Mouse PrimPol Outperforms Its Human Counterpart as a Robust DNA Primase
Abstract
1. Introduction
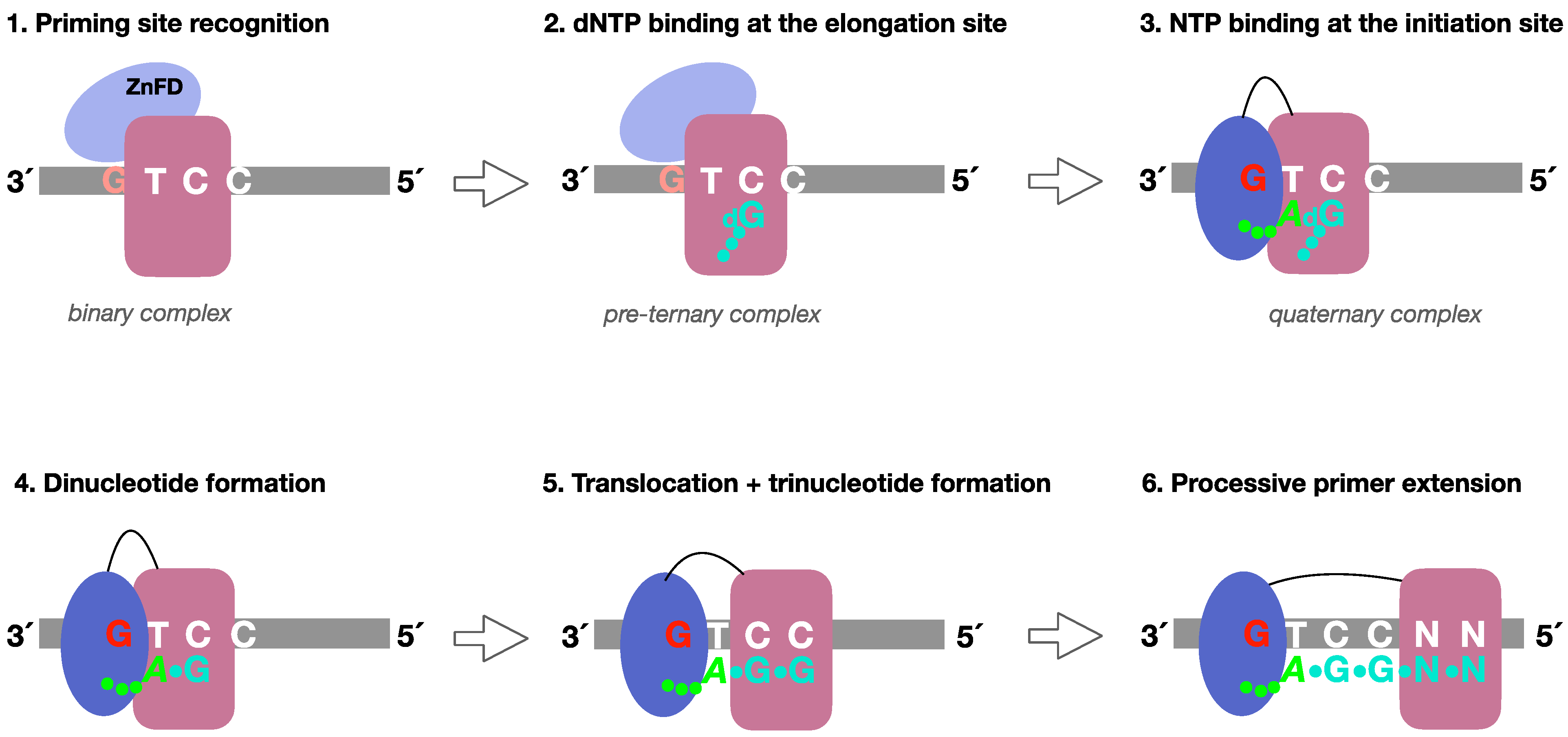
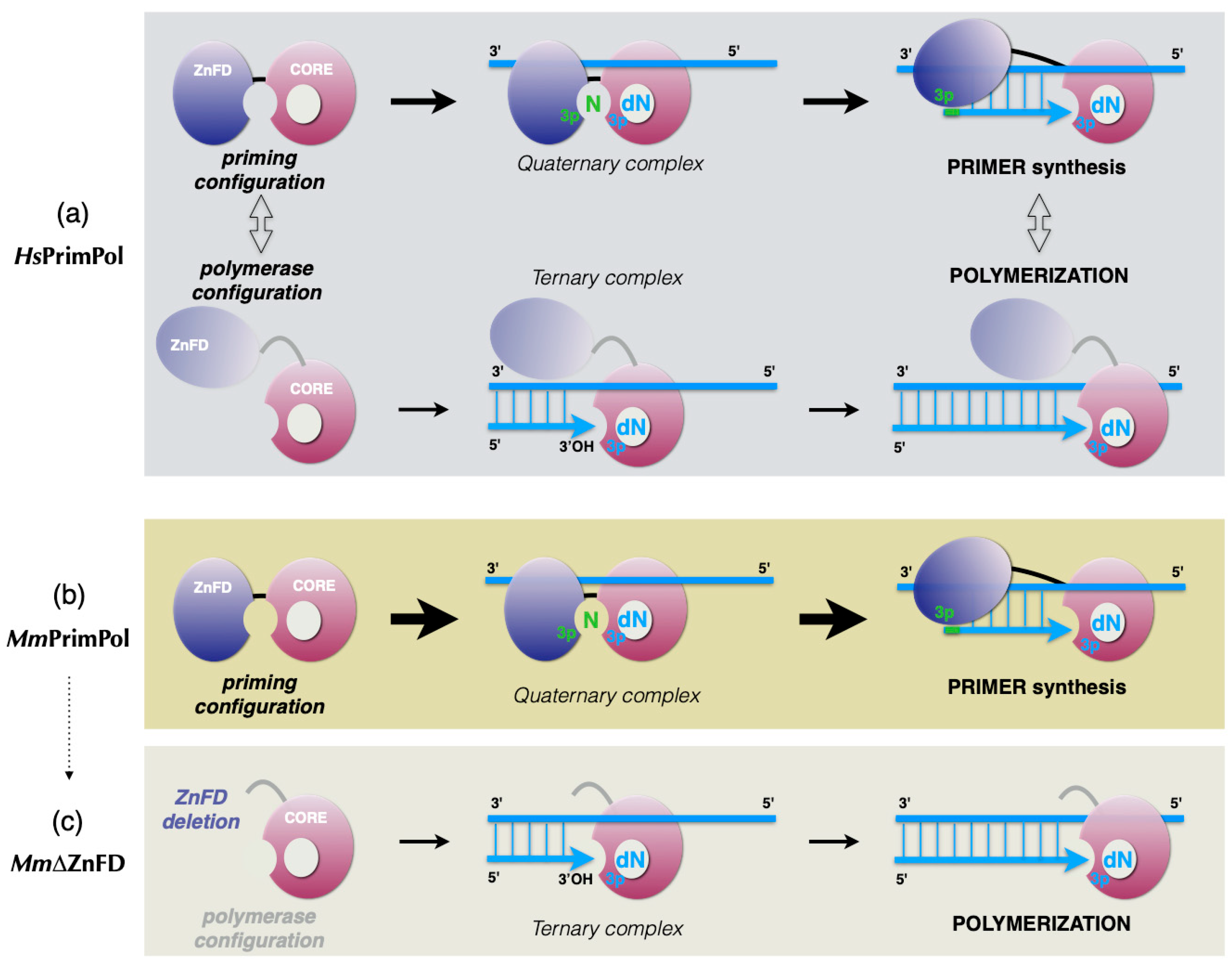
- (1)
- Formation of a PrimPol/ssDNA binary complex.
- (2)
- Further interaction with the incoming 3′-deoxynucleotide (pre-ternary complex).
- (3)
- The ZnFD is mobilized to facilitate binding and selection of the 5′-nucleoside triphosphate (preferentially a ribonucleotide), that will become the first nucleotide of the newly synthesized primer.
- (4)
- Once the 5′-nucleotide (acting as a primer) and the 3′-nucleotide are selected and a quaternary complex is formed, catalysis of the initial dimer occurs.
- (5)
- A maintained interaction of the ZnFD with the 5′-terminal triphosphate is essential for the subsequent translocation and insertion event to form a trimer.
- (6)
- Further elongation of the primer occurs processively, i.e., with no dissociation, thanks to the sustained interaction of the ZnFD with the initiating nucleotide. When the primer reaches an optimal length, PrimPol dissociates and the primer is extended by the replicative DNA polymerase.
2. Results
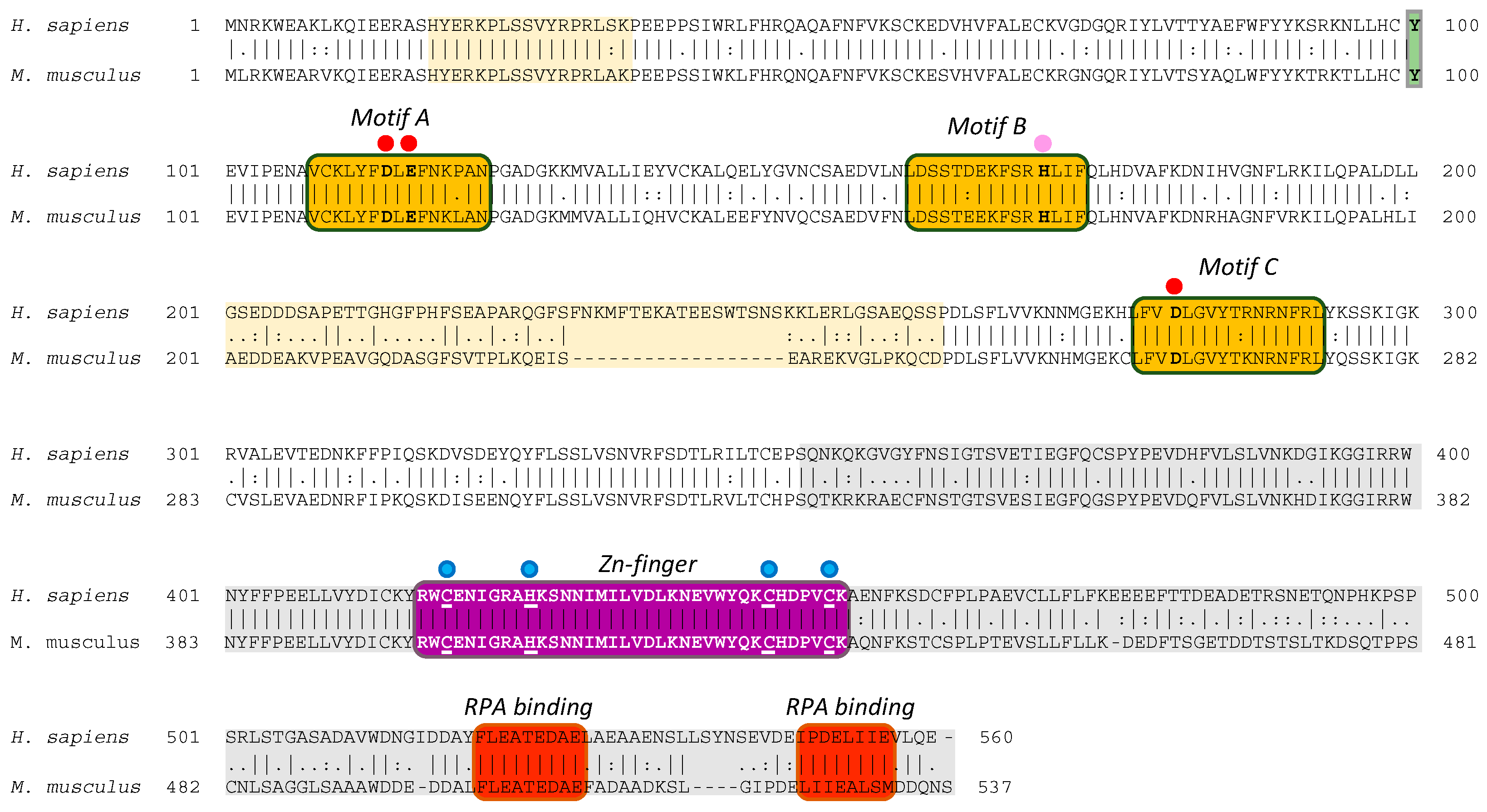
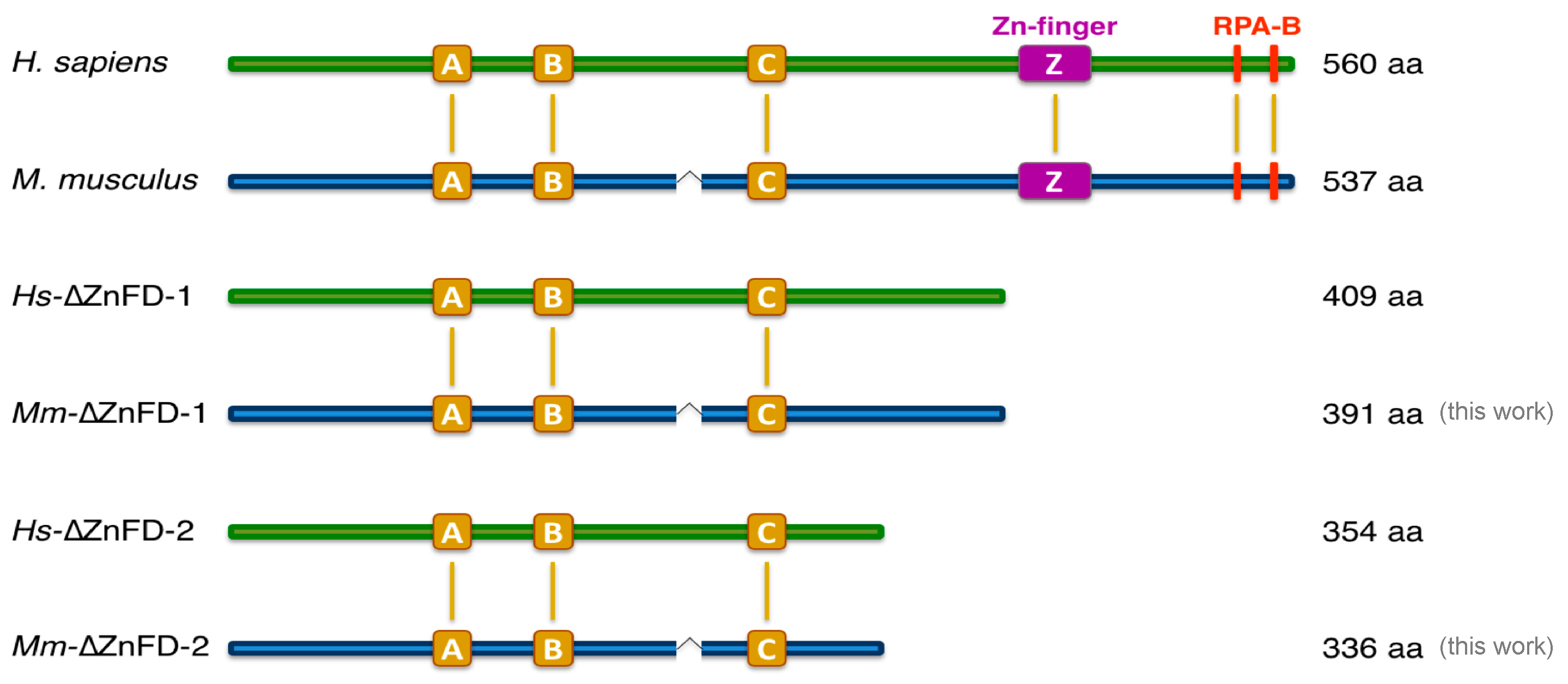
2.1. The Mouse and Human PrimPol Share High Amino Acid Sequence Similarity
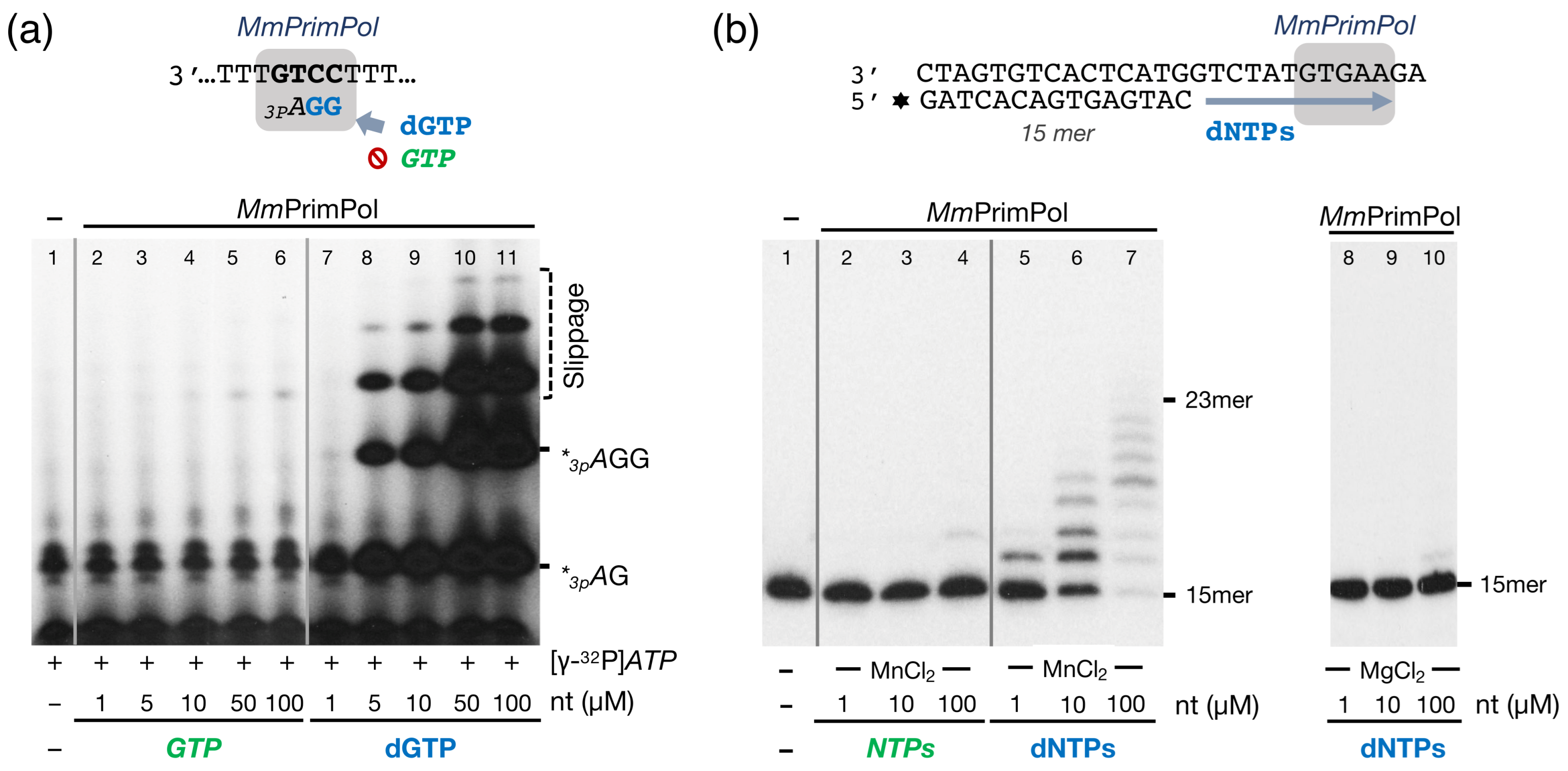
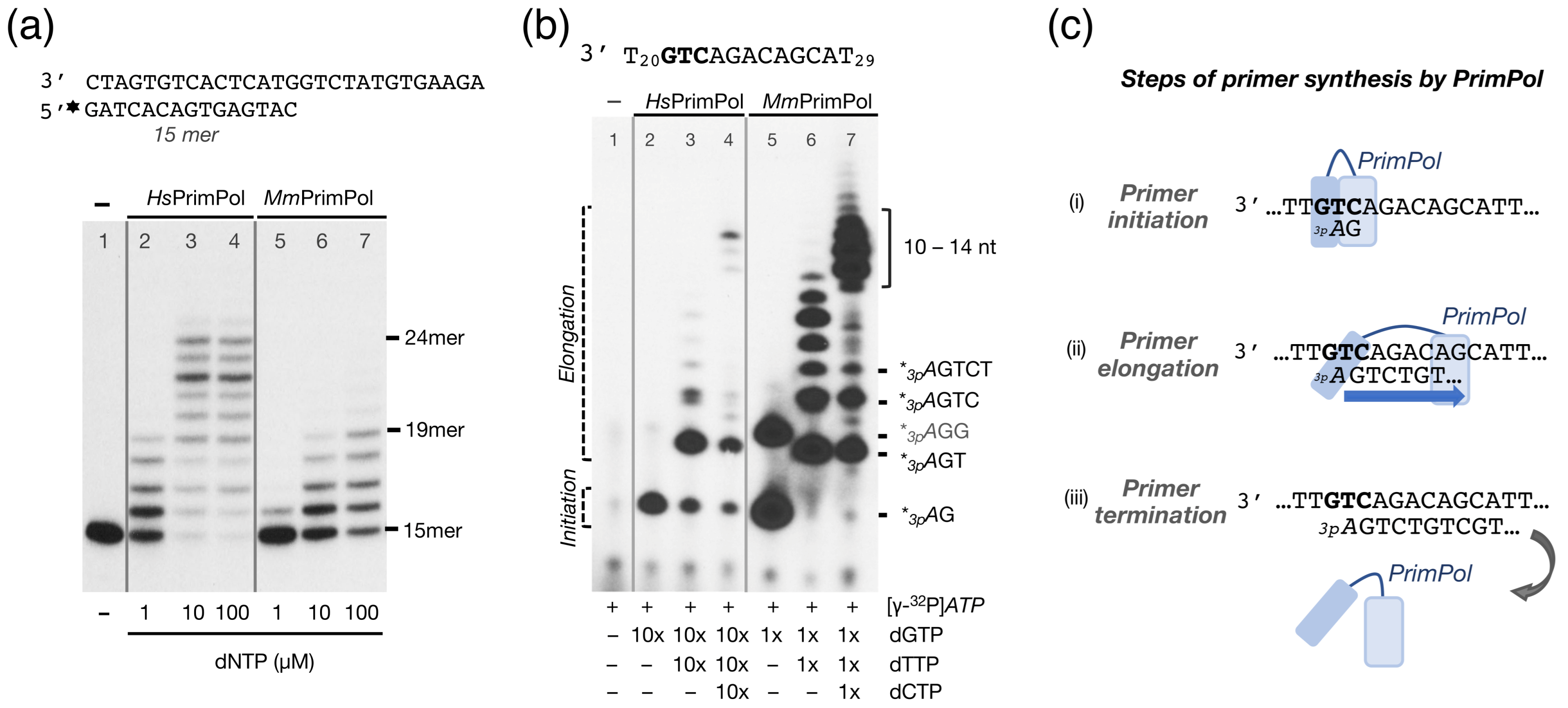
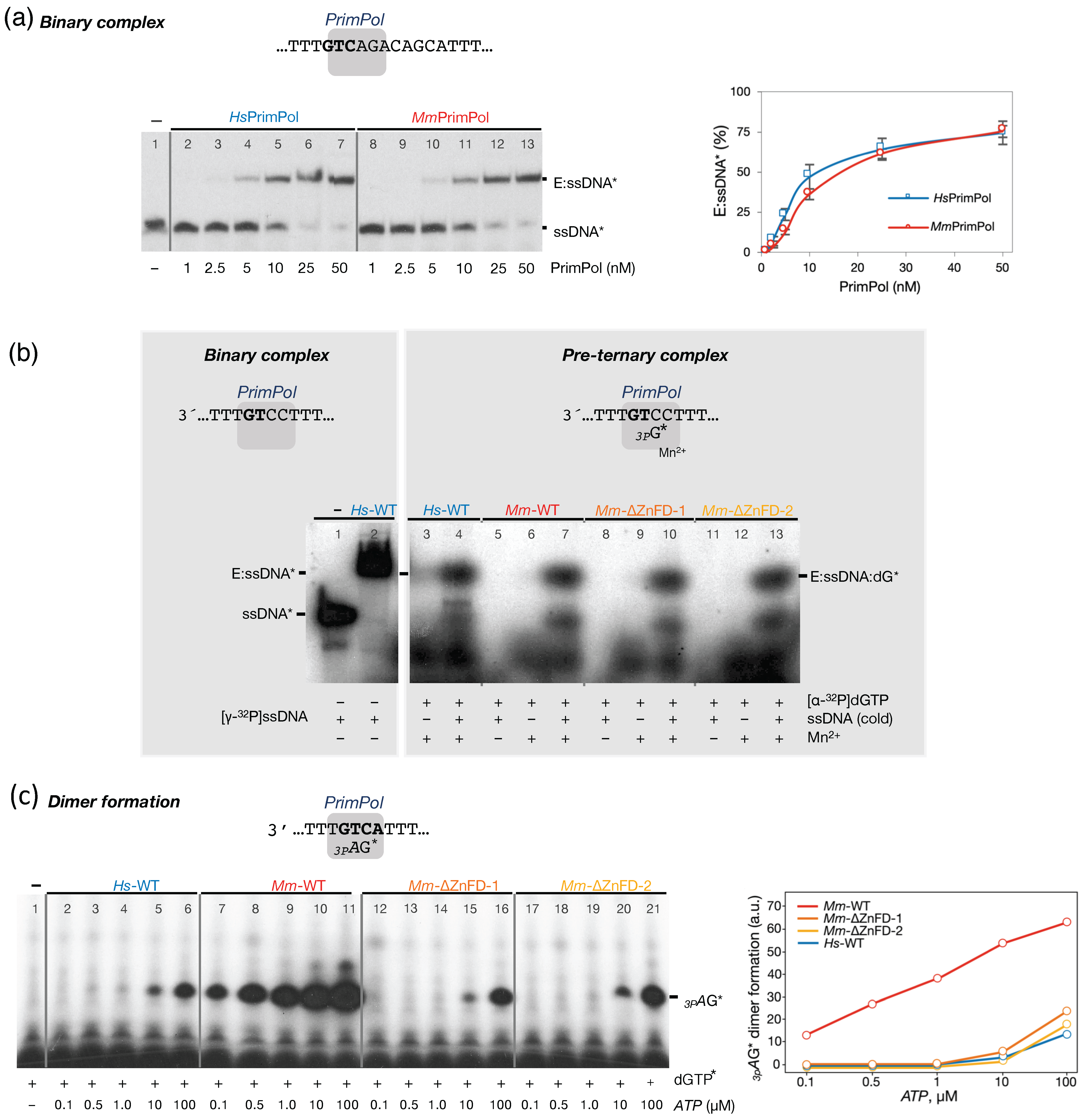
2.2. The Mouse PrimPol Is a True DNA Primase
2.3. The Mouse PrimPol Has a Strong Preference to Initiate and Complete Primer Synthesis, but Not to Extend Pre-Existing Primer Chains
2.4. The Zinc-Finger Domain of the Mouse PrimPol Brakes Its DNA Polymerase Activity but Is Essential for Its Strong DNA Primase Activity
2.5. The Mouse and Human PrimPols Form Similar Binary and Pre-Ternary Complexes as Primase Intermediates
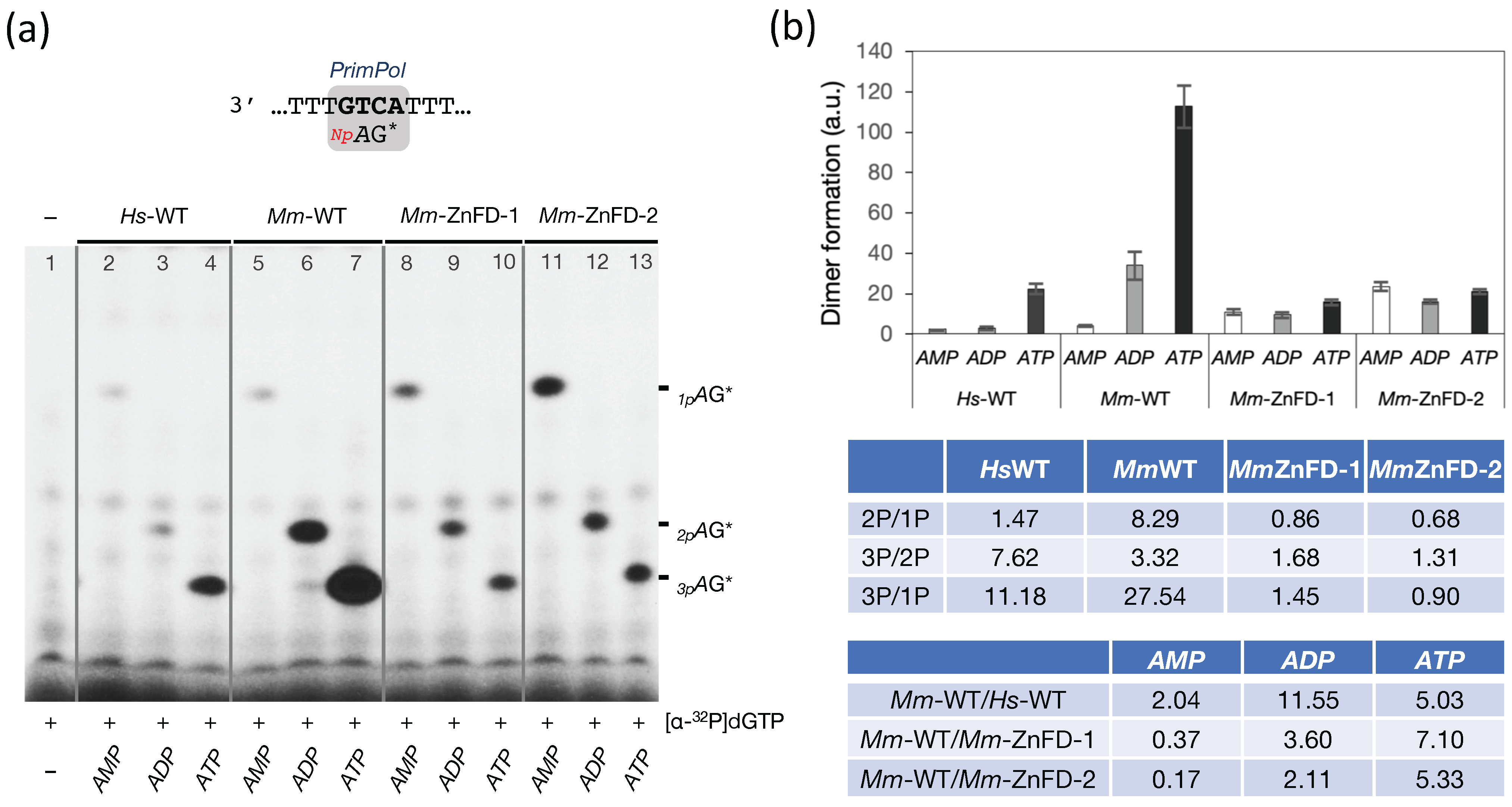
2.6. The Zn-Finger Domain of the Mouse PrimPol Confers High Affinity for the Initiating 5′-Ribonucleotide
2.7. The Zn-Finger Domain of the Mouse PrimPol Likely Interacts with the β- and γ-Phosphate Moiety of the 5′-Initiating Ribonucleotide
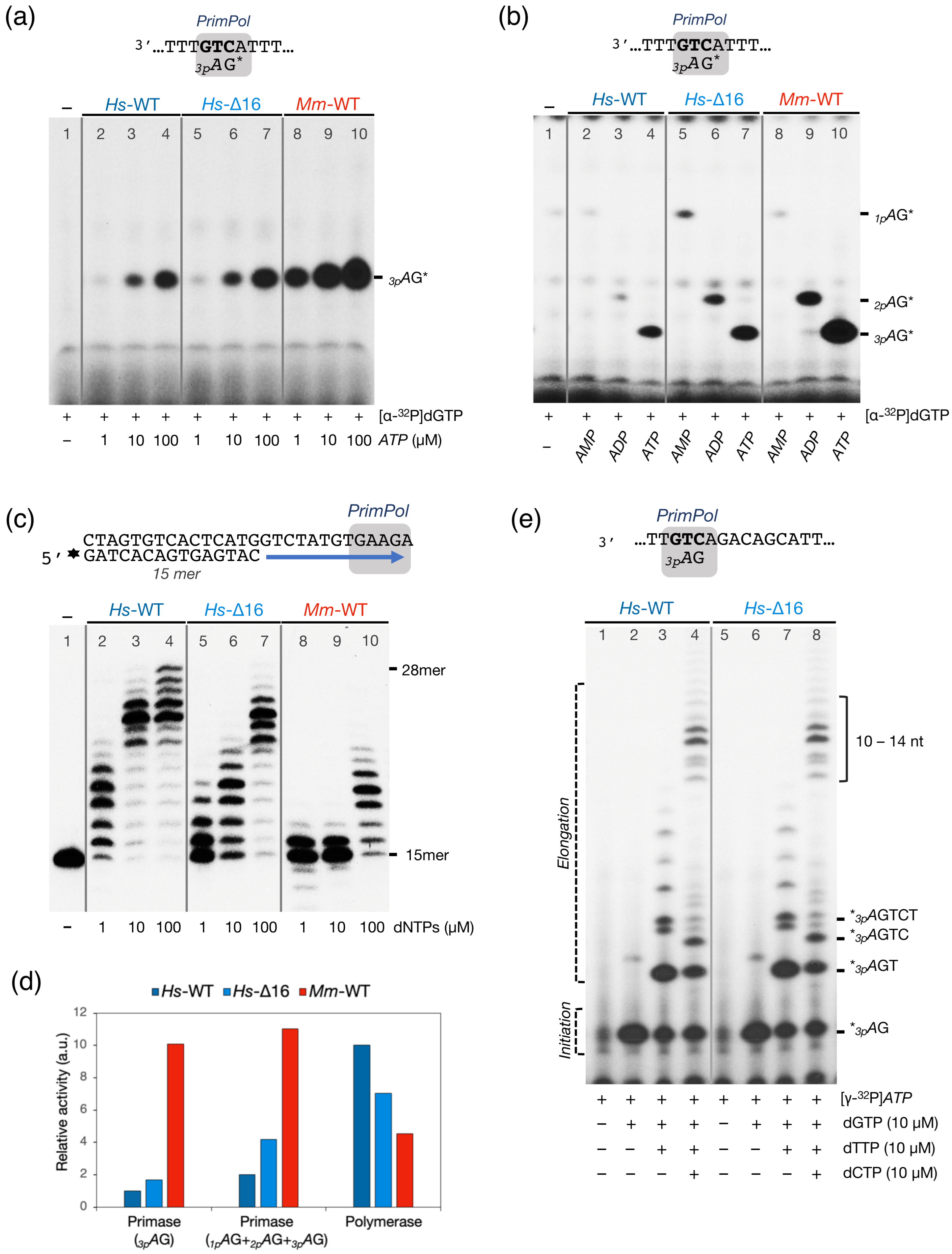
2.8. Deletion of HsPrimPol Flexible Region (∆231–246) Alters Its DNA Polymerase-DNA Primase Balance
3. Discussion
3.1. Initial Closing and Progressive Mobilization of the ZnFD During DNA Primer Synthesis: A Model
3.2. Mouse PrimPol: Need for a Strong Primase?
4. Materials and Methods
4.1. Reagents
4.2. Oligonucleotides
4.3. Cloning the Mouse PrimPol and Mutant Generation
4.4. Purification of the Human and Mouse PrimPol Variants
4.5. Polymerase Assay on Specific Primer: Template Molecules
4.6. Primase Assay on Specific Oligonucleotide Templates
4.7. EMSA for PrimPol/ssDNA Binary Complex
4.8. EMSA for PrimPol/ssDNA/dNTP Pre-Ternary Complex
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HsPrimPol SOFTWARE VERSION INCLUDED | Homo sapiens PrimPol |
| MmPrimPol | Mus musculus PrimPol |
| AEP | Archaeo-Eukaryotic Primases |
| ZnFD | Zinc Finger Domain |
| dNTPs | deoxynucleoside triphosphates |
| NTPs | ribonucleoside triphosphates |
References
- Frick, D.N.; Richardson, C.C. DNA Primases. Annu. Rev. Biochem. 2001, 70, 39–80. [Google Scholar] [CrossRef] [PubMed]
- Laosirieix, S.; Pellegrini, L.; Bell, S. The Promiscuous Primase. Trends Genet. 2005, 21, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, R.D.; Stengel, G. Mechanism and Evolution of DNA Primases. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Bergsch, J.; Allain, F.H.-T.; Lipps, G. Recent Advances in Understanding Bacterial and Archaeoeukaryotic Primases. Curr. Opin. Struct. Biol. 2019, 59, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Stodola, J.L.; Burgers, P.M. Mechanism of Lagging-Strand DNA Replication in Eukaryotes. In DNA Replication; Advances in Experimental Medicine and, Biology; Masai, H., Foiani, M., Eds.; Springer Singapore: Singapore, 2017; Volume 1042, pp. 117–133. [Google Scholar] [CrossRef]
- Baranovskiy, A.; Tahirov, T. Elaborated Action of the Human Primosome. Genes 2017, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M. Origin and Evolution of the Archaeo-Eukaryotic Primase Superfamily and Related Palm-Domain Proteins: Structural Insights and New Members. Nucleic Acids Res. 2005, 33, 3875–3896. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, S.; Reyes, A.; Martínez-Jiménez, M.I.; Chocrón, E.S.; Mourón, S.; Terrados, G.; Powell, C.; Salido, E.; Méndez, J.; Holt, I.J.; et al. PrimPol, an Archaic Primase/Polymerase Operating in Human Cells. Mol. Cell 2013, 52, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Mourón, S.; Rodriguez-Acebes, S.; Martínez-Jiménez, M.I.; García-Gómez, S.; Chocrón, S.; Blanco, L.; Méndez, J. Repriming of DNA Synthesis at Stalled Replication Forks by Human PrimPol. Nat. Struct. Mol. Biol. 2013, 20, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.A.; Sastre-Moreno, G.; Perpiñá, C.; Guerra, S.; Martínez-Jiménez, M.I.; Blanco, L. The Invariant Glutamate of Human PrimPol DxE Motif Is Critical for Its Mn2+-Dependent Distinctive Activities. DNA Repair 2019, 77, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Lou, J.; Xia, Y.; Su, B.; Liu, T.; Cui, J.; Sun, Y.; Lou, H.; Huang, J. hPrimpol1/CCDC111 Is a Human DNA Primase-polymerase Required for the Maintenance of Genome Integrity. EMBO Rep. 2013, 14, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Ruiz, C.; Blanco, L.; Martínez-Jiménez, M.I. 3′dNTP Binding Is Modulated during Primer Synthesis and Translesion by Human PrimPol. Int. J. Mol. Sci. 2023, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.; Díaz-Talavera, A.; Calvo, P.A.; Blanco, L.; Martínez-Jiménez, M.I. Human PrimPol Discrimination against Dideoxynucleotides during Primer Synthesis. Genes 2021, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Talavera, A.; Calvo, P.A.; González-Acosta, D.; Díaz, M.; Sastre-Moreno, G.; Blanco-Franco, L.; Guerra, S.; Martínez-Jiménez, M.I.; Méndez, J.; Blanco, L. A Cancer-Associated Point Mutation Disables the Steric Gate of Human PrimPol. Sci. Rep. 2019, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Keen, B.A.; Jozwiakowski, S.K.; Bailey, L.J.; Bianchi, J.; Doherty, A.J. Molecular Dissection of the Domain Architecture and Catalytic Activities of Human PrimPol. Nucleic Acids Res. 2014, 42, 5830–5845. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, M.I.; Calvo, P.A.; García-Gómez, S.; Guerra-González, S.; Blanco, L. The Zn-Finger Domain of Human PrimPol Is Required to Stabilize the Initiating Nucleotide during DNA Priming. Nucleic Acids Res. 2018, 46, 4138–4151. [Google Scholar] [CrossRef] [PubMed]
- Guilliam, T.A.; Brissett, N.C.; Ehlinger, A.; Keen, B.A.; Kolesar, P.; Taylor, E.M.; Bailey, L.J.; Lindsay, H.D.; Chazin, W.J.; Doherty, A.J. Molecular Basis for PrimPol Recruitment to Replication Forks by RPA. Nat. Commun. 2017, 8, 15222. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Weller, S.K. A Mutation in the C-terminal Putative Zn2+ Finger Motif of UL52 Severely Affects the Biochemical Activities of the HSV-1 Helicase-Primase Subcomplex. J. Biol. Chem. 1999, 274, 8068–8076. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Carrington-Lawrence, S.D.; Bai, P.; Weller, S.K. Mutations in the Putative Zinc-Binding Motif of UL52 Demonstrate a Complex Interdependence between the UL5 and UL52 Subunits of the Human Herpes Simplex Virus Type 1 Helicase/Primase Complex. J. Virol. 2005, 79, 9088–9096. [Google Scholar] [CrossRef] [PubMed]
- Quinet, A.; Tirman, S.; Jackson, J.; Šviković, S.; Lemaçon, D.; Carvajal-Maldonado, D.; González-Acosta, D.; Vessoni, A.T.; Cybulla, E.; Wood, M.; et al. PRIMPOL-Mediated Adaptive Response Suppresses Replication Fork Reversal in BRCA-Deficient Cells. Mol. Cell 2020, 77, 461–474.e9. [Google Scholar] [CrossRef] [PubMed]
- Piberger, A.L.; Bowry, A.; Kelly, R.D.W.; Walker, A.K.; González-Acosta, D.; Bailey, L.J.; Doherty, A.J.; Méndez, J.; Morris, J.R.; Bryant, H.E.; et al. PrimPol-Dependent Single-Stranded Gap Formation Mediates Homologous Recombination at Bulky DNA Adducts. Nat. Commun. 2020, 11, 5863. [Google Scholar] [CrossRef] [PubMed]
- Mellor, C.; Nassar, J.; Šviković, S.; Sale, J.E. PRIMPOL Ensures Robust Handoff between On-the-Fly and Post-Replicative DNA Lesion Bypass. Nucleic Acids Res. 2024, 52, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.P.M.; Thada, V.; Zhao, R.; Krishnamoorthy, A.; Leser, M.; Lindsey Rose, K.; Cortez, D. CHK1 Phosphorylates PRIMPOL to Promote Replication Stress Tolerance. Sci. Adv. 2022, 8, eabm0314. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, D.; Jozwiakowski, S.K.; Romanello, M.; Guilbaud, G.; Guilliam, T.A.; Bailey, L.J.; Sale, J.E.; Doherty, A.J. PrimPol Is Required for Replicative Tolerance of G Quadruplexes in Vertebrate Cells. Mol. Cell 2016, 61, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-Muñumer, R.; Forslund, J.M.E.; Goffart, S.; Pfeiffer, A.; Stojkovič, G.; Carvalho, G.; Al-Furoukh, N.; Blanco, L.; Wanrooij, S.; Pohjoismäki, J.L.O. PrimPol Is Required for Replication Reinitiation after mtDNA Damage. Proc. Natl. Acad. Sci. USA 2017, 114, 11398–11403. [Google Scholar] [CrossRef] [PubMed]
- Rechkoblit, O.; Gupta, Y.K.; Malik, R.; Rajashankar, K.R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Structure and Mechanism of Human PrimPol, a DNA Polymerase with Primase Activity. Sci. Adv. 2016, 2, e1601317. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, N.A.; Ramirez-Aguilar, K.A.; Urban, M.; Kuchta, R.D. Herpes Simplex Virus-1 Helicase−Primase: Roles of Each Subunit in DNA Binding and Phosphodiester Bond Formation. Biochemistry 2009, 48, 10199–10207. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.; Rudd, S.G.; Jozwiakowski, S.K.; Bailey, L.J.; Soura, V.; Taylor, E.; Stevanovic, I.; Green, A.J.; Stracker, T.H.; Lindsay, H.D.; et al. PrimPol Bypasses UV Photoproducts during Eukaryotic Chromosomal DNA Replication. Mol. Cell 2013, 52, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Stojkovič, G.; Makarova, A.V.; Wanrooij, P.H.; Forslund, J.; Burgers, P.M.; Wanrooij, S. Oxidative DNA Damage Stalls the Human Mitochondrial Replisome. Sci. Rep. 2016, 6, 28942. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, W.; Morehouse, N.; Tree, M.O.; Zhao, L. Divalent Cations Alter the Rate-Limiting Step of PrimPol-Catalyzed DNA Elongation. J. Mol. Biol. 2019, 431, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Sheaff, R.J.; Kuchta’, R.D. Mechanism of Calf Thymus DNA Primase: Slow Initiation, Rapid Polymerization, and Intelligent Terminationt. Biochemistry 1993, 32, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Li, A.W.H.; Zabrady, K.; Bainbridge, L.J.; Zabrady, M.; Naseem-Khan, S.; Berger, M.B.; Kolesar, P.; Cisneros, G.A.; Doherty, A.J. Molecular Basis for the Initiation of DNA Primer Synthesis. Nature 2022, 605, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Zerbe, L.K.; Kuchta, R.D. The P58 Subunit of Human DNA Primase Is Important for Primer Initiation, Elongation, and Counting. Biochemistry 2002, 41, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Baranovskiy, A.G.; Zhang, Y.; Suwa, Y.; Gu, J.; Babayeva, N.D.; Pavlov, Y.I.; Tahirov, T.H. Insight into the Human DNA Primase Interaction with Template-Primer. J. Biol. Chem. 2016, 291, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.; Calvo, P.A.; Diaz-Talavera, A.; Carvalho, G.; Calero, N.; Martínez-Carrón, A.; Velázquez-Ruiz, C.; Villadangos, S.; Guerra, S.; Martínez-Jiménez, M.I. Mechanism of DNA Primer Synthesis by Human PrimPol. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 45, pp. 289–310. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, G.; Guerra, S.; Martínez-Jiménez, M.I.; Blanco, L. Mouse PrimPol Outperforms Its Human Counterpart as a Robust DNA Primase. Int. J. Mol. Sci. 2025, 26, 6947. https://doi.org/10.3390/ijms26146947
Carvalho G, Guerra S, Martínez-Jiménez MI, Blanco L. Mouse PrimPol Outperforms Its Human Counterpart as a Robust DNA Primase. International Journal of Molecular Sciences. 2025; 26(14):6947. https://doi.org/10.3390/ijms26146947
Chicago/Turabian StyleCarvalho, Gustavo, Susana Guerra, María I. Martínez-Jiménez, and Luis Blanco. 2025. "Mouse PrimPol Outperforms Its Human Counterpart as a Robust DNA Primase" International Journal of Molecular Sciences 26, no. 14: 6947. https://doi.org/10.3390/ijms26146947
APA StyleCarvalho, G., Guerra, S., Martínez-Jiménez, M. I., & Blanco, L. (2025). Mouse PrimPol Outperforms Its Human Counterpart as a Robust DNA Primase. International Journal of Molecular Sciences, 26(14), 6947. https://doi.org/10.3390/ijms26146947






