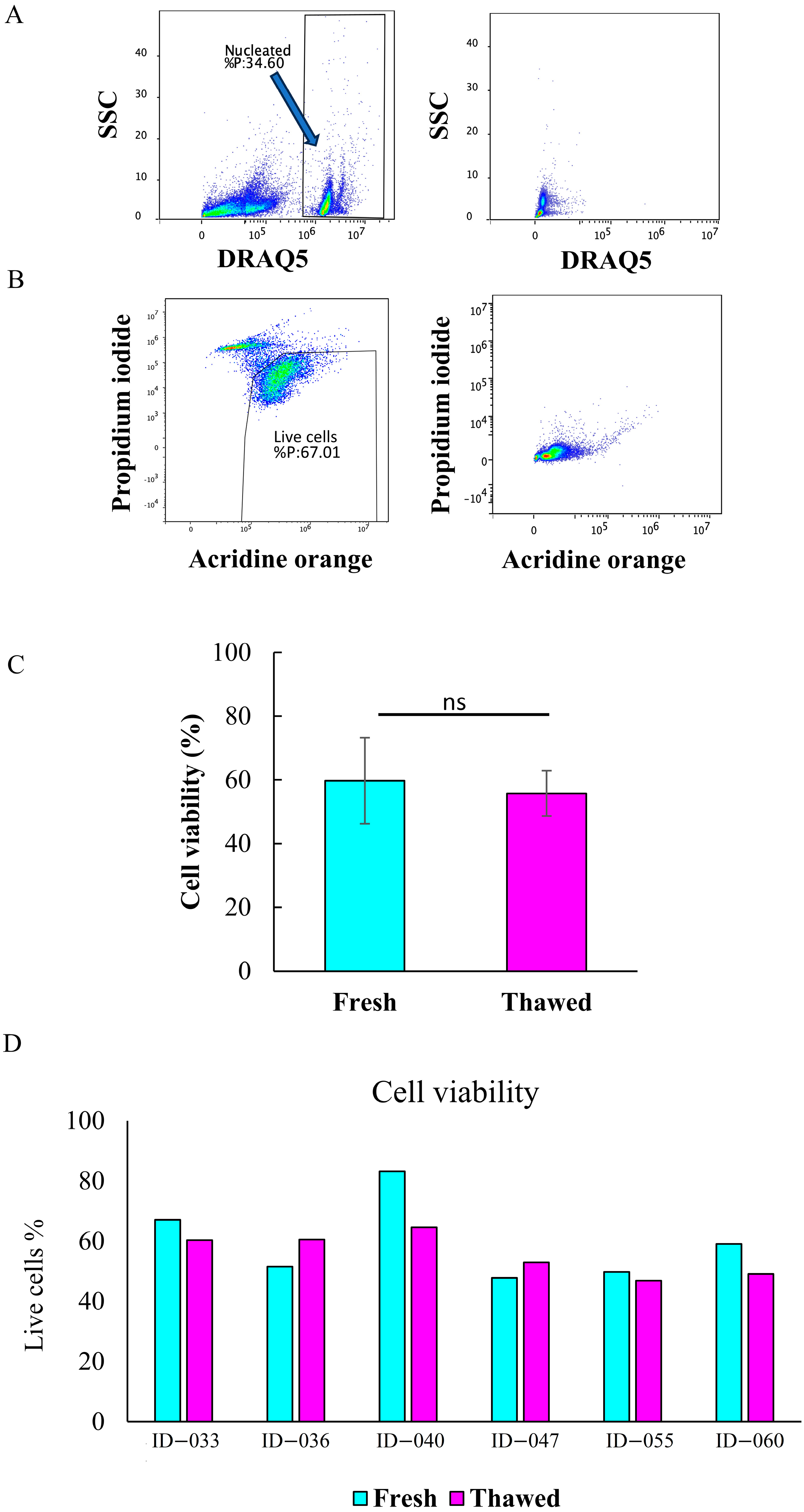
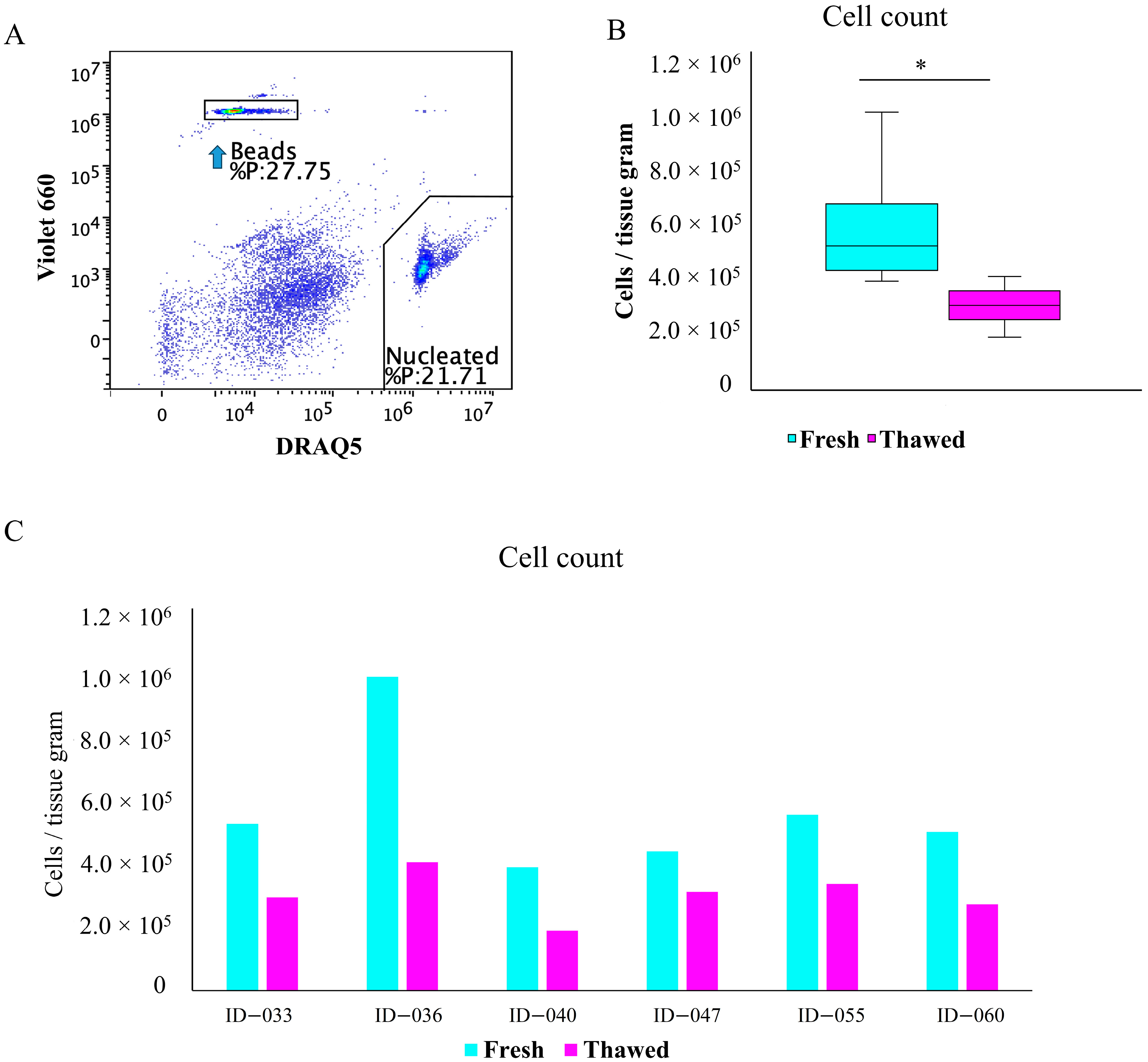
2.2. Total Cell Viability and Count
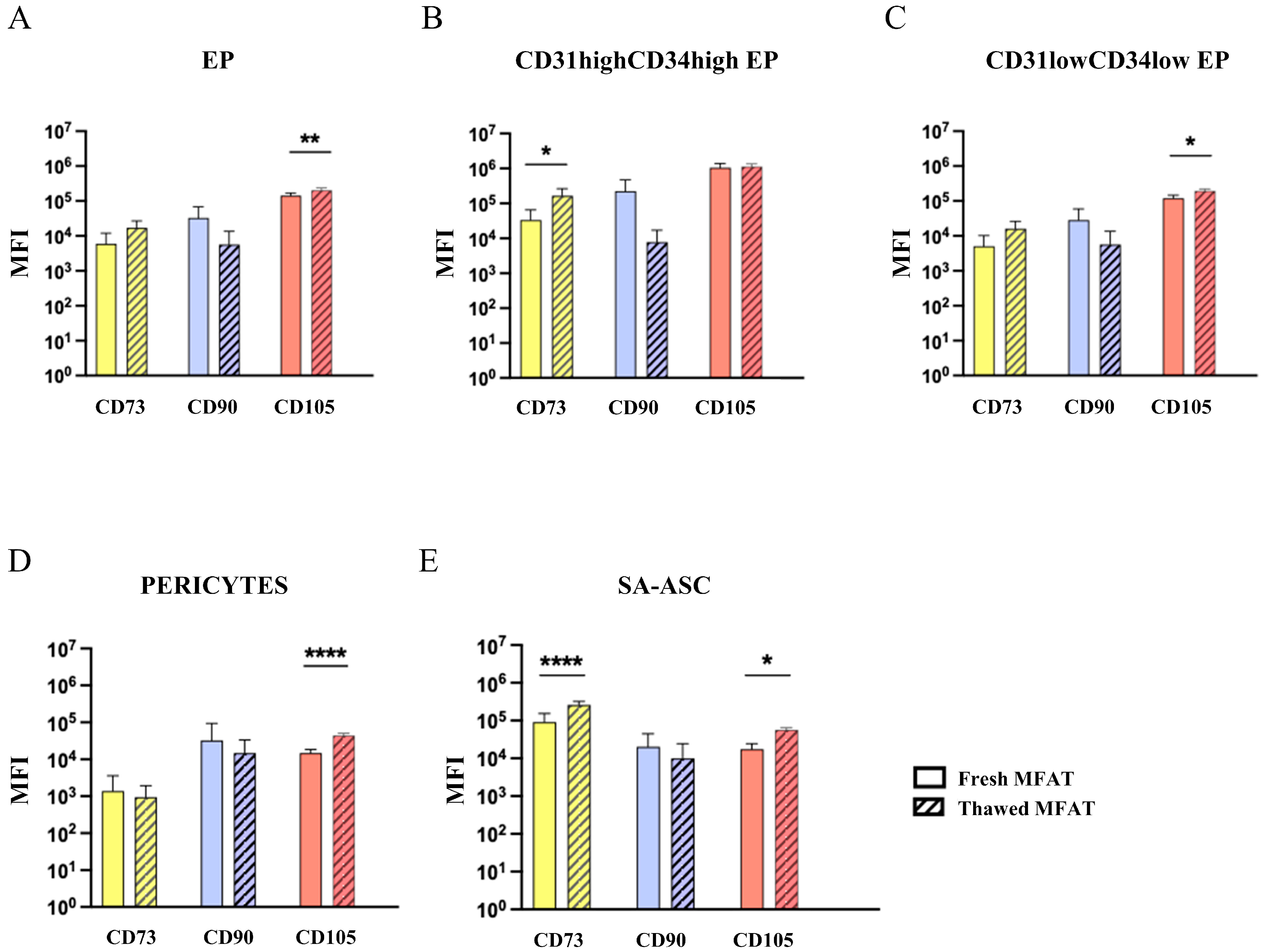
2.3. Mesenchymal Stromal Progenitor Cell-Associated Markers
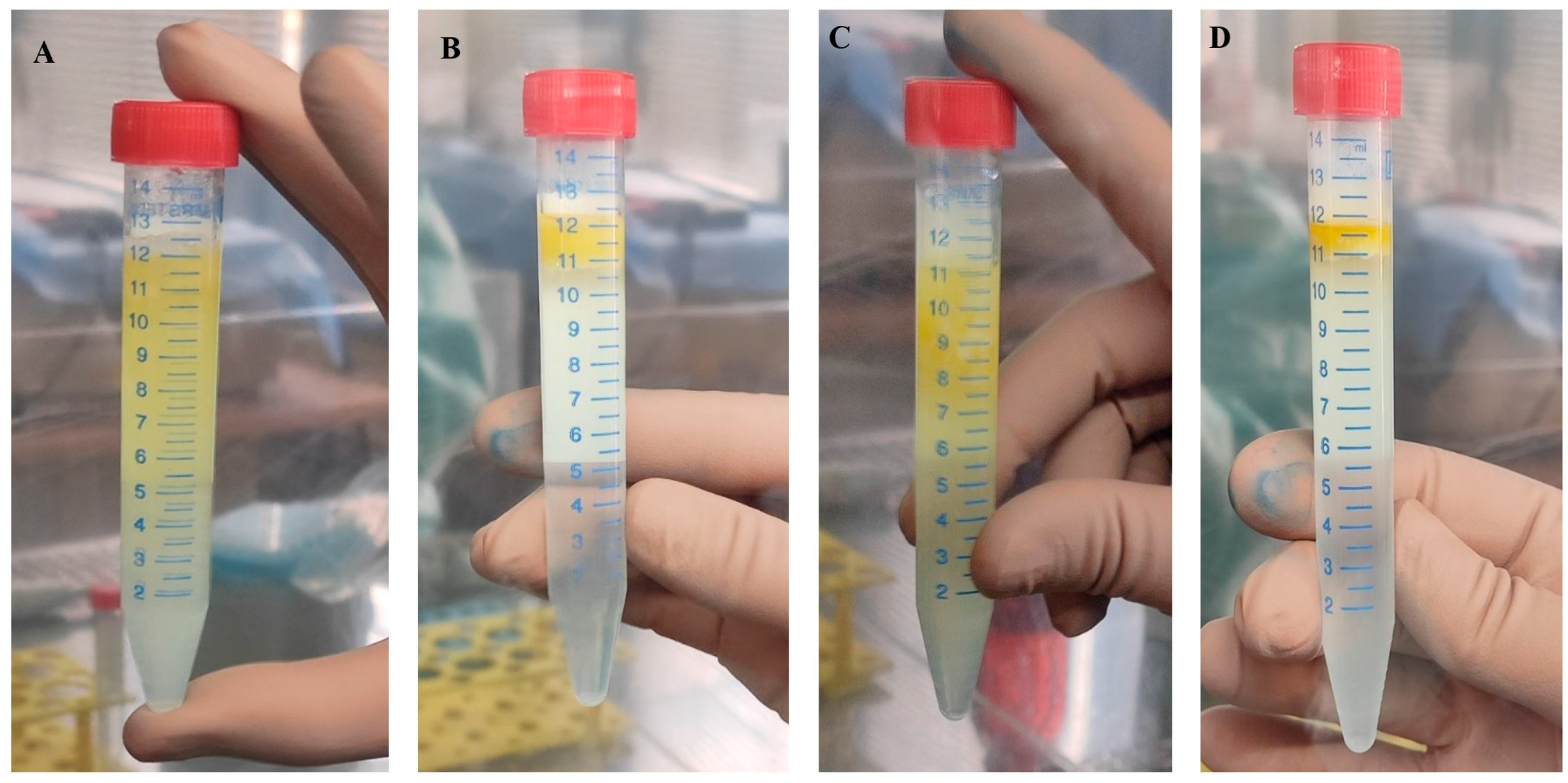
2.4. Morphological Evaluation of Cryopreserved Adipose Tissue
2.5. Analysis of DMSO Residue in MFAT Preparation After Cryopreservation
2.6. Microbiological Evaluation of MFAT Preparation
3. Discussion

4. Materials and Methods
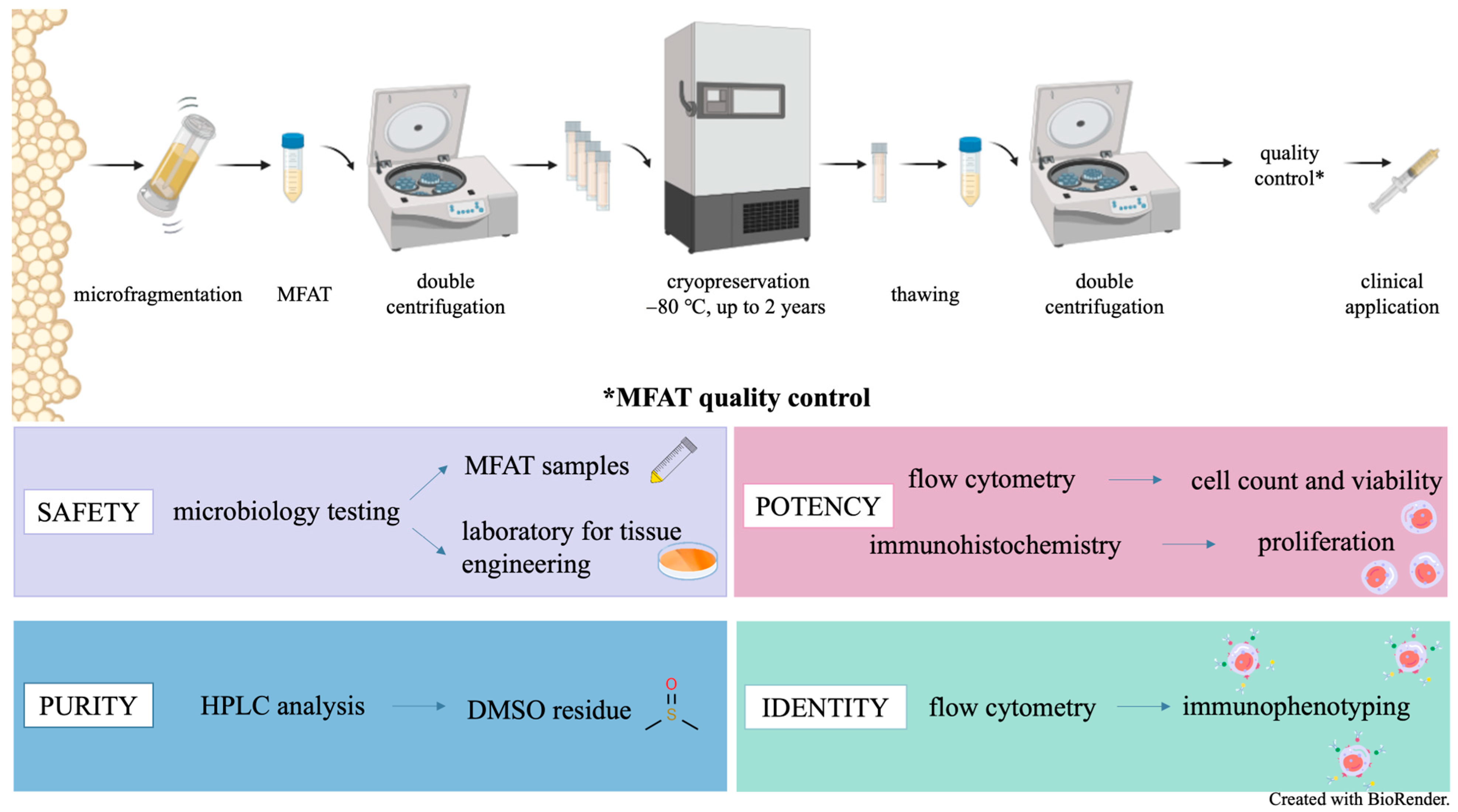
4.1. Study Design
4.2. Initial Examination and Procurement
4.3. Cryopreservation
4.4. Thawing and Preparation of MFAT Batch
4.5. Quality Control
4.5.1. Microbiological Testing
4.5.2. Immunohistochemistry
4.5.3. Reverse Phase High-Performance Liquid Chromatography (RP HPLC) for Estimation of Residual DMSO
4.5.4. SVF Cell Isolation for the Flow Cytometry Analyses
4.5.5. Flow Cytometry Immunophenotyping of SVF from MFAT
4.5.6. Flow Cytometry Determination of SVF Cell Viability
4.5.7. Flow Cytometry Determination of SVF Cell Count
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Felthaus, O.; Prantl, L. Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects. Cells 2025, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- European Committee (Partial Agreement) on Organ Transplantation. Guide to the Quality and Safety of TISSUES AND CELLS for Human Application European Committee (Partial Agreement) on Organ Transplantation (CD-P-TO); The European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2022. [Google Scholar]
- Coleman, S.R. Long-Term Survival of Fat Transplants: Controlled Demonstrations. Aesth Plast. Surg. 2020, 44, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; et al. Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. Front. Immunol. 2022, 13, 918565. [Google Scholar] [CrossRef] [PubMed]
- Casado, S.; Del Val Toledo Lobo, M.; Paíno, C.L. Dynamics of Plasma Membrane Surface Related to the Release of Extracellular Vesicles by Mesenchymal Stem Cells in Culture. Sci. Rep. 2017, 7, 6767. [Google Scholar] [CrossRef] [PubMed]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef] [PubMed]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Van Thanh, V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Bagge, J.; Hölmich, P.; Hammer, F.A.; Nehlin, J.O.; Vomstein, K.; Blønd, L.; Hölmich, L.R.; Barfod, K.W. Successful Isolation of Viable Stem Cells from Cryopreserved Microfragmented Human Adipose Tissue from Patients with Knee Osteoarthritis—A Comparative Study of Isolation by Tissue Explant Culture and Enzymatic Digestion. J. Exp. Orthop. 2023, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Encarnacion Ramirez, M.d.J.; Engelgard, M.; Igorevich, E.I.; Saporiti, A.; Valentinovich Kotenko, K.; Montemurro, N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina 2023, 59, 2090. [Google Scholar] [CrossRef] [PubMed]
- Díaz Rodríguez, R.; Van Hoeck, B.; De Gelas, S.; Blancke, F.; Ngakam, R.; Bogaerts, K.; Jashari, R. Determination of Residual Dimethylsulfoxide in Cryopreserved Cardiovascular Allografts. Cell Tissue Bank. 2017, 18, 263–270. [Google Scholar] [CrossRef] [PubMed]
- González Porto, S.A.; Domenech, N.; González Rodríguez, A.; Avellaneda Oviedo, E.M.; Blanco, F.J.; Arufe Gonda, M.C.; Álvarez Jorge, Á.; Sánchez Ibañez, J.; Rendal Vázquez, E. The Addition of Albumin Improves Schwann Cells Viability in Nerve Cryopreservation. Cell Tissue Bank. 2018, 19, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape in Vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2006/86/EC of 24 October 2006 Implementing Directive 2004/23/EC of the European Parliament and of the Council as Regards Traceability Requirements, Notification of Serious Adverse Reactions and Events and Certain Technical Requirements for the Coding, Processing, Preservation, Storage and Distribution of Human Tissues and Cells. Off. J. Eur. Union 2006, 294, 32–50.
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 4968724. [Google Scholar] [CrossRef] [PubMed]
- Polancec, D.; Zenic, L.; Hudetz, D.; Boric, I.; Jelec, Z.; Rod, E.; Vrdoljak, T.; Skelin, A.; Plecko, M.; Turkalj, M.; et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Zenic, L.; Polancec, D.; Hudetz, D.; Jelec, Z.; Rod, E.; Vidovic, D.; Staresinic, M.; Sabalic, S.; Vrdoljak, T.; Petrovic, T.; et al. Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes 2021, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.S.; Muise, A.; Harris, D.T. Patient Use of Autologous Cryopreserved Intact Adipose Tissue from Lipoaspirate. AIMS Cell Tissue Eng. 2017, 1, 224–235. [Google Scholar] [CrossRef]
- Favaretto, F.; Compagnin, C.; Cogliati, E.; Montagner, G.; Dell’Antonia, F.; Berna, G.; Vettor, R.; Milan, G.; Trojan, D. Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. Int. J. Mol. Sci. 2023, 24, 8190. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B. Knee Osteoarthritis Diagnosis, Treatment and Associated Factors of Progression: Part II. Casp. J. Intern. Med. 2011, 2, 249–255. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Maličev, E.; Jazbec, K. An Overview of Mesenchymal Stem Cell Heterogeneity and Concentration. Pharmaceuticals 2024, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.E.S.; Iminitoff, M.; Williams, E.; Damani, T.; Jackson-Patel, V.; Fan, V.; James, J.; Dunbar, P.R.; Feisst, V.; Sheppard, H.M. Ex Vivo Human Adipose Tissue Derived Mesenchymal Stromal Cells (ASC) Are a Heterogeneous Population That Demonstrate Rapid Culture-Induced Changes. Front. Pharmacol. 2020, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Donnenberg, A.D. Pericytes: A Universal Adult Tissue Stem Cell? Cytom. Part A 2012, 81 A, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C. Adipose-Derived Mesenchymal Stromal/Stem Cells: An Update on Their Phenotype in Vivo and in Vitro. World J. Stem Cells 2014, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Rubin, J.P.; Donnenberg, A.D. Mesenchymal Markers on Human Adipose Stem/Progenitor Cells. Cytom. Part A 2013, 83 A, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Gornicka, A.; Berk, M.P.; Thapaliya, S.; Dixon, L.J.; Kashyap, S.; Schauer, P.R.; Feldstein, A.E. Adipocyte Apoptosis, a Link between Obesity, Insulin Resistance, and Hepatic Steatosis. J. Biol. Chem. 2010, 285, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.; Janowicz, K.; Moncrieff, L.; Dompe, C.; Strauss, E.; Kocherova, I.; Nawrocki, M.J.; Kruszyna, Ł.; Wasiatycz, G.; Antosik, P.; et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020, 21, 3790. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.M.; Wofford, J.D.; Wall, D.A. Comparison of Cord Blood Thawing Methods on Cell Recovery, Potency, and Infusion. Transfusion 2010, 50, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef] [PubMed]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Jeleč, Ž.; Rod, E.; Borić, I.; Plečko, M.; Primorac, D. The Future of Cartilage Repair. In Personalized Medicine in Healthcare Systems: Legal, Medical and Economic Implications; Bodiroga-Vukobrat, N., Rukavina, D., Pavelić, K., Sander, G.G., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2019; pp. 375–411. [Google Scholar]
- Publications Office of the European Union. Regulation (EU) 2024/1938 of the European Parliament and of the Council of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/ECText with EEA Relevance; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- ISO 15189; Medical Laboratories—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2022.


| Samples | Patient ID | Samples Procedure | Specimen | % DMSO |
|---|---|---|---|---|
| Sample 1 | 023 | 1. washing of MFAT | 10% HPA | 0.7191 |
| 2. washing of MFAT | 1% HPA | 0.0226 | ||
| Sample 2 | 028 | 1. washing of MFAT | 10% HPA | 0.7944 |
| 2. washing of MFAT | 1% HPA | 0.0271 | ||
| Sample 3 | 033 | 1. washing of MFAT | 10% HPA | 0.5594 |
| 2. washing of MFAT | 1% HPA | 0.0156 | ||
| Sample 4 | 040 | 1. washing of MFAT | 10% HPA | 0.6274 |
| 2. washing of MFAT | 1% HPA | 0.0129 | ||
| Sample 5 | 036 | 1. washing of MFAT | 10% HPA | 0.8671 |
| 2. washing of MFAT | 1% HPA | 0.0354 | ||
| Sample 6 | 047 | 1. washing of MFAT | 10% HPA | 0.8802 |
| 2. washing of MFAT | 1% HPA | 0.0382 | ||
| Sample 7 | 051 | 1. washing of MFAT | 10% HPA | 0.8881 |
| 2. washing of MFAT | 1% HPA | 0.0410 |
| Analysis | Sample Type | Specimen |
|---|---|---|
| Microbiological sterility | Laboratory environment | Air, contact, fingers |
| MFAT | Adipose tissue | |
| Aqueous phase of MFAT | 1. washing of MFAT 10% HPA | |
| 2. washing of MFAT 1% HPA | ||
| DMSO residue | Aqueous phase of MFAT | 1. washing of MFAT 10% HPA |
| 2. washing of MFAT 1% HPA | ||
| Immunohistochemistry | MFAT | Adipose tissue |
| Cell viability | MFAT | Adipose tissue |
| Cell number | MFAT | Adipose tissue |
| Immunophenotyping | MFAT | Adipose tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zekušić, M.; Brlek, P.; Zenić, L.; Molnar, V.; Ledinski, M.; Bujić Mihica, M.; Štimac, A.; Halassy, B.; Ramić, S.; Puljić, D.; et al. Cryopreservation and Validation of Microfragmented Adipose Tissue for Autologous Use in Knee Osteoarthritis Treatment. Int. J. Mol. Sci. 2025, 26, 6969. https://doi.org/10.3390/ijms26146969
Zekušić M, Brlek P, Zenić L, Molnar V, Ledinski M, Bujić Mihica M, Štimac A, Halassy B, Ramić S, Puljić D, et al. Cryopreservation and Validation of Microfragmented Adipose Tissue for Autologous Use in Knee Osteoarthritis Treatment. International Journal of Molecular Sciences. 2025; 26(14):6969. https://doi.org/10.3390/ijms26146969
Chicago/Turabian StyleZekušić, Marija, Petar Brlek, Lucija Zenić, Vilim Molnar, Maja Ledinski, Marina Bujić Mihica, Adela Štimac, Beata Halassy, Snježana Ramić, Dominik Puljić, and et al. 2025. "Cryopreservation and Validation of Microfragmented Adipose Tissue for Autologous Use in Knee Osteoarthritis Treatment" International Journal of Molecular Sciences 26, no. 14: 6969. https://doi.org/10.3390/ijms26146969
APA StyleZekušić, M., Brlek, P., Zenić, L., Molnar, V., Ledinski, M., Bujić Mihica, M., Štimac, A., Halassy, B., Ramić, S., Puljić, D., Vučemilo, T., Tremolada, C., Sabalić, S., Karli, D. C., Tsoukas, D., & Primorac, D. (2025). Cryopreservation and Validation of Microfragmented Adipose Tissue for Autologous Use in Knee Osteoarthritis Treatment. International Journal of Molecular Sciences, 26(14), 6969. https://doi.org/10.3390/ijms26146969









