Intermolecular Charge Transfer Induced Sensitization of Yb3+ in β-Diketone Coordination Compounds with Excellent Luminescence Efficiency
Abstract
1. Introduction
2. Results and Discussion
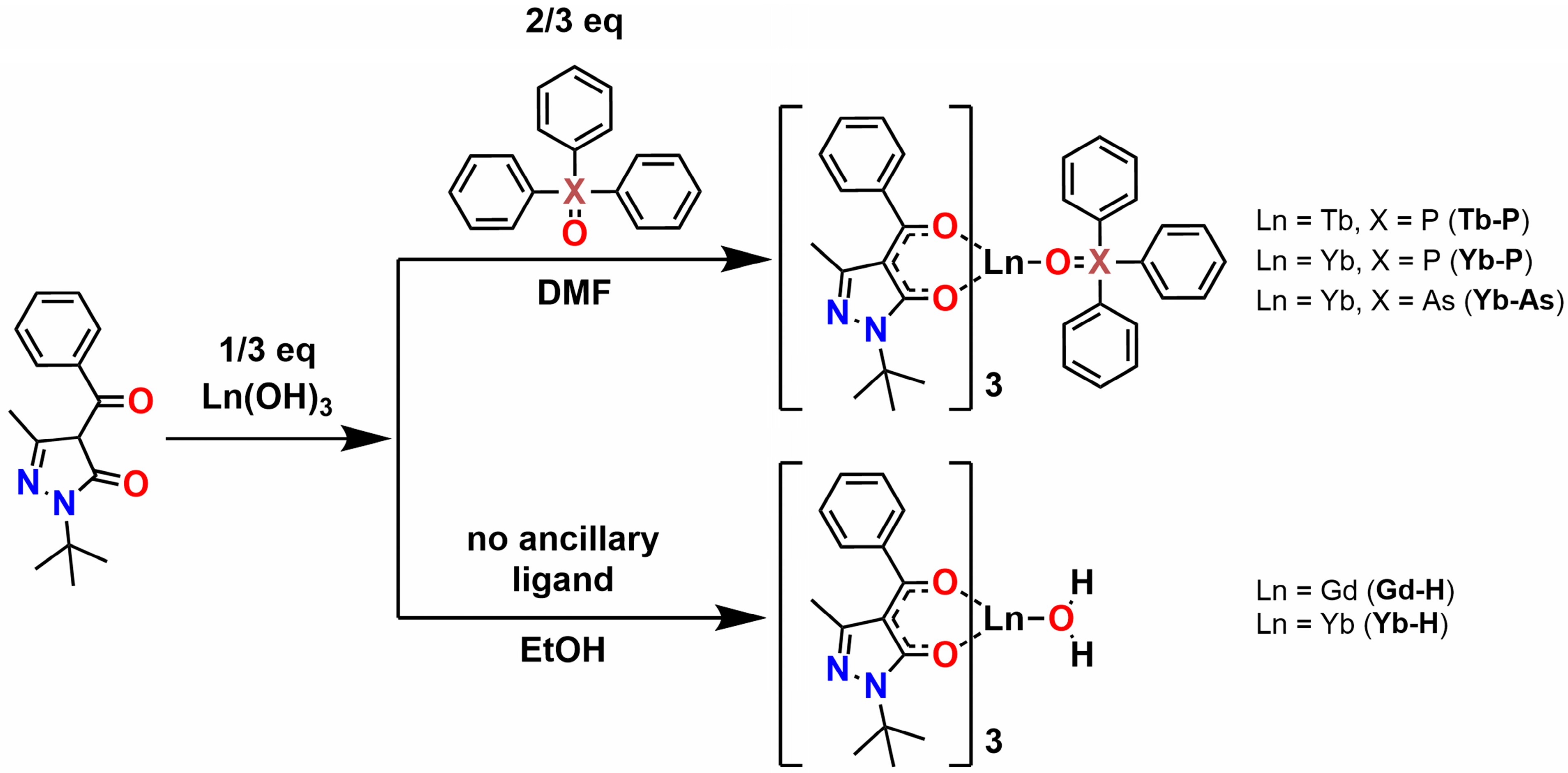
2.1. Synthesis and Crystal Structure
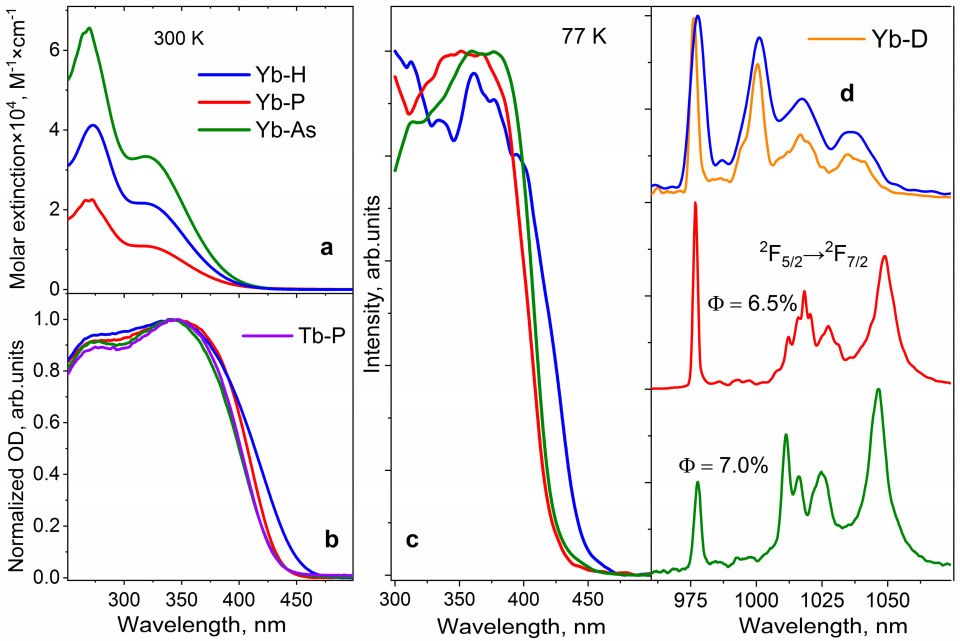
2.2. Luminescent Properties
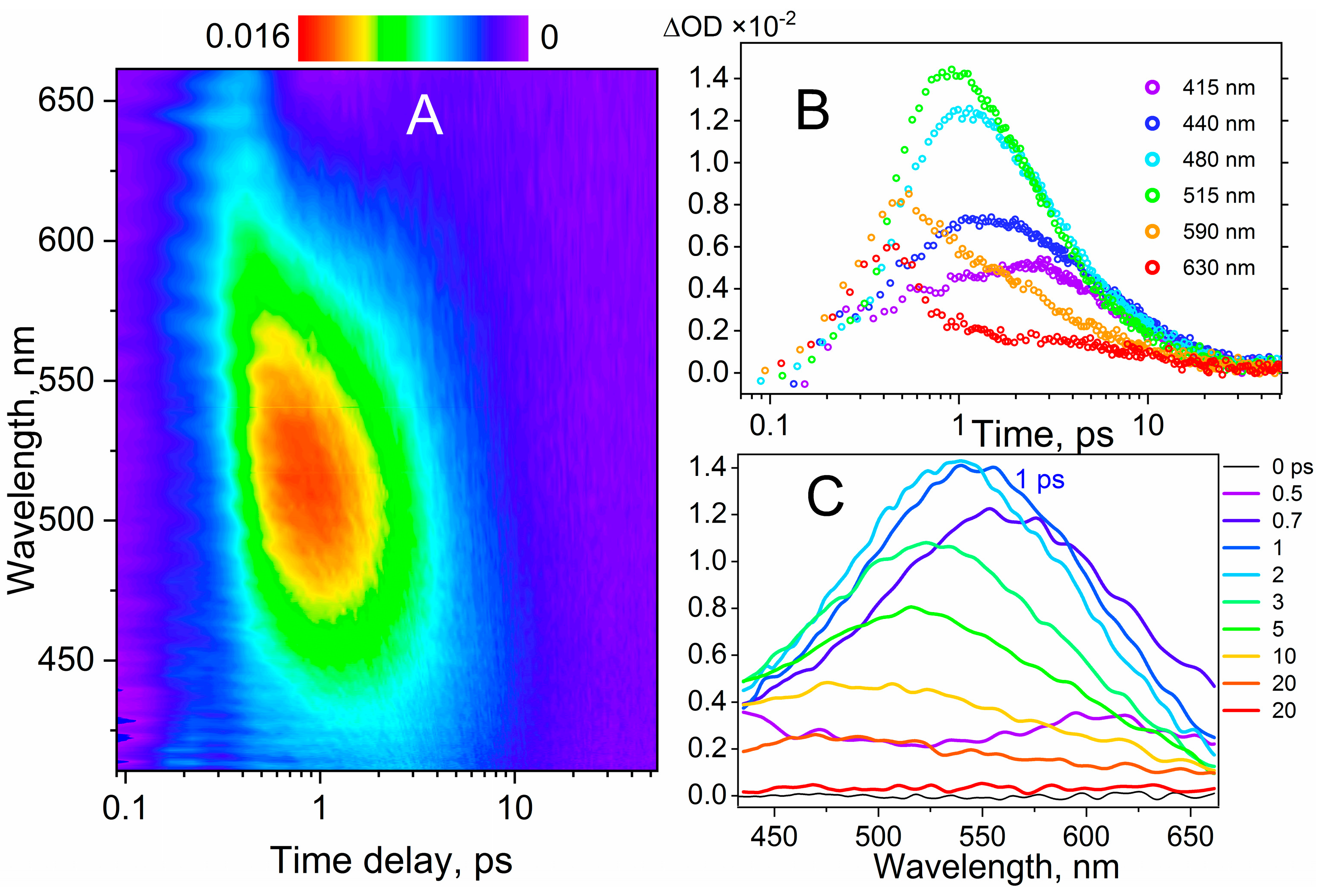
2.3. Charge Transfer Studies
3. Methods and Materials
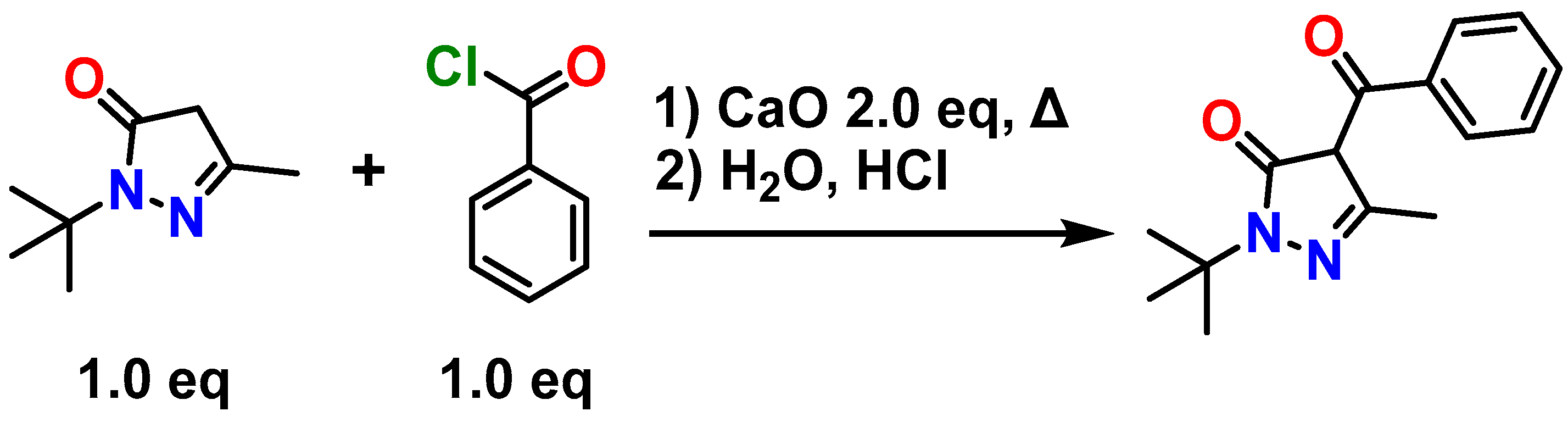
3.1. Synthesis of 4-Benzoyl-2-(tert-butyl)-5-methyl-2,4-dihydro-3H-pyrazol-3-one
3.2. Synthesis of Complexes
3.2.1. [Gd(QtBu)3(H2O)] (Gd-H)
3.2.2. [Yb(QtBu)3(H2O)] (Yb-H)
3.2.3. [Tb(QtBu)3(TPPO)] (Tb-P)
3.2.4. [Yb(QtBu)3(TPPO)] (Yb-P)
3.2.5. [Yb(QtBu)3(TPAO)] (Yb-As)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, Z.; Aderne, R.E.; Kai, J.; Chavarria, H.I.P.; Cremona, M. Ytterbium β-Diketonate Complexes for near Infra-Red Organic Light-Emitting Devices. Thin Solid Film. 2016, 620, 34–42. [Google Scholar] [CrossRef]
- Ahmed, Z.; Aderne, R.E.; Kai, J.; Resende, J.A.L.C.; Cremona, M. Synthesis and NIR-Optoelectronic Properties of a Seven-Coordinate Ytterbium Tris β-Diketonate Complex with C3 Geometrical Structure. Polyhedron 2016, 117, 518–525. [Google Scholar] [CrossRef]
- Bettencourt-Dias, A. Small Molecule Luminescent Lanthanide Ion Complexes—Photophysical Characterization and Recent Developments. Curr. Org. Chem. 2007, 11, 1460–1480. [Google Scholar] [CrossRef]
- Bhat, S.A.; Iftikhar, K. Synthesis and NIR Photoluminescence Studies of Novel Yb(III) Complexes of Asymmetric Perfluoryl β-Diketone. J. Lumin. 2019, 208, 334–341. [Google Scholar] [CrossRef]
- Biju, S.; Raj, D.B.A.; Reddy, M.L.P.; Kariuki, B.M. Synthesis, Crystal Structure, and Luminescent Properties of Novel Eu3+ Heterocyclic β-Diketonate Complexes with Bidentate Nitrogen Donors. Inorg. Chem. 2006, 45, 10651–10660. [Google Scholar] [CrossRef] [PubMed]
- Borda, P.; Crease, A.E.; Legzdins, P. The Determination of Lanthanides in Organometallic Complexes by the Closed Oxygen Flask Method. Microchem. J. 1973, 18, 172–177. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Bünzli, J.G. Rising Stars in Science and Technology: Luminescent Lanthanide Materials. Eur. J. Inorg. Chem. 2017, 2017, 5058–5063. [Google Scholar] [CrossRef]
- Metlin, M.T.; Goryachii, D.O.; Aminev, D.F.; Datskevich, N.P.; Korshunov, V.M.; Metlina, D.A.; Pavlov, A.A.; Mikhalchenko, L.V.; Kiskin, M.A.; Garaeva, V.V.; et al. Bright Yb3+ Complexes for Efficient Pure Near-Infrared OLEDs. Dye. Pigment. 2021, 195, 109701. [Google Scholar] [CrossRef]
- Abad Galán, L.; Wada, S.; Cameron, L.; Sobolev, A.N.; Hasegawa, Y.; Zysman-Colman, E.; Ogden, M.I.; Massi, M. Photophysical Investigation of Near Infrared Emitting Lanthanoid Complexes Incorporating Tris(2-Naphthoyl)Methane as a New Antenna Ligand. Dalton Trans. 2019, 48, 3768–3776. [Google Scholar] [CrossRef] [PubMed]
- Tsvirko, M.P.; Meshkova, S.B.; Venchikov, V.Y.; Topilova, Z.M.; Bol’shoi, D.V. Determination of Contributions of Various Molecular Groups to Nonradiative Deactivation of Electronic Excitation Energy in β-Diketonate Complexes of Ytterbium(III). Opt. Spectrosc. 2001, 90, 669–673. [Google Scholar] [CrossRef]
- Mara, D.; Artizzu, F.; Smet, P.F.; Kaczmarek, A.M.; Van Hecke, K.; Van Deun, R. Vibrational Quenching in Near-Infrared Emitting Lanthanide Complexes: A Quantitative Experimental Study and Novel Insights. Chem. Eur. J. 2019, 25, 15944–15956. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Ke, X.-S.; Hu, J.-Y.; Liu, Y.-W.; Ma, F.; Sun, H.-L.; Zhang, J.-L. Bioinspired Orientation of β-Substituents on Porphyrin Antenna Ligands Switches Ytterbium(III) NIR Emission with Thermosensitivity. Inorg. Chem. 2017, 56, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Tang, J.; Liu, Y.-W.; Jing, J.; Sun, Y.; Zhang, J.-L. Highly Luminescent, Biocompatible Ytterbium(III) Complexes as near-Infrared Fluorophores for Living Cell Imaging. Chem. Sci. 2018, 9, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Ning, Y.; Meng, Y.-S.; Zhang, J.; Wu, Z.-Y.; Gao, S.; Zhang, J.-L. Highly Near-IR Emissive Ytterbium(III) Complexes with Unprecedented Quantum Yields. Chem. Sci. 2017, 8, 2702–2709. [Google Scholar] [CrossRef] [PubMed]
- Kokozay, V.N.; Vassilyeva, O.Y.; Makhankova, V.G. Direct Synthesis of Heterometallic Complexes. In Direct Synthesis of Metal Complexes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–237. ISBN 978-0-12-811061-4. [Google Scholar]
- Femoni, C.; Iapalucci, M.C.; Ruggieri, S.; Zacchini, S. From Mononuclear Complexes to Molecular Nanoparticles: The Buildup of Atomically Precise Heterometallic Rhodium Carbonyl Nanoclusters. Acc. Chem. Res. 2018, 51, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.J. Shedding Light on the Structural and Luminescent Properties of Lanthanoid β-Triketonate Complexes. Master’s Thesis, Curtin University, Perth, Australia, 2019. [Google Scholar]
- Li, N.; Li, Y.; Wu, X.; Zhu, C.; Xie, J. Radical Deuteration. Chem. Soc. Rev. 2022, 51, 6291–6306. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kitagawa, Y.; Hasegawa, Y.; Imoto, H.; Naka, K. Drastic Enhancement of Photosensitized Energy Transfer Efficiency of a Eu(III) Complex Driven by Arsenic. Inorg. Chem. 2021, 60, 8605–8612. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Metlina, D.A.; Kompanets, V.O.; Melnikov, A.A.; Freire, R.O.; Silva, G.S.; Chekalin, S.V.; Taydakov, I.V. Ultrafast Dynamics and Relaxation Pathways in Eu(III) Complexes with Fluorinated-Diketonate Ligands. Dye. Pigment. 2023, 218, 111474. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Yakushev, I.A.; Polikovskiy, T.A.; Taydakov, I.V.; Drozdov, A.A.; Marchetti, F.; Pettinari, C. Self-Assembly of a Two-Dimensional Coordination Polymer Based on Silver and Lanthanide Tetrakis-Acylpyrazolonates: An Efficient New Strategy for Suppressing Ligand-to-Metal Charge Transfer Quenching of Europium Luminescence. Polymers 2023, 15, 867. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, N.A.; Lemport, P.S.; Polikovskiy, T.A.; Tsorieva, A.V.; Metlin, M.T.; Taydakov, I.V.; Zonov, R.V.; Lyssenko, K.A.; Vokuev, M.F.; Rodin, I.A.; et al. Large Energy Gap between Singlet and Triplet States Is No Longer a Problem: Intermediate Charge Transfer State Boosts Overall Quantum Yield up to 67% in Eu3+ Complexes. Rare Met. 2025, 44, 4279–4293. [Google Scholar] [CrossRef]
- Avagyan, N.A.; Lemport, P.S.; Polikovskiy, T.A.; Tsorieva, A.V.; Metlin, M.T.; Taydakov, I.V.; Zonov, R.V.; Lyssenko, K.A.; Vokuev, M.F.; Rodin, I.A.; et al. 4,7-Substituted 1,10-Phenanthroline-2,9-Dicarboxamides: Photophysics of Ligands and Their Complexes with the Eu–Gd–Tb Triad. Dalton Trans. 2024, 53, 14469–14480. [Google Scholar] [CrossRef] [PubMed]
- Faustino, W.M.; Malta, O.L.; De Sá, G.F. Intramolecular Energy Transfer through Charge Transfer State in Lanthanide Compounds: A Theoretical Approach. J. Chem. Phys. 2005, 122, 054109. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, E.; Carneiro Neto, A.N.; Trush, V.A.; Malta, O.L.; Jerzykiewicz, L.; Amirkhanov, V.M.; Legendziewicz, J.; Gawryszewska, P. Spectroscopic Aspects for the Yb3+ Coordination Compound with a Large Energy Gap between the Ligand and Yb3+ Excited States. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121072. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone Ligands: Synthesis, Structures, Metal Coordination Chemistry and Applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A. Lanthanide Acylpyrazolonates: Synthesis, Properties and Structural Features. Russ. Chem. Rev. 2012, 81, 1159–1169. [Google Scholar] [CrossRef]
- Zhao, Z.; Bian, M.; Lin, C.; Fu, X.; Yu, G.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Efficient Green OLEDs Achieved by a Terbium(III) Complex with Photoluminescent Quantum Yield Close to 100%. Sci. China Chem. 2021, 64, 1504–1509. [Google Scholar] [CrossRef]
- Girotto, E.; Pereira, A.; Arantes, C.; Cremona, M.; Bortoluzzi, A.J.; Salla, C.A.M.; Bechtold, I.H.; Gallardo, H. Efficient Terbium Complex Based on a Novel Pyrazolone Derivative Ligand Used in Solution-Processed OLEDs. J. Lumin. 2019, 208, 57–62. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Korshunov, V.M.; Metlin, M.T.; Metlina, D.A.; Kiskin, M.A.; Aminev, D.F.; Datskevich, N.P.; Drozdov, A.A.; Pettinari, C.; Marchetti, F.; et al. Towards Bright Dysprosium Emitters: Single and Combined Effects of Environmental Symmetry, Deuteration, and Gadolinium Dilution. Dye. Pigment. 2022, 199, 110078. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, J.; Zhu, C.; Han, B.; Liu, Y.; Yin, Z.; Li, F.; Liu, C.-M. An Intense Luminescent Dy(III) Single-Ion Magnet with the Acylpyrazolonate Ligand Showing Two Slow Magnetic Relaxation Processes. New J. Chem. 2018, 42, 16992–16998. [Google Scholar] [CrossRef]
- Shi, M.; Li, F.; Yi, T.; Zhang, D.; Hu, H.; Huang, C. Tuning the Triplet Energy Levels of Pyrazolone Ligands to Match the 5D0 Level of Europium(III). Inorg. Chem. 2005, 44, 8929–8936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, Q.; Huang, C.; Yao, G.; Umetani, S.; Matsui, M.; Ying, L.; Yu, A.; Zhao, X. Room-Temperature Fluorescence, Phosphorescence and Crystal Structures of 4-Acyl Pyrazolone Lanthanide Complexes: Ln(L)3·2H2O. Polyhedron 1997, 16, 1381–1389. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Belousov, Y.A.; Lyssenko, K.A.; Varaksina, E.; Drozdov, A.A.; Marchetti, F.; Pettinari, R.; Pettinari, C. Synthesis, Phosphorescence and Luminescence Properties of Novel Europium and Gadolinium Tris-Acylpyrazolonate Complexes. Inorganica Chim. Acta 2020, 502, 119279. [Google Scholar] [CrossRef]
- Polikovskiy, T.; Korshunov, V.; Gontcharenko, V.; Kiskin, M.; Belousov, Y.; Pettinari, C.; Taydakov, I. Dynamics of the Ligand Excited States Relaxation in Novel β-Diketonates of Non-Luminescent Trivalent Metal Ions. Int. J. Mol. Sci. 2023, 24, 8131. [Google Scholar] [CrossRef] [PubMed]
- Belousov, Y.A.; Drozdov, A.A.; Shikin, D.D.; Tombesi, A.; Pettinari, R.; Marchetti, F.; Pettinari, C. Rare Earth Acylpyrazolonates-Synthesis, Structure, Luminescent Properties and Applications. Eur. J. Inorg. Chem. 2025, 28, e202400568. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Pekareva, I.S.; Bünzli, J.-C.G. Intermolecular Interactions as Actors in Energy-Transfer Processes in Lanthanide Complexes with 2,2′-Bipyridine. J. Phys. Chem. B 2009, 113, 9265–9277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Mahiya, K.; Iftikhar, K. Structures and Pure Near-Infrared Photophysics of Erbium and Ytterbium(III) Complexes Incorporating Fluorinated β-Diketone and Neutral Unidentate Ligands. New J. Chem. 2020, 44, 13172–13181. [Google Scholar] [CrossRef]
- Ahmed, Z.; Iftikhar, K. Sensitization of Visible and NIR Emitting Lanthanide(III) Ions in Noncentrosymmetric Complexes of Hexafluoroacetylacetone and Unsubstituted Monodentate Pyrazole. J. Phys. Chem. A 2013, 117, 11183–11201. [Google Scholar] [CrossRef] [PubMed]
- Jinnai, K.; Kabe, R.; Adachi, C. A Near-Infrared Organic Light-Emitting Diode Based on an Yb(III) Complex Synthesized by Vacuum Co-Deposition. Chem. Commun. 2017, 53, 5457–5460. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, A.; Gumy, F.; Bünzli, J.-C.G. Intrinsic Quantum Yields and Radiative Lifetimes of Lanthanide Tris(Dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Congiu, M.; Alamiry, M.; Moudam, O.; Ciorba, S.; Richardson, P.R.; Maron, L.; Jones, A.C.; Richards, B.S.; Robertson, N. Preparation and Photophysical Studies of [Ln(Hfac)3DPEPO], Ln = Eu, Tb, Yb, Nd, Gd; Interpretation of Total Photoluminescence Quantum Yields. Dalton Trans. 2013, 42, 13537. [Google Scholar] [CrossRef] [PubMed]
- Shavaleev, N.M.; Scopelliti, R.; Gumy, F.; Bünzli, J.-C.G. Surprisingly Bright Near-Infrared Luminescence and Short Radiative Lifetimes of Ytterbium in Hetero-Binuclear Yb−Na Chelates. Inorg. Chem. 2009, 48, 7937–7946. [Google Scholar] [CrossRef] [PubMed]
- Deiters, E.; Gumy, F.; Bünzli, J.G. Acridone-Benzimidazole Ring-Fused Ligands: A New Class of Sensitizers of Lanthanide Luminescence via Low-Energy Excitation. Eur. J. Inorg. Chem. 2010, 2010, 2723–2734. [Google Scholar] [CrossRef]
- Tsorieva, A.V.; Korshunov, V.M.; Metlin, M.T.; Vlasova, T.S.; Gontcharenko, V.E.; Metlina, D.A.; Kompanets, V.O.; Chekalin, S.V.; Taydakov, I.V. Halogenated Dibenzoylmethane Eu3+ Complexes as Spectroscopic Markers: A Pioneering Photobleaching Strategy for Counterfeit Applications and Controlling Luminescence Efficiency. Mater. Chem. Front. 2025, 9, 809–817. [Google Scholar] [CrossRef]
- Tsaryuk, V.I.; Zhuravlev, K.P. Role of LMCT States in Luminescence Excitation Processes in Europium Indolecarboxylates. Opt. Spectrosc. 2023, 131, 1103–1111. [Google Scholar] [CrossRef]
- Faustino, W.M.; Malta, O.L.; Teotonio, E.E.S.; Brito, H.F.; Simas, A.M.; De Sá, G.F. Photoluminescence of Europium(III) Dithiocarbamate Complexes: Electronic Structure, Charge Transfer and Energy Transfer. J. Phys. Chem. A 2006, 110, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- De Mello DonegÁ, C.; Ribeiro, S.J.L.; Gon Çalves, R.R.; Blasse, G. Luminescence and Non-Radiative Processes in Lanthanide Squarate Hydrates. J. Phys. Chem. Solids 1996, 57, 1727–1734. [Google Scholar] [CrossRef]
- Phelan, J.P.; Ellman, J.A. Catalytic Enantioselective Addition of Pyrazol-5-ones to Trisubstituted Nitroalkenes with an N-Sulfinylurea Organocatalyst. Adv. Synth. Catal. 2016, 358, 1713–1718. [Google Scholar] [CrossRef]
- Jensen, B.S.; Meier, H.; Lundquist, K.; Refn, S. The Synthesis of 1-Phenyl-3-Methyl-4-Acyl-Pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, Y.; Li, H.; Gao, T.; Yan, P. Visible Light Sensitized Near-Infrared Luminescence of Ytterbium via ILCT States in Quadruple-Stranded Helicates. Dalton Trans. 2019, 48, 4026–4034. [Google Scholar] [CrossRef] [PubMed]


| Compound | Medium | τobs, μs | Φ, % | Reference |
|---|---|---|---|---|
| [Yb(QtBu)3(TPAO)] | Powder | 35 | 7.0 a | This work |
| [Yb(QtBu)3(TPPO)] | 31 | 6.5 a | ||
| [Yb(tta)3(dpso)]·H2O | CHCl3 | – | 2.40 b | [1] |
| [Yb(tta)3(dbso)]·H2O | 1.41 b | |||
| [Yb(tta)3(bga)]·H2O | 1.33 b | |||
| [Yb(tta)3(TPPO)]·H2O | CHCl3 | – | 1.92 b | [2] |
| Na[YbL4] | Powder | Radiative lifetime (930 μs) | 1.3 a | [26] |
| [Yb(hfaa)3(Hind)2] | Powder | 13.4 | 0.55 a | [39] |
| [Yb(hfaa)3(pz)2] | CHCl3 | 47 | 3.1 b | [40] |
| Yb(DBM)3 (DPEPO) | Anhydrous DMSO | 42.6 | 0.4 b | [41] |
| Na3[Yb(dpa)3] | Tris-HCl | 2.23 | 1.5 b | [42] |
| [Yb(hfac)3DPEPO] | CH2Cl2 | 22 | 2 a | [43] |
| [NaYb(Lq)4]·H2O | Powder | 19 | 3.1 a | [44] |
| [NaYb(LqF)4] | 20 | 3.3 a | ||
| [NaYb(LqMe)4] | 22 | 3.7 a | ||
| [Yb(LAB2)3] | Powder CHCl3/MeOH | 24.8 16.5 | 1.6 a 1.1 a | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polikovskiy, T.A.; Shikin, D.D.; Korshunov, V.M.; Gontcharenko, V.E.; Metlin, M.T.; Datskevich, N.P.; Islamov, M.M.; Kompanets, V.O.; Chekalin, S.V.; Belousov, Y.A.; et al. Intermolecular Charge Transfer Induced Sensitization of Yb3+ in β-Diketone Coordination Compounds with Excellent Luminescence Efficiency. Int. J. Mol. Sci. 2025, 26, 6814. https://doi.org/10.3390/ijms26146814
Polikovskiy TA, Shikin DD, Korshunov VM, Gontcharenko VE, Metlin MT, Datskevich NP, Islamov MM, Kompanets VO, Chekalin SV, Belousov YA, et al. Intermolecular Charge Transfer Induced Sensitization of Yb3+ in β-Diketone Coordination Compounds with Excellent Luminescence Efficiency. International Journal of Molecular Sciences. 2025; 26(14):6814. https://doi.org/10.3390/ijms26146814
Chicago/Turabian StylePolikovskiy, Trofim A., Daniil D. Shikin, Vladislav M. Korshunov, Victoria E. Gontcharenko, Mikhail T. Metlin, Nikolay P. Datskevich, Marat M. Islamov, Victor O. Kompanets, Sergey V. Chekalin, Yuriy A. Belousov, and et al. 2025. "Intermolecular Charge Transfer Induced Sensitization of Yb3+ in β-Diketone Coordination Compounds with Excellent Luminescence Efficiency" International Journal of Molecular Sciences 26, no. 14: 6814. https://doi.org/10.3390/ijms26146814
APA StylePolikovskiy, T. A., Shikin, D. D., Korshunov, V. M., Gontcharenko, V. E., Metlin, M. T., Datskevich, N. P., Islamov, M. M., Kompanets, V. O., Chekalin, S. V., Belousov, Y. A., & Taydakov, I. V. (2025). Intermolecular Charge Transfer Induced Sensitization of Yb3+ in β-Diketone Coordination Compounds with Excellent Luminescence Efficiency. International Journal of Molecular Sciences, 26(14), 6814. https://doi.org/10.3390/ijms26146814






