Hepatic Inflammation Primes Vascular Dysfunction Following Treatment with LPS in a Murine Model of Pediatric Fatty Liver Disease
Abstract
1. Introduction
2. Results
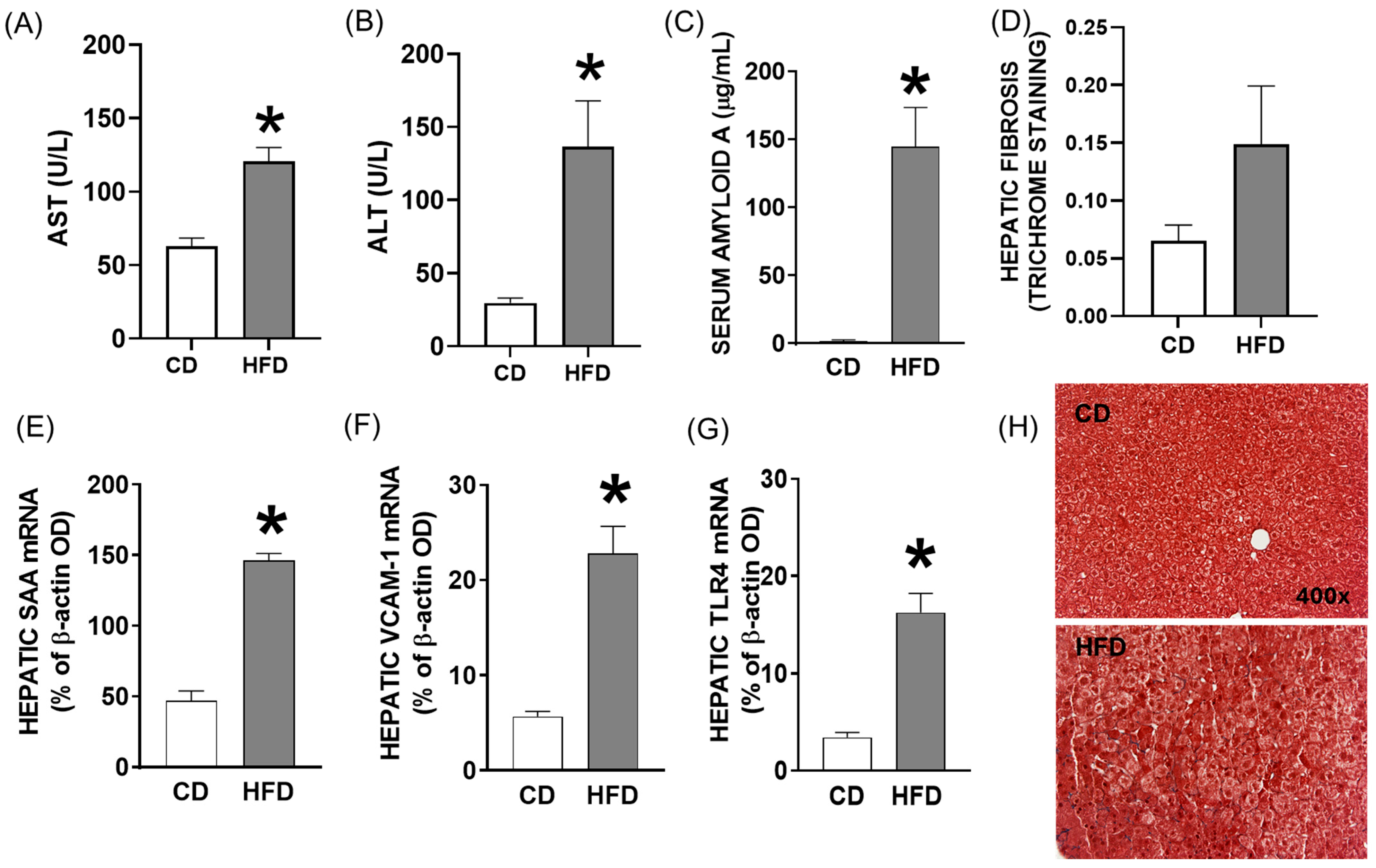
2.1. Young Mice Fed a High-Saturated-Fat and High-Cholesterol Diet (HFD) Exhibit Characteristics of Pediatric Fatty Liver Disease
2.2. Elevated Levels of Hepatic Enzymes and Inflammatory Mediators in Blood and Hepatic Inflammatory Response in Young Mice Fed a High-Fat Diet for Four Weeks
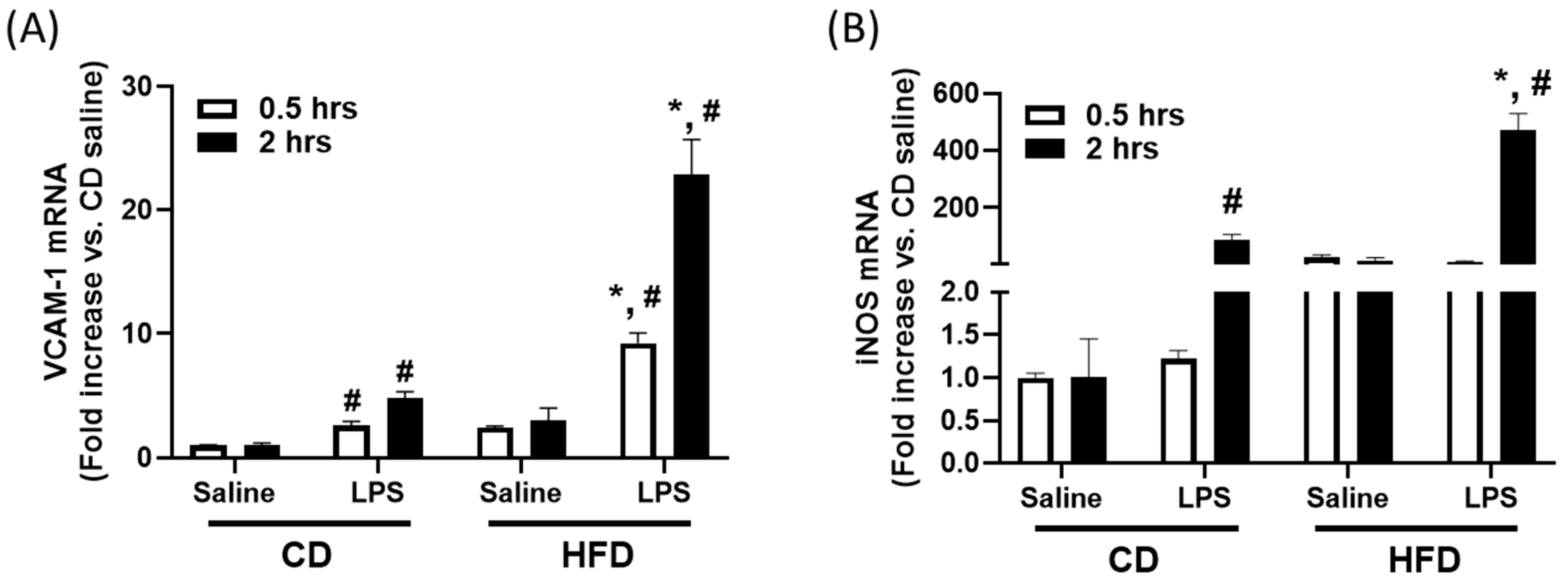
2.3. Pre-Exposure to High-Fat Diet in Young Mice Primes Hepatic Immune Response to LPS, Potentially Contributing to Vascular Dysfunction
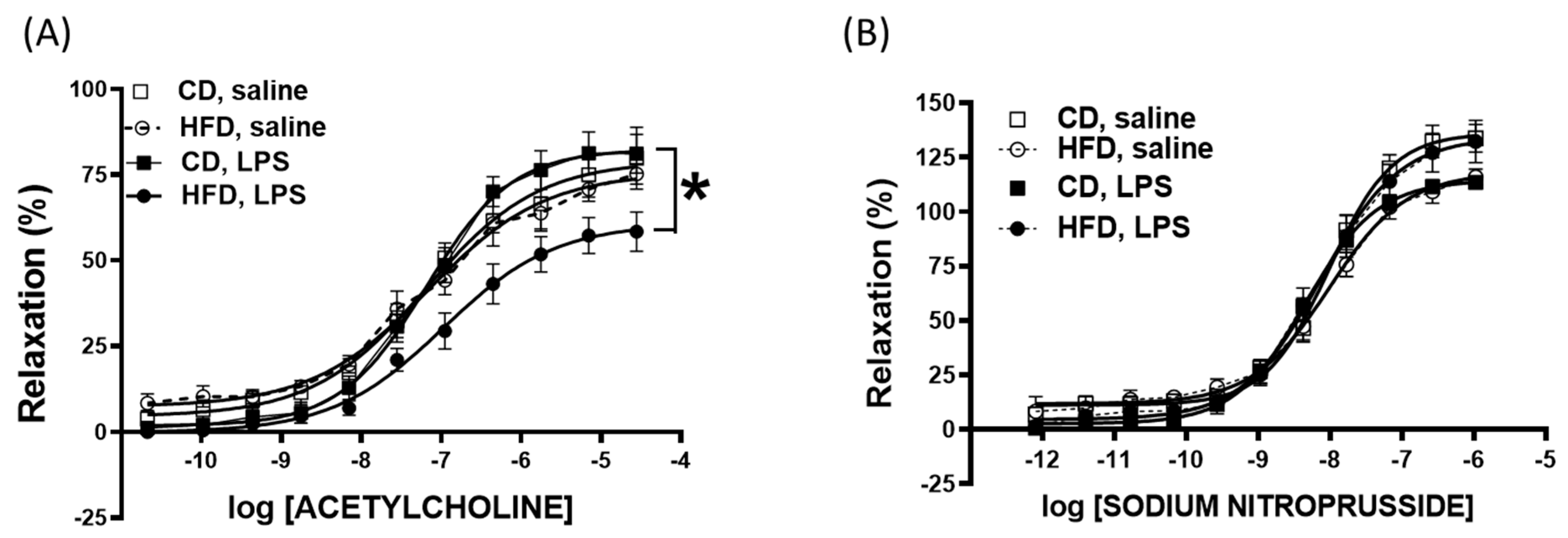
2.4. Impaired Vascular Function After Nonlethal LPS Challenge in Young Mice Fed a High-Fat Diet
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Plasma Measurements
4.3. Quantification of Fibrosis in Liver
4.4. Tissue mRNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
4.5. Isolated Vascular Function in Isolated Aortic Ring Segments
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Le, M.H.; Barakat, M.T.; Cheung, R.C.; Nguyen, M.H. The Changing Epidemiology of Liver Disease Among US Children and Adolescents From 1999 to 2016. Am. J. Gastroenterol. 2021, 116, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Trejo, A.M.; Vetvicka, V.; Vistejnova, L.; Kralickova, M.; Montufar, E.B. Hepatocyte and immune cell crosstalk in non-alcoholic fatty liver disease. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Kalsch, A.I.; Scharnagl, H.; Kleber, M.E.; Windpassinger, C.; Sattler, W.; Leipe, J.; Kramer, B.K.; Marz, W.; Malle, E. Long- and short-term association of low-grade systemic inflammation with cardiovascular mortality in the LURIC study. Clin. Res. Cardiol. 2020, 109, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.P.; Peña, K.; Burjonrappa, S. The Obesity Paradox in the Pediatric Trauma Patient. J. Pediatr. Surg. 2024, 59, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Prince, N.J.; Brown, K.L.; Mebrahtu, T.F.; Parslow, R.C.; Peters, M.J. Weight-for-age distribution and case-mix adjusted outcomes of 14,307 paediatric intensive care admissions. Intensive Care Med. 2014, 40, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, I.; Bodilly, L.; Kaplan, J. The Impact of Obesity on Critical Illnesses. Shock 2021, 56, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hartjes, K.; Shah, U.; Khalili, H.; Arnell, H.; Ludvigsson, J.F. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J. Hepatol. 2021, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Liu, B.; Li, Y.; Wang, Y.; Miskovsky, J.; Gaitanis, M.; Promrat, K.; Wu, W.C. Impact of Non-Alcoholic Fatty Liver Disease on Sepsis Inpatient Outcomes: A Nationwide Sample Analysis (2000–2019). J. Clin. Med. 2024, 13, 5737. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, L.; Comoretto, R.; Gregori, D.; Da Dalt, L.; Amigoni, A.; Daverio, M. Sepsis Prognostic Scores Accuracy in Predicting Adverse Outcomes in Children With Sepsis Admitted to the Pediatric Intensive Care Unit From the Emergency Department: A 10-Year Single-Center Experience. Pediatr. Emerg. Care 2023, 39, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J.; Lowry, S.F. The role of cytokine mediators in septic shock. Adv. Surg. 1990, 23, 21–56. [Google Scholar] [PubMed]
- Shi, Q.; Vandeberg, J.F.; Jett, C.; Rice, K.; Leland, M.M.; Talley, L.; Kushwaha, R.S.; Rainwater, D.L.; Vandeberg, J.L.; Wang, X.L. Arterial endothelial dysfunction in baboons fed a high-cholesterol, high-fat diet. Am. J. Clin. Nutr. 2005, 82, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Wolfort, R.M.; Stokes, K.Y.; Granger, D.N. CD4+ T lymphocytes mediate hypercholesterolemia-induced endothelial dysfunction via a NAD(P)H oxidase-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2619–H2626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, P.; Petersen, T.L.; Zhang, L.; Yan, K.; Thompson, N.E. Using Aggregate Vasoactive-Inotrope Scores to Predict Clinical Outcomes in Pediatric Sepsis. Front. Pediatr. 2022, 10, 778378. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, T.; Rose, J.L.; Stevens, R.L.; Hoyt, D.G. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J. Inflamm. 2007, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sohn, I.; Ahn, J.I.; Lee, K.H.; Lee, Y.S.; Lee, Y.S. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene 2004, 340, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, L.; Phan, J.; Strauss, M.; Tafuri, S.; Reue, K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J. Biol. Chem. 2003, 278, 42774–42784. [Google Scholar] [CrossRef] [PubMed]
- Vinue, A.; Herrero-Cervera, A.; Gonzalez-Navarro, H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients 2018, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Morganti, A.A.; Zervoudakis, I.; Letcher, R.L.; Romney, B.M.; Von Oeyon, P.; Papera, S.; Sealey, J.E.; Laragh, J.H. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am. J. Med. 1980, 68, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Groner, J.A.; Joshi, M.; Bauer, J.A. Pediatric precursors of adult cardiovascular disease: Noninvasive assessment of early vascular changes in children and adolescents. Pediatrics 2006, 118, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, P.; Predieri, B.; Madeo, S.; Lami, F.; Iughetti, L. Longitudinal evaluation of endothelial markers in children and adolescents with familial hypercholesterolemia. Acta Biomed. 2021, 92, e2021343. [Google Scholar]
- Faraj, T.A.; Stover, C.; Erridge, C. Dietary Toll-Like Receptor Stimulants Promote Hepatic Inflammation and Impair Reverse Cholesterol Transport in Mice via Macrophage-Dependent Interleukin-1 Production. Front. Immunol. 2019, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 2007, 100, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Kox, W.J. Endothelium function in sepsis. Inflamm. Res. 2000, 49, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yang, Q.; Yuan, K.; Loustalot, F.; Fang, J.; Daniels, S.R.; Hong, Y. Non-high-density lipoprotein cholesterol: Distribution and prevalence of high serum levels in children and adolescents: United States National Health and Nutrition Examination Surveys, 2005–2010. J. Pediatr. 2014, 164, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kit, B.K.; Carroll, M.D.; Lacher, D.A.; Sorlie, P.D.; DeJesus, J.M.; Ogden, C. Trends in serum lipids among US youths aged 6 to 19 years, 1988–2010. JAMA 2012, 308, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.J.; Chiamori, N.; Golub, O.J.; Berkman, S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am. J. Clin. Pathol. 1960, 34, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Wattanapitayakul, S.K.; Weinstein, D.M.; Holycross, B.J.; Bauer, J.A. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J. 2000, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Shoemaker, R.; Alsiraj, Y.; Murphy, M.; Gibbons, T.E.; Bauer, J.A. Hepatic Inflammation Primes Vascular Dysfunction Following Treatment with LPS in a Murine Model of Pediatric Fatty Liver Disease. Int. J. Mol. Sci. 2025, 26, 6802. https://doi.org/10.3390/ijms26146802
Huang H, Shoemaker R, Alsiraj Y, Murphy M, Gibbons TE, Bauer JA. Hepatic Inflammation Primes Vascular Dysfunction Following Treatment with LPS in a Murine Model of Pediatric Fatty Liver Disease. International Journal of Molecular Sciences. 2025; 26(14):6802. https://doi.org/10.3390/ijms26146802
Chicago/Turabian StyleHuang, Hong, Robin Shoemaker, Yasir Alsiraj, Margaret Murphy, Troy E. Gibbons, and John A. Bauer. 2025. "Hepatic Inflammation Primes Vascular Dysfunction Following Treatment with LPS in a Murine Model of Pediatric Fatty Liver Disease" International Journal of Molecular Sciences 26, no. 14: 6802. https://doi.org/10.3390/ijms26146802
APA StyleHuang, H., Shoemaker, R., Alsiraj, Y., Murphy, M., Gibbons, T. E., & Bauer, J. A. (2025). Hepatic Inflammation Primes Vascular Dysfunction Following Treatment with LPS in a Murine Model of Pediatric Fatty Liver Disease. International Journal of Molecular Sciences, 26(14), 6802. https://doi.org/10.3390/ijms26146802





