The “Bagno dell’Acqua” Lake as a Novel Mars-like Analogue: Prebiotic Syntheses of PNA and RNA Building Blocks and Oligomers
Abstract
1. Introduction
2. Results
2.1. Role of Microbialite in Prebiotic Chemical Reactions
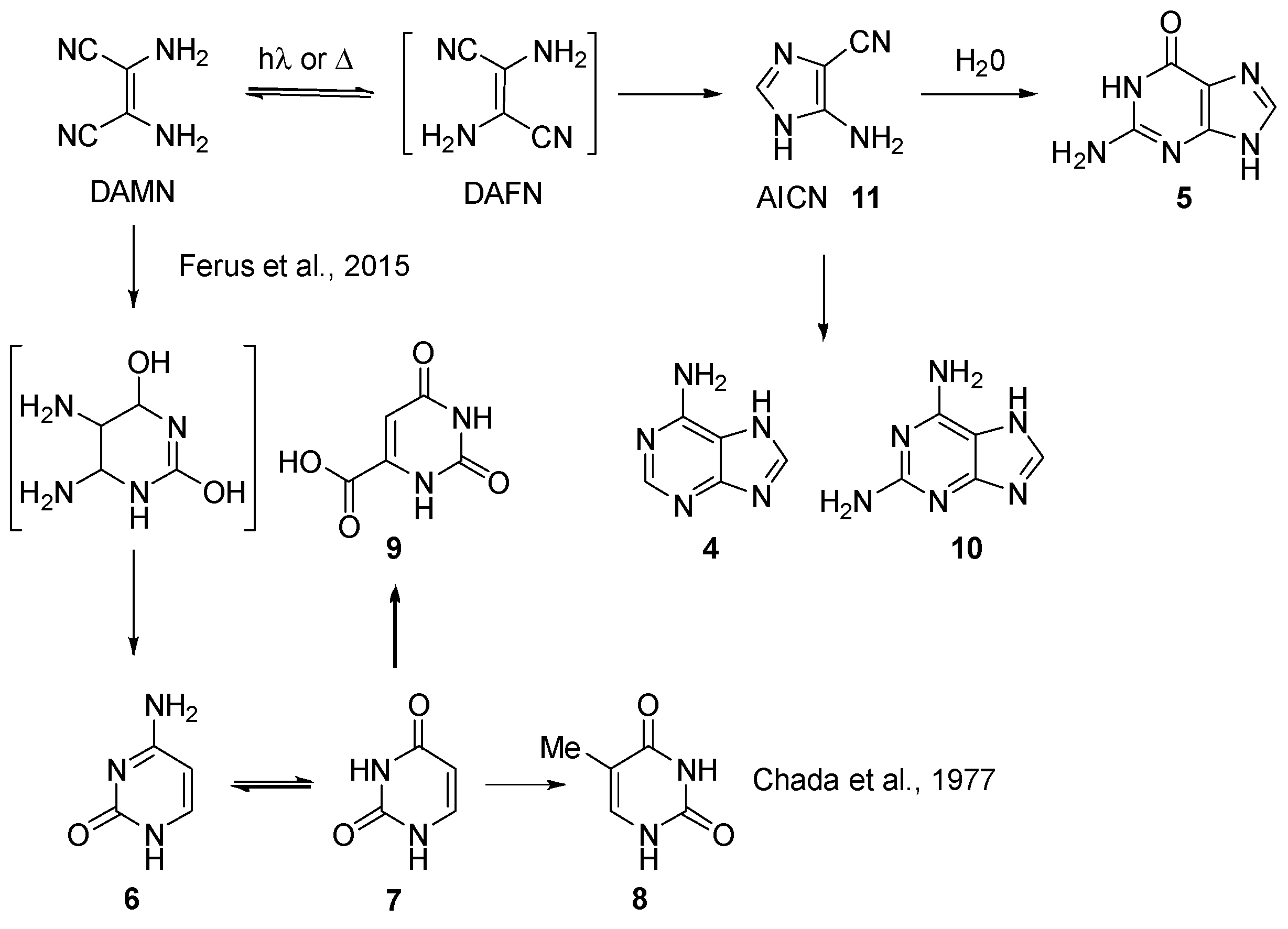
2.1.1. Nucleobases and Analogues
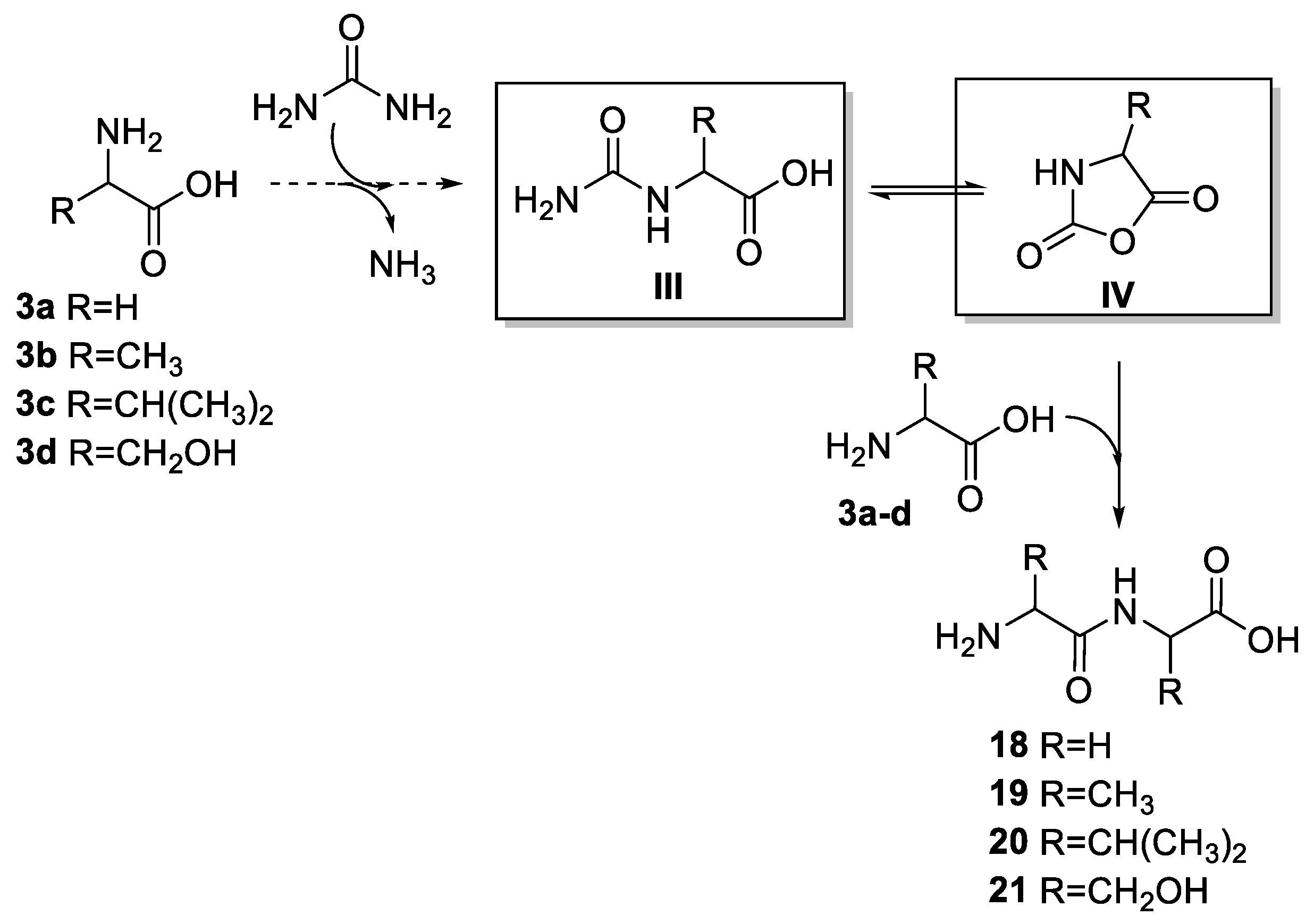
2.1.2. PNA’s Building Blocks
2.1.3. Peptides and Miscellanea
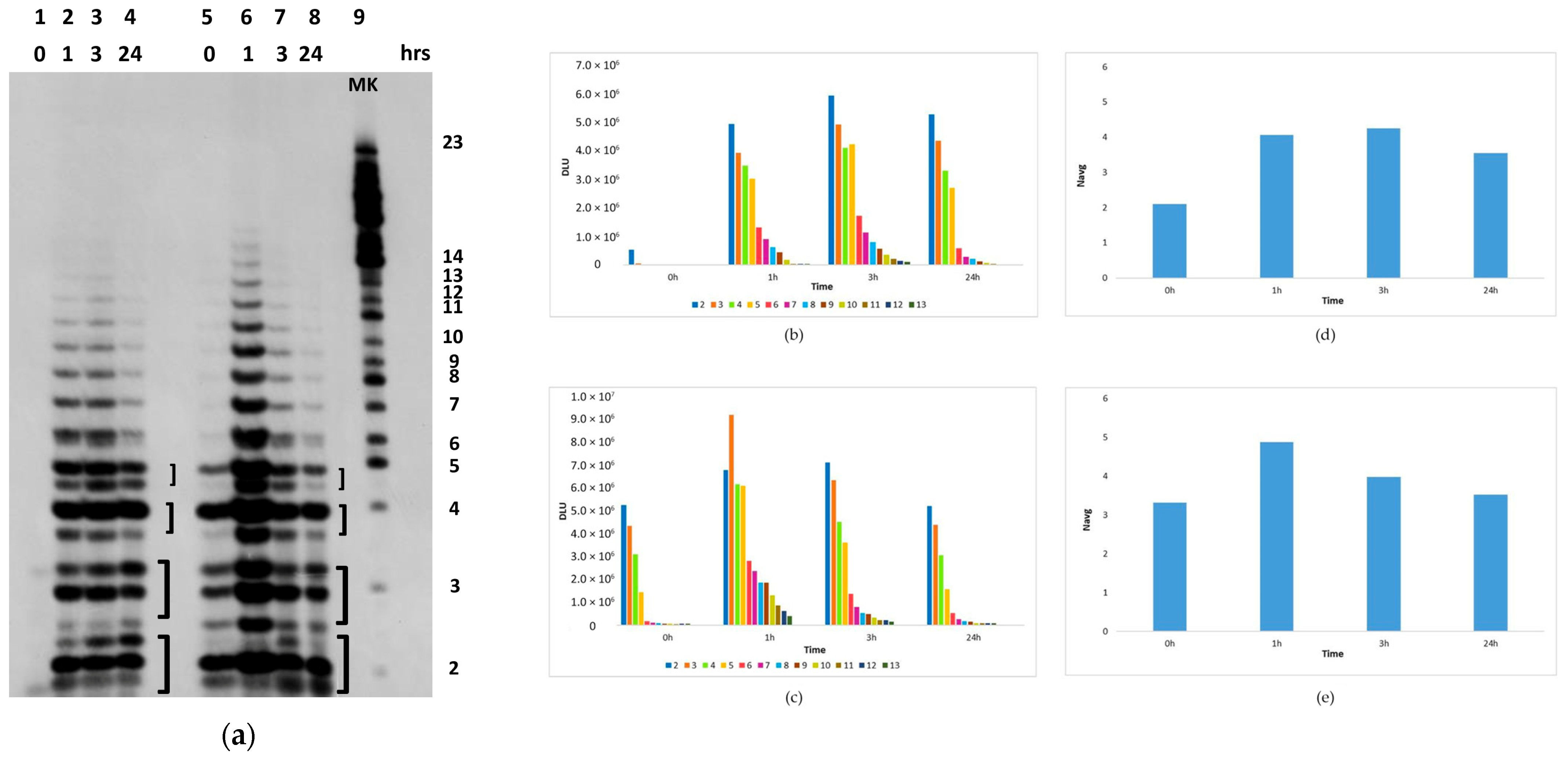
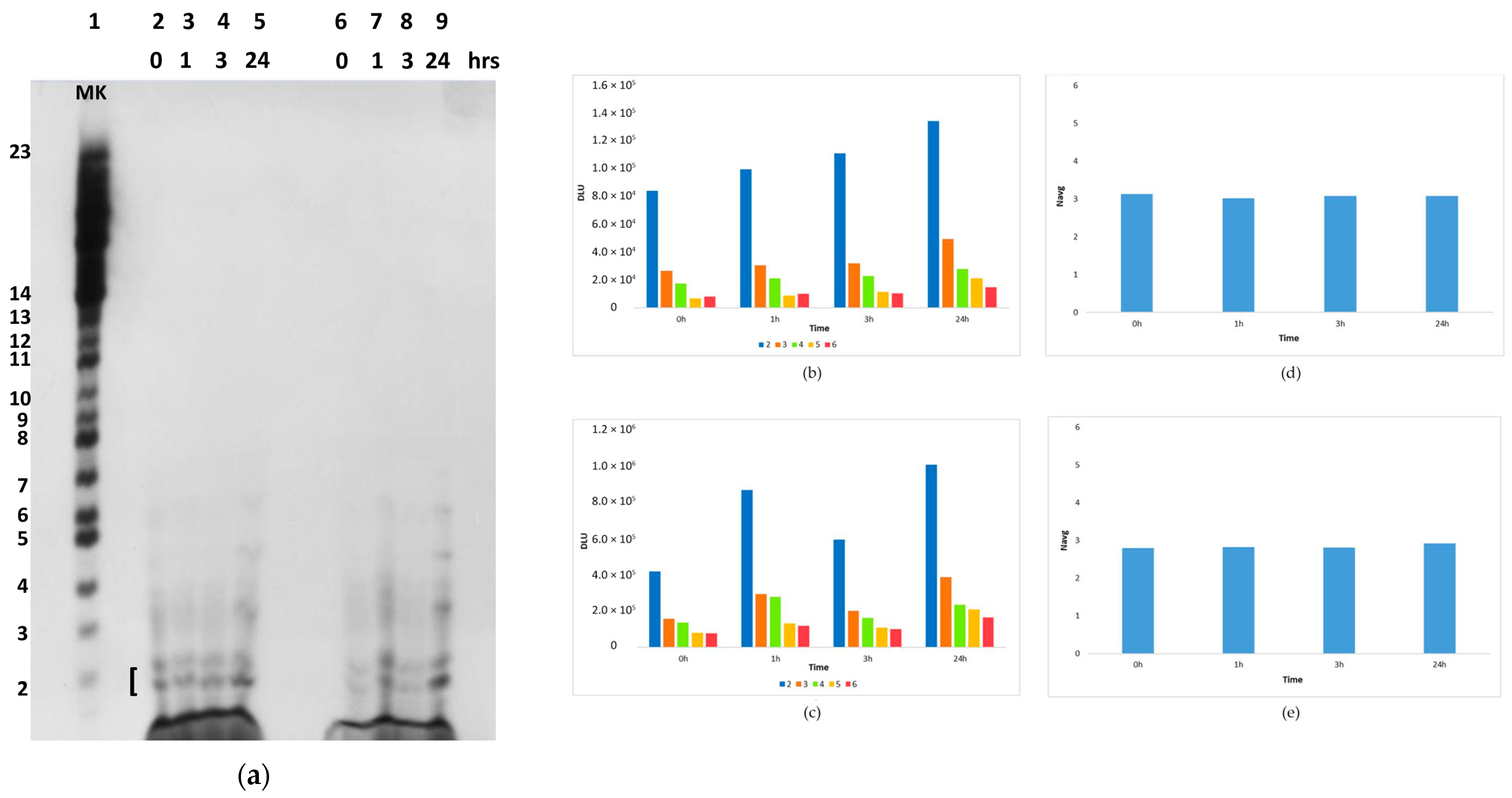
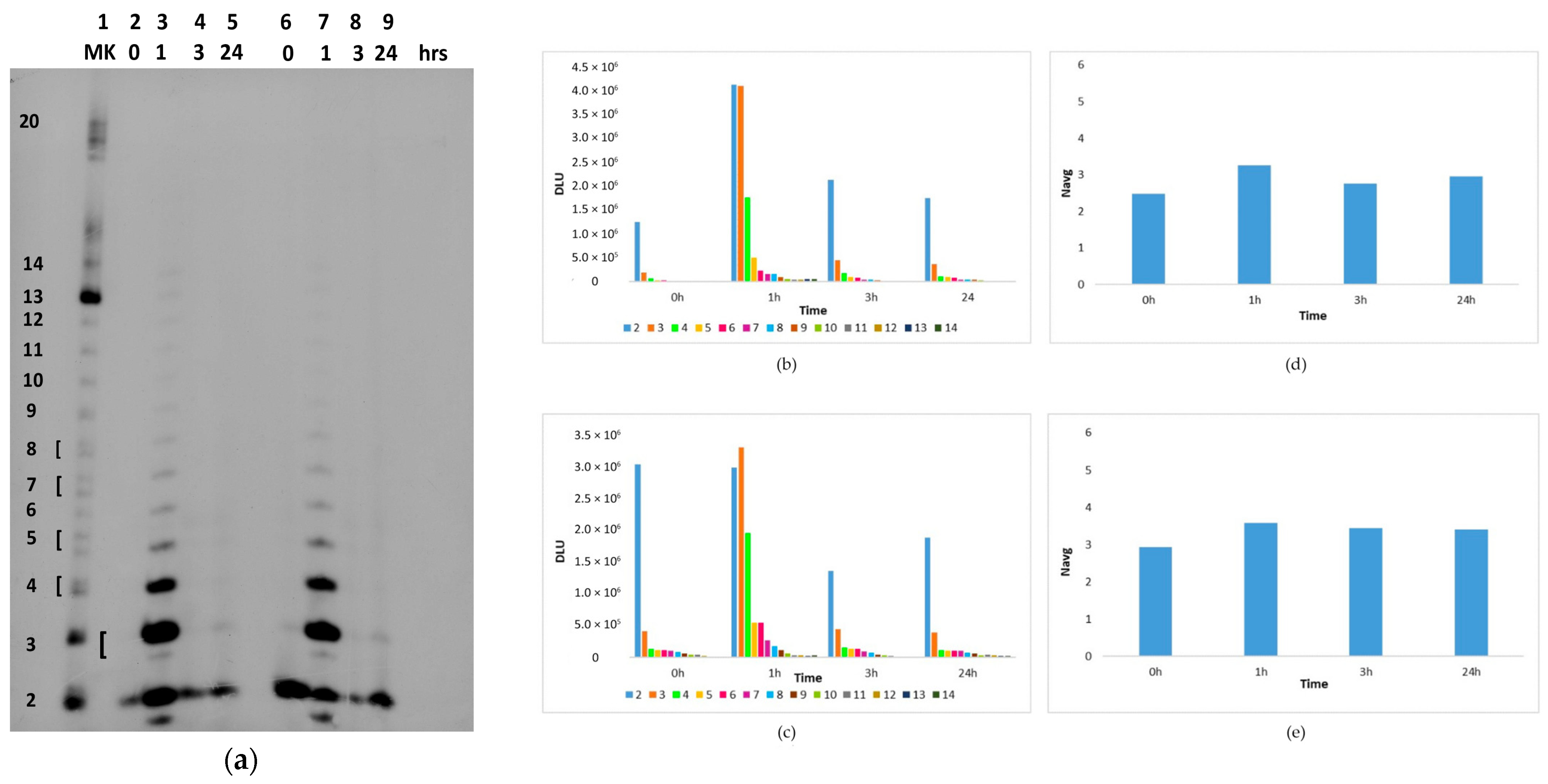
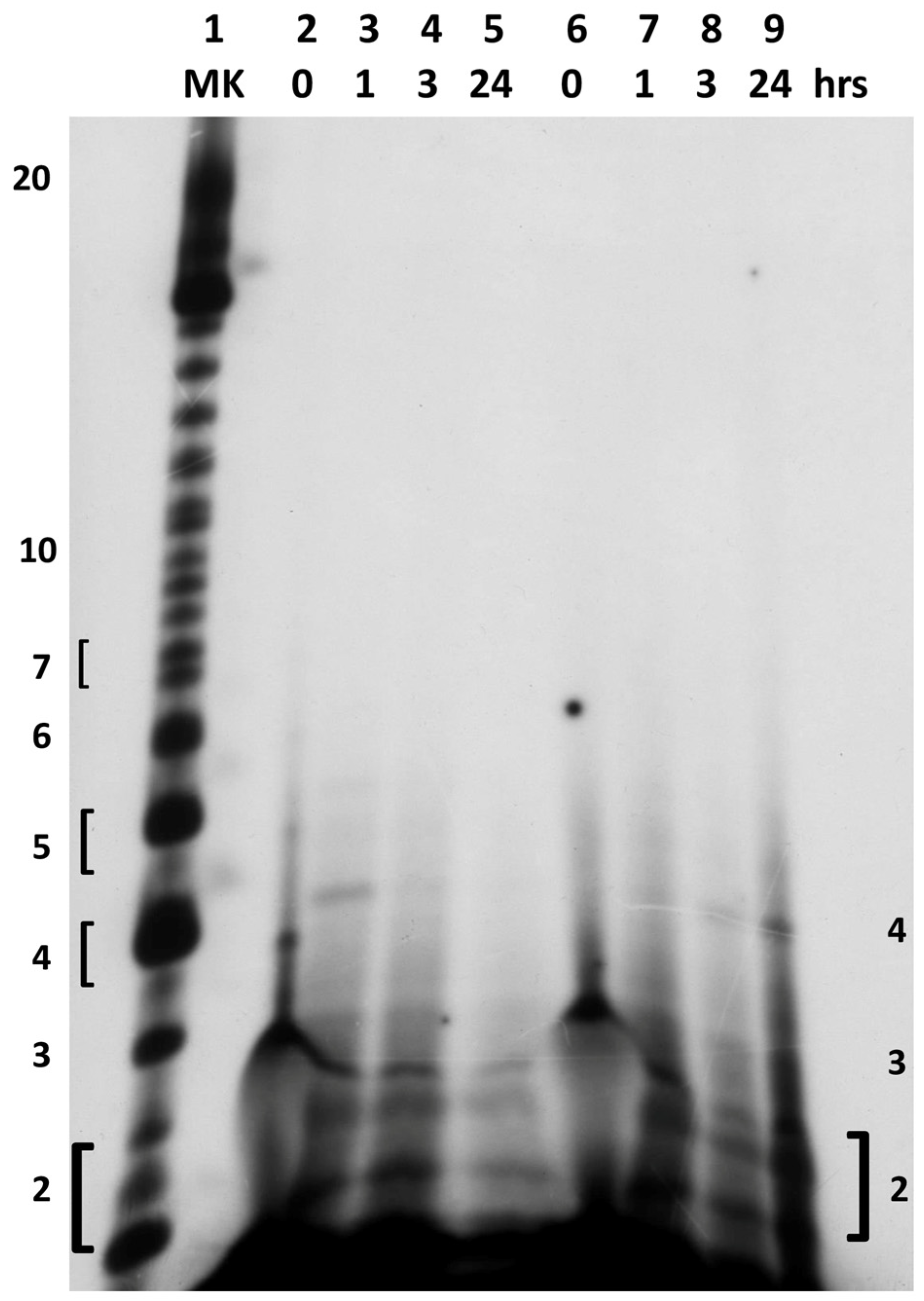
2.2. In Situ Abiotic Polymerization of cGMP
2.2.1. Water Chemistry of the Bagno dell’Acqua Lake
2.2.2. The Polymerization Reaction
2.2.3. Effect of Sunlight on the Polymerization Reaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Microbialite Powder
4.3. Setting of the Reaction
4.4. Procedure for the Derivatization of the Sample
4.5. Analysis of Reaction Products
4.6. Polymerization of 3′,5′ cGMP
4.7. 5′ Terminal Labeling of the RNA Oligomers Formed in the Polymerization Experiments
4.8. Polyacrylamide Gel Electrophoresis
4.9. Image Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RNA | RiboNucleic Acid |
| PNA | Peptide Nucleic Acid |
| cGMP | 3′,5′-cyclic guanosine monophosphate |
| DAMN | DiAminoMaleoNitrile |
| AICN | 4-AminoImidazole-5-CarboNitrile |
| DAFN | DiAminoFumaroNitrile |
| AMN | AminoMalonoNitrile |
| Gly | Glycine |
| Ala | L-Alanine |
| Val | L-Valine |
| Ser | L-Serine |
| Phe | L-Phenylalanine |
| Tyr | L-Tyrosine |
| NCA | N-CarboxyAmide |
| HPLC | High Performance Liquid Chromatography |
| PAGE | PolyAcrylamide Gel Electrophoresis |
| DLU | Digital Light Units |
| Navg | average length in Nucleotide units |
| Tris | Tris(hydroxymethyl)amino methane |
| DBU | 1,8-DiazaBicyclo[5.4.0]Undec-7-ene |
| BSTFA | N, N-Bis-trimethylSilyl TriFluoroAcetamide |
| ATP | adenosine triphosphate |
| cpm | counts per minute |
| TBE | Tris Borate Ethylenediaminetetraacetic acid |
References
- Spray, J.G.; Thompson, L.M.; Biren, M.B.; O’Connell-Cooper, C. The Manicouagan impact structure as a terrestrial analogue site for lunar and martian planetary science. Planet. Space Sci. 2010, 58, 538–551. [Google Scholar] [CrossRef]
- West, M.D.; Clarke, J.D.; Thomas, M.; Pain, C.F.; Walter, M.R. The geology of Australian Mars analogue sites. Planet. Space Sci. 2010, 58, 447–458. [Google Scholar] [CrossRef]
- Szponar, N.; Brazelton, W.J.; Schrenk, M.O.; Bower, D.M.; Steele, A.; Morrill, P.L. Geochemistry of a continental site of serpentinization, the Tablelands Ophiolite, Gros Morne National Park: A Mars analogue. Icarus 2013, 224, 286–296. [Google Scholar] [CrossRef]
- Preston, L.J.; Dartnell, L.R. Planetary habitability: Lessons learned from terrestrial analogues. Int. J. Astrobiol. 2014, 13, 81–98. [Google Scholar] [CrossRef]
- Martin, P.E.; Ehlmann, B.L.; Thomas, N.H.; Wiens, R.C.; Hollis, J.J.R.; Beegle, L.W.; Bhartia, R.; Clegg, S.M.; Blaney, D.L. Studies of a lacustrine-volcanic Mars analog field site with Mars-2020-like instruments. Earth Space Sci. 2020, 7, e2019EA000720. [Google Scholar] [CrossRef]
- Cloutis, E.; Stromberg, J.; Applin, D.; Connell, S.; Kubanek, K.; Kuik, J.; Lechowicz, A.; Parkinson, A.; Ramirez, M.; Turenne, N.; et al. The Lake St. Martin impact structure (Manitoba, Canada): A simulated rover exploration of a sulfate-bearing impact crater. Planet. Space Sci. 2021, 208, 105336. [Google Scholar] [CrossRef]
- Dypvik, H.; Hellevang, H.; Krzesińska, A.; Sætre, C.; Viennet, J.C.; Bultel, B.; Dwijesh, R.; Poulet, F.; Loizeau, D.; Veneranda, M.; et al. The Planetary Terrestrial Analogues Library (PTAL)–An exclusive lithological selection of possible Martian earth analogues. Planet. Space Sci. 2021, 208, 105339. [Google Scholar] [CrossRef]
- Ferrari, M.; De Angelis, S.; Frigeri, A.; Bruschini, E.; Gomez, F.; De Sanctis, M.C. In situ measurement and sampling of acidic alteration products at Río Tinto in support of the scientific activity of the Ma_MISS instrument. Front. Astron. Space Sci. 2023, 10, 1197724. [Google Scholar] [CrossRef]
- Mahood, G.A.; Hildreth, W. Geology of the peralkaline volcano at Pantelleria, Strait of Sicily. Bull. Volcanol. 1986, 48, 143–172. [Google Scholar] [CrossRef]
- Civetta, L.; Cornette, Y.; Crisci, G.; Gillot, P.Y.; Orsi, G.; Requeiros, C.S. Geology, geochronology and chemical evolution of the island of Pantelleria. Geol. Mag. 1984, 121, 541–562. [Google Scholar] [CrossRef]
- Aiuppa, A.; D’Alessandro, W.; Gurrieri, S.; Madonia, P.; Parello, F. Hydrologic and geochemical survey of the lake “Specchio di Venere” (Pantelleria island, Southern Italy). Environ. Geol. 2007, 53, 903–913. [Google Scholar] [CrossRef]
- Baliva, A.; Marinangeli, L.; Piluso, E.; Ori, G.G.; Ruscito, V. Minero-Petrographic Studies of a Possible Martian Analogue Environment: Specchio DI Venere Lake, Pantelleria Island (Italy); American Astronomical Society: Washington, DC, USA, 1999; DPS meeting #31, id.47.04. [Google Scholar]
- Dongarrá, G.; Hauser, S.; Alaimi, R.; Carapezza, M.; Tonani, F. Hot waters on Pantelleria island. Geochemical features and preliminary geothermal investigations. Geothermics 1983, 12, 49–63. [Google Scholar] [CrossRef]
- Cangemi, M.; Bellanca, A.S.; Hopkinson, L.; Mapelli, F.; Neri, R. The genesis of actively growing siliceous stromatolites: Evidence from Lake Specchio di Venere, Pantelleria Island. Italy Chem. Geol. 2010, 276, 318–330. [Google Scholar] [CrossRef]
- Ingrassia, M.; Conte, A.M.; Perinelli, C.; Aldega, L.; Di Bella, L.; Mazzoni, C.; Fazi, S.; Falese, F.G.; Ruspandini, T.; Piacentini, A.; et al. Experimental vs. Natural Mineral Precipitation in Modern Microbialites: The Case Study of the Alkaline Bagno Dell’acqua Lake (Pantelleria Island, Italy). Minerals 2024, 14, 1013. [Google Scholar] [CrossRef]
- Cangemi, M.; Censi, P.; Reimer, A.; D’Alessandro, W.; Hause-Reitner, D.; Madonia, P.; Oliveri, Y.; Pecoraino, G.; Reitner, J. Carbonate precipitation in the alkaline lake Specchio di Venere (Pantelleria Island, Italy) and the possible role of microbial mats. Appl. Geochem. 2016, 67, 168–176. [Google Scholar] [CrossRef]
- Singh, D.; Singh, P.; Nidhi, R.; Mukherjee, S. Investigation of mineral assemblages in a newly identified endorheic playa near Huygens basin on Mars and their astrobiological implications. Icarus 2022, 372, 114757. [Google Scholar] [CrossRef]
- Hays, L.E.; Graham, H.V.; Des Marais, D.J.; Hausrath, E.M.; Horgan, B.; McCollom, T.M.; Parenteau, M.N.; Potter-McIntyre, S.L.; Williams, A.J.; Lynch, K.L. Biosignature preservation and detection in Mars analog environments. Astrobiology 2017, 17, 363–400. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.M. Surface area control of organic carbon accumulation in continental shelf sediments. Geochim. Cosmochim. Acta 1994, 58, 1271–1284. [Google Scholar] [CrossRef]
- Hughes, C.M.; Rice, M.S.; Barnhart, C.J.; Swanson, T.E.; Pfeiffer, A.M.; Goudge, T.A. Sources of clay-rich sediment in Eberswalde crater, Mars with implications for biopreservation potential. J. Geophys. Res. Planets 2023, 128, e2022JE007545. [Google Scholar] [CrossRef]
- Salvatore, M.R.; Goudge, T.A.; Bramble, M.S.; Edwards, C.S.; Bandfield, J.L.; Amador, E.S.; Mustard, J.F.; Christensen, P.R. Bulk mineralogy of the NE Syrtis and Jezero crater regions of Mars derived through thermal infrared spectral analyses. Icarus 2018, 301, 76–96. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F. An in-situ record of major environmental transitions on early Mars at Northeast Syrtis Major. Geophys. Res. Lett. 2012, 39, L11202. [Google Scholar] [CrossRef]
- Squyres, S.W.; Arvidson, R.E.; Ruff, S.; Gellert, R.; Morris, R.V.; Ming, D.W.; Crumpler, L.; Farmer, J.D.; Des Marais, D.J.; Yen, A. Detection of Silica-Rich Deposits on Mars. Science 2008, 320, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Quantin, C.; Thollot, P.; Loizeau, D.; Ody, A.; Lozach, L. Oxia Planum, a clay-laden landing site proposed for the ExoMars Rover Mission: Aqueous mineralogy and alteration scenarios. In Proceedings of the 47th Lunar and Planetary Science Conference, Woodlands, TX, USA, 21–25 March 2016; LPI Contribution No. 1903. p. 2064. [Google Scholar]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian Surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291–315. [Google Scholar] [CrossRef]
- Bruschini, E.; Ferrari, M.; Mazzoni, C.; Fazi, S.; Latino Chiocci, F.; Mazzini, I.; Costanzo, G.; De Angelis, S.; De Sanctis, M.C.; Altieri, F.; et al. Preliminary spectroscopic investigation of a potential Mars analog site: Lake Bagno dell’Acqua, Pantelleria, Italy. Planet. Space Sci. 2024, 245, 105893. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Saladino, R.; Delfino, I.; García-Ruiz, J.M.; Di Mauro, E. Prebiotic Organic Chemistry of Formamide and the Origin of Life in Planetary Conditions: What We Know and What Is the Future. Int. J. Mol. Sci. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of Mineral Surfaces in Prebiotic Chemical Evolution. In Silico Quantum Mechanical Studies. Life 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Costanzo, G.; Negri, R.; Di Mauro, E. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide: Implications for the origin of life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciambecchini, U.; Ciciriello, F.; Costanzo, G.; Di Mauro, E. Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites. ChemBioChem 2004, 5, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Rich, A. Horizons in Biochemistry—Albert Szenl-Gyorgyi Dedicatory Volume; Academic Press, Inc.: New York, NY, USA, 1962; pp. 103–126. [Google Scholar]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Cech, T.R. “The RNA worlds in context”. Cold Spring Harb. Perspect. Biol. 2012, 4, a006742. [Google Scholar] [CrossRef] [PubMed]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, H.; Wochner, A.; Holliger, P. Freeze-thaw cycles as drivers of complex ribozyme assembly. Nat. Chem. 2015, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Montmorillonite Catalysis of 30–50 Mer Oligonucleotides: Laboratory Demonstration of Potential Steps in the Origin of the RNA World. Orig. Life Evol. Biosph. 2002, 32, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ferris, J.P. One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis. J. Am. Chem. Soc. 2006, 128, 8914–8919. [Google Scholar] [CrossRef] [PubMed]
- Weimann, B.J.; Lohrmann, R.; Orgel, L.E.; Schneide, H.; Sulston, J.E. Template-directed synthesis with adenosine-5′-phosphorimidazolide. Science 1968, 161, 387. [Google Scholar] [CrossRef] [PubMed]
- Verlander, M.S.; Lohrmann, R.; Orgel, L.E. Catalysts for the self-polymerization of adenosine cyclic 2′,3′-phosphate. J. Mol. Evol. 1973, 2, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Pino, S.; Ciciriello, F.; Di Mauro, E. Generation of Long RNA Chains in Water. J. Biol. Chem. 2009, 284, 33206–33216. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Šponer, J.E.; Šponer, J.; Cirigliano, A.; Benedetti, P.; Giliberti, V.; Polito, R.; Di Mauro, E. Sustainability and Chaos in the Abiotic Polymerization of 3′,5′ Cyclic Guanosine Monophosphate: The Role of Aggregation. ChemSystemChem 2020, 3, e2000011. [Google Scholar] [CrossRef]
- Di Giulio, M. On the RNA world: Evidence in favor of an early ribonucleopeptide world. J. Mol. Evol. 1997, 45, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Van der Gulik, P.T.S.; Speijer, D. How Amino Acids and Peptides Shaped the RNA World. Life 2015, 5, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Charles, W.C. What RNA World? Why a Peptide/RNA Partnership Merits Renewed Experimental Attention. Life 2015, 5, 294–320. [Google Scholar] [CrossRef] [PubMed]
- Mancin, E.; Capecchi, E.; Botta, L.; Bizzarri, B.M. Multicomponent Synthesis of C(8)-Substituted Purine Building Blocks of Peptide Nucleic Acids from Prebiotic Compounds. ChemistryOpen 2024, 13, e202400265. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Fanelli, A.; Cesarini, S.; Saladino, R. A Three-Way Regioselective Synthesis of Amino Acid Decorated Imidazole, Purine and Pyrimidine Derivatives by Multicomponent Chemistry Starting from Prebiotic Diaminomaleonitrile. Eur. J. Org. Chem. 2022, e202200598. [Google Scholar] [CrossRef]
- Pieck, J.C.; Kuch, D.; Grolle, F.; Linne, U.; Haas, C.; Carell, T. PNA-based reagents for the direct and site-specific synthesis of thymine dimer lesions in genomic DNA. J. Am. Chem. Soc. 2006, 128, 1404–1405. [Google Scholar] [CrossRef] [PubMed]
- Carell, T.; Brandmayr, C.; Hienzsch, A.; Müller, M.; Pearson, D.; Reiter, V.; Thoma, I.; Thumbs, P.; Wagner, M. Structure and function of noncanonical nucleobases. Angew. Chem. Int. Ed. Engl. 2012, 51, 7110–7131. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Bizzarri, B.M.; Di Mauro, E. Determinism of formamide-based biogenic prebiotic reactions. Phys. Life Rev. 2024, 51, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, C.; Piacentini, A.; Di Bella, L.; Aldega, L.; Perinelli, C.; Conte, A.M.; Ingrassia, M.; Ruspandini, T.; Bonfanti, A.; Caraba, B.; et al. Carbonate precipitation and phosphate trapping by microbialite isolates from an alkaline insular lake (Bagno dell’Acqua, Pantelleria Island, Italy). Front. Microbiol. 2024, 15, 1391968. [Google Scholar] [CrossRef] [PubMed]
- Visek, W.J.; Shoemaker, J.D. Orotic acid, arginine, and hepatotoxicity. J. Am. Coll. Nutr. 1986, 5, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kirnos, M.D.; Khudyakov, I.Y.; Alexandrushkina, N.I.; Vanyushin, B.F. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature 1977, 270, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Šponer, J.E.; Szabla, R.; Góra, R.W.; Saitta, A.M.; Pietrucci, F.; Saija, F.; Di Mauro, E.; Saladino, R.; Ferus, M.; Civiš, S.; et al. Prebiotic synthesis of nucleic acids and their building blocks at the atomic level-merging models and mechanisms from advanced computations and experiments. Phys. Chem. Chem. Phys. 2016, 18, 20047–20066. [Google Scholar] [CrossRef] [PubMed]
- Pino, S.; Sponer, J.E.; Costanzo, G.; Saladino, R.; Di Mauro, E. From formamide to RNA, the path is tenuous but continuous. Life 2015, 5, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, W.H.; Mochel, W.E. Mechanism of the Photoöxidation of Amides. J. Am. Chem. Soc. 1959, 81, 3000–3005. [Google Scholar] [CrossRef]
- Begland, R.W.; Hartter, D.R.; Jones, F.N.; Sam, D.J.; Sheppard, W.A.; Webster, O.W.; Weigert, F.J. Hydrogen cyanide chemistry. VIII. New chemistry of diaminomaleonitrile. Heterocyclic synthesis. J. Org. Chem. 1974, 39, 2341–2350. [Google Scholar] [CrossRef]
- Ferus, M.; Nesvorný, D.; Šponer, J.; Kubelík, P.; Michalčíková, R.; Shestivská, V.; Šponer, J.E.; Civiš, S. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 2015, 112, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Chada, M.S.; Subbaraman, A.S.; Kazi, Z.A.; Choughuley, A.S. A possible prebiotic synthesis of thymine: Uracil-formaldehyde-formic acid reaction. Biosystems 1977, 9, 73–80. [Google Scholar] [CrossRef]
- Julian, R.R.; Hodyss, R.; Kinnear, B.; Jarrold, M.; Beauchamp, J.L. Nanocrystalline Aggregation of Serine Detected by Electrospray Ionization Mass Spectrometry: Origin of the Stable Homochiral Gas-Phase Serine Octamer. J. Phys. Chem. B 2002, 106, 1219–1228. [Google Scholar] [CrossRef][Green Version]
- Nainyte, M.; Meller, F.; Ganazzoli, G.; Chan, C.Y.; Crisp, A.; Globisch, D.; Carell, T. Amino Acid Modified RNA Bases as Building Blocks of an Early Earth RNA-Peptide World. Chem. Eur. J. 2020, 26, 14856–14860. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.A.; Domeyer, D.M.; Scior, T.R.; Albrecht, W.; Hauser, D.R. Synthesis and biological testing of purine derivatives as potential ATP-competitive kinase inhibitors. J. Med. Chem. 2005, 48, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Toraya, T.; Kida, T.; Tanaka, S.; Matsushita, M.; Tsurukai, T.; Shiotsuka, H. Synthesis of Some 1-Methyladenine Analogs and Their Biological Activities on Starfish Oocyte Maturation. Biosci. Biotechnol. Biochem. 1998, 62, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.C.; Chern, J.W.; Lin, G.S.; Wang, A.H. Unusual conformational flexibility in N1-substituted uncommon purine nucleosides. Crystal structure of 1-allyl-isoguanosine and 1-allyl-xanthosine. FEBS Lett. 1992, 297, 4–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mas, I.; Hortelano, C.; Ruiz-Bermejo, M.; De la Fuente, J.L. Highly efficient melt polymerization of diaminomaleonitrile. Eur. Polym. J. 2021, 143, 110185. [Google Scholar] [CrossRef]
- Slavova, S.; Enchev, V. Self-catalytic mechanism of prebiotic reactions: From formamide to purine bases. Int. J. Quantum Chem. 2020, 120, e26362. [Google Scholar] [CrossRef]
- Collet, H.; Bied, C.; Mion, L.; Taillades, J.; Commeyras, A. A new simple and quantitative synthesis of α-aminoacid-N-carboxyanhydrides (oxazolidines-2,5-dione). Tetrahedron Lett. 1996, 37, 9043–9046. [Google Scholar] [CrossRef]
- Taillades, J.; Collet, H.; Garrel, L.; Beuzelin, I.; Boiteau, L.; Choukroun, H.; Commeyras, A. N-Carbamoyl Amino Acid Solid–Gas Nitrosation by NO/NOx: A New Route to Oligopeptides via α-Amino Acid N-Carboxyanhydride. Prebiotic Implications. J. Mol. Evol. 1999, 48, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, S.; Delanghe, J.R.; Speeckaert, R.; Van Biesen, W.; Speeckaert, M.M. Mechanisms and consequences of carbamoylation. Nat. Rev. Nephrol. 2017, 13, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Saladino, R.; Botta, G.; Giorgi, A.; Scipioni, A.; Pino, S.; Di Mauro, E. Generation of RNA Molecules by a Base-Catalysed Click-Like Reaction. ChemBioChem 2012, 13, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Šponer, J.E.; Šponer, J.; Giorgi, A.; Di Mauro, E.; Pino, S.; Costanzo, G. Untemplated Nonenzymatic Polymerization of 3′,5′ cGMP: A Plausible Route to 3′,5′-Linked Oligonucleotides in Primordia. J. Phys. Chem. B 2015, 119, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Morasch, M.; Mast, C.B.; Langer, J.K.; Schilcher, P.; Braun, D. Dry polymerization of 3′,5′-cyclic GMP to long strands of RNA. ChemBioChem 2014, 15, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Šponer, J.E.; Šponer, J.; Nováková, O.; Brabec, V.; Šedo, O.; Zdráhal, Z.; Costanzo, G.; Pino, S.; Saladino, R.; Di Mauro, E. Emergence of the first catalytic oligonucleotides in a formamide-based origin scenario. Chem.-Eur. J. 2016, 22, 3572–3586. [Google Scholar] [CrossRef] [PubMed]
- Foerstner, H. Nota preliminare sulla geologia dell’isola di Pantelleria secondo gli studi fatti negli anni 1874 e 1881. Boll. R. Com. Geol. Ital. 1881, 12, 532–556. [Google Scholar]
- Duchi, V.; Campana, M.E.; Minissale, A.; Thompson, M. Geochemistry of thermal fluids on the volcanic isle of Pantelleria, southern Italy. Appl. Geochem. 1994, 9, 147–160. [Google Scholar] [CrossRef]
- Azzaro, E.; Badalamenti, F.; Dongarrà, G.; Hauser, S. Geochemical and mineralogical studies of lake Specchio di Venere, Pantelleria island, Italy. Chem. Geol. 1983, 40, 149–165. [Google Scholar] [CrossRef]
- Bocchi, G.; Gabbianelli, G.; Lanzafame, G.; Lucchini, F.; Rabbi, E.; Rossi, P.L. Relazione sui rilievi eseguiti sul Lago di Venere, Pantelleria. Bull. Ital. Natl. Group Volcanol. 1988, 4, 63–73. [Google Scholar]
- D’Alessandro, W.; Dongarra, G.; Gurrieri, S.; Parello, F.; Valenza, M. Geochemical characterization of naturally occurring fluids on the Island of Pantelleria (Italy). Min. Petrogr. Acta 1994, 37, 91–102. [Google Scholar]
- Parello, F.; Allard, P.; D’Alessandro, W.; Federico, C.; Jean-Baptiste, P.; Catani, O. Isotope geochemistry of Pantelleria volcanic fluids. Sicily Channel rift: A mantle volatile end-member for volcanism in Southern Europe. Earth Planet. Sci. Lett. 2000, 180, 325–339. [Google Scholar] [CrossRef]
- Jácome Paz, M.; Inguaggiato, S.; Taran, Y.; Vita, F.; Pecoraino, G. Carbon dioxide emissions from Specchio di Venere, Pantelleria, Italy. Bull. Volcanol. 2016, 78, 29. [Google Scholar] [CrossRef]
- Cohen, Z.R.; Ding, D.; Zhou, L.; DasGupta, S.; Haas, S.; Sinclair, K.P.; Todd, Z.R.; Black, R.A.; Szostak, J.W.; Catling, D.C. Natural soda lakes provide compatible conditions for RNA and membrane function that could have enabled the origin of life. PNAS Nexus 2024, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Yamaguchi, E. Synthesis of well-defined thermoresponsive polyphosphoester macroinitiators using organocatalysts. Macromolecules 2010, 43, 2664–2666. [Google Scholar] [CrossRef]
- Steinbach, T.; Ritz, S.; Wurm, F.R. Water-soluble poly(phosphonate)s via living ring-opening polymerization. ACS Macro Lett. 2014, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Soukup, G.A.; Breaker, R.R. Relationship between internucleotide linkage geometry and the stability of RNA. RNA 1999, 5, 1308–1325. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, P.; Oivanen, M.; Lonnberg, H. Interconversion and phosphoester hydrolysis of 2′, 5′- and 3′, 5′- dinucleoside mono-phosphates: Kinetics and mechanisms. J. Org. Chem. 1991, 56, 5396–5401. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Dibrova, D.V.; Bychkov, A.Y. Origin of the RNA world in cold Hadean geothermal fields enriched in zinc and potassium: Abiogenesis as a positive fallout from the Moon-forming impact? Life 2025, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Sasselov, D.D. Influence of the UV Environment on the Synthesis of Prebiotic Molecules. Astrobiology 2016, 16, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Barks, H.L.; Buckley, R.; Grieves, G.A.; Di Mauro, E.; Hud, N.V.; Orlando, T.M. Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: Relaxation of the requirements for prebiotic purine nucleobase formation. Chembiochem 2010, 11, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, A.A.; Zhang, Y.; de Vries, M.S.; Kohler, B. Life in the light: Nucleic acid photoproperties as a legacy of chemical evolution. Phys. Chem. Chem. Phys. 2016, 18, 24228–24238. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, L.; Richterb, C.; Albini, A. Solar light induced carbon–carbon bond formation via TiO2 photocatalysis. Chem. Commun. 1998, 7, 805–806. [Google Scholar] [CrossRef]



| Entry | Product | RT (a) | Amount of Product (μg/mg) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 + 2 + 3a (A) | 1 + 2 + 3b (B) | 1 + 2 + 3c (C) | 1 + 2 + 3d (D) | 1 + 2 + 3e (E) | 1 + 2 + 3f (F) | |||
| Nucleobases and analogues | ||||||||
| 1 |  4 | 12.87 (d) | 1.26 | 0.26 | 1.65 | 4.78 | 0.33 | 0.44 |
| 2 | 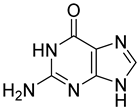 5 | 21.04 (d) | 9.3 | 6.57 | 1.46 | 2.90 | 1.49 | 0.98 |
| 3 | 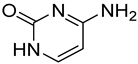 6 | 11.20 (d) | 0.65 | 1.72 | 0.32 | 6.47 | 0.16 | 0.45 |
| 4 | 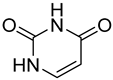 7 | 9.82 (c) | / | 0.66 | 8.22 | 12.05 | 3.43 | 2.52 |
| 5 |  8 | 7.50 (b) | 1.98 | 1.11 | 8.89 | 5.88 | 5.77 | 7.39 |
| 6 | 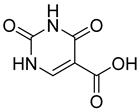 9 | 10.46 (d) | 1.05 | 0.11 | 1.23 | 5.26 | 0.12 | 0.27 |
| 7 | 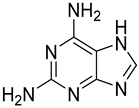 10 | 4.65 (d) | 0.31 | Traces | Traces | Traces | Traces | Traces |
| 8 |  11 | 6.81 (d) | / | 1.30 | / | / | / | / |
| PNA’s building blocks | ||||||||
| 9 |  12 | 16.89 (c) | 0.70 | / | / | / | / | / |
| 10 | 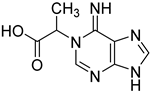 13 | 11.06 | / | 1.83 | / | / | / | / |
| 11 | 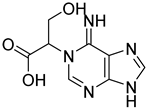 14 | 13.49 | / | / | / | 0.04 | / | / |
| 12 | 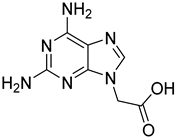 15 | 10.11 (d) | 1.02 | / | / | / | / | / |
| 13 | 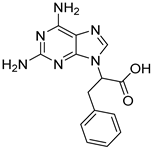 16 | 9.79 | / | / | / | / | 2.29 | / |
| 14 | 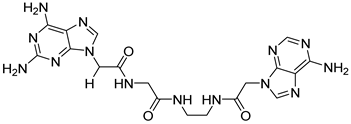 17 | 20.28 | 0.45 | / | / | / | / | / |
| Peptides and miscellaneous | ||||||||
| 15 | 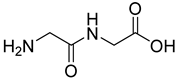 18 | 6.99 (e) | 122 | / | / | / | / | / |
| 16 | 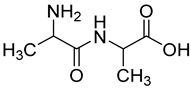 19 | 4.34 (d) | / | 315.04 | / | / | / | / |
| 17 |  20 | 8.61 (c) | / | / | 5.57 | / | / | / |
| 18 |  21 | 10.98 (c) | / | / | / | 4.10 | / | / |
| 19 | 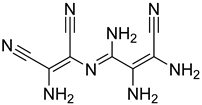 22 | 7.27 (b) | 59.5 | 21.61 | 4.81 | 5.93 | 3.41 | 3.45 |
| 20 |  23 | 8.55 (b) | 163 | / | / | 18.55 | / | / |
| 21 | 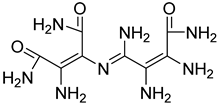 24 | 13.30 (c) | 1.05 | 2.75 | 1.41 | 0.53 | / | / |
| 22 | 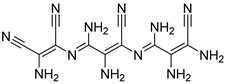 25 | 12.08 (c) | 320.6 | 242.41 | 194.20 | 164.98 | 124.89 | 168.33 |
| 23 | 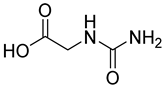 26 | 6.20 (b) | 25.34 | / | / | / | / | / |
| 24 | 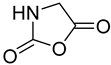 27 | 8.33 (c) | 49.53 | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubertini, V.; Mancin, E.; Bruschini, E.; Ferrari, M.; Piacentini, A.; Fazi, S.; Mazzoni, C.; Bizzarri, B.M.; Saladino, R.; Costanzo, G. The “Bagno dell’Acqua” Lake as a Novel Mars-like Analogue: Prebiotic Syntheses of PNA and RNA Building Blocks and Oligomers. Int. J. Mol. Sci. 2025, 26, 6952. https://doi.org/10.3390/ijms26146952
Ubertini V, Mancin E, Bruschini E, Ferrari M, Piacentini A, Fazi S, Mazzoni C, Bizzarri BM, Saladino R, Costanzo G. The “Bagno dell’Acqua” Lake as a Novel Mars-like Analogue: Prebiotic Syntheses of PNA and RNA Building Blocks and Oligomers. International Journal of Molecular Sciences. 2025; 26(14):6952. https://doi.org/10.3390/ijms26146952
Chicago/Turabian StyleUbertini, Valentina, Eleonora Mancin, Enrico Bruschini, Marco Ferrari, Agnese Piacentini, Stefano Fazi, Cristina Mazzoni, Bruno Mattia Bizzarri, Raffaele Saladino, and Giovanna Costanzo. 2025. "The “Bagno dell’Acqua” Lake as a Novel Mars-like Analogue: Prebiotic Syntheses of PNA and RNA Building Blocks and Oligomers" International Journal of Molecular Sciences 26, no. 14: 6952. https://doi.org/10.3390/ijms26146952
APA StyleUbertini, V., Mancin, E., Bruschini, E., Ferrari, M., Piacentini, A., Fazi, S., Mazzoni, C., Bizzarri, B. M., Saladino, R., & Costanzo, G. (2025). The “Bagno dell’Acqua” Lake as a Novel Mars-like Analogue: Prebiotic Syntheses of PNA and RNA Building Blocks and Oligomers. International Journal of Molecular Sciences, 26(14), 6952. https://doi.org/10.3390/ijms26146952