Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review
Abstract
1. Introduction
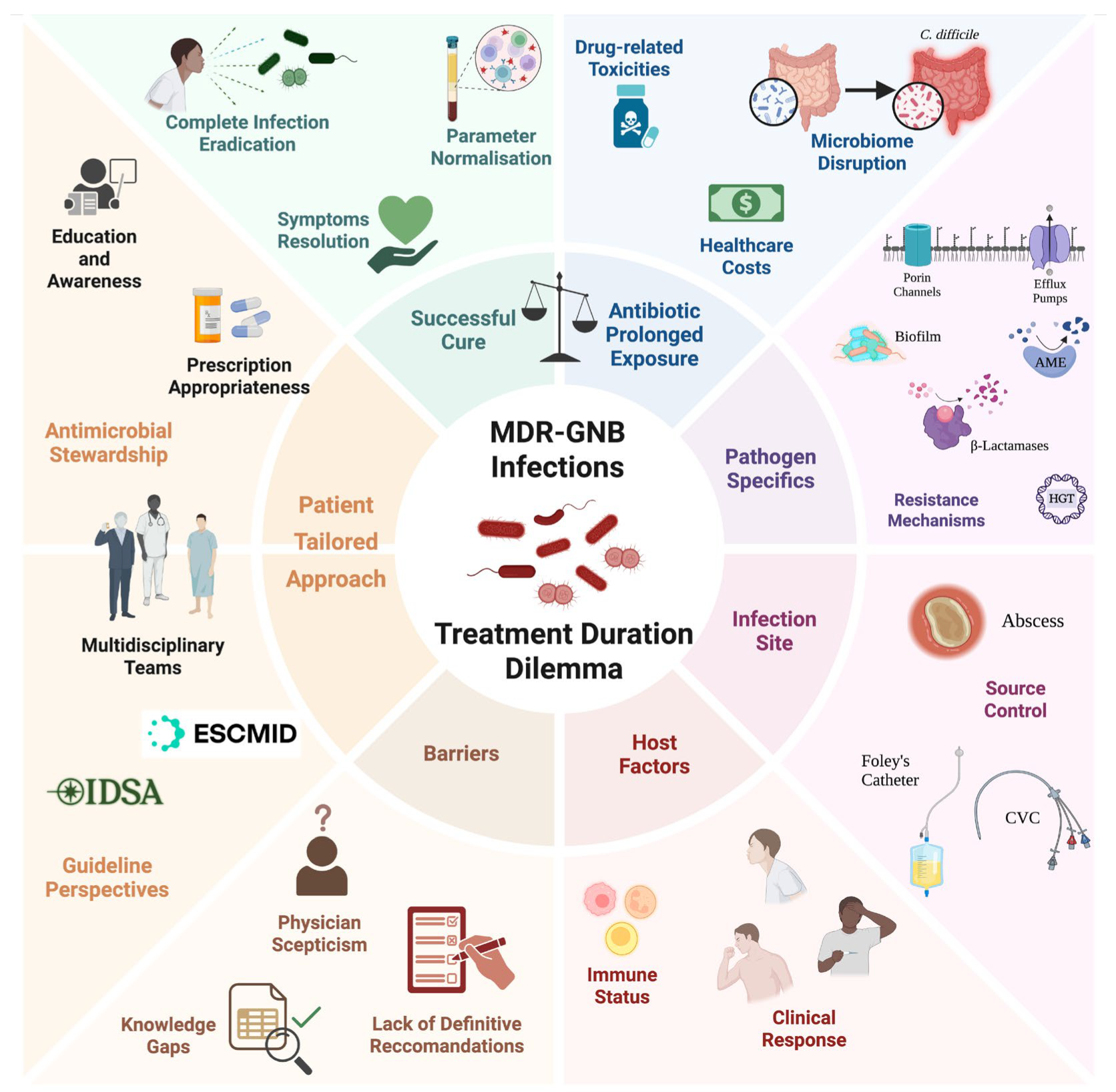
2. The Duration Dilemma
3. Methods
4. The MDR-GNB Landscapes
5. Guideline Perspectives
5.1. IDSA AMR Guidance (Versions 2020–2024)
5.2. ESCMID Guidelines (2021)
5.3. Comparison and Other Guidelines
6. Evidence Review: Short Versus Long Antibiotic Courses
7. Nuances in Duration Decisions for Specific MDR Pathogens
8. Key Factors Guiding Individualised Therapy Duration
9. Knowledge Gaps and Future Directions
10. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- About Antimicrobial Resistance|Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/antimicrobial-resistance/about/index.html (accessed on 22 June 2025).
- About Gram-Negative Bacteria|Gram-Negative Bacteria|CDC. Available online: https://www.cdc.gov/gram-negative-bacteria/about/index.html (accessed on 22 June 2025).
- Thaden, J.T.; Pogue, J.M.; Kaye, K.S. Role of Newer and Re-Emerging Older Agents in the Treatment of Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Virulence 2017, 8, 403–416. [Google Scholar] [CrossRef]
- Marino, A.; Maniaci, A.; Lentini, M.; Ronsivalle, S.; Nunnari, G.; Cocuzza, S.; Parisi, F.M.; Cacopardo, B.; Lavalle, S.; La Via, L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia 2025, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Facts and Stats|Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/facts-stats/index.html (accessed on 22 June 2025).
- Epidemiology of MDROs|Infection Control|CDC. Available online: https://www.cdc.gov/infection-control/hcp/mdro-management/epidemiology.html (accessed on 22 June 2025).
- Background|Infection Control|CDC. Available online: https://www.cdc.gov/infection-control/hcp/mdro-management/background.html (accessed on 22 June 2025).
- Marino, A.; Stracquadanio, S.; Bellanca, C.M.; Augello, E.; Ceccarelli, M.; Cantarella, G.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Oral Fosfomycin Formulation in Bacterial Prostatitis: New Role for an Old Molecule-Brief Literature Review and Clinical Considerations. Infect. Dis. Rep. 2022, 14, 621–634. [Google Scholar] [CrossRef]
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef] [PubMed]
- 2019 Antibiotic Resistance Threats Report|Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 22 June 2025).
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO Bacterial Priority Pathogens List 2024: A Prioritisation Study to Guide Research, Development, and Public Health Strategies against Antimicrobial Resistance. Lancet Infect. Dis. 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- de Waele, J.J.; Martin-Loeches, I. Optimal Duration of Antibiotic Treatment in Gram-Negative Infections. Curr. Opin. Infect. Dis. 2018, 31, 606–611. [Google Scholar] [CrossRef]
- Royer, S.; Demerle, K.M.; Dickson, R.P.; Prescott, H.C. Shorter versus Longer Courses of Antibiotics for Infection in Hospitalized Patients: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2018, 13, 336–342. [Google Scholar] [CrossRef]
- Corona, A.; De Santis, V.; Agarossi, A.; Prete, A.; Cattaneo, D.; Tomasini, G.; Bonetti, G.; Patroni, A.; Latronico, N. Antibiotic Therapy Strategies for Treating Gram-Negative Severe Infections in the Critically Ill: A Narrative Review. Antibiotics 2023, 12, 1262. [Google Scholar] [CrossRef]
- Mo, Y.; Tan, W.C.; Cooper, B.S. Antibiotic Duration for Common Bacterial Infections—A Systematic Review. JAC Antimicrob. Resist. 2025, 7, dlae215. [Google Scholar] [CrossRef]
- Armand-Lefèvre, L.; Angebault, C.; Barbier, F.; Hamelet, E.; Defrance, G.; Ruppé, E.; Bronchard, R.; Lepeule, R.; Lucet, J.C.; Mniai, A.E.; et al. Emergence of Imipenem-Resistant Gram-Negative Bacilli in Intestinal Flora of Intensive Care Patients. Antimicrob. Agents Chemother. 2013, 57, 1488–1495. [Google Scholar] [CrossRef]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic Resistance: What Is so Special about Multidrug-Resistant Gram-Negative Bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Ling, W.; Furuya-Kanamori, L.; Ezure, Y.; Harris, P.N.A.; Paterson, D.L. Adverse Clinical Outcomes Associated with Infections by Enterobacterales Producing ESBL (ESBL-E): A Systematic Review and Meta-Analysis. JAC Antimicrob. Resist. 2021, 3, dlab068. [Google Scholar] [CrossRef]
- Ramatla, T.; Mafokwane, T.; Lekota, K.; Monyama, M.; Khasapane, G.; Serage, N.; Nkhebenyane, J.; Bezuidenhout, C.; Thekisoe, O. “One Health” Perspective on Prevalence of Co-Existing Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia Coli and Klebsiella Pneumoniae: A Comprehensive Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 88. [Google Scholar] [CrossRef]
- Thaden, J.T.; Lewis, S.S.; Hazen, K.C.; Huslage, K.; Fowler, V.G.; Moehring, R.W.; Chen, L.F.; Jones, C.D.; Moore, Z.S.; Sexton, D.J.; et al. Rising Rates of Carbapenem-Resistant Enterobacteriaceae in Community Hospitals: A Mixed-Methods Review of Epidemiology and Microbiology Practices in a Network of Community Hospitals in the Southeastern United States. Infect. Control Hosp. Epidemiol. 2014, 35, 978–983. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas Aeruginosa with Difficult-to-Treat Resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- WHO. Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 22 June 2025).
- Zhang, S.; Di, L.; Qi, Y.; Qian, X.; Wang, S. Treatment of Infections Caused by Carbapenem-Resistant Acinetobacter Baumannii. Front. Cell Infect. Microbiol. 2024, 14, 1395260. [Google Scholar] [CrossRef]
- Marino, A.; Augello, E.; Stracquadanio, S.; Bellanca, C.M.; Cosentino, F.; Spampinato, S.; Cantarella, G.; Bernardini, R.; Stefani, S.; Cacopardo, B.; et al. Unveiling the Secrets of Acinetobacter Baumannii: Resistance, Current Treatments, and Future Innovations. Int. J. Mol. Sci. 2024, 25, 6814. [Google Scholar] [CrossRef]
- Cantón, R.; Ruiz-Garbajosa, P. Treatment Guidelines for Multidrug-Resistant Gram-Negative Microorganisms. Rev. Esp. Quimioter. 2023, 36, 46–51. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter Baumannii, and Stenotrophomonas Maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; Marino, A.; De Vito, A.; Giovagnorio, F.; Stracquadanio, S.; Colpani, A.; Di Bella, S.; Madeddu, G.; Parisi, S.G.; Stefani, S.; et al. Rare or Unusual Non-Fermenting Gram-Negative Bacteria: Therapeutic Approach and Antibiotic Treatment Options. Antibiotics 2025, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- IDSA. Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. 2024. Available online: https://www.idsociety.org/practice-guideline/amr-guidance/ (accessed on 22 June 2025).
- Soto, C.L.; Hsu, A.J.; Lee, J.H.; Dzintars, K.; Choudhury, R.; Jenkins, T.C.; McCreary, E.K.; Quartuccio, K.S.; Stohs, E.J.; Zimmerman, M.; et al. Identifying Effective Durations of Antibiotic Therapy for the Treatment of Carbapenem-Resistant Enterobacterales Bloodstream Infections: A Multicenter Observational Study. Clin. Infect. Dis. 2024, 78, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Claeys, K.C.; Justo, J.A.; Heil, E.L. No Crystal Ball? Using Risk Factors and Scoring Systems to Predict Extended-Spectrum Beta-Lactamase Producing Enterobacterales (ESBL-E) and Carbapenem-Resistant Enterobacterales (CRE) Infections. Curr. Infect. Dis. Rep. 2022, 24, 147–158. [Google Scholar] [CrossRef]
- Heil, E.L.; Bork, J.T.; Abbo, L.M.; Barlam, T.F.; Cosgrove, S.E.; Davis, A.; Ha, D.R.; Jenkins, T.C.; Kaye, K.S.; Lewis, J.S.; et al. Optimizing the Management of Uncomplicated Gram-Negative Bloodstream Infections: Consensus Guidance Using a Modified Delphi Process. Open Forum Infect. Dis. 2021, 8, ofab434. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Sader, H.S.; Duncan, L.R.; Arends, S.J.R.; Carvalhaes, C.G.; Castanheira, M. Antimicrobial Activity of Aztreonam-Avibactam and Comparator Agents When Tested against a Large Collection of Contemporary Stenotrophomonas Maltophilia Isolates from Medical Centers Worldwide. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Kaye, K.S.; Shorr, A.F.; Wunderink, R.G.; Du, B.; Poirier, G.E.; Rana, K.; Miller, A.; Lewis, D.; O’Donnell, J.; Chen, L.; et al. Efficacy and Safety of Sulbactam–Durlobactam versus Colistin for the Treatment of Patients with Serious Infections Caused by Acinetobacter Baumannii–Calcoaceticus Complex: A Multicentre, Randomised, Active-Controlled, Phase 3, Non-Inferiority Clinical Trial (ATTACK). Lancet Infect. Dis. 2023, 23, 1072–1084. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- GRADE Handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 23 June 2025).
- World Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2022. Available online: https://books.google.co.jp/books?hl=en&lr=&id=UHgOEQAAQBAJ&oi=fnd&pg=PR6&dq=2021+Antibacterial+agents+in+clinical+and+preclinical+development:+an+overview+and+analysis&ots=coT6TDPKoB&sig=Wjxm-lRLZnG526S_URNsrqqh-L4#v=onepage&q=2021%20Antibacterial%20agents%20in%20clinical%20and%20preclinical%20development%3A%20an%20overview%20and%20analysis&f=false (accessed on 15 June 2025).
- Scudeller, L.; Righi, E.; Chiamenti, M.; Bragantini, D.; Menchinelli, G.; Cattaneo, P.; Giske, C.G.; Lodise, T.; Sanguinetti, M.; Piddock, L.J.V.; et al. Systematic Review and Meta-Analysis of in Vitro Efficacy of Antibiotic Combination Therapy against Carbapenem-Resistant Gram-Negative Bacilli. Int. J. Antimicrob. Agents 2021, 57, 106344. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- ESCMID. The Update of the 2021 European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli. Available online: https://www.escmid.org/guidelines-journals/guidelines/guidelines-in-development/the-update-of-the-2021-european-society-of-clinical-microbiology-and-infectious-diseases-escmid-guidelines-for-the-treatment-of-infections-caused-by-multidrug-resistant-gram-negative-bacilli/ (accessed on 23 June 2025).
- ESCMID. Guidelines in Development. Available online: https://www.escmid.org/guidelines-journals/guidelines/guidelines-in-development/ (accessed on 23 June 2025).
- Lawandi, A.; Yek, C.; Kadri, S.S. IDSA Guidance and ESCMID Guidelines: Complementary Approaches toward a Care Standard for MDR Gram-Negative Infections. Clin. Microbiol. Infect. 2022, 28, 465–469. [Google Scholar] [CrossRef]
- Yahav, D.; Paul, M.; Van Nieuwkoop, C.; Huttner, A. Is Shorter Always Better? The Pros and Cons of Treating Gram-Negative Bloodstream Infections with 7 Days of Antibiotics. JAC Antimicrob. Resist. 2022, 4, dlac058. [Google Scholar] [CrossRef]
- Dudoignon, E.; Caméléna, F.; De Tymowski, C.; Lafaurie, M.; Dépret, F. Shorter Versus Longer Course of Antibiotic Therapy for Gram-Negative Bacteremia: Time for a Tailored Duration? Clin. Infect. Dis. 2024, 79, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.A.; van Heijl, I.; van Werkhoven, C.H.; Islam, J.; Hendriks-Spoor, K.D.; Bielicki, J.; Bonten, M.J.M.; Walker, A.S.; Llewelyn, M.J.; Harbarth, S.; et al. The Quality of Studies Evaluating Antimicrobial Stewardship Interventions: A Systematic Review. Clin. Microbiol. Infect. 2019, 25, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. The Maxwell Finland Lecture: For the Duration—Rational Antibiotic Administration in an Era of Antimicrobial Resistance and Clostridium Difficile. Clin. Infect. Dis. 2008, 46, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hensgens, M.P.M.; Goorhuis, A.; Dekkers, O.M.; Kuijper, E.J. Time Interval of Increased Risk for Clostridium Difficile Infection after Exposure to Antibiotics. J. Antimicrob. Chemother. 2012, 67, 742–748. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete Recovery and Individualized Responses of the Human Distal Gut Microbiota to Repeated Antibiotic Perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The Future of Antibiotics and Resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef]
- Spellberg, B. The New Antibiotic Mantra—”shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef]
- Shorter Is Better|Mysite. Available online: https://www.bradspellberg.com/shorter-is-better (accessed on 23 June 2025).
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-Negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef]
- Von Dach, E.; Albrich, W.C.; Brunel, A.S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-Reactive Protein-Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients with Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020, 323, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Montero-Mateos, E.; Praena-Segovia, J.; León-Jiménez, E.; Natera, C.; López-Cortés, L.E.; Valiente, L.; Rosso-Fernández, C.M.; Herrero, M.; Aller-García, A.I.; et al. Seven-versus 14-Day Course of Antibiotics for the Treatment of Bloodstream Infections by Enterobacterales: A Randomized, Controlled Trial. Clin. Microbiol. Infect. 2022, 28, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, A.; von Dach, E.; Molina, J.; Franceschini, E.; Koppel, F.; Yelin, D.; Dishon-Benattar, Y.; Mussini, C.; Rodríguez-Baño, J.; Cisneros, J.M.; et al. Duration of Antibiotic Treatment for Gram-Negative Bacteremia—Systematic Review and Individual Participant Data (IPD) Meta-Analysis. EClinicalMedicine 2023, 55, 101750. [Google Scholar] [CrossRef] [PubMed]
- The BALANCE Investigators, for the Canadian Critical Care Trials Group; Association of Medical Microbiology and Infectious Disease Canada Clinical Research Network; Australian and New Zealand Intensive Care Society Clinical Trials Group. Australasian Society for Infectious Diseases Clinical Research Network Antibiotic Treatment for 7 versus 14 Days in Patients with Bloodstream Infections. N. Engl. J. Med. 2025, 392, 1065–1078. [Google Scholar] [CrossRef]
- Lee, T.C.; Prosty, C.J.; Fralick, M.; Huttner, A.; McDonald, E.G.; Molina, J.; Paul, M.; Pinto, R.; Rishu, A.; von Dach, E.; et al. Seven vs Fourteen Days of Antibiotics for Gram-Negative Bloodstream Infection: A Systematic Review and Noninferiority Meta-Analysis. JAMA Netw. Open 2025, 8, e251421. [Google Scholar] [CrossRef]
- Rodrigues, R.D.; Garcia, R.C.L.; Bittencourt, G.A.; Waichel, V.B.; Garcia, E.C.L.; Rigatto, M.H. Antimicrobial Therapy Duration for Bloodstream Infections Caused by Pseudomonas Aeruginosa or Acinetobacter Baumannii-Calcoaceticus Complex: A Retrospective Cohort Study. Antibiotics 2023, 12, 538. [Google Scholar] [CrossRef]
- Dimopoulou, D.; Moschopoulos, C.D.; Dimopoulou, K.; Dimopoulou, A.; Berikopoulou, M.M.; Andrianakis, I.; Tsiodras, S.; Kotanidou, A.; Fragkou, P.C. Duration of Antimicrobial Treatment in Adult Patients with Pneumonia: A Narrative Review. Antibiotics 2024, 13, 1078. [Google Scholar] [CrossRef]
- Daghmouri, M.A.; Dudoignon, E.; Chaouch, M.A.; Baekgaard, J.; Bougle, A.; Leone, M.; Deniau, B.; Depret, F. Comparison of a Short versus Long-Course Antibiotic Therapy for Ventilator-Associated Pneumonia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. EClinicalMedicine 2023, 58, 101880. [Google Scholar] [CrossRef]
- Albin, O.R.; Kaye, K.S.; Mccreary, E.K.; Pogue, J.M. Less Is More? Antibiotic Treatment Duration in Pseudomonas Aeruginosa Ventilator-Associated Pneumonia. Clin. Infect. Dis. 2023, 76, 745–749. [Google Scholar] [CrossRef]
- Haddad, S.F.; Allaw, F.; Kanj, S.S. Duration of Antibiotic Therapy in Gram-Negative Infections with a Particular Focus on Multidrug-Resistant Pathogens. Curr. Opin. Infect. Dis. 2022, 35, 614–620. [Google Scholar] [CrossRef]
- Truong, C.N.; Chin-Beckford, N.; Vega, A.; DeRonde, K.; Simon, J.; Abbo, L.M.; Rosa, R.; Vu, C.A. Duration of Antibiotic Therapy for Multidrug Resistant Pseudomonas Aeruginosa Pneumonia: Is Shorter Truly Better? BMC Infect. Dis. 2024, 24, 911. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Trautner, B.; Amundson, C.; Kuskowski, M.; Johnson, J.R. Effect of 7 vs. 14 Days of Antibiotic Therapy on Resolution of Symptoms among Afebrile Men with Urinary Tract Infection: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 326, 324–331. [Google Scholar] [CrossRef]
- Otero, J.Á.; Lamas Ferreiro, J.L.; Rivo, A.S.; Piñeiro, F.M.; González, L.G.; de Salamanca Holzinger, I.E.; Cavero, J.; Conde, I.R.; Soneira, M.F.; de la Fuente Aguado, J. Treatment Duration of Complicated Urinary Tract Infections by Extended-Spectrum Betalactamases Producing Enterobacterales. PLoS ONE 2020, 15, e0237365. [Google Scholar] [CrossRef]
- McAteer, J.; Lee, J.H.; Cosgrove, S.E.; Dzintars, K.; Fiawoo, S.; Heil, E.L.; Kendall, R.E.; Louie, T.; Malani, A.N.; Nori, P.; et al. Defining the Optimal Duration of Therapy for Hospitalized Patients with Complicated Urinary Tract Infections and Associated Bacteremia. Clin. Infect. Dis. 2023, 76, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-Abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg. Infect. 2010, 11, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Blot, S.; De Waele, J.J.; Vogelaers, D. Essentials for Selecting Antimicrobial Therapy for Intra-Abdominal Infections. Drugs 2012, 72, e17–e32. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of Short-Course Antimicrobial Therapy for Intraabdominal Infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef]
- Ra, J.H.; Rattan, R.; Patel, N.J.; Bhattacharya, B.; Butts, C.A.; Gupta, S.; Asfaw, S.H.; Como, J.J.; Sahr, S.M.; Bugaev, N. Duration of Antimicrobial Treatment for Complicated Intra-Abdominal Infections after Definitive Source Control: A Systematic Review, Meta-Analysis, and Practice Management Guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2023, 95, 603–612. [Google Scholar] [CrossRef]
- Patel, K.; Maguigan, K.L.; Loftus, T.J.; Mohr, A.M.; Shoulders, B.R. Optimal Antibiotic Duration for Bloodstream Infections Secondary to Intraabdominal Infection. J. Surg. Res. 2021, 260, 82–87. [Google Scholar] [CrossRef]
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Atanasov, B.; Augustin, G.; et al. WSES/GAIS/SIS-E/WSIS/AAST Global Clinical Pathways for Patients with Intra-Abdominal Infections. World J. Emerg. Surg. 2021, 16, 49. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults: A Randomized Trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.; Grant, C.; Cooke, R.P.D.; Dempsey, G. Short-Course versus Prolonged-Course Antibiotic Therapy for Hospital-Acquired Pneumonia in Critically Ill Adults. Cochrane Database Syst. Rev. 2015, 2015, CD007577. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Poulakou, G.; Pneumatikos, I.A.; Armaganidis, A.; Kollef, M.H.; Matthaiou, D.K. Short- vs Long-Duration Antibiotic Regimens for Ventilator-Associated Pneumonia: A Systematic Review and Meta-Analysis. Chest 2013, 144, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Matthaiou, D.K. Duration of Therapy of Ventilator-Associated Pneumonia. Curr. Opin. Infect. Dis. 2016, 29, 218–222. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, E45–E67. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Nagavci, B.; Aliberti, S.; Antonelli, M.; Bassetti, M.; Bos, L.D.; Chalmers, J.D.; Derde, L.; de Waele, J.; et al. ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Severe Community-Acquired Pneumonia. Intensiv. Care Med. 2023, 49, 615–632. [Google Scholar] [CrossRef]
- van Nieuwkoop, C.; van der Starre, W.E.; Stalenhoef, J.E.; van Aartrijk, A.M.; van der Reijden, T.J.K.; Vollaard, A.M.; Delfos, N.M.; van’t Wout, J.W.; Blom, J.W.; Spelt, I.C.; et al. Treatment Duration of Febrile Urinary Tract Infection: A Pragmatic Randomized, Double-Blind, Placebo-Controlled Non-Inferiority Trial in Men and Women. BMC Med. 2017, 15, 70. [Google Scholar] [CrossRef]
- Germanos, G.J.; Trautner, B.W.; Zoorob, R.J.; Salemi, J.L.; Drekonja, D.; Gupta, K.; Grigoryan, L. No Clinical Benefit to Treating Male Urinary Tract Infection Longer Than Seven Days: An Outpatient Database Study. Open Forum Infect. Dis. 2019, 6, ofz216. [Google Scholar] [CrossRef]
- Optimizing Antibiotics in Patients with Intra-Abdominal Infections—Global Alliance for Infections in Surgery. Available online: https://infectionsinsurgery.org/optimizing-antibiotics-use-in-patients-with-intra-abdomional-infections/ (accessed on 27 June 2025).
- Le Berre, C.; Degrendel, M.; Houard, M.; Benetazzo, L.; Vachée, A.; Georges, H.; Wallet, F.; Patoz, P.; Bortolotti, P.; Nseir, S.; et al. Optimizing Antibiotic Treatment Duration for ESBL-Producing Enterobacteriaceae Bacteremia in ICU: A Multicentric Retrospective Cohort Study. Antibiotics 2025, 14, 358. [Google Scholar] [CrossRef]
- Tansarli, G.S.; Andreatos, N.; Pliakos, E.E.; Mylonakis, E. A Systematic Review and Meta-Analysis of Antibiotic Treatment Duration for Bacteremia Due to Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global Epidemiology of CTX-M β-Lactamases: Temporal and Geographical Shifts in Genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Fitzpatrick, M.A.; Suda, K.J.; Smith, B.M.; Gonzalez, B.; Jones, M.; Schweizer, M.L.; Evans, M.; Evans, C.T. Comparative Effectiveness of Antibiotic Therapy for Carbapenem-Resistant Enterobacterales (CRE) Bloodstream Infections in Hospitalized US Veterans. JAC Antimicrob. Resist. 2022, 4, dlac106. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Campanella, E.; Stracquadanio, S.; Calvo, M.; Migliorisi, G.; Nicolosi, A.; Cosentino, F.; Marletta, S.; Spampinato, S.; Prestifilippo, P.; et al. Ceftazidime/Avibactam and Meropenem/Vaborbactam for the Management of Enterobacterales Infections: A Narrative Review, Clinical Considerations, and Expert Opinion. Antibiotics 2023, 12, 1521. [Google Scholar] [CrossRef]
- Ni, W.; Han, Y.; Liu, J.; Wei, C.; Zhao, J.; Cui, J.; Wang, R.; Liu, Y. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e3126. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, J.; Sun, S.; Deng, S. Mortality-Related Risk Factors and Novel Antimicrobial Regimens for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review. Infect. Drug Resist. 2022, 15, 6907–6926. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef]
- Shields, R.K.; Paterson, D.L.; Tamma, P.D. Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter Baumannii-Calcoaceticus Complex Infections. Clin. Infect. Dis. 2023, 76, S179–S193. [Google Scholar] [CrossRef]
- Serapide, F.; Guastalegname, M.; Gullì, S.P.; Lionello, R.; Bruni, A.; Garofalo, E.; Longhini, F.; Trecarichi, E.M.; Russo, A. Antibiotic Treatment of Carbapenem-Resistant Acinetobacter Baumannii Infections in View of the Newly Developed β-Lactams: A Narrative Review of the Existing Evidence. Antibiotics 2024, 13, 506. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, E.S. Optimizing Treatment for Carbapenem-Resistant Acinetobacter Baumannii Complex Infections: A Review of Current Evidence. Infect. Chemother. 2024, 56, 171–187. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. Short-Course versus Long-Course Colistin for Treatment of Carbapenem-Resistant a. baumannii Cancer Patient. Antibiotics 2021, 10, 484. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-Resistant Acinetobacter Baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Patamatamkul, S. Possible Reluctance to Shorten Antibiotic Duration in Gram-Negative Bacteremia and Limitations of Mortality-Based Outcomes: The Need to Prioritize Clinical-Microbiologic Recurrence in Future Trials—Insights from the “Bacteremia Antibiotic Length Actually Needed for Clinical Effectiveness” (BALANCE) Trial. IJID Reg. 2025, 15, 100639. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.T.; Tamma, P.D.; Pan, Q.; Doi, Y.; Daneman, N. Survey of Infectious Diseases Providers Reveals Variability in Duration of Antibiotic Therapy for the Treatment of Gram-Negative Bloodstream Infections. JAC Antimicrob. Resist. 2022, 4, dlac005. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Arenzana-Seisdedos, A.; De Waele, J.; Kollef, M.H. To Which Extent Can We Decrease Antibiotic Duration in Critically Ill Patients? Expert Rev. Clin. Pharmacol. 2017, 10, 1215–1223. [Google Scholar] [CrossRef]
- Rossel, A.; Zandberg, K.P.M.; Albrich, W.C.; Huttner, A. How Representative Is a Point-of-Care Randomized Trial? Clinical Outcomes of Patients Excluded from a Point-of-Care Randomized Controlled Trial Evaluating Antibiotic Duration for Gram-Negative Bacteraemia: A Multicentre Prospective Observational Cohort Study. Clin. Microbiol. Infect. 2022, 28, 297.e1–297.e6. [Google Scholar] [CrossRef]
- Pseudomonas Aeruginosa Infections Treatment & Management: Approach Considerations, Medical Care, Surgical Care. Available online: https://emedicine.medscape.com/article/226748-treatment#d5 (accessed on 27 June 2025).
- Shamsrizi, P.; Gladstone, B.P.; Carrara, E.; Luise, D.; Cona, A.; Bovo, C.; Tacconelli, E. Variation of Effect Estimates in the Analysis of Mortality and Length of Hospital Stay in Patients with Infections Caused by Bacteria-Producing Extended-Spectrum Beta-Lactamases: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e030266. [Google Scholar] [CrossRef]
- Tamma, P.D.; Conley, A.T.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Amoah, J.; Avdic, E.; Tolomeo, P.; Wise, J.; Subudhi, S.; et al. Association of 30-Day Mortality with Oral Step-Down vs Continued Intravenous Therapy in Patients Hospitalized with Enterobacteriaceae Bacteremia. JAMA Intern. Med. 2019, 179, 316–323. [Google Scholar] [CrossRef]
- Punjabi, C.; Tien, V.; Meng, L.; Deresinski, S.; Holubar, M. Oral Fluoroquinolone or Trimethoprim-Sulfamethoxazole vs ß-Lactams as Step-Down Therapy for Enterobacteriaceae Bacteremia: Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2019, 6, ofz364. [Google Scholar] [CrossRef]
- Roger, C. Understanding Antimicrobial Pharmacokinetics in Critically Ill Patients to Optimize Antimicrobial Therapy: A Narrative Review. J. Intensiv. Med. 2024, 4, 287–298. [Google Scholar] [CrossRef]
- Tanaka, R. Pharmacokinetic Variability and Significance of Therapeutic Drug Monitoring for Broad-Spectrum Antimicrobials in Critically Ill Patients. J. Pharm. Health Care Sci. 2025, 11, 21. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Hammond, N.E.; Brett, S.J.; Cotta, M.O.; De Waele, J.J.; Devaux, A.; Di Tanna, G.L.; Dulhunty, J.M.; Elkady, H.; Eriksson, L.; et al. Prolonged vs Intermittent Infusions of β-Lactam Antibiotics in Adults with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. JAMA 2024, 332, 638. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z. Do Prolonged Infusions of β-Lactam Antibiotics Improve Outcomes in Critically Ill Patients with Sepsis? It Is Time to Say Yes. Crit. Care 2024, 28, 380. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Brett, S.J.; Waele, J.J.; Rajbhandari, D.; Billot, L.; Cotta, M.O.; Davis, J.S.; Finfer, S.; Hammond, N.E.; Knowles, S.; et al. Continuous vs Intermittent β-Lactam Antibiotic Infusions in Critically Ill Patients with Sepsis: The BLING III Randomized Clinical Trial. JAMA 2024, 332, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Chen, J.; Bai, C.; Sun, D.; Qiu, Y.; Teng, M.; Dong, Y. Efficacy, Safety, and Therapeutic Drug Monitoring of Polymyxin B Sulfate and Colistin Sulfate in Critically Ill Patients: A Real-World Retrospective Study. Front. Pharmacol. 2024, 15, 1466888. [Google Scholar] [CrossRef]
- Takahashi, N.; Kondo, Y.; Kubo, K.; Egi, M.; Kano, K.I.; Ohshima, Y.; Nakada, T. aki Efficacy of Therapeutic Drug Monitoring-Based Antibiotic Regimen in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Intensiv. Care 2023, 11, 48. [Google Scholar] [CrossRef]
- Kubo, K.; Sakuraya, M.; Sugimoto, H.; Takahashi, N.; Kano, K.I.; Yoshimura, J.; Egi, M.; Kondo, Y. Benefits and Harms of Procalcitonin- or C-Reactive Protein-Guided Antimicrobial Discontinuation in Critically Ill Adults with Sepsis: A Systematic Review and Network Meta-Analysis*. Crit. Care Med. 2024, 52, e522–e534. [Google Scholar] [CrossRef]
- Dark, P.; Hossain, A.; Mcauley, D.F.; Brealey, D.; Carlson, G.; Clayton, J.C.; Felton, T.W.; Ghuman, B.K.; Gordon, A.C.; Hellyer, T.P.; et al. Biomarker-Guided Antibiotic Duration for Hospitalized Patients with Suspected Sepsis: The ADAPT-Sepsis Randomized Clinical Trial. JAMA 2025, 333, 682–693. [Google Scholar] [CrossRef]
- Imlay, H.; Laundy, N.C.; Forrest, G.N.; Slavin, M.A. Shorter Antibiotic Courses in the Immunocompromised: The Impossible Dream? Clin. Microbiol. Infect. 2023, 29, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Flokas, M.E.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-Producing Enterobacteriaceae in Paediatric Urinary Tract Infections: A Systematic Review and Meta-Analysis. J. Infect. 2016, 73, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, G.; Brigante, G.; Giacobbe, D.R.; Maraolo, A.E.; Gona, F.; Falcone, M.; Giannella, M.; Grossi, P.; Pea, F.; Rossolini, G.M.; et al. Diagnosis and Management of Infections Caused by Multidrug-Resistant Bacteria: Guideline Endorsed by the Italian Society of Infection and Tropical Diseases (SIMIT), the Italian Society of Anti-Infective Therapy (SITA), the Italian Group for Antimicrobial Stewardship (GISA), the Italian Association of Clinical Microbiologists (AMCLI) and the Italian Society of Microbiology (SIM). Int. J. Antimicrob. Agents 2022, 60, 106611. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.A.; Branche, A.; Neeser, O.L.; Wirz, Y.; Haubitz, S.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; et al. Procalcitonin-Guided Antibiotic Treatment in Patients with Positive Blood Cultures: A Patient-Level Meta-Analysis of Randomized Trials. Clin. Infect. Dis. 2019, 69, 388–396. [Google Scholar] [CrossRef]
- Kim, C.J. Current Status of Antibiotic Stewardship and the Role of Biomarkers in Antibiotic Stewardship Programs. Infect. Chemother. 2022, 54, 674–698. [Google Scholar] [CrossRef]
- Goldenberg, S. Molecular-Based Diagnostics, Including Future Trends. Medicine 2013, 41, 663–666. [Google Scholar] [CrossRef]
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Peterson, L.E.; Musser, J.M. Integrating Rapid Diagnostics and Antimicrobial Stewardship Improves Outcomes in Patients with Antibiotic-Resistant Gram-Negative Bacteremia. J. Infect. 2014, 69, 216–225. [Google Scholar] [CrossRef]
- Goff, D.A.; Kullar, R.; Bauer, K.A.; File, T.M. Eight Habits of Highly Effective Antimicrobial Stewardship Programs to Meet the Joint Commission Standards for Hospitals. Clin. Infect. Dis. 2017, 64, 1134–1139. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Petzold, E.; Kreiswirth, B.N.; Bonomo, R.A.; Banerjee, R.; Lautenbach, E.; Evans, S.R.; Hanson, K.E.; Klausner, J.D.; Patel, R.; et al. Advancing Diagnostics to Address Antibacterial Resistance: The Diagnostics and Devices Committee of the Antibacterial Resistance Leadership Group. Clin. Infect. Dis. 2017, 64, S41–S47. [Google Scholar] [CrossRef][Green Version]
- Beganovic, M.; McCreary, E.K.; Mahoney, M.V.; Dionne, B.; Green, D.A.; Timbrook, T.T. Interplay between Rapid Diagnostic Tests and Antimicrobial Stewardship Programs among Patients with Bloodstream and Other Severe Infections. J. Appl. Lab. Med. 2019, 3, 601–616. [Google Scholar] [CrossRef]
- Banerjee, R.; Komarow, L.; Virk, A.; Rajapakse, N.; Schuetz, A.N.; Dylla, B.; Earley, M.; Lok, J.; Kohner, P.; Ihde, S.; et al. Randomized Trial Evaluating Clinical Impact of RAPid IDentification and Susceptibility Testing for Gram-Negative Bacteremia: RAPIDS-GN. Clin. Infect. Dis. 2021, 73, E39–E46. [Google Scholar] [CrossRef]
- Spellberg, B.; Rice, L.B. The Shorter Is Better Movement: Past, Present, Future. Clin. Microbiol. Infect. 2023, 29, 141–142. [Google Scholar] [CrossRef]
| Pathogen/ Resistance Type | IDSA Approach | ESCMID Approach | Key Differences/Notes |
|---|---|---|---|
| ESBL-E/3GCephRE | Preferred: Carbapenems (ertapenem for cystitis/mild; meropenem/imipenem for others). Alternatives: Consider fluoroquinolones, TMP-SMX, nitrofurantoin, and fosfomycin based on susceptibility and site (esp. UTI). Piperacillin-tazobactam non-inferiority data debated, potentially less reliable for severe BSI. | Severe Infection: Carbapenem (imipenem/meropenem recommended; ertapenem possible for BSI without shock). Low-risk/Non-severe: Suggests piperacillin-tazobactam, amoxicillin-clavulanate, or quinolone. TMP-SMX is considered for non-severe cUTI. | ESCMID more permissive of β-lactam/β-lactamase inhibitors for non-severe infections than IDSA, which emphasises carbapenems more broadly. Neither provides specific duration guidance [23]. |
| CRE | Preferred (KPC/OXA-48):
Ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam. Preferred (MBL): Ceftazidime-avibactam + aztreonam, and cefiderocol. Alternatives (UTI/IAI): Aminoglycosides, cefiderocol, tigecycline/eravacycline, and polymyxins (cystitis only) based on susceptibility/site. | Severe (KPC/OXA-48): Meropenem-vaborbactam or ceftazidime-avibactam if active. Severe (MBL/Resistant to others): Conditionally recommends cefiderocol. Suggests ceftazidime-avibactam + aztreonam. Non-severe : Consider older agents if active (e.g., aminoglycosides for cUTI). Combination therapy is suggested if susceptible only to older agents or new agents are unavailable. | General alignment on newer agents for severe infections. IDSA provides more tiered options. Neither provides specific duration guidance [23]. ESCMID used GRADE [37]. |
| DTR-P. aeruginosa/CRPA | Preferred: Ceftolozane-tazobactam, ceftazidime-avibactam, and imipenem-relebactam. Cefiderocol an alternative. Aminoglycosides (amikacin/tobramycin) alternative for UTI. High-dose extended-infusion traditional β-lactams (e.g., cefepime) if susceptible. Cefiderocol is preferred for MBL strains. | Severe: Suggests ceftolozane-tazobactam if active. Cefiderocol is suggested for cUTI. Non-severe: Use older active agents. Combination therapy (two active drugs) is suggested if relying on polymyxins, aminoglycosides, or fosfomycin for severe infection. | IDSA provides more options with newer agents. ESCMID emphasises ceftolozane-tazobactam. Neither provides specific duration guidance [23]. WHO moved CRPA from Critical to High Priority [24]. |
| CRAB | Preferred: Sulbactam-durlobactam (+carbapenem background). Alternative: High-dose ampicillin-sulbactam (e.g., 27 g/day) + ≥ 1 other active agent (e.g., polymyxin, minocycline, tigecycline/eravacycline, and cefiderocol). | HAP/VAP (Sulbactam-S):
Suggests ampicillin-sulbactam. Sulbactam-R: Suggests polymyxin or high-dose tigecycline if active. Cefiderocol, polymyxin-meropenem, and polymyxin-rifampin combinations are not recommended. Severe: Suggests a combination of ≥2 active agents (polymyxin, aminoglycoside, tigecycline, and sulbactam). | IDSA strongly favours sulbactam-based regimens (new agent preferred) [23]. ESCMID recommendations are more varied based on susceptibility and site, cautioning against some combinations. Neither provides specific duration guidance [37]. |
| Infection Site | Key Study/Evidence Source (Snippets) | Typical Durations Compared | Main Finding (Short vs. Long) | Key Outcomes Assessed | Major Caveats/Limitations |
|---|---|---|---|---|---|
| BSI | Yahav et al. [54], Von Dach et al. [55], Molina et al. [56], The BALANCE Investigators et al. [58]. | 7 d vs. 14 d | Non-inferiority of 7 d | Mortality (30/90 d), relapse, LOS, readmission, and adverse events | Primarily uncomplicated Enterobacterales BSI (often urinary source); adequate source control and clinical stability required; MDR pathogens, immunocompromised, andcritically ill often excluded/underrepresented; clinician reluctance [59]. |
| HAP/ VAP | Chastre et al. [75], Pugh et al. [76], Dimopoulos et al. [77,78], IDSA/ATS, ERS/ESCMID Guidelines [79,80,81,82]. | 7–8 d vs. 10–15 d | Non-inferiority of 7–8 d (overall) | Mortality, clinical cure, recurrence/relapse, and antibiotic-free days | Higher recurrence/relapse risk noted with short course for NF-GNB VAP [61]; optimal duration for MDR NF-GNB VAP remains debated [64]. |
| cUTIs/ Pyelonephritis | van Nieuwkoop et al. [83], Drekonja et al. [66], Germanos et al. [84]. | 5–7 d vs. 10–14 d | Non-inferiority of 7 d often found for Enterobacterales (including ESBL-E) and clinically stable patients | Symptom resolution, mortality, and recurrence/reinfection | Longer duration often used for P. aeruginosa [64]; 7 d efficacy in cUTI + bacteraemia may depend on IV/high bioavailability agents [68]; prostatitis requires longer therapy [64]; limited data for CRE/DTR-P. aeruginosa. |
| IAIs | Sawyer et al. [71], Sartelli et al. [74]. | Fixed 4 d vs. 8 d (until clinical resolution) | Non-inferiority of fixed 4d | Composite (SSI, recurrent IAI, and death) | Requires adequate source control [85]; primarily community-acquired IAI studied [72]; limited data for MDR-GNB IAI or incomplete source control [73]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Augello, E.; Bellanca, C.M.; Cosentino, F.; Stracquadanio, S.; La Via, L.; Maniaci, A.; Spampinato, S.; Fadda, P.; Cantarella, G.; et al. Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review. Int. J. Mol. Sci. 2025, 26, 6905. https://doi.org/10.3390/ijms26146905
Marino A, Augello E, Bellanca CM, Cosentino F, Stracquadanio S, La Via L, Maniaci A, Spampinato S, Fadda P, Cantarella G, et al. Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review. International Journal of Molecular Sciences. 2025; 26(14):6905. https://doi.org/10.3390/ijms26146905
Chicago/Turabian StyleMarino, Andrea, Egle Augello, Carlo Maria Bellanca, Federica Cosentino, Stefano Stracquadanio, Luigi La Via, Antonino Maniaci, Serena Spampinato, Paola Fadda, Giuseppina Cantarella, and et al. 2025. "Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review" International Journal of Molecular Sciences 26, no. 14: 6905. https://doi.org/10.3390/ijms26146905
APA StyleMarino, A., Augello, E., Bellanca, C. M., Cosentino, F., Stracquadanio, S., La Via, L., Maniaci, A., Spampinato, S., Fadda, P., Cantarella, G., Bernardini, R., Cacopardo, B., & Nunnari, G. (2025). Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review. International Journal of Molecular Sciences, 26(14), 6905. https://doi.org/10.3390/ijms26146905










