Inhibitory Effects of Vandetanib on Catecholamine Synthesis in Rat Pheochromocytoma PC12 Cells
Abstract
1. Introduction
2. Results
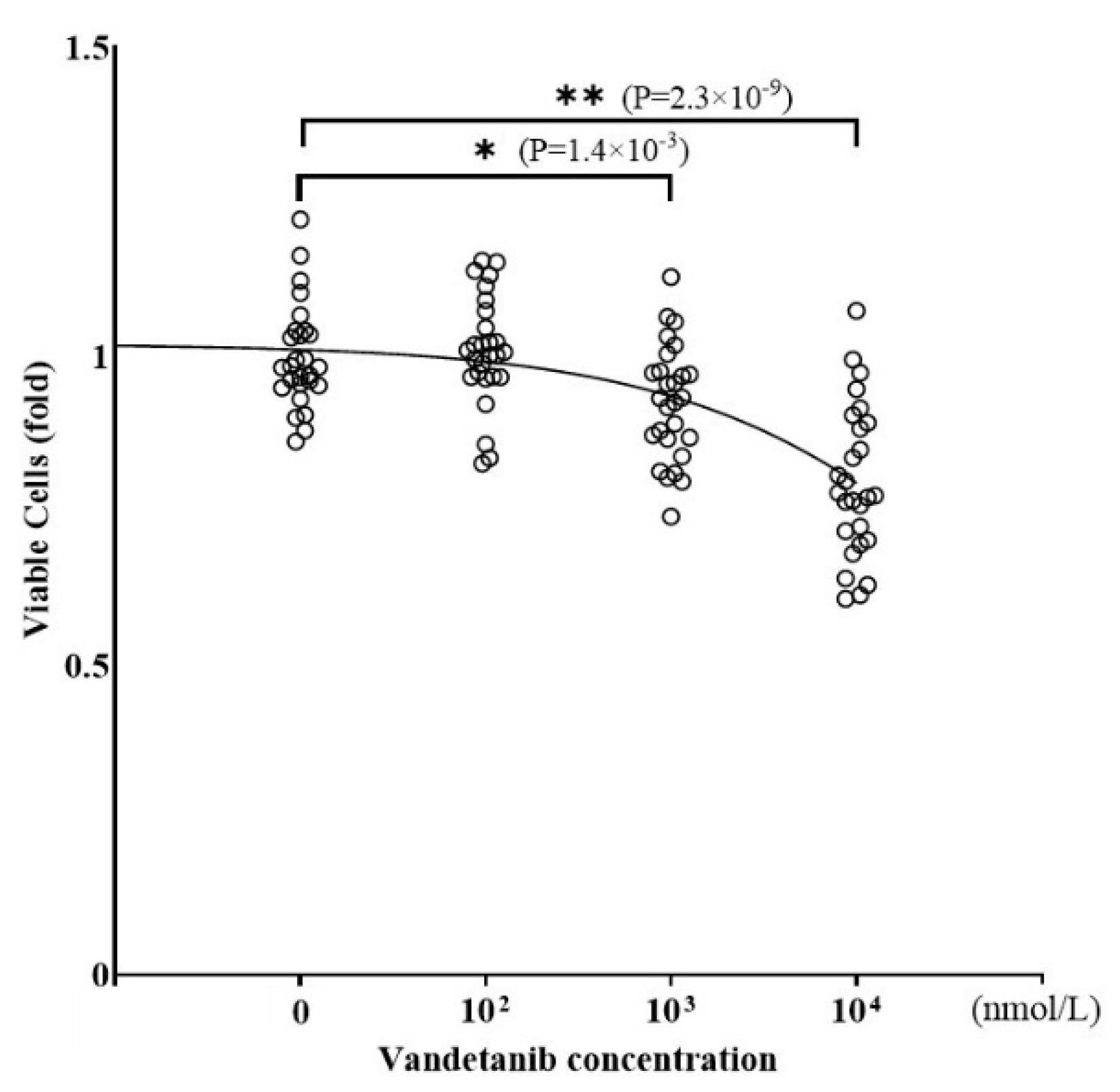
2.1. Effects of Vandetanib on Cell Proliferation and Catecholamine Synthesis in PC12
2.2. Effects of RET Knockdown on Catecholamine Synthesis in PC12 Cells
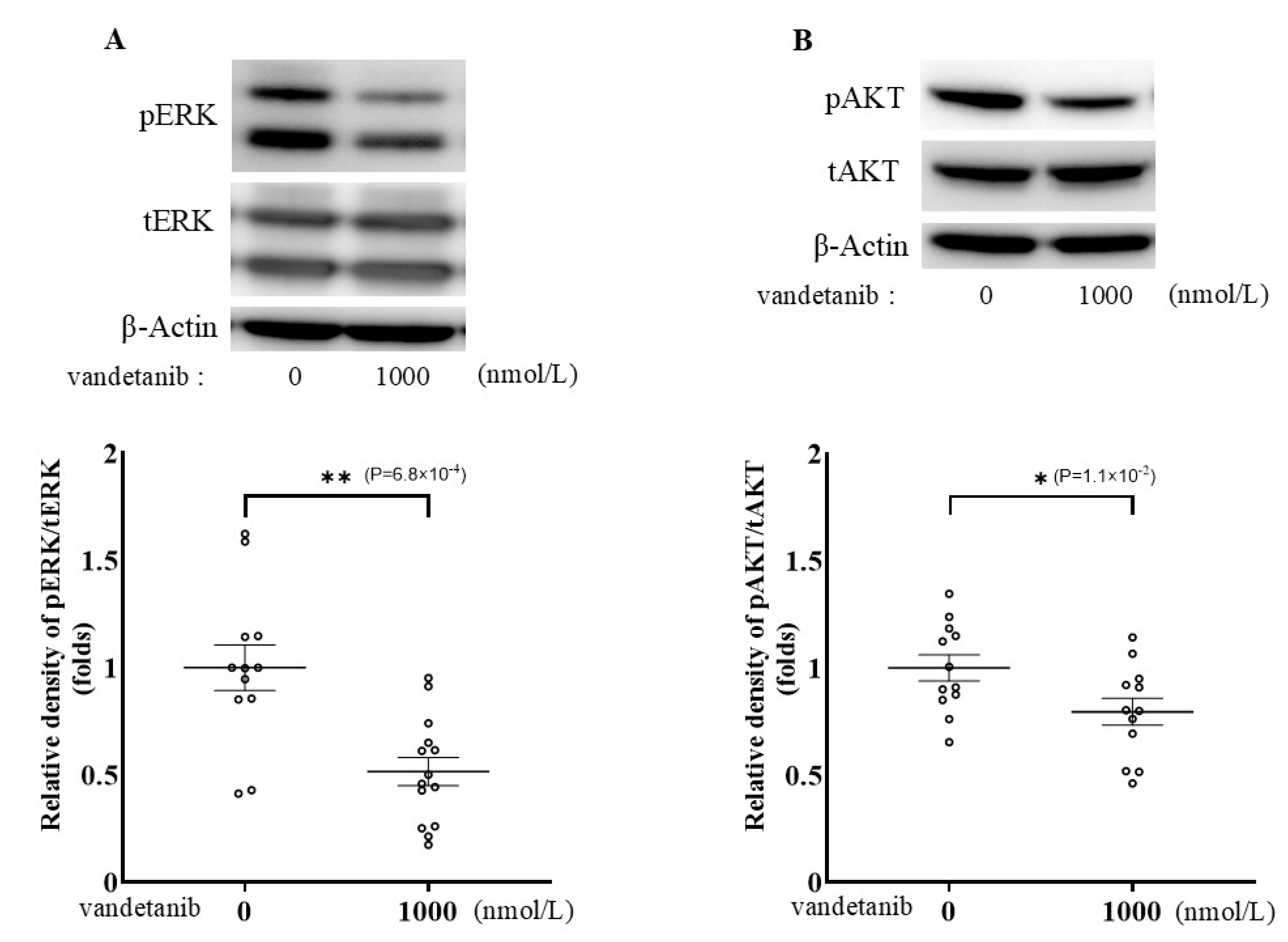
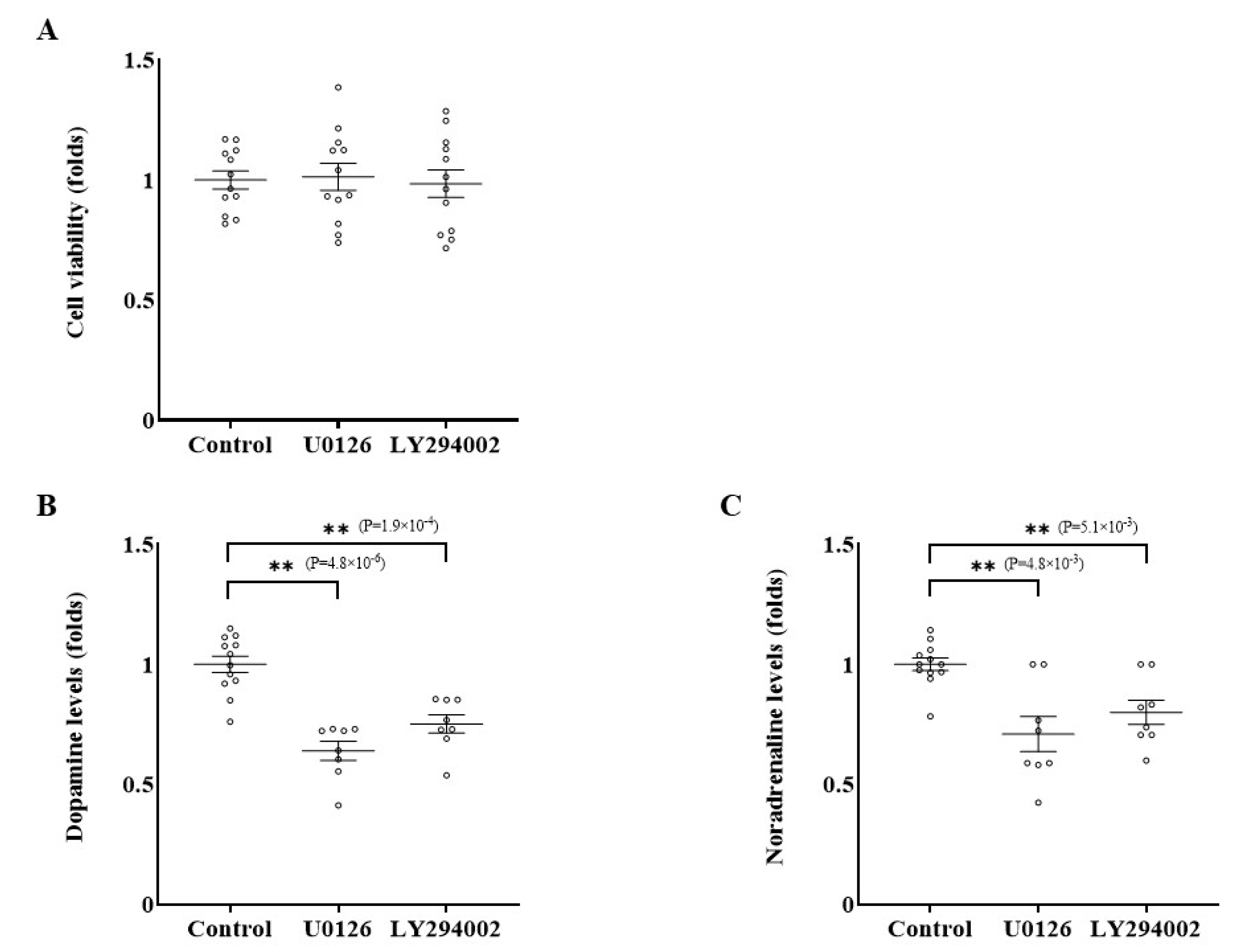
2.3. Effects of Inhibition of RET-ERK and RET-AKT Pathways on Catecholamine Synthesis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection of Small Interfering RNA
4.3. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction Analysis
4.4. Cell Viability Assay
4.5. Catecholamine Assay
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RET | Rearranged during transfection |
| MTC | Medullary thyroid cancer |
| PCC | Pheochromocytoma |
| TKI | Tyrosine kinase inhibitor |
| PI3K | Phosphatidylinositol 3-kinase |
| NSCLC | Non-small-cell lung cancer |
| VEGFR | Vascular endothelial growth factor receptor |
| PDGFR | Platelet-derived growth factor receptor |
| EGFR | Epidermal growth factor receptor |
| FDA | US Food and Drug Administration |
| EMA | European Medicines Agency |
| TCGA | The Cancer Genome Atlas |
| ERK | Extracellular signal-regulated kinase |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| MIBG | Iodine-131 metaiodobenzylguanidine |
| CVD | Cyclophosphamide, vincristine, and dacarbazine |
| DMEM | Dulbecco’s modified Eagle’s Medium |
| FCS | Fetal calf serum |
| HS | Horse serum |
| siRNA | Small interfering RNA |
| RT | Reverse transcription |
| PCR | Polymerase chain reaction |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
References
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Rev. Cancer 2014, 14, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Pachnis, V.; Mankoo, B.; Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 1993, 119, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- King, K.S.; Pacak, K. Familial pheochromocytomas and paragangliomas. Mol. Cell Endocrinol. 2014, 386, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Soda, M.; Togashi, Y.; Suzuki, R.; Sakata, S.; Hatano, S.; Asaka, R.; Hamanaka, W.; Ninomiya, H.; Uehara, H.; et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012, 18, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Elisei, R. RET/PTC Translocations and Clinico-Pathological Features in Human Papillary Thyroid Carcinoma. Front. Endocrinol. 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Knowles, P.P.; Murray-Rust, J.; Kjaer, S.; Scott, R.P.; Hanrahan, S.; Santoro, M.; Ibanez, C.F.; McDonald, N.Q. Structure and chemical inhibition of the RET tyrosine kinase domain. J. Biol. Chem. 2006, 281, 33577–33587. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.G.; Ardini, E.; Locati, L.D.; Greco, A.; Licitra, L.; Pierotti, M.A. RET inhibition: Implications in cancer therapy. Expert. Opin. Ther. Targets 2013, 17, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Favier, J.; Amar, L.; Gimenez-Roqueplo, A.P. Paraganglioma and phaeochromocytoma: From genetics to personalized medicine. Nat. Rev. Endocrinol. 2015, 11, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.L.; Strong, E.A.; Wang, T.S.; Evans, D.B.; Clarke, C.N. Screening guidelines and recommendations for patients at high risk of developing endocrine cancers. J. Surg. Oncol. 2020, 121, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Thosani, S.; Ayala-Ramirez, M.; Palmer, L.; Hu, M.I.; Rich, T.; Gagel, R.F.; Cote, G.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. The Characterization of Pheochromocytoma and Its Impact on Overall Survival in Multiple Endocrine Neoplasia Type 2. J. Clin. Endocrinol. Metab. 2013, 98, E1813–E1819. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Goichot, B.; Berruti, A.; Hadoux, J.; Moalla, S.; Laboureau, S.; Nölting, S.; de la Fouchardière, C.; Kienitz, T.; Deutschbein, T.; et al. Sunitinib for metastatic progressive phaeochromocytomas and paragangliomas: Results from FIRSTMAPPP, an academic, multicentre, international, randomised, placebo-controlled, double-blind, phase 2 trial. Lancet 2024, 403, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Fazeli, S.; Román-Gonzalez, A. Antiangiogenic therapies for pheochromocytoma and paraganglioma. Endocr.-Relat. Cancer 2020, 27, R239–R254. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Habra, M.A.; Campbell, M.T.; Tamsen, G.; Cruz-Goldberg, D.; Long, J.; Bassett, R.; Dantzer, R.; Balderrama-Brondani, V.; Varghese, J.; et al. Cabozantinib in patients with unresectable and progressive metastatic phaeochromocytoma or paraganglioma (the Natalie Trial): A single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 658–667. [Google Scholar] [CrossRef]
- Deschler-Baier, B.; Konda, B.; Massarelli, E.; Hu, M.I.; Wirth, L.J.; Xu, X.; Wright, J.; Clifton-Bligh, R.J. Clinical Activity of Selpercatinib in RET-mutant Pheochromocytoma. J. Clin. Endocrinol. Metab. 2025, 110, e600. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Chougnet, C.N.; Habra, M.A.; Palmer, J.L.; Leboulleux, S.; Cabanillas, M.E.; Caramella, C.; Anderson, P.; Al Ghuzlan, A.; Waguespack, S.G.; et al. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J. Clin. Endocrinol. Metab. 2012, 97, 4040–4050. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://jglobal.jst.go.jp/detail?JGLOBAL_ID=201802226714209958 (accessed on 12 July 2025).
- Nolting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, D.; De Falco, V.; Tamburrino, A.; Coluzzi, S.; Troncone, G.; Chiappetta, G.; Ciardiello, F.; Tortora, G.; Fagin, J.A.; Ryan, A.J.; et al. The tyrosine kinase inhibitor ZD6474 blocks proliferation of RET mutant medullary thyroid carcinoma cells. Endocr. Relat. Cancer 2011, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Blenis, J.; Yuan, J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc. Natl. Acad. Sci. USA 2008, 105, 6584–6589. [Google Scholar] [CrossRef] [PubMed]
- Nölting, S.; Grossman, A.B. Signaling pathways in pheochromocytomas and paragangliomas: Prospects for future therapies. Endocr. Pathol. 2012, 23, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Mweempwa, A.; Xu, H.; Vissers, J.H.A.; Tothill, R.W.; Pattison, A.D.; Fellowes, A.P.; Thomas, D.M.; Richardson, G.; Hicks, R.J.; Grimmond, S.M.; et al. Novel RET Fusion RET-SEPTIN9 Predicts Response to Selective RET Inhibition With Selpercatinib in Malignant Pheochromocytoma. JCO Precis. Oncol. 2021, 5, 1160–1165. [Google Scholar] [CrossRef]
- Morimoto, E.; Inagaki, K.; Komatsubara, M.; Terasaka, T.; Itoh, Y.; Fujisawa, S.; Sasaki, E.; Nishiyama, Y.; Hara, T.; Wada, J. Effects of Wnt-beta-Catenin Signaling and Sclerostin on the Phenotypes of Rat Pheochromocytoma PC12 Cells. J. Endocr. Soc. 2022, 6, bvac121. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Rpl19 | CTGAAGGTCAAAGGGAATGTG | GGACAGAGTCTTGATGATCTC |
| Ret | GTCCAGTCCAACAACAACTC | AGTTCTCCACGCAAACTTTC |
| Kdr | AAGTTGTTTGTCCAACATCTGG | CCGTCTTTTAGTACAATGCCTG |
| Flt4 | GGACCTTGTCTGCTACAGTTTC | CAGTAAAATGTTCCGAGCAGC |
| Egfr | ACCTATCTGCACCATCGAC | TGGAGAATTCGAGAATCAACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, Y.; Inagaki, K.; Terasaka, T.; Morimoto, E.; Ishii, T.; Yamaoka, K.; Fujisawa, S.; Wada, J. Inhibitory Effects of Vandetanib on Catecholamine Synthesis in Rat Pheochromocytoma PC12 Cells. Int. J. Mol. Sci. 2025, 26, 6927. https://doi.org/10.3390/ijms26146927
Itoh Y, Inagaki K, Terasaka T, Morimoto E, Ishii T, Yamaoka K, Fujisawa S, Wada J. Inhibitory Effects of Vandetanib on Catecholamine Synthesis in Rat Pheochromocytoma PC12 Cells. International Journal of Molecular Sciences. 2025; 26(14):6927. https://doi.org/10.3390/ijms26146927
Chicago/Turabian StyleItoh, Yoshihiko, Kenichi Inagaki, Tomohiro Terasaka, Eisaku Morimoto, Takahiro Ishii, Kimitomo Yamaoka, Satoshi Fujisawa, and Jun Wada. 2025. "Inhibitory Effects of Vandetanib on Catecholamine Synthesis in Rat Pheochromocytoma PC12 Cells" International Journal of Molecular Sciences 26, no. 14: 6927. https://doi.org/10.3390/ijms26146927
APA StyleItoh, Y., Inagaki, K., Terasaka, T., Morimoto, E., Ishii, T., Yamaoka, K., Fujisawa, S., & Wada, J. (2025). Inhibitory Effects of Vandetanib on Catecholamine Synthesis in Rat Pheochromocytoma PC12 Cells. International Journal of Molecular Sciences, 26(14), 6927. https://doi.org/10.3390/ijms26146927






