Molecular Mechanisms of Microvascular Obstruction and Dysfunction in Percutaneous Coronary Interventions: From Pathophysiology to Therapeutics—A Comprehensive Review
Abstract
1. Introduction
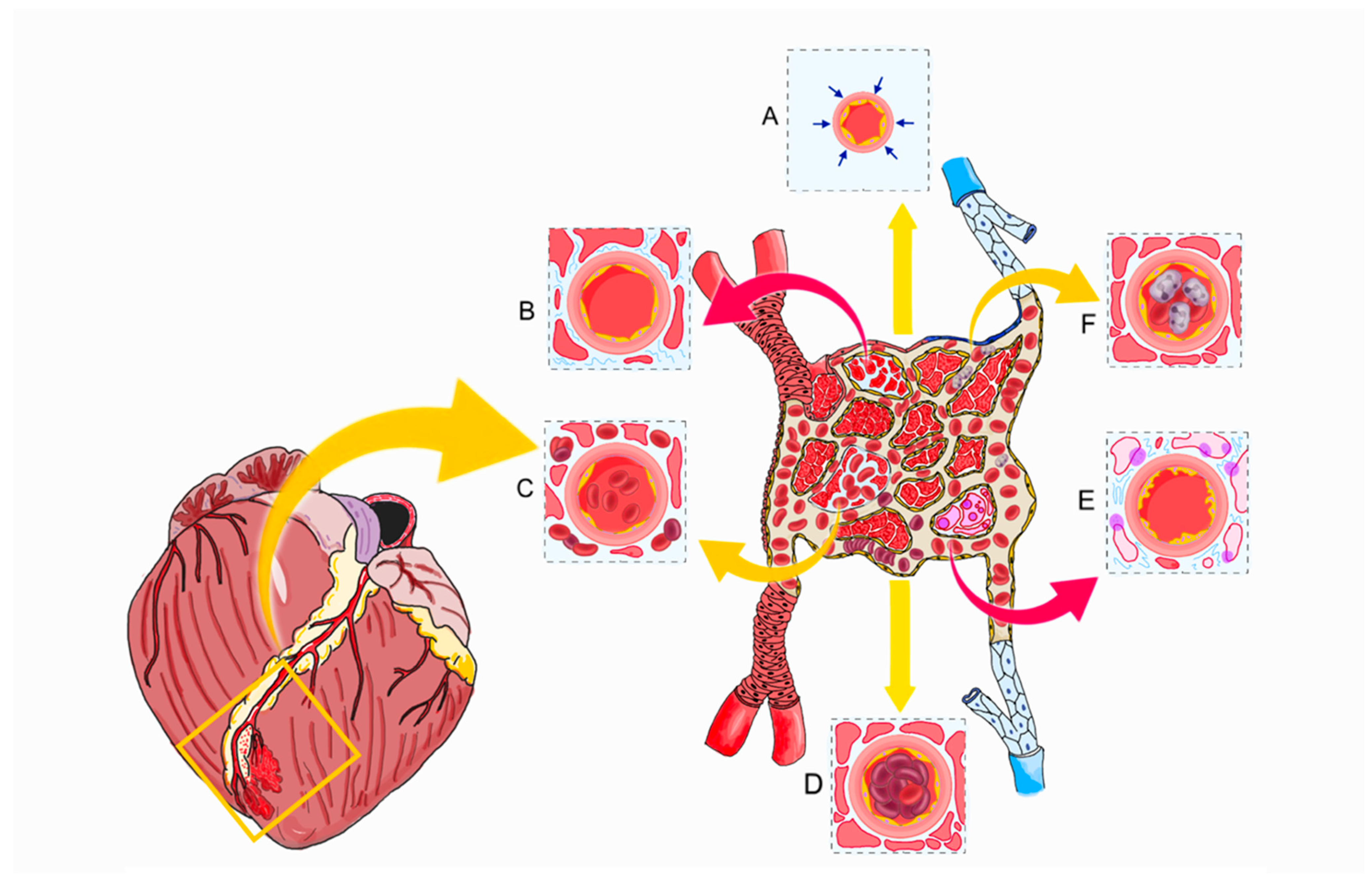
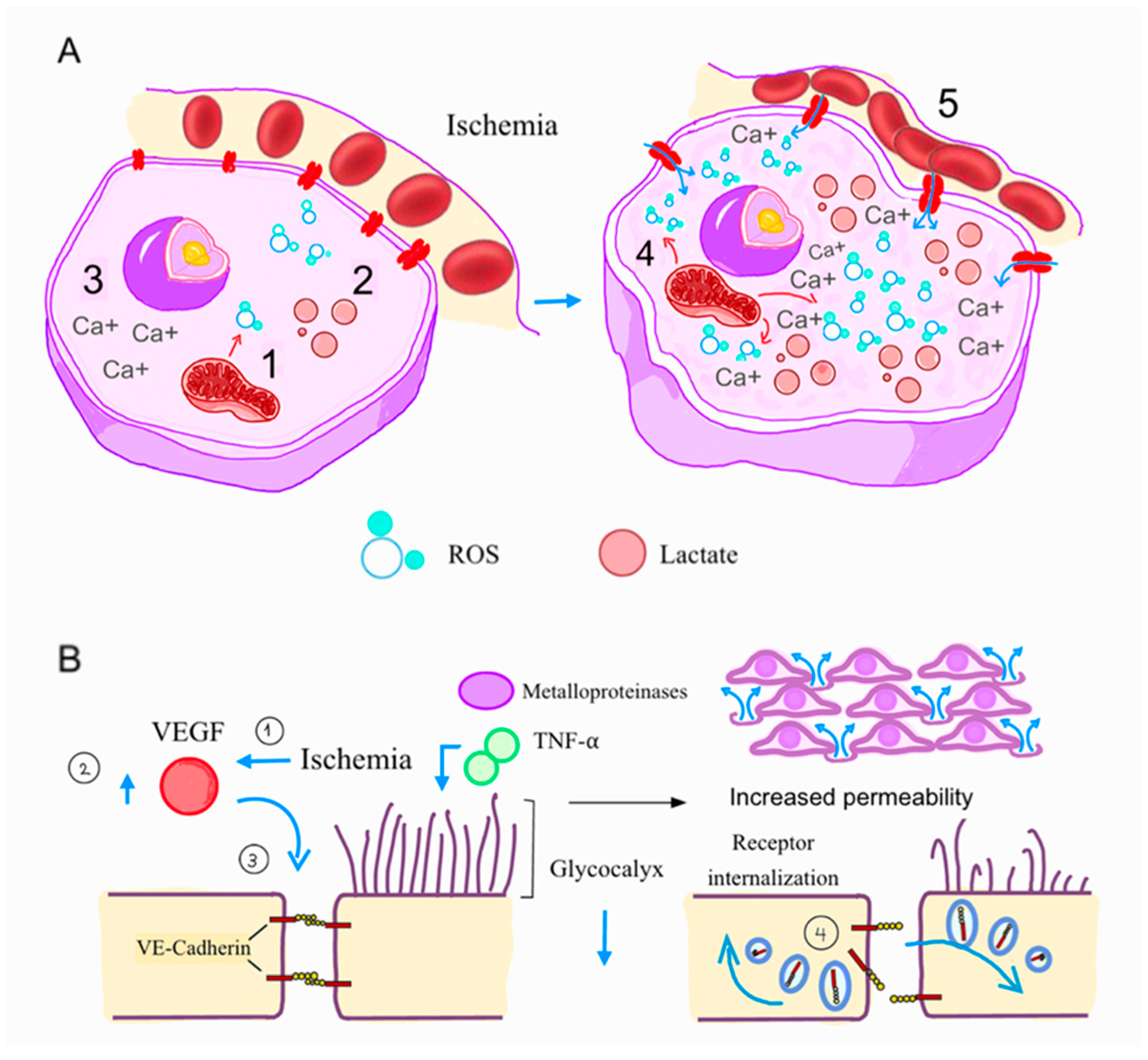
2. Pathophysiology
3. Diagnosis
4. Treatment (Figure 4)
4.1. Pharmacological Treatment with Antithrombotic Agents
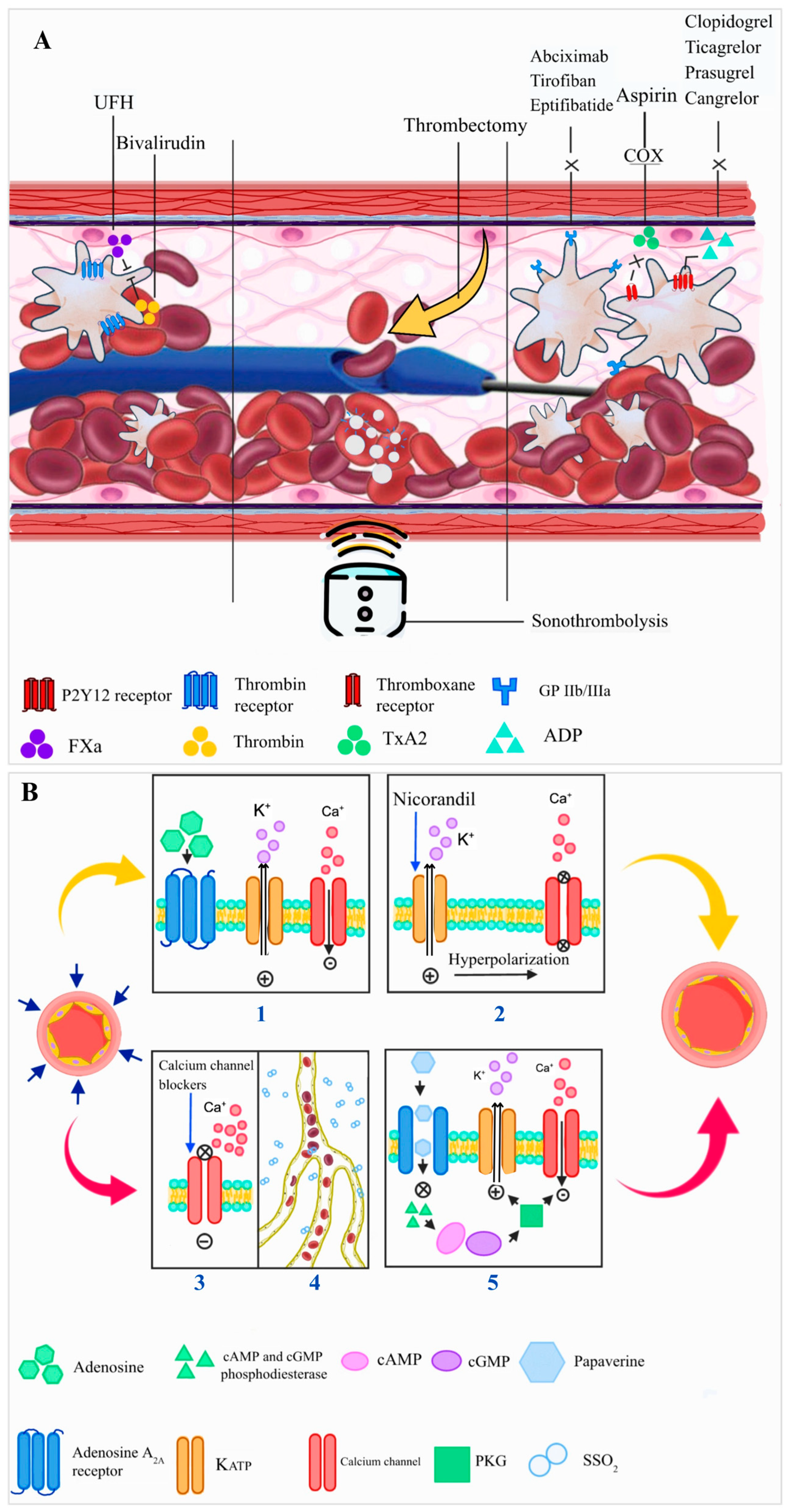
4.2. PCI Technique and Adjunctive Devices
5. Enhancing Microvascular Function and Reducing Ischemia–Reperfusion Injury
5.1. Anti-Inflammatory Drugs
5.2. Pharmacological Treatment of Established Non-Reflow During PCI
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krumholz, H.M.; Normand, S.L.T.; Wang, Y. Twenty-Year Trends in Outcomes for Older Adults with Acute Myocardial Infarction in the United States. JAMA Netw. Open 2019, 2, e191938. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Eeckhout, E.; Kern, M.J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 2001, 22, 729–739. [Google Scholar] [CrossRef]
- Van Kranenburg, M.; Magro, M.; Thiele, H.; De Waha, S.; Eitel, I.; Cochet, A.; Cottin, Y.; Atar, D.; Buser, P.; Wu, E.; et al. Prognostic Value of Microvascular Obstruction and Infarct Size, as Measured by CMR in STEMI Patients. JACC Cardiovasc. Imaging 2014, 7, 930–939. [Google Scholar] [CrossRef]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef]
- Yang, L.; Cong, H.; Lu, Y.; Chen, X.; Liu, Y. Prediction of no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine 2020, 99, E20152. [Google Scholar] [CrossRef] [PubMed]
- Mangiacapra, F.; Bressi, E.; Di Gioia, G.; Pellicano, M.; Di Serafino, L.; Peace, A.J.; Bartunek, J.; Morisco, C.; Wijns, W.; De Bruyne, B.; et al. Coronary microcirculation and peri-procedural myocardial injury during elective percutaneous coronary intervention. Int. J. Cardiol. 2020, 306, 42–46. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Cassese, S.; Xhepa, E.; Joner, M.; Sager, H.B.; Kufner, S.; Laugwitz, K.L.; Schunkert, H.; Kastrati, A. Coronary no-reflow and adverse events in patients with acute myocardial infarction after percutaneous coronary intervention with current drug-eluting stents and third-generation P2Y12 inhibitors. Clin. Res. Cardiol. 2024, 113, 1006–1016. [Google Scholar] [CrossRef]
- Uddin, M.; Mir, T.; Khalil, A.; Mehar, A.; Gomez-Pineiro, E.; Babu, M.A.; Sheikh, M.; Soubani, A.; Saydain, G.; Afonso, L. ST-Elevation Myocardial Infarction Outcomes: A United States Nationwide Emergency Departments Cohort Study. J. Emerg. Med. 2022, 62, 306–315. [Google Scholar] [CrossRef]
- Gandhi, S.; Garratt, K.N.; Li, S.; Wang, T.Y.; Bhatt, D.L.; Davis, L.L.; Zeitouni, M.; Kontos, M.C. Ten-Year Trends in Patient Characteristics, Treatments, and Outcomes in Myocardial Infarction from National Cardiovascular Data Registry Chest Pain-MI Registry. Circ. Cardiovasc. Qual. Outcomes 2022, 15, E008112. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Ito, H. Microvasculature in Acute Myocardial Ischemia: Part I—Evolving Concepts in Pathophysiology, Diagnosis, and Treatment. Circulation 2004, 109, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nan, D.; Lu, Y.; Niu, Z.; Ren, Y.; Qu, X.; Huang, Y.; Jin, H. Microcirculation No-Reflow Phenomenon after Acute Ischemic Stroke. Eur. Neurol. 2023, 86, 85–94. [Google Scholar] [CrossRef]
- Jennings, R.B.; Reimer, K.A. The cell biology of acute myocardial ischemia. Annu. Rev. Med. 1991, 42, 225–246. [Google Scholar] [CrossRef]
- Sharma, G.P.; Varley, K.G.; Kim, S.W.; Barwinsky, J.; Cohen, M.; Dhalla, N.S. Alterations in energy metabolism and ultrastructure upon reperfusion of the ischemic myocardium after coronary occlusion. Am. J. Cardiol. 1975, 36, 234–243. [Google Scholar] [CrossRef]
- King, L.M.; Opie, L.H. Glucose and glycogen utilisation in myocardial ischemia-Changes in metabolism and consequences for the myocyte. Mol. Cell. Biochem. 1998, 180, 3–26. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.R.C.; Fiolet, J.W.T.; Vanbavel, E.; Coronel, R.; Opthof, T.; Spaan, J.A.E.; Janse, M.J. Intracellular Ca2+, Intercellular Electrical Coupling, and Mechanical Activity in Ischemic Rabbit Papillary Muscle Effects of Preconditioning and Metabolic Blockade. Circ. Res. 1996, 79, 237–246. [Google Scholar] [CrossRef]
- Miklós, Z.; Ivanics, T.; Roemen, T.H.M.; Van Der Vusse, G.J.; Dézsi, L.; Szekeres, M.; Kemecsei, P.; Tóth, A.; Kollai, M.; Ligeti, L. Time related changes in calcium handling in the isolated ischemic and reperfused rat heart. Mol. Cell. Biochem. 2003, 250, 115–124. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.J.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Mahmoud, A.M.; Le Master, E.; Levitan, I.; Phillips, S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, 647–663. [Google Scholar] [CrossRef]
- Østergaard, L.; Kristiansen, S.B.; Angleys, H.; Frøkiær, J.; Hasenkam, J.M.; Jespersen, S.N.; Bøtker, H.E. The role of capillary transit time heterogeneity in myocardial oxygenation and ischemic heart disease. Basic Res. Cardiol. 2014, 109, H647–H663. [Google Scholar] [CrossRef]
- Chappell, D.; Dörfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef]
- Van den Berg, B.M.; Vink, H.; Spaan, J.A.E. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar] [CrossRef]
- Patel, V.G.; Brayton, K.M.; Mintz, G.S.; Maehara, A.; Banerjee, S.; Brilakis, E.S. Intracoronary and noninvasive imaging for prediction of distal embolization and periprocedural myocardial infarction during native coronary artery percutaneous intervention. Circ. Cardiovasc. Imaging 2013, 6, 1102–1114. [Google Scholar] [CrossRef]
- Kumar, R.; Qayyum, D.; Ahmed, I.; Rai, L.; Mir, A.; Awan, R.; Naseer, A.B.; Basit, A.; Sial, J.A.; Saghir, T.; et al. Predilation Ballooning in High Thrombus Laden STEMIs: An Independent Predictor of Slow Flow/No-Reflow in Patients Undergoing Emergent Percutaneous Coronary Revascularization. J. Interv. Cardiol. 2023, 2023, 4012361. [Google Scholar] [CrossRef]
- Mccully, J.D.; Wakiyama, H.; Hsieh, Y.J.; Jones, M.; Levitsky, S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2004, 286, H1923–H1935. [Google Scholar] [CrossRef]
- Prech, M.; Marszałek, A.; Schröder, J.; Filas, V.; Lesiak, M.; Jemielity, M.M.; Araszkiewicz, A.; Grajek, S. Apoptosis as a Mechanism for the Elimination of Cardiomyocytes After Acute Myocardial Infarction. Am. J. Cardiol. 2010, 105, 1240–1245. [Google Scholar] [CrossRef]
- Valikeserlis, I.; Athanasiou, A.A.; Stakos, D. Cellular mechanisms and pathways in myocardial reperfusion injury. Coron. Artery Dis. 2021, 32, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Mathey, D.G.; Schofer, J.; Kuck, K.H.; Beil, U.; Kloppel, G. Transmural, haemorrhagic myocardial infarction after intracoronary streptokinase. Clinical, angiographic, and necropsy findings. Heart 1982, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Tarikuz Zaman, A.K.M.; Spees, J.L.; Sobel, B.E. Attenuation of cardiac vascular rhexis. Coron. Artery Dis. 2013, 24, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Robbers, L.F.H.J.; Eerenberg, E.S.; Teunissen, P.F.A.; Jansen, M.F.; Hollander, M.R.; Horrevoets, A.J.G.; Knaapen, P.; Nijveldt, R.; Heymans, M.W.; Levi, M.M.; et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur. Heart J. 2013, 34, 2346–2353. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: Relation to microvascular obstruction and prognostic significance. Circ. Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef]
- Yoshino, S.; Cilluffo, R.; Best, P.J.M.; Atkinson, E.J.; Aoki, T.; Cunningham, J.M.; De Andrade, M.; Choi, B.J.; Lerman, L.O.; Lerman, A. Single nucleotide polymorphisms associated with abnormal coronary microvascular function. Coron. Artery Dis. 2014, 25, 281–289. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Mehilli, J.; Schulz, S.; Iijima, R.; Keta, D.; Byrne, R.A.; Pache, J.; Seyfarth, M.; Schömig, A.; Kastrati, A. Prognostic Significance of Epicardial Blood Flow Before and After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2008, 52, 512–517. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Ikushima, M.; Kawano, S.; Okamura, A.; Asano, K.; Kuroda, T.; Tanaka, K.; Masuyama, T.; Hori, M.; et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 1–7. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular magnetic resonance in acute st-segment-elevation myocardial infarction: Recent advances, controversies, and future directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Bulluck, H.; Foin, N.; Tan, J.W.; Low, A.F.; Sezer, M.; Hausenloy, D.J. Invasive Assessment of the Coronary Microcirculation in Reperfused ST-Segment-Elevation Myocardial Infarction Patients: Where Do We Stand? Circulation: Cardiovascular Interventions. Circ. Cardiovasc. Interv. 2017, 10, e004373. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.A.P.; Murphy, C.A.; Petrie, C.J.; Martin, T.N.; Balmain, S.; Clements, S.; Steedman, T.; Wagner, G.S.; Dargie, H.J.; McMurray, J.J.V. Microvascular Obstruction Remains a Portent of Adverse Remodeling in Optimally Treated Patients with Left Ventricular Systolic Dysfunction After Acute Myocardial Infarction. Circ. Cardiovasc. Imaging 2010, 3, 360–367. [Google Scholar] [CrossRef]
- Nijveldt, R.; Hofman, M.B.M.; Hirsch, A.; Beek, A.M.; Umans, V.A.W.M.; Algra, P.R.; Piek, J.J.; van Rossum, A.C. Assessment of Microvascular Obstruction and Prediction of Short-term Remodeling after Acute Myocardial Infarction: Cardiac MR Imaging Study. Radiology 2009, 250, 363–370. [Google Scholar] [CrossRef]
- Scarsini, R.; Portolan, L.; Della Mora, F.; Marin, F.; Mainardi, A.; Ruzzarin, A.; Levine, M.B.; Banning, A.P.; Ribichini, F.; Garcia Garcia, H.M.; et al. Angiography-Derived and Sensor-Wire Methods to Assess Coronary Microvascular Dysfunction in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2023, 16, 965–981. [Google Scholar] [CrossRef]
- Maznyczka, A.M.; Oldroyd, K.G.; McCartney, P.; McEntegart, M.; Berry, C. The Potential Use of the Index of Microcirculatory Resistance to Guide Stratification of Patients for Adjunctive Therapy in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 951–966. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients with STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Zhang, Y.; Zhang, K.J.; Hu, K.; Shi, Z.; Ma, L.K. The coronary angiography-derived index of microcirculatory resistance predicts perioperative myocardial injury in stable coronary artery disease patients undergoing PCI. Heliyon 2024, 10, e35240. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef]
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Ribichini, F.; Ferreira, V.M.; Channon, K.M.; et al. Angiography-derived index of microcirculatory resistance (IMRangio) as a novel pressure-wire-free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef]
- Duan, Y.; Yin, Q.; Yang, Y.; Miao, H.; Han, S.; Chi, Q.; Lv, H.; Lu, Y.; Zhou, Y. Integrating angio-IMR and CMR-assessed microvascular obstruction for improved risk stratification of STEMI patients. Sci. Rep. 2025, 15, 5470. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigoli, E.; Leonardi, S.; Rothenbühler, M.; Gagnor, A.; Calabrò, P.; Garducci, S.; Rubartelli, P.; Briguori, C.; Andò, G.; et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N. Engl. J. Med. 2015, 373, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, Z.; Qin, L.; Wang, M.; Wang, X.; Zhang, H.; Liu, Y.; Li, Y.; Jia, Z.; Liu, L.; et al. Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A randomised trial. Lancet 2022, 400, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Valgimigli, M.; Erlinge, D.; Han, Y.; Steg, P.G.; Stables, R.H.; Frigoli, E.; James, S.K.; Li, Y.; Goldstein, P.; et al. Bivalirudin vs. Heparin Anticoagulation in STEMI: Confirmation of the BRIGHT-4 Results. J. Am. Coll. Cardiol. 2024, 84, 1512–1524. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, Z.; Xu, B.; Chen, B.; Ge, H.; Ding, S.; Pu, J. Impact of Bivalirudin on Ischemia/Reperfusion Injury in Patients with Reperfused STEMI Assessed by Cardiac Magnetic Resonance. Pharmaceuticals 2024, 17, 196. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. Available online: http://www.nejm.org/doi/abs/10.1056/NEJMoa0706482 (accessed on 7 July 2025). [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Khan, J.N.; Greenwood, J.P.; Nazir, S.A.; Lai, F.Y.; Dalby, M.; Curzen, N.; Hetherington, S.; Kelly, D.J.; Blackman, D.; Peebles, C.; et al. Infarct size following treatment with second-versus third-generation P2Y12 antagonists in patients with multivessel coronary disease at ST-segment elevation myocardial infarction in the CvLPRIT study. J. Am. Heart Assoc. 2016, 5, e003403. [Google Scholar] [CrossRef]
- Xu, J.; Lo, S.; Mussap, C.J.; French, J.K.; Rajaratnam, R.; Kadappu, K.; Premawardhana, U.; Nguyen, P.; Juergens, C.P.; Leung, D.Y. Impact of Ticagrelor Versus Clopidogrel on Coronary Microvascular Function After Non–ST-Segment–Elevation Acute Coronary Syndrome. Circ. Cardiovasc. Interv. 2022, 15, e011419. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, K.; Kim, Y.D. Comparison between ticagrelor and clopidogrel on myocardial blood flow in patients with acute coronary syndrome, using 13 N-ammonia positron emission tomography. Am. Heart J. 2020, 222, 121–130. [Google Scholar] [CrossRef]
- Scanavini-Filho, M.A.; Berwanger, O.; Matthias, W.; Aguiar, M.O.; Chiang, H.P.; Azevedo, L.; Baracioli, L.M.; Lima, F.G.; Furtado, R.H.M.; Dalcoquio, T.F.; et al. Effects of Ticagrelor and Clopidogrel on Coronary Microcirculation in Patients with Acute Myocardial Infarction. Adv. Ther. 2022, 39, 1832–1843. [Google Scholar] [CrossRef]
- Ubaid, S.; Ford, T.J.; Berry, C.; Murray, H.M.; Wrigley, B.; Khan, N.; Thomas, M.R.; Armesilla, A.L.; Townend, J.N.; Khogali, S.S.; et al. Cangrelor versus Ticagrelor in Patients Treated with Primary Percutaneous Coronary Intervention: Impact on Platelet Activity, Myocardial Microvascular Function and Infarct Size: A Randomized Controlled Trial. Thromb. Haemost. 2019, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Abtan, J.; Steg, P.G.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Abnousi, F.; Prats, J.; Deliargyris, E.N.; et al. Efficacy and Safety of Cangrelor in Preventing Periprocedural Complications in Patients with Stable Angina and Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2016, 9, 1905–1913. [Google Scholar] [CrossRef]
- Bulluck, H.; Chong, J.H.; Bryant, J.; Annathurai, A.; Chai, P.; Chan, M.; Chawla, A.; Chin, C.Y.; Chung, Y.C.; Gao, F.; et al. Effect of Cangrelor on Infarct Size in ST-Segment-Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial (The PITRI Trial). Circulation 2024, 150, 91–101. [Google Scholar] [CrossRef]
- Beavers, C.J.; Effoe, S.A.; Dobesh, P.P. Selatogrel: A Novel Subcutaneous P2Y12 Inhibitor. J. Cardiovasc. Pharmacol. 2022, 79, 161–167. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2025, 85, 2135–2237. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Saraei, R.; Jurczyk, D.; Fach, A.; Hambrecht, R.; Wienbergen, H.; Frerker, C.; Schmidt, T.; Allali, A.; Joost, A.; et al. Glycoprotein IIb/IIIa Inhibitors in Acute Myocardial Infarction and Angiographic Microvascular Obstruction: The REVERSE-FLOW Trial. Eur. Heart J. 2024, 45, 5058–5067. [Google Scholar] [CrossRef] [PubMed]
- Latter, J.; McCartney, P.; Berry, C. Bailout glycoprotein IIb/IIIa inhibitors for coronary microvascular obstruction: Guideline-indicated, but does harm outweigh benefit? Eur. Heart J. 2024, 45, 5068–5070. [Google Scholar] [CrossRef]
- Rikken, S.A.O.F.; Bor, W.L.; Selvarajah, A.; Zheng, K.L.; Hack, A.P.; Gibson, C.M.; Granger, C.B.; Bentur, O.S.; Coller, B.S.; van ’t Hof, A.W.J.; et al. Prepercutaneous coronary intervention Zalunfiban dose-response relationship to target vessel blood flow at initial angiogram in st-elevation myocardial infarction—A post hoc analysis of the cel-02 phase IIa study. Am. Heart J. 2023, 262, 75–82. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.J.; Yu, C.W.; Kim, Y.M.; Hong, S.J.; Park, J.H.; Choi, R.K.; Choi, Y.J.; Park, J.S.; Kim, T.H.; et al. INNOVATION study (Impact of Immediate Stent Implantation Versus Deferred Stent Implantation on Infarct Size and Microvascular Perfusion in Patients with ST-Segment-Elevation Myocardial Infarction). Circ. Cardiovasc. Interv. 2016, 9, e004101. [Google Scholar] [CrossRef]
- Belle, L.; Motreff, P.; Mangin, L.; Rangé, G.; Marcaggi, X.; Marie, A.; Ferrier, N.; Dubreuil, O.; Zemour, G.; Souteyrand, G.; et al. Comparison of Immediate with Delayed Stenting Using the Minimalist Immediate Mechanical Intervention Approach in Acute ST-Segment-Elevation Myocardial Infarction: The MIMI Study. Circ. Cardiovasc. Interv. 2016, 9, e003388. [Google Scholar] [CrossRef]
- Kelbæk, H.; Høfsten, D.E.; Køber, L.; Helqvist, S.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; De Backer, O.; et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): An open-label, randomised controlled trial. Lancet 2016, 387, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, B.; Wang, J.; Zhao, Y.; Yang, R.; Du, H.; Jiang, J.; Jin, C.; Xiong, E. Deferred versus immediate stenting in patients with ST-segment elevation myocardial infarction: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e004838. [Google Scholar] [CrossRef]
- Lee, J.M.; Rhee, T.M.; Chang, H.; Ahn, C.; Park, T.K.; Yang, J.H.; Song, Y.B.; Choi, S.H.; Gwon, H.C.; Hahn, J.Y. Deferred versus conventional stent implantation in patients with acute ST-segment elevation myocardial infarction: An updated meta-analysis of 10 studies. Int. J. Cardiol. 2017, 230, 509–517. [Google Scholar] [CrossRef]
- Carrick, D.; Oldroyd, K.G.; McEntegart, M.; Haig, C.; Petrie, M.C.; Eteiba, H.; Hood, S.; Owens, C.; Watkins, S.; Layland, J.; et al. A randomized trial of deferred stenting versus immediate stenting to prevent No- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J. Am. Coll. Cardiol. 2014, 63, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Lønborg, J.; Engstrøm, T.; Ahtarovski, K.A.; Nepper-Christensen, L.; Helqvist, S.; Vejlstrup, N.; Kyhl, K.; Schoos, M.M.; Ghotbi, A.; Göransson, C.; et al. Myocardial Damage in Patients with Deferred Stenting After STEMI A DANAMI-3-DEFER Substudy. J. Am. Coll. Cardiol. 2017, 69, 2794–2804. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhandari, M.; Vishwakarma, P.; Sethi, R. Deferred Stenting for Heavy Thrombus Burden During Percutaneous Coronary Intervention for ST-Elevation MI. Eur. Cardiol. Rev. 2021, 16, e08. [Google Scholar] [CrossRef]
- Baim, D.S.; Wahr, D.; George, B.; Leon, M.B.; Greenberg, J.; Cutlip, D.E.; Kaya, U.; Popma, J.J.; Ho, K.K.L.; Kuntz, R.E. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 2002, 105, 1285–1290. [Google Scholar] [CrossRef]
- Paul, T.K.; Bhatheja, S.; Panchal, H.B.; Zheng, S.; Banerjee, S.; Rao, S.V.; Guzman, L.; Beohar, N.; Zhao, D.; Mehran, R.; et al. Outcomes of Saphenous Vein Graft Intervention with and Without Embolic Protection Device: A Comprehensive Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2017, 10, e005538. [Google Scholar] [CrossRef]
- Jin, B.; Dong, X.H.; Zhang, C.; Li, Y.; Shi, H.M. Distal protection devices in primary percutaneous coronary intervention of native coronary artery lesions: A meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2012, 28, 871–876. [Google Scholar] [CrossRef]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.; JGLde Smet, B.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.-S.; Suurmeijer, A.J.; et al. Thrombus Aspiration during Primary Percutaneous Coronary Intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized Trial of Primary PCI with or without Routine Manual Thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; James, S.; Džavík, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus Aspiration in ST-Segment–Elevation Myocardial Infarction. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Mathews, S.J.; Parikh, S.A.; Wu, W.; Metzger, D.C.; Chambers, J.W.; Ghali, M.G.H.; Sumners, M.J.; Kolski, B.C.; Pinto, D.S.; Dohad, S. Sustained Mechanical Aspiration Thrombectomy for High Thrombus Burden Coronary Vessel Occlusion: The Multicenter CHEETAH Study. Circ. Cardiovasc. Interv. 2023, 16, E012433. [Google Scholar] [CrossRef] [PubMed]
- Mathias, W.; Tsutsui, J.M.; Tavares, B.G.; Fava, A.M.; Aguiar, M.O.D.; Borges, B.C.; Oliveira, M.T.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; et al. Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2832–2842. [Google Scholar] [CrossRef]
- Aguiar, M.O.D.; Tavares, B.G.; Tsutsui, J.M.; Fava, A.M.; Borges, B.C.; Oliveira, M.T.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; Chiang, H.P.; et al. Sonothrombolysis Improves Myocardial Dynamics and Microvascular Obstruction Preventing Left Ventricular Remodeling in Patients with ST Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2020, 13, E009536. [Google Scholar] [CrossRef]
- Saku, K.; Kakino, T.; Arimura, T.; Sunagawa, G.; Nishikawa, T.; Sakamoto, T.; Kishi, T.; Tsutsui, H.; Sunagawa, K. Left Ventricular Mechanical Unloading by Total Support of Impella in Myocardial Infarction Reduces Infarct Size, Preserves Left Ventricular Function, and Prevents Subsequent Heart Failure in Dogs. Circ. Heart Fail. 2018, 11, e004397. [Google Scholar] [CrossRef]
- Benenati, S.; Crimi, G.; Macchione, A.; Giachero, C.; Pescetelli, F.; Balbi, M.; Porto, I.; Vercellino, M. Mechanical Unloading of the Left Ventricle before Coronary Reperfusion in Preclinical Models of Myocardial Infarction without Cardiogenic Shock: A Meta-Analysis. J. Clin. Med. 2022, 11, 4913. [Google Scholar] [CrossRef]
- Kapur, N.K.; Alkhouli, M.A.; DeMartini, T.J.; Faraz, H.; George, Z.H.; Goodwin, M.J.; Hernandez-Montfort, J.A.; Iyer, V.S.; Josephy, N.; Kalra, S.; et al. Unloading the Left Ventricle Before Reperfusion in Patients with Anterior ST-Segment-Elevation Myocardial Infarction: A Pilot Study Using the Impella CP. Circulation 2019, 139, 337–346. [Google Scholar] [CrossRef]
- Kapur, N.K.; Pahuja, M.; Kochar, A.; Karas, R.H.; Udelson, J.E.; Moses, J.W.; Stone, G.W.; Aghili, N.; Faraz, H.; O’Neill, W.W. Delaying reperfusion plus left ventricular unloading reduces infarct size: Sub-analysis of DTU-STEMI pilot study. Cardiovasc. Revasc. Med. 2024, 60, 11–17. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Kawano, S.; Okamura, A.; Kurotobi, T.; Date, M.; Inoue, K.; Fujii, K. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur. Heart J. 2006, 27, 534–539. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.; Choi, D.; Lee, C.J.; Lee, S.H.; Ko, Y.G.; Hong, M.K.; Kim, B.K.; Oh, S.J.; Jeon, D.W.; et al. Efficacy of High-Dose Atorvastatin Loading Before Primary Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction. The STATIN STEMI Trial. JACC Cardiovasc. Interv. 2010, 3, 332–339. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Huse, C.; Anstensrud, A.K.; Michelsen, A.E.; Ueland, T.; Broch, K.; Woxholt, S.; Woxholt, S.; Yang, K.; Sharma, K.; Tøllefsen, I.M.; et al. Interleukin-6 inhibition in ST-elevation myocardial infarction: Immune cell profile in the randomised ASSAIL-MI trial. eBioMedicine 2022, 80, 104013. [Google Scholar] [CrossRef]
- Lieder, H.R.; Braczko, F.; Gedik, N.; Stroetges, M.; Heusch, G.; Kleinbongard, P. Cardioprotection by post-conditioning with exogenous triiodothyronine in isolated perfused rat hearts and isolated adult rat cardiomyocytes. Basic Res. Cardiol. 2021, 116, 27. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.I.; Grigoriou, K.P.; Trikas, A.G.; Alexopoulos, N.A.; Mourouzis, I.S. Translating thyroid hormone into clinical practice: Lessons learned from the post-hoc analysis on data available from the ThyRepair study. Front. Endocrinol. 2024, 15, 1405251. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wang, S.; Qi, D. Effects of Cyclosporine on Reperfusion Injury in Patients: A Meta-Analysis of Randomized Controlled Trials. Oxid. Med. Cell. Longev. 2015, 2015, 287058. [Google Scholar] [CrossRef]
- Adlam, D.; Zarebinski, M.; Uren, N.G.; Ptaszynski, P.; Oldroyd, K.G.; Munir, S.; Zaman, A.; Contractor, H.; Kiss, R.G.; Édes, I.; et al. A Randomized, double-blind, dose ranging clinical trial of intravenous FDY-5301 in acute STEMI patients undergoing primary PCI. Int. J. Cardiol. 2022, 347, 1–7. [Google Scholar] [CrossRef]
- Bartorelli, A.L. Hyperoxemic Perfusion for Treatment of Reperfusion Microvascular Ischemia in Patients with Myocardial Infarction. Am. J. Cardiovasc. Drugs 2003, 3, 253–263. [Google Scholar] [CrossRef]
- Richard Spears, J.; Prcevski, P.; Xu, R.; Li, L.; Brereton, G.; DiCarli, M.; Spanta, A.; Crilly, R.; Lavine, S.; vander Heide, R. Aqueous Oxygen Attenuation of Reperfusion Microvascular Ischemia in a Canine Model of Myocardial Infarction. ASAIO J. 2003, 49, 716–720. [Google Scholar] [CrossRef]
- Kaluza, G.L.; Creech, J.L.; Furer, A.; Afari, M.E.; Milewski, K.; Yi, G.H.; Cheng, Y.; Conditt, G.B.; McGregor, J.C.; Blum, D.; et al. Chronic myocardial and coronary arterial effects of intracoronary supersaturated oxygen therapy in swine with normal and ischemic-reperfused myocardium. Sci. Rep. 2022, 12, 5785. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Martin, J.L.; De Boer, M.J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Falah, B.; Kotinkaduwa, L.N.; Schonning, M.J.; Redfors, B.; de Waha, S.; Granger, C.B.; Maehara, A.; Eitel, I.; Thiele, H.; Stone, G.W. Microvascular Obstruction in Patients with Anterior STEMI Treated with Supersaturated Oxygen. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101356. [Google Scholar] [CrossRef]
- Zeymer, U.; Hassinger, F.; Bramlage, P.; Schafer, A.; Westermann, D.; Thiele, H. Hyperoxemic oxygen therapy in patients with acute anterior myocardial infarction: HOT-AAMI—Design and rationale of a randomized trial. Am. Heart J. 2025, 286, 35–44. [Google Scholar] [CrossRef]
- Yang, X.M.; Proctor, J.B.; Cui, L.; Krieg, T.; Downey, J.M.; Cohen, M.V. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J. Am. Coll. Cardiol. 2004, 44, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Mancardi, D.; Raimondo, S.; Geuna, S.; Pagliaro, P. The paradigm of postconditioning to protect the heart: Molecular Medicine. J. Cell. Mol. Med. 2008, 12, 435–458. [Google Scholar] [CrossRef]
- Staat, P.; Rioufol, G.; Piot, C.; Cottin, Y.; Cung, T.T.; L’Huillier, I.; Aupetit, J.F.; Bonnefoy, E.; Finet, G.; André-Fouët, X.; et al. Postconditioning the human heart. Circulation 2005, 112, 2143–2148. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Clemmensen, P.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning During Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2017, 2, 490. [Google Scholar] [CrossRef]
- Chong, J.; Bulluck, H.; Fw Ho, A.; Boisvert, W.A.; Hausenloy, D.J. Chronic remote ischemic conditioning for cardiovascular protection. Cond. Med. 2019, 2, 164–169. [Google Scholar]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Vanezis, A.P.; Arnold, J.R.; Rodrigo, G.; Lai, F.Y.; Debiec, R.; Nazir, S.; Khan, J.N.; Ng, L.L.; Chitkara, K.; Coghlan, J.G.; et al. Daily remote ischaemic conditioning following acute myocardial infarction: A randomised controlled trial. Heart 2018, 104, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.L.; Herring, M.J.; Kloner, R.A. Delayed treatment with hypothermia protects against the no-reflow phenomenon despite failure to reduce infarct size. J. Am. Heart Assoc. 2013, 2, e004234. [Google Scholar] [CrossRef] [PubMed]
- Farissi, M.E.; Keulards, D.C.J.; van’t Veer, M.; Zelis, J.M.; Berry, C.; de Bruyne, B.; Engstrøm, T.; Fröbert, O.; Piroth, Z.; Oldroyd, K.G.; et al. Selective intracoronary hypothermia in patients with ST-elevation myocardial infarction. Rationale and design of the EURO-ICE trial. EuroIntervention 2021, 16, 1444–1446. [Google Scholar] [CrossRef]
- Syeda, B.; Schukro, C.; Heinze, G.; Modaressi, K.; Glogar, D.; Maurer, G.; Mohl, W. The salvage potential of coronary sinus interventions: Meta-analysis and pathophysiologic consequences. J. Thorac. Cardiovasc. Surg. 2004, 127, 1703–1712. [Google Scholar] [CrossRef]
- Khattab, A.A.; Stieger, S.; Kamat, P.J.; Vandenberghe, S.; Bongoni, A.; Stone, G.W.; Seiler, C.; Meier, B.; Hess, O.M.; Rieben, R. Effect of pressure-controlled intermittent coronary sinus occlusion (picso) on myocardial ischaemia and reperfusion in a closed-chest porcine model. EuroIntervention 2013, 9, 398–406. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar]
- De Maria, G.L.; Greenwood, J.P.; Zaman, A.G.; Carrié, D.; Coste, P.; Valgimigli, M.; Behan, M.; Berry, C.; Erglis, A.; Panoulas, V.F.; et al. Pressure-Controlled Intermittent Coronary Sinus Occlusion (PiCSO) in Acute Myocardial Infarction: The PiCSO-AMI-I Trial. Circ. Cardiovasc. Interv. 2024, 17, E013675. [Google Scholar] [CrossRef]
- Zhang, Y.; Wernly, B.; Cao, X.; Mustafa, S.J.; Tang, Y.; Zhou, Z. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic Res. Cardiol. 2021, 116, 22. [Google Scholar] [CrossRef]
- Cohen, M.V.; Downey, J.M. Adenosine: Trigger and mediator of cardioprotection. Basic Res. Cardiol. 2008, 103, 203–215. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Sato, H.; Williams, M.W.; Fernandez, A.Z.; Vinten-Johansen, J. Adenosine A2-receptor activation inhibits neutrophil-mediated injury to coronary endothelium. Am. J. Physiol.-Heart Circ. Physiol. 1996, 271, H1456–H1464. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Niccoli, G.; Rigattieri, S.; Rosaria De Vita, M.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; Ranieri De Caterina, A.; La Torre, G.; Paolo Lo Schiavo, Y.Y.; et al. Open-Label, Randomized, Placebo-Controlled Evaluation of Intracoronary Adenosine or Nitroprusside After Thrombus Aspiration During Primary Percutaneous Coronary Intervention for the Prevention of Microvascular Obstruction in Acute Myocardial Infarction The REOPEN-AMI Study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [PubMed]
- Kumbhani, D.J.; De Lemos, J.A. Finding an effective treatment for microvascular obstruction in STEMI: A road to perdition? Eur. Heart J. 2016, 37, 1920–1922. [Google Scholar] [CrossRef]
- Niccoli, G.; Spaziani, C.; Crea, F. Left ventricular remodeling and 1-year clinical follow-Up of the REOPEN-AMI trial. J. Am. Coll. Cardiol. 2014, 63, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Forman, M.B.; Gibbons, R.J.; Ross, A.M.; Alexander, R.W.; Stone, G.W. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: The AMISTAD-2 trial. Eur. Heart J. 2006, 27, 2400–2405. [Google Scholar] [CrossRef]
- Nazir, S.A.; McCann, G.P.; Greenwood, J.P.; Kunadian, V.; Khan, J.N.; Mahmoud, I.Z.; Blackman, D.J.; Been, M.; Abrams, K.R.; Shipley, L.; et al. Strategies to attenuate micro-vascular obstruction during P-PCI: The randomized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur. Heart J. 2016, 37, 1910–1919. [Google Scholar] [CrossRef]
- Aetesam-Ur-Rahman, M.; Brown, A.J.; Jaworski, C.; Giblett, J.P.; Zhao, T.X.; Braganza, D.M.; Clarke, S.C.; Agrawal, B.S.K.; Bennett, M.R.; West, N.E.J.; et al. Adenosine-induced coronary steal is observed in patients presenting with st-segment-elevation myocardial infarction. J. Am. Heart Assoc. 2021, 10, e019899. [Google Scholar] [CrossRef]
- Bulluck, H.; Sirker, A.; Loke, Y.K.; Garcia-Dorado, D.; Hausenloy, D.J. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: An updated meta-analysis of randomized controlled trials. Int. J. Cardiol. 2016, 202, 228–237. [Google Scholar] [CrossRef]
- Laborante, R.; Bianchini, E.; Restivo, A.; Ciliberti, G.; Galli, M.; Vergallo, R.; Rodolico, D.; Zito, A.; Princi, G.; Leone, A.M.; et al. Adenosine as adjunctive therapy in acute coronary syndrome: A meta-Analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 173–182. [Google Scholar] [CrossRef]
- Abdelaziz, H.K.; Elkilany, W.; Khalid, S.; Sabet, S.; Saad, M. Efficacy and safety of intracoronary verapamil versus sodium nitroprusside for the prevention of microvascular obstruction during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Coron. Artery Dis. 2017, 28, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, Y.; Dong, W.; Jiang, Z.C.; Li, T.; Cheng, L.Q.; Zou, Y.T.; Jiang, X.S.; Zhou, H.; Xin, A.; et al. Effects of Nicorandil Administration on Infarct Size in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: The CHANGE Trial. J. Am. Heart Assoc. 2022, 11, e026232. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, A.; Yamamoto, K.; Hirose, E.; Kobayashi, Y.; Takafuji, H.; Sano, F.; Matsumoto, K.; Ohara, M.; Yoshioka, R.; Takinami, H.; et al. Nicorandil prevents microvascular dysfunction resulting from PCI in patients with stable angina pectoris: A randomised study. EuroIntervention 2014, 9, 1050–1058. [Google Scholar] [CrossRef]
- Xu, L.; Wang, L.; Li, K.; Zhang, Z.; Sun, H.; Yang, X. Nicorandil prior to primary percutaneous coronary intervention improves clinical outcomes in patients with acute myocardial infarction: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2019, 13, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Stone, P.H.; Muller, J.E.; Braunwald, E. Calcium Channel Blocking Agents in the Treatment of Cardiovascular Disorders. Part I: Basic and Clinical Electrophysiologic Effects. Ann. Intern. Med. 1980, 93, 875–885. [Google Scholar] [CrossRef]
- Vijayalakshmi, K. Prospective, randomised, controlled trial to study the effect of intracoronary injection of verapamil and adenosine on coronary blood flow during percutaneous coronary intervention in patients with acute coronary syndromes. Heart 2006, 92, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, E011408. [Google Scholar] [CrossRef]
- Wilson, R.F.; White, C.W. Intracoronary papaverine: An ideal coronary vasodilator for studies of the coronary circulation in conscious humans. Circulation 1986, 73, 444–451. [Google Scholar] [CrossRef]
- Ashrafi, S.; Alam, S.; Sultana, A.; Raj, A.; Emon, N.U.; Richi, F.T. Papaverine: A Miraculous Alkaloid from Opium and Its Multimedicinal Application. Molecules 2023, 28, 3149. [Google Scholar] [CrossRef]
- De Bruyne, B.; Pijls, N.H.J.; Barbato, E.; Bartunek, J.; Bech, J.W.; Wijns, W.; Heyndrickx, G.R. Intracoronary and intravenous adenosine 5′-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation 2003, 107, 1877–1883. [Google Scholar] [CrossRef]
- Mizukami, T.; Sonck, J.; Gallinoro, E.; Kodeboina, M.; Canvedra, A.; Nagumo, S.; Bartunek, J.; Wyffels, E. Duration of hyperemia with intracoronary administration of papaverine. J. Am. Heart Assoc. 2021, 10, e018562. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Sato, H.; Tateishi, H.; Kawagoe, T.; Shimatani, Y.; Kurisu, S.; Sakai, K. Attenuation of the no-reflow phenomenon after coronary angioplasty for acute myocardial infarction with intracoronary papaverine. Am. Heart J. 1996, 132, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Thrane, P.G.; Olesen, K.K.W.; Thim, T.; Gyldenkerne, C.; Mortensen, M.B.; Kristensen, S.D.; Maeng, M. Mortality Trends After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2023, 82, 999–1010. [Google Scholar] [CrossRef]
- Bulluck, H.; Zheng, H.; Chan, M.Y.; Foin, N.; Foo, D.C.; Lee, C.W.; Lim, S.T.; Sahlen, A.; Tan, H.C.; Tan, J.W.; et al. Independent Predictors of Cardiac Mortality and Hospitalization for Heart Failure in a Multi-Ethnic Asian ST-segment Elevation Myocardial Infarction Population Treated by Primary Percutaneous Coronary Intervention. Sci. Rep. 2019, 9, 10072. [Google Scholar] [CrossRef] [PubMed]

| Intervention | Mechanism | Evidence Summary |
|---|---|---|
| Antithrombotic Therapy | ||
| Anticoagulants (UFH, Bivalirudin) | Inhibit thrombin activity and Factor Xa; reduce thrombus propagation and embolization risk during PCI. | Mixed results from RCT. Bivalirudin reduces major bleeding compared to UFH, with mixed effects on thrombotic outcomes. May reduce CMVO [51,52,53,54]. |
| Oral P2Y12 Inhibitors (Ticagrelor, Prasugrel) | Inhibit ADP-mediated platelet aggregation by blocking P2Y12 receptor; enhance platelet inhibition vs. clopidogrel. | RCTs demonstrated ischemic benefit of potent inhibitors compared to clopidogrel and is recommended by guidelines. Conflicting evidence regarding CMVO assessed by CMR [55,56,58,60]. |
| Parenteral P2Y12 Inhibitors (Cangrelor) | Reversibly blocks P2Y12 receptor; rapid onset/offset allows periprocedural platelet inhibition. | PITRI trial: no additional CMVO benefit on top of ticagrelor [63]. |
| GP IIb/IIIa Inhibitors (abciximab, tirofiban, eptifibatide) | Block fibrinogen binding and final common pathway of platelet aggregation; used in bailout no-reflow scenarios. | REVERSE-FLOW: reduced MVO but did not decrease infarct size; increases risk of non-fatal bleeding events [66]. |
| Inflammation and Endothelial Protection | ||
| Statins | Stabilize endothelium via pleiotropic effects; reduce oxidative stress, inflammation, and preserve glycocalyx integrity. | Meta-analyses and RCTs show lower MVO incidence and preserved LV function with high-dose statins [92,93]. |
| IL-6 Inhibitor (Tocilizumab) | Blocks IL-6 receptor, reducing systemic and local inflammation; modulates infarct healing and limits edema. | ASSAIL-MI: Small RCT showed an increase in myocardial salvage and reduction in CMVO [95]. |
| Device-Based Mechanical Strategies | ||
| Supersaturated Oxygen Therapy (SSO2) | Delivers high-dissolved O2 post-PCI; reduces capillary edema and enhances microvascular perfusion. | AMIHOT I/II: reduced infarct size and CMVO [102,104]. |
| Thrombectomy (Manual/Mechanical) | Physically removes thrombus from culprit vessel; targets macro- and microembolization. | Manual: TOTAL/TASTE: no MACE benefit; not routinely recommended. Mechanical: CHEETAH trial: improved flow in high thrombus burden [81,82,83,84]. |
| Sonothrombolysis | Microbubble-induced thrombus disruption via ultrasound cavitation; enhances clot resolution. | Small RCT enhanced recanalization and reduced infarct size [85]. |
| Mechanical Unloading | Reduces myocardial oxygen demand pre-reperfusion; limits infarct size and MVO. | DTU-STEMI: delayed reperfusion after LV unloading reduced infarct size/area at risk [90]. A larger RCT finished enrolling. (NCT03947619). |
| Pressure-controlled Coronary Sinus Occlusion (PiCSO) | Increases venous pressure cyclically to redistribute flow, reduce edema, and activate endothelial recovery. | Failed to demonstrate a reduction in infarct size, CMVO, or intramyocardial hemorrhage [117,118]. |
| Pharmacological established no-reflow treatment | ||
| Papaverine | Inhibits phosphodiesterase → ↑cAMP/cGMP → smooth muscle relaxation; potent microvascular vasodilator. | Small study: 56% decrease in TIMI frame count [142]. |
| Adenosine | Activates A2A receptor → vasodilation; also inhibits neutrophil adhesion and ROS production. | Heterogenous results. Intracoronary moderate–high dose of adenosine may improve CMVO and LVEF. Caution with advanced heart block and hemodynamic effects. Evidence from RCTs [123,126]. |
| Nicorandil | Dual KATP channel opener and NO donor; preconditions myocardium and reduces microvascular resistance. | Improved coronary blood flow and cardiac systolic function. Also improved prognosis [132,133,134]. |
| Calcium Channel Blockers | Inhibit L-type Ca2+ channels in smooth muscle; attenuate vasospasm and improve flow. | Compared to placebo, verapamil improved coronary flow. Compared to adenosine, verapamil also caused a higher incidence of transient heart block. Compared to nitroprusside, verapamil resulted in significant improvements in CMVO, with fewer adverse events [131,136]. |
| Epinephrine | Stimulates β2-receptors on microvasculature → vasodilation; used in refractory no-reflow. | COAR trial: improved angiographic criteria of coronary no-reflow [137]. |
| Nitroprusside | Releases NO directly; dilates both arterial and venous vasculature, including microvessels. | Did not show benefits compared to verapamil [123,127,131]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau, A.M.; Silva, P.G.; Mejía, H.P.G.; Granada, J.F.; Kaluza, G.L.; Burkhoff, D.; Abizaid, T.; Pileggi, B.; Freire, A.F.D.; Godinho, R.R.; et al. Molecular Mechanisms of Microvascular Obstruction and Dysfunction in Percutaneous Coronary Interventions: From Pathophysiology to Therapeutics—A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 6835. https://doi.org/10.3390/ijms26146835
Nicolau AM, Silva PG, Mejía HPG, Granada JF, Kaluza GL, Burkhoff D, Abizaid T, Pileggi B, Freire AFD, Godinho RR, et al. Molecular Mechanisms of Microvascular Obstruction and Dysfunction in Percutaneous Coronary Interventions: From Pathophysiology to Therapeutics—A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(14):6835. https://doi.org/10.3390/ijms26146835
Chicago/Turabian StyleNicolau, Andre M., Pedro G. Silva, Hernan Patricio G. Mejía, Juan F. Granada, Grzegorz L. Kaluza, Daniel Burkhoff, Thiago Abizaid, Brunna Pileggi, Antônio F. D. Freire, Roger R. Godinho, and et al. 2025. "Molecular Mechanisms of Microvascular Obstruction and Dysfunction in Percutaneous Coronary Interventions: From Pathophysiology to Therapeutics—A Comprehensive Review" International Journal of Molecular Sciences 26, no. 14: 6835. https://doi.org/10.3390/ijms26146835
APA StyleNicolau, A. M., Silva, P. G., Mejía, H. P. G., Granada, J. F., Kaluza, G. L., Burkhoff, D., Abizaid, T., Pileggi, B., Freire, A. F. D., Godinho, R. R., Campos, C. M., de Brito, F. S., Jr., Abizaid, A., & Melo, P. H. C. (2025). Molecular Mechanisms of Microvascular Obstruction and Dysfunction in Percutaneous Coronary Interventions: From Pathophysiology to Therapeutics—A Comprehensive Review. International Journal of Molecular Sciences, 26(14), 6835. https://doi.org/10.3390/ijms26146835





