1. Introduction
One of the greatest innovations of the 20th century, plastics have become indispensable to human existence because of their advantageous qualities. Since 2000, global plastic production has doubled from approximately 200 million tonnes (Mt) to ~400 Mt in 2019 [
1]. Unfortunately, because plastics are not biodegradable, plastic trash builds up in landfills and the ocean, which causes major environmental issues [
2].
On a global scale, the recycling rate for plastic garbage stands at a modest 18%, with a slightly higher proportion of 24% being submitted to burning. The other 58% of plastic waste is either deposited in landfills or released into the natural environment, resulting in the long-term buildup and persistence of plastics [
3]. Plastics can be classified into two distinct groups: biodegradable plastics, which can come from either natural or synthetic sources and possess the ability to decompose through the action of microorganisms, and nondegradable plastics, which are derived from petrochemicals and exhibit a higher molecular weight due to the repetitive arrangement of small monomer units, commonly known as synthetic polymers. Illustrations of the latter classification include polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyethylene terephthalate (PET), and other plastics [
4].
1.1. PET
With an annual output of around 50 Mt, PET has become one of the most used synthetic polymers since its invention in the 1940s and has emerged as an important contributor to the growing problem of plastic waste. The primary cause of its propensity for degradation is largely attributed to the existence of hydrolysable ester linkages in its composition [
5].
Physical and chemical approaches have been implemented to degrade plastic waste, such as ultraviolet (UV) treatment, physical stress, hydrolysis, and ammonolysis [
6]. However, these approaches all have some limitations. For example, physical treatments degrade plastics into smaller fragments, thus will not significantly reduce the plastic entering the landfill flow, and the additives in the plastic will also be degraded to release substances that may affect the environment [
7]. Chemical treatment requires the use of solvents that are not environmentally friendly and always result in the release of byproducts such as carbon monoxide (CO), carbon dioxide (CO
2), terephthalic acid, anhydrides, carboxylic acids, and esters [
8].
To confront the imminent peril of plastic accumulation in marine and terrestrial environments, it is crucial to implement efficient biodegradative methods that alleviate the burden of plastic in the environment.
1.2. PETase from Ideonella sakaiensis
PET does not biodegrade well and instead accumulates as garbage. It was recently discovered that bacteria and fungi possess thermophilic hydrolases, which are best suited for catalysing PET hydrolysis at elevated temperatures. Remarkably, enzymes derived from
Ideonella sakaiensis were reported to digest PET well at room temperature [
9].
PETase, derived from
I. sakaiensis, also identified as
IsPETase, is an enzyme that has similarities to cutinase. It has attracted attention as a potentially effective and innovative approach to completely break down the polymer into its fundamental constituent units.
IsPETase exhibits increased activity at ambient temperatures and acts specifically on highly crystalline PET [
10], while the high-resolution (0.92 Å) X-Ray crystal structure of
IsPETase provided more information on the enzyme’s working mechanism and suggested that it is not fully optimised for crystalline PET degradation [
11].
There are concerns about the structural and sequence characteristics that enable
IsPETase to outperform its homologues at lower temperatures, since
IsPETase can break down PET films at ambient temperature, whereas other hydrolases require a high temperature to promote PET hydrolysis [
9]. Although with these advantages, the practical implementation of
IsPETase in the biodegradation of PET is hindered by its low solubility [
12], highlighting the need to screen more similar enzymes to make
IsPETase a more feasible target to be engineered for PET’s biodegradation.
This review provides a focused synthesis of recent advances in the discovery and engineering of PET-degrading enzymes, particularly homologues of IsPETase. We explore innovative in silico and AI-driven screening strategies, evaluate diverse enzymatic activity assays, and critically assess recombinant expression systems across bacterial, yeast, and algal hosts. Emphasis is placed on enhancing enzyme solubility, secretion, and activity through molecular and host-specific engineering approaches. By highlighting both the progress and persistent challenges in PETase development, this review aims to guide future research toward scalable, sustainable solutions for enzymatic PET biodegradation and recycling.
2. Screening IsPETase Homologues
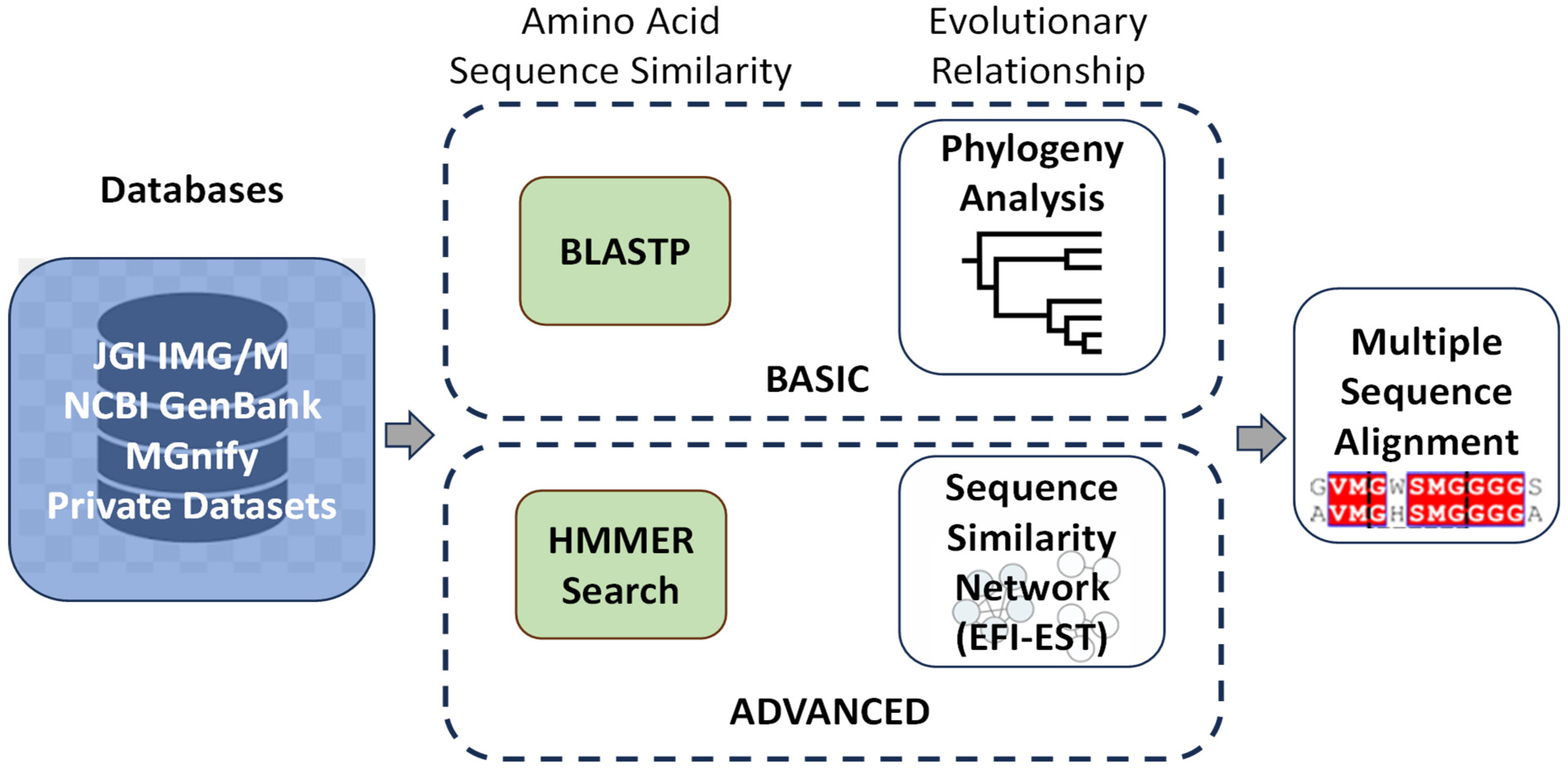
2.1. In Silico Screening
2.1.1. Amino Acid Sequence-Based Screening
Several research teams have employed in silico-based methodologies to screen enzymes exhibiting a high level of similarity to
IsPETase, sourced from a diverse range of microorganisms or environmental samples. The objective of these efforts is to identify potential candidates for further genetic engineering. Researchers hope to accelerate the degradation process of PET [
13,
14,
15]. These screening approaches, as outlined in
Figure 1, offer a promising pathway towards the development of more effective biocatalysts for environmental applications in plastic waste management.
Studies employing the strategies outlined in
Figure 1 have been published. However, only a tiny fraction of these investigations resulted in confirmed enzymatic activity of the newly identified candidates, leaving further validation still required [
14,
16,
17,
18,
19].
Many studies have utilised the NCBI (National Center for Biotechnology Information;
http://ncbi.nlm.nih.gov) database for enzyme screening, employing a variety of in silico strategies. These range from simpler methods such as BLSTP [
15] to more advanced techniques like PSI-BLAST [
19,
20]. These approaches are particularly appealing to researchers without extensive bioinformatic expertise, as they are relatively straightforward to implement. Remarkably, they have proven effective in identifying promising enzyme candidates, including novel enzymes whose activities have been experimentally validated.
For instance, a more comprehensive strategy was adopted by searching across multiple databases, including NCBI, UniProt, and the JGI Integrated Microbial Genomes and Microbiomes (JGI/IMG) database [
14]. The researchers employed an
IsPETase-specific Hidden Markov Model (HMM)-based search algorithm, which enabled the discovery of novel
IsPETase homologues. Similarly, the MGnify database, another resource for microbiome data, was explored using an HMM-based approach [
17]. Both studies successfully identified enzymes that exhibited activity against PET, highlighting the efficiency of this search technique in uncovering enzymes with plastic-degrading capabilities.
In contrast to the broad database-wide searches, other researchers have focused their efforts on specific groups of microorganisms within databases. For example, the basic BLASTP strategy was applied to 52 Streptomyces genome sequences obtained from NCBI, which included 23 terrestrial and 29 marine isolates. The work targeted approach led to the discovery of an
IsPETase-like enzyme, whose activity was confirmed through experimental assays [
16]. On the other hand, BLAST searches were conducted across 7375 terrestrial metagenomic samples in the JGI/IMG database [
13] (
https://img.jgi.doe.gov/). While homologues closely related to
IsPETase were identified, the activity of these candidates was not tested, leaving their potential for PET degradation unverified.
These diverse strategies demonstrate the adaptability and potential of in silico screening in identifying promising enzymes for further study in PET biodegradation research.
2.1.2. Artificial Intelligence (AI)-Driven and Structure-Based Screening
In addition to engineering IsPETase, AI tools have also been employed for PETase screening. However, the limited number of experimentally confirmed PETases presents a challenge for effective model training. To address this limitation, existing studies have devised various strategies to enhance training datasets, thereby mitigating the need for additional PETase sequences.
One machine learning (ML) model, employing the support vector machine method, was trained using a dataset of PETase homologues derived from thermal metagenomes. The homologues were collected through in silico screening based on amino acid sequences [
21], and the ML-assisted screening resulted in the identification of 74 candidate enzymes. Of these, 51 were successfully expressed in
Escherichia coli, and 37 exhibited detectable PET hydrolytic activity, including 23 enzymes that had not been previously reported.
In a separate study, a Generative Adversarial Network (GAN) was utilised for data augmentation. By employing a pre-trained deep Evolutionary Scale Language Model (ESM), potential PET-degrading enzymes were identified from a custom-built metagenomic library. Ultimately, 21 novel potential PET-degrading enzymes were identified through structure analysis, though enzymatic activity assays were not conducted to confirm their functionality [
22].
To enhance the accuracy of in silico screening strategies, structural features were considered during the screening phase, rather than being assessed solely at the confirmation stage. In one study, novel PETases were identified from the AlphaFold protein structure database. Candidate enzymes were selected based on the presence of key
IsPETase features, including an aspartic acid residue at positions 43–49 and a histidine residue at positions 71–81, following the Ser-Met-Gly-Gly-Gly motif. These candidates were further refined using the catalytic triad C-α mutual distance criterion, yielding an initial selection of 4594 putative PET hydrolases. Subsequent analysis, including sequence similarity network construction and multiple sequence alignment, narrowed this to a final pool of 107 candidate sequences. Although the most promising candidate, LSPET4, did not surpass
IsPETase in activity, mutated
IsPETase based on the structure feature of LSPET4 demonstrated a 9.32-fold increase in
IsPETase hydrolytic activity at 45 °C [
23].
Another study developed a novel PETase discovery pipeline by integrating protein language models with a structural representation tree. The most promising enzyme identified,
KbPETase, exhibited twice the activity of
IsPETase at 30 °C. Notably, when compared with FastPETase, the most active
IsPETase mutant with the optimal catalytic performance at 50 °C,
KbPETase still displayed a catalytic efficiency 1.3 times greater than that of FastPETase [
24].
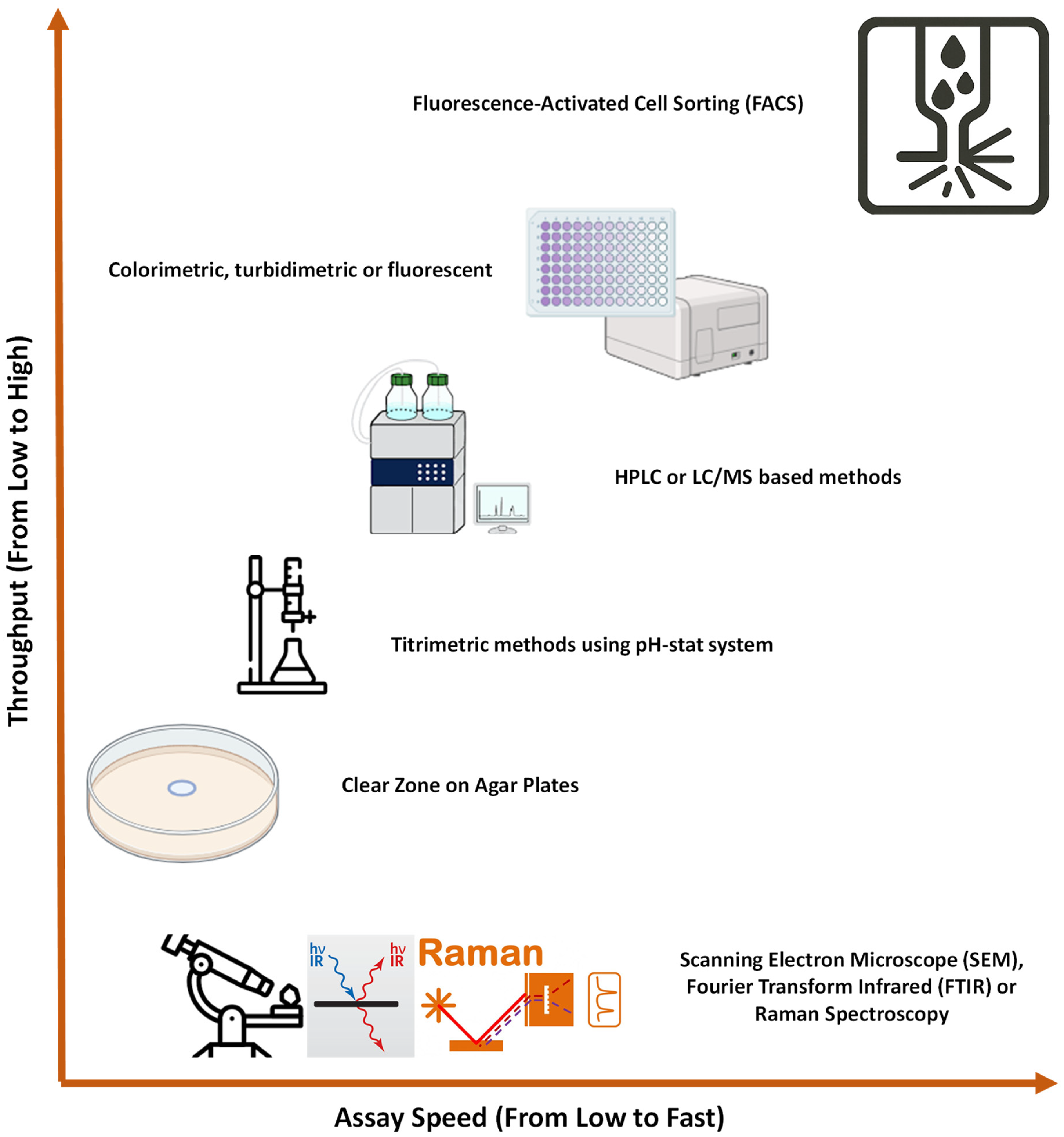
2.2. Assays to Screen PETase
Researchers typically combine in silico approaches with PET-degrading activity assays to screen novel PETases. This dual approach allows them to identify enzymes that are structurally similar to
IsPETases and capable of degrading PET. The most commonly used assays for measuring PET-degrading activity are depicted in
Figure 2. These screening techniques vary in scale from low to ultra-high throughput, depending on the context. High and ultra-high throughput assays are generally employed when screening engineered enzymes resulting from mutation or directed evolution [
25,
26,
27,
28,
29]. However, when it comes to screening
IsPETase homologues from environmental samples or databases like NCBI, medium or even low-throughput assays are often more suitable due to the low number of hits from in silico searching. These lower throughput methods strike a balance between efficiency and affordability, making them a more practical choice for such exploratory studies, where fewer samples may be initially tested [
14,
30].
Building on this framework, researchers tailor their choice of assay based on the nature of the PETase candidates and the experimental constraints. Agar plate-based methods, for example, provide a straightforward and visual means of assessing polymer degradation but are limited by their low throughput [
14,
30]. Titrimetric methods, including the use of pH-stat systems, provide a moderate-throughput alternative for quantifying acid release during PET hydrolysis [
31,
32]. HPLC or LC/MS-based assays offer higher specificity and sensitivity, enabling detailed quantification of degradation products, though they require more time and instrumentation [
20,
33,
34].
For high-throughput applications, colorimetric, turbidimetric, or fluorescent assays are widely used due to their compatibility with microplate formats and automation [
25,
28,
35]. At the ultra-high-throughput level, Fluorescence-Activated Cell Sorting (FACS) allows for rapid screening of vast enzyme libraries, particularly in yeast surface display systems [
29].
While not typically used for routine screening, scanning electron microscope (SEM), Fourier Transform Infrared spectroscopy (FTIR) and Raman spectroscopy serve as valuable tools in specialised contexts [
36,
37,
38]. These methods are slower, more costly, and lower in throughput, but they provide unique insights into enzyme–substrate interactions and the degradation of crystalline PET. As such, they are often employed to complement biochemical assays in mechanistic or structural studies.
3. Heterologous Expression Platforms to Screen PETases
Beyond screening for novel homologues,
IsPETase itself has been engineered to enhance its properties. Challenges such as inefficient enzyme secretion and cytoplasmic stress have led to the development of secretion expression and surface display platforms in diverse hosts [
39]. These advanced platforms facilitate the rapid screening of enzyme variants, eliminating the need for cell lysis and avoiding the formation of inclusion bodies, thereby streamlining the screening process [
12,
40,
41].
The successful expression of IsPETase and its homologues in heterologous systems is critical for both fundamental research and industrial applications. Various microbial hosts, including bacteria, yeasts, and microalgae, have been explored for this purpose, each offering distinct advantages and limitations. For example, E. coli is widely used due to its rapid growth and well-established genetic tools, while Bacillus subtilis offers efficient secretion capabilities. Yeasts such as Pichia pastoris and Saccharomyces cerevisiae provide eukaryotic post-translational modifications, and microalgae offer environmentally sustainable platforms for enzyme production. In the following subsections, we examine host-specific strategies that have been developed to enhance PETase expression, solubility, and secretion in these systems.
3.1. Bacteria
3.1.1. E. coli
Most studies that have expressed
IsPETase or its homologues in
E. coli have utilised pET-series vectors and BL21 (DE3) or derived strains, such as BL21 (DE3)-T1R [
42] and BL21-Gold (DE3) [
26], with IPTG added to induce expression.
As an alternative to the widely used
E. coli BL21 (DE3),
E. coli SHuffle T7 was engineered for the intracellular expression of
IsPETase
Mut, an active mutant of
IsPETase. This alternative was chosen due to the propensity of proteins with disulfide linkages to misfold and accumulate as inclusion bodies during overexpression, a phenomenon initially observed with the expression vectors pBAD and pET30a [
43,
44]. This was corroborated by a separate study in which
IsPETase was expressed in multiple
E. coli hosts, with SHuffle T7 Express demonstrating the highest enzymatic activity. However, this advantage was not observed for FastPETase (N233K/R224Q/S121E/D186H/R280A) [
45] and Hot-PETase [
46], two variants of
IsPETase [
47]. An additional noteworthy finding indicated that the formation of inclusion bodies during the overexpression of
IsPETase
Mut in
E. coli was primarily attributed to protein aggregation driven by interactions among hydrophobic regions, rather than the presence of disulfide bonds [
12]. Consequently, the precise role of SHuffle T7 as an expression host requires further elucidation.
A novel, growth-decoupled
E. coli strain, enGenes Biotech’s e
x-press V2, was employed in one study to express the PET-degrading enzyme polyester hydrolyse Leipzig 7 (PHL7) [
48]. In this system, induction with arabinose inhibits
E. coli host cell RNA polymerase, thereby arresting cell growth and reallocating cellular resources to enhance recombinant protein production [
49]. PET-degrading activity was detected in the unpurified fermentation supernatant; however, enGenes e
x-press V2 was not benchmarked against established
E. coli host strains [
48].
Enhanced Secretion System
A frequently encountered challenge in expressing IsPETase in E. coli is its low solubility. Current research has explored various strategies to address this limitation.
The Sec-dependent pathway is associated with post- and co-translational translocation of pre-folded polypeptides across the inner membrane [
26,
42]. Signal peptides employing the Sec-dependent pathway are frequently utilised, including PelB, PhoA, OmpC, OmpF, OmpA, and MalE [
50]. Among these, PelB has found widespread commercial use in pET vector expression systems, such as pET20b, 22b, and 26b [
50], making it a convenient starting point for enhancing
IsPETase secretion.
Employing this approach,
IsPETase was cloned into pET22b(+), which contains the DNA sequence for PelB, and subsequently generated a library via standard error-prone PCR (epPCR) to induce mutations in PelB. Using an assay to evaluate the ability to degrade 4-
pNPA (
para-nitrophenyl acetate), they identified mutants
IsPETase with significantly enhanced activity. The increased PETase concentration in the supernatant correlated with improved activity, exhibiting at least 1.7-fold enhancement across various assays [
26].
In another study, multiple Sec-dependent signal peptides, including MalE, LamB, and SurA, were tested and demonstrated that LamB, a maltoporin involved in maltose transportation, facilitated the production of significantly higher levels of extracellular
IsPETase in
E. coli [
42].
Further advancements in the heterologous expression of
IsPETase in
E. coli were achieved by modifying signal peptides with N-terminal enhancers. These enhancers, derived from the endogenous signal peptide of β-fructofuranosidase (β-FFase) originating from
Arthrobacter arilaitensis NJEM01, proved effective in achieving extracellular synthesis of
IsPETase with increased enzymatic activity [
51].
An intriguing finding in this area arises from the use of native signal peptides. When an
IsPETase homologue from
Streptomyces sp. SM14 was expressed in
E. coli with its native signal peptide; the enzyme was successfully exported, as confirmed by the polycaprolactone (PCL) plate clearing assay [
16]. This result underscores the potential of leveraging native signal peptides for effective extracellular expression and secretion in heterologous systems.
Cell Surface Display (CSD) System
Microbial cell surface display systems enable peptides and proteins to be presented on the surface of microbial cells by fusing them with anchoring motifs [
57]. The outer membrane-bound fatty acid transporter (FadL) from
E. coli was previously demonstrated to be effective for an esterase enzyme display on the surface of
E. coli [
58]. When FadL was co-expressed with the CSD system,
IsPETase exhibited activity in the tributyrin plate clearing assay and demonstrated enhanced thermostability [
40].
Building on the advancements in CSD systems, the PgsA protein from
B. subtilis was employed as an anchoring protein for the heterologous expression of
IsPETase in
E. coli [
59]. PgsA is part of the enzyme complex that synthesises poly-γ-glutamic acid (PGA) in
B. subtilis and has been fused with lipase B from
Candida antarctica (CalB) to display the enzyme in an active form on the cell surface of
E. coli [
60]. The fused protein was expressed in
E. coli, and it was confirmed that the active form of the
IsPETase was present on the cell surface of
E. coli [
59].
Increasing Membrane Permeability
Colicin families offer the functional diversity of unique cell-killing mechanisms that allow the simultaneous release of recombinant enzymes from cells. Of over 20 colicins, the lysis gene from Colicin E7 was effectively reported to enhance the secretion of intracellular recombinant enzymes from
E. coli [
61]. An
IsPETase homologue screened from metagenome analysis, F148, was co-expressed with Colicin E7 in
E. coli BL21 (DE3). Compared with the control strain only expressing F148, during the same period, nearly 3-fold degradation products could be observed, since Colicin E7 enabled protein release simultaneously by obstructing the outer membrane through phospholipase activation [
62].
In another study, a comparable strategy was adopted by fusing
IsPETase with
Thermobifida fusca cutinase, an
IsPETase homologue previously reported to degrade phospholipids and thereby increase cell membrane permeability [
63]. This approach successfully enhanced enzyme secretion, providing another example of facilitating secretion through the modulation of cell membrane permeability [
64].
3.1.2. B. subtilis
B. subtilis is widely recognised as a robust biotechnological workhorse for the heterologous expression of enzymes. Its non-pathogenic nature and designation as a GRAS (generally regarded as safe) microorganism render it fully compliant with regulatory requirements for industrial applications [
65,
66]. Similar strategies to those employed in
E. coli have been adapted for use in
B. subtilis to enhance the extracellular expression of
IsPETases.
Enhanced Secretion System
The use of five
Bacillus-derived signal peptides for expressing
IsPETase was investigated in
B. subtilis WB600, a strain derived from
B. subtilis 168 with six protease genes knocked out [
67]. The signal peptides tested were amy from
Bacillus amyloliquefaciens; Sac and AprE, both from
B. subtilis 168; apr and BprA, both from
Bacillus licheniformis WX-02. Among these, the signal peptide amy (SPamy) demonstrated the most significant effect, increasing enzyme activity in the cell culture supernatant nearly four-fold [
68].
In contrast to studies on
IsPETase expression in
E. coli, which consistently reported that the Sec-dependent secretion pathway enhances
IsPETase secretion, research on
B. subtilis has yielded conflicting results. In a separate study, the expression of
IsPETase in
B. subtilis 168 was examined using three Sec signal peptides (AmyE, Bpr, and SacB) and two Tat signal peptides (PhoD and YwbN), which direct proteins through the twin-arginine translocation (Tat) pathway. However, none of these signal peptides improved
IsPETase secretion compared to its native signal peptide [
69]. Thusfurther research is required to elucidate the discrepancies observed in the findings of these studies.
Chaperone Overexpression
In
B. subtilis CBS2, the hrcA gene, a negative regulator of the dnaK and groESL operons [
70,
71], was inactivated. However, the protein titre of
IsPETase was not improved in the chaperone-overexpressing strain. In contrast, a PET hydrolase from the bacterium HR29 (
BhrPETase) exhibited an approximately 20-fold increase in production [
72].
3.2. Yeast
3.2.1. S. cerevisiae
Surface display systems have been adopted to enhance the extracellular concentration of
IsPETase. The anchoring protein GCW51 was successfully utilised to display
IsPETase on the surface of yeast [
73]. In a more recent study, a novel surface display platform was developed based on
S. cerevisiae spores to display FastPETase [
41].
Unlike the cell wall of vegetative
S. cerevisiae cells, which consists of a two-layer structure comprising β-glucan and mannan, the spore wall features a four-layer structure composed of mannan, β-glucan, chitosan, and dityrosine. Displaying proteins on the surface of
S. cerevisiae spores offers distinct advantages over traditional cell surface display, as it eliminates the need for transmembrane transport. Furthermore, the unique structure of the spore wall imparts resistance to environmental stresses, as demonstrated by a study, where the displayed FastPETase exhibited significantly improved thermostability [
45].
Yeast surface display systems provide a promising approach to producing IsPETase, offering the advantages of bypassing enzyme purification, resulting in substantial time and cost savings, and facilitating the reuse of the biocatalyst.
3.2.2. Yarrowia lipolytica
Y. lipolytica has been identified as a potential host for the expression of
IsPETase, owing to its ability to assimilate atypical carbon sources, allowing for the assimilation of degradation products from PET [
74]. Following the successful expression of
IsPETase in
Y. lipolytica, the same group optimised its expression by testing various pre-treatment methods [
75]. However, in comparison to other host systems, research in
Y. lipolytica has yet to progress towards a deeper understanding and optimisation at the molecular biology level.
3.2.3. P. pastoris
The methylotrophic yeast
P. pastoris is widely recognised as a versatile workhorse for the production of heterologous proteins in industrial applications [
76,
77]. This expression system has been shown to support efficient secretory expression, and its post-translational glycosylation further enhances expression yields by increasing the resistance of glycoproteins to proteolytic degradation [
78,
79]. In another study,
IsPETase was successfully expressed in
P. pastoris, demonstrating significant improvements in the enzyme’s specific activity and thermostability both for native
IsPETase and FastPETase [
80].
In another study, a chaperone co-expression strategy was employed in
P. pastoris to enhance the production of the FastPETase variant, FastPETase-212/277. A panel of chaperones was evaluated, including protein disulphide isomerase (PDI), thiol oxidase (Ero1), the bZIP transcription factor HAC1, and proprotein convertase Kex2. Among these, Ero1 was identified as the most effective in boosting expression [
81].
3.3. Microalgae
Microalgae present a compelling option for the synthesis of recombinant plastic-degrading enzymes and various environmental applications, owing to their ubiquitous presence in aquatic systems, minimal production of endotoxins, and ability to grow without the need for supplemental organic carbon [
82]. In a study, the photosynthetic microalga
Phaeodactylum tricornutum was employed to secrete a modified PETase, PETaseR280A, into the surrounding culture medium for PET degradation. The gene encoding PETaseR280A was co-expressed with the signal peptide of
P. tricornutum alkaline phosphatase. The secreted enzyme demonstrated PET-degrading activity, confirming successful expression and secretion [
83]. However, the application of
P. tricornutum is constrained by its specific growth requirements, including low temperatures, silica as a nutrient, and high salinity.
To overcome these limitations,
Chlamydomonas reinhardtii, a GRAS-designated freshwater microalga, was employed in a subsequent study for the expression of wild-type
IsPETase.
C. reinhardtii was selected due to its faster growth rate and freshwater habitat [
84]. While the enzyme’s activity was confirmed, the authors did not specify whether a signal peptide was employed to assist the secretion, and enzyme activity was assessed using cell lysates.
In another study,
C. reinhardtii was employed as the host organism, with the original signal peptide retained to target the recombinant protein to the thylakoid lumen. This study differed by integrating the
IsPETase gene into the plastome at a specific site, restoring a photosynthesis-essential gene. This restoration allowed antibiotic-free selection following gene integration [
85].
In summary, these findings underscore the potential of bioengineered microalgae for producing IsPETase and homologues, offering promising applications in bioremediation strategies for PET-polluted aquatic environments.
4. Microbial Expression Systems for IsPETases and Homologues
The exploration of microbial platforms for IsPETase expression and the advancement of in silico analyses, screening assays, and novel enzyme discovery strategies underscore the rapid progress in this field.
4.1. Prokaryotic Hosts
Microbial hosts, particularly
E. coli (
Table 1), remain the backbone for heterologous expression, offering high yields and a broad toolkit for genetic manipulation. Sec-dependent secretion pathways, fusion tags, and chaperone co-expression strategies have been instrumental in improving the solubility, stability, and extracellular secretion of
IsPETase. Recent integrative approaches, such as combining vesicle nucleating peptides with chaperone systems, have further advanced production levels. However, limitations such as protein misfolding and inclusion body formation necessitate continued optimisation and host-specific engineering.
CSD systems represent a complementary approach, allowing IsPETase localisation on the cell membrane and bypassing purification steps. The use of anchor proteins such as FadL and PgsA in E. coli has resulted in enhanced enzyme activity and thermostability. While promising, further investigation into the stability, scalability, and cost-effectiveness of CSD systems is essential to translate these findings into industrial applications.
In
B. subtilis, signal peptide optimisation, such as the use of amy from
B. amyloliquefaciens, has demonstrated success in increasing extracellular
IsPETase production. However, conflicting results regarding Sec- and Tat-dependent pathways underscore the complexity of secretion mechanisms in this host. Comparative molecular studies are needed to elucidate these discrepancies and fully harness
B. subtilis as an efficient expression host with a secretion system. Only the strategies of enhancing the secretion system with signal peptides and co-expressing chaperones have been explored in
B. subtilis to date (
Table 1). Several fusion tags have been developed to facilitate the secretion of target proteins in
B. subtilis, including cellulase [
86], SUMO3 [
87], Spy [
88], and lysyl tRNA synthetase [
89]. Further studies are anticipated to improve
IsPETase secretion and extracellular production in this expression host.
As
B. subtilis spores can be readily engineered to display heterologous antigens or proteins on their surface, the CSD system has been adapted for spore-surface display in
B. subtilis [
90]. Regarding the final strategy, which has been successfully employed in
E. coli, thereby increasing membrane permeability to enhance protein secretion, there are also successful examples in
B. subtilis. For instance, it has been demonstrated that the addition of Triton X-100 increased the secretion level of γ-cyclodextrin glycosyltransferase by 21.5%, an effect attributed to enhanced cell membrane permeability [
91].
Thus, future research could focus on advancing B. subtilis as an expression host for IsPETases through the development of fusion tag technology, spore–surface display systems, and strategies for modulating cell membrane permeability.
4.2. Eukaryotic Hosts
Eukaryotic hosts, including yeasts and microalgae, offer unique advantages due to their advanced post-translational modification systems and regulatory compliance for industrial applications.
P. pastoris has shown significant potential, with glycosylation improving enzyme stability and chaperone co-expression strategies enhancing yields [
81]. Yeast surface display systems, particularly those employing
S. cerevisiae spores, have innovatively addressed the challenges of purification and environmental stress resistance [
92]. Microalgae, such as
P. tricornutum and
C. reinhardtii, have emerged as promising platforms for
IsPETase production in aquatic environments [
93,
94]. However, challenges related to scalability, nutrient requirements, and environmental conditions must be addressed to maximise the potential of these hosts. Furthermore, only a limited number of strategies have been tested in these systems to enhance
IsPETase secretion, leaving considerable potential for the exploration and implementation of alternative approaches.
4.3. Expression and Production of IsPETase and Homologues in Scaled-Up Systems
The industrial-scale application of IsPETase and homologues hinges on the development of robust, high-yield expression systems capable of producing active enzymes under fermentor conditions. While significant strides have been made in engineering IsPETase variants with enhanced activity and thermostability, translating these advances into scalable bioprocesses remains a key challenge.
Among the various microbial hosts explored,
E. coli remains the most widely used due to its rapid growth, well-characterised genetics, and ease of manipulation. However,
P. pastoris has demonstrated superior capabilities in protein secretion and post-translational modifications. Notably, co-expression of the chaperone Ero1 in
P. pastoris, combined with a high-cell-density fed-batch fermentation strategy in a 30-L bioreactor, enabled the production of up to 3.23 g/L of secreted FastPETase [
81]. This represents one of the highest reported yields for the expression of
IsPETase and homologues in a eukaryotic system. Although this system demonstrated excellent productivity, it required complex media and extended cultivation times to achieve optimal results.
In
E. coli, the highest yield to date was also achieved for FastPETase, using a synergistic strategy that combined co-expression of cognate chaperones (DnaK/DnaJ from
I. sakaiensis) with fusion of VNp6. This approach enabled the secretion of over 2 g/L FastPETase in a 5 L fed-batch fermenter [
56].
Despite these promising results, several limitations persist. One of the most significant challenges is the lack of standardisation in reporting outcomes. Many studies use enzyme activity or PET degradation efficiency as proxies for expression levels, which allows researchers to avoid time-consuming purification procedures [
95]. While this approach is practical, it complicates direct comparison across studies. This inconsistency in metrics, combined with variations in substrate type, reaction conditions, and quantification methods, hampers reproducibility and comparative assessment.
5. Conclusions
In summary, the combination of advanced expression systems, in silico methods, screening assays, and extracellular production strategies is accelerating the development of IsPETase for PET degradation. However, the field still faces challenges, including the scalability of production systems, discrepancies in secretion efficiency across hosts, and the need for standardised screening protocols. Interdisciplinary approaches that integrate synthetic biology, computational modelling, and environmental microbiology hold the key to overcoming these challenges and advancing the application of IsPETase for sustainable bioremediation. Additionally, exploring the potential of diverse microbial systems could provide novel insights and enhance the robustness of PET degradation strategies. Continued investment in these areas will be crucial for developing effective and scalable solutions to address plastic pollution.
Furthermore, while laboratory-scale advancements in PETase engineering are promising, their translation to industrial-scale applications presents significant challenges. These include the need for cost-effective and high-yield fermentation systems, robust enzyme stability under process conditions, and integration into existing waste management infrastructures. Although various microbial hosts have been explored for the expression of IsPETase and its homologues, including E. coli, B. subtilis, yeasts, and microalgae, comparative studies remain limited, and each system presents distinct advantages and constraints. E. coli is the most widely used host due to its rapid growth and genetic tractability, but issues such as inclusion body formation and limited secretion efficiency persist. B. subtilis offers better secretion potential but yields inconsistent results across signal peptide systems. Yeasts like P. pastoris provide post-translational modifications and improved enzyme stability, while microalgae offer sustainable platforms but are still in early development stages. Despite these advances, the state-of-the-art in IsPETase expression under fermentor conditions is still emerging. Limitations such as low solubility, inefficient folding, and suboptimal secretion continue to hinder large-scale application. Regulatory hurdles such as the approval of genetically modified organisms (GMOs) for environmental use, biosafety concerns, and compliance with international standards also pose barriers to implementation. Addressing these issues will require interdisciplinary collaboration among biotechnologists, process engineers, and policymakers to ensure that PETase-based biodegradation strategies are not only scientifically viable but also scalable, safe, and regulatory-compliant.
Author Contributions
Conceptualization, H.G.; investigation, T.S.O., S.S.I., M.D. and H.G.; formal analysis, M.D. and H.G.; writing—original draft, T.S.O. and S.S.I.; writing—review, M.D. and H.G.; editing, M.D. and H.G.; supervision, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 4-pNA | Para-nitrophenyl acetate |
| β-FFase | β-fructofuranosidase |
| AI | Artificial intelligence |
| BHET | Bis(2-hydroxyethyl) terephthalate |
| CBM66 | Carbohydrate-binding module 66 |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CSD | Cell surface display |
| ESM | Evolutionary scale language model |
| epPCR | Error-prone PCR |
| FACS | Fluorescence-activated cell sorting |
| FTIR | Fourier transform infrared spectroscopy |
| GAN | Generative adversarial network |
| GMO | Genetically modified organism |
| GRAS | Generally regarded as safe |
| HMM | Hidden Markov model |
| HPLC | High-performance liquid chromatography |
| JGI/IMG | JGI integrated microbial genomes and microbiomes |
| LC | Liquid chromatography |
| ML | Machine learning |
| Mt | Million tonnes |
| NCBI | National Center for Biotechnology Information |
| PCL | Polycaprolactone |
| PDI | Protein disulphide isomerase |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PGA | poly-γ-glutamic acid |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| SEM | Scanning electron microscope |
| SUMO | Small ubiquitin-related modifier |
| Tat | Twin-arginine translocation |
| UV | Ultraviolet |
| VNp | Vesicle nucleating peptide |
References
- Cowger, W.; Willis, K.A.; Bullock, S.; Conlon, K.; Emmanuel, J.; Erdle, L.M.; Eriksen, M.; Farrelly, T.A.; Hardesty, B.D.; Kerge, K.; et al. Global Producer Responsibility for Plastic Pollution. Sci. Adv. 2024, 10, eadj8275. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastic Degradation by Microbes: A Sustainable Approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Bronscheuer, U.T. Feeding on Plastic. Science 2016, 351, 1154–1155. [Google Scholar] [CrossRef]
- Zaini, N.; Kasmuri, N.; Mojiri, A.; Kindaichi, T.; Nayono, S.E. Plastic Pollution and Degradation Pathways: A Review on the Treatment Technologies. Heliyon 2024, 10, e28849. [Google Scholar] [CrossRef] [PubMed]
- Grewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for Degradation of Plastic Polymers Floating in the Marine Environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener Routes for Recycling of Polyethylene Terephthalate. Egypt. J. Pet. 2016, 1, 53–64. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A Bacterium that Degrades and Assimilates Poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Palm, G.J.; Reisky, L.; Bottcher, D.; Muller, H.; Michels, E.A.P.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structures of the Plastic-Degrading Ideonella sakaiensis MHETase Bound to a Substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; Omari, K.E.; et al. Characterization and Engineering of a Plastic-Degrading Aromatic Polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef]
- Aer, L.; Jiang, Q.; Gul, I.; Qi, Z.; Feng, J.; Tang, L. Overexpression and Kinetic Analysis of Ideonella sakaiensis PETase for Polyethylene Terephthalate (PET) Degradation. Environ. Res. 2022, 212 Pt D, 113472. [Google Scholar] [CrossRef]
- Karunatillaka, I.; Jaroszewski, L.; Godzik, A. Novel Putative Polyethylene Terephthalate (PET) Plastic Degrading Enzymes from the Environmental Metagenome. Proteins Struct. Funct. Bioinf. 2022, 90, 504–511. [Google Scholar] [CrossRef]
- Zhang, H.; Perez-Garcia, P.; Dierkes, R.F.; Applegate, V.; Schumacher, J.; Chibani, C.M.; Sternagel, S.; Preuss, L.; Weigert, S.; Schmeisser, C.; et al. The Bacterioidetes Aequorivita sp. and Kaistella jeonii Produce Promiscuous Esterases with PET-Hydrolyzing Activity. Front. Microbiol. 2022, 12, 803896. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Grewall, A.; Hooda, S. In silico Approach for Identification of Polyethylene Terephthalate Hydrolase (PETase)-Like Enzymes. Bioremediation J. 2023, 27, 311–323. [Google Scholar] [CrossRef]
- Almeida, E.L.; Rincon, A.F.C.; Jackson, S.A.; Dobson, A.D.W. In silico Screening and Heterologous Expression of a Polyethylene Terephthalate Hydrolase (PETase)-Like Enzyme (SM14est) with Polycaprolactone (PCL)-Degrading Activity, from the Marine Sponge-Derived Strain Streptomyces sp. SM14. Front. Microbiol. 2019, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Eiamthong, B.; Meesawat, P.; Wongsatit, T.; Jitdee, J.; Sangsri, R.; Patchsung, M.; Aphicho, K.; Suraritdechachai, S.; Huguenin-Dezot, N.; Tang, S.; et al. Discovery and Genetic Code Expansion of a Polyethylene Terephthalate (PET) Hydrolase from the Human Saliva Metagenome for the Degradation and Bio-Functionalization of PET. Angew. Chem. Int. Ed. Engl. 2022, 134, e202203061. [Google Scholar] [CrossRef]
- Ho, N.H.E.; Effendi, S.S.W.; Ting, W.-W.; Yi, Y.-C.; Yu, J.-Y.; Chang, J.-S.; Ng, I.-S. Heterologous Expression and Characterization of Aquabacterium parvum Lipase, a Close Relative of Ideonella sakaiensis PETase in Escherichia coli. Biochem. Eng. J. 2023, 197, 108985. [Google Scholar] [CrossRef]
- Liu, X.; Jin, J.; Sun, H.; Li, S.; Zhang, F.; Yu, X.; Cao, Q.; Song, Y.; Li, N.; Lu, Z.; et al. Perspectives on the Microorganisms with the Potentials of PET-Degradation. Front. Microbiol. 2025, 16, 134532. [Google Scholar] [CrossRef]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.-Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Structural Insight into Molecular Mechanism of Poly(ethylene terephthalate) Degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef]
- Erickson, E.; Gado, J.E.; Avilan, L.; Bratti, F.; Brizendine, R.K.; Cox, P.A.; Gill, R.; Graham, R.; Kim, D.-J.; Konig, G.; et al. Sourcing Thermotolerant Poly(ethylene terephthalate) Hydrolase Scaffolds from Natural Diversity. Nat. Commun. 2022, 13, 7850. [Google Scholar] [CrossRef]
- Jahanshahi, D.A.; Barzani, M.R.R.; Bahram, M.; Ariaeenejad, S.; Kavousi, K. Metagenomic Exploration and Computational Prediction of Novel Enzymes for Polyethylene Terephthalate Degradation. Ecotoxicol. Environ. Saf. 2025, 289, 117640. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, C.; Li, B.; Zhu, L.; Ming, D.; Jiang, L. Structure-Guided Discovery and Rational Design of a New Poly(ethylene terephthalate) Hydrolase from AlphaFold Protein Structure Database. J. Hazard. Mater. 2024, 480, 136389. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhong, B.; Zheng, L.; Huang, R.; Jiang, S.; Li, M.; Tan, P.; Hong, L. Harnessing Protein Language Model for Structure-Based Discovery of Highly Efficient and Robust PET Hydrolases. Nat. Commun. 2025, 16, 6211. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, L.; Liu, H.; Li, Q.; Xu, G.; Zhang, Y.; Guan, F.; Zhang, Y.; Zhang, W.; Wu, N.; et al. Protein Engineering of a Stable IsPETase for PET Plastic Degradation by Premuse. Int. J. Biol. Macromol. 2021, 180, 667–676. [Google Scholar] [CrossRef]
- Shi, L.; Liu, H.; Gao, S.; Weng, Y.; Zhu, L. Enhanced Extracellular Production of IsPETase in Escherichia coli via Engineering of the pelB Signal Peptide. J. Agric. Food Chem. 2021, 69, 2245–2252. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. A High-Throughput Expression and Screening Platform for Applications-Driven PETase Engineering. Biotechnol. Bioeng. 2023, 120, 1000–1014. [Google Scholar] [CrossRef]
- Liu, K.; Xu, Z.; Zhao, Z.; Chen, Y.; Chai, Y.; Ma, L.; Li, S. A Dual Fluorescence Assay Enables High-Throughput Screening for Poly(ethylene terephthalate) Hydrolases. ChemSusChem 2023, 16, e202202019. [Google Scholar] [CrossRef]
- Cribari, M.A.; Unger, M.J.; Unarta, I.C.; Ogorek, A.N.; Huang, X.; Martell, J.D. Ultraghigh-Throughput Directed Evolution of Polymer-Degrading Enzymes Using Yeast Display. J. Am. Chem. Soc. 2023, 145, 27380–27389. [Google Scholar] [CrossRef]
- Brott, S.; Pfaff, L.; Schuricht, J.; Schwarz, J.-N.; Bottcher, D.; Badenhorst, C.P.S.; Wei, R.; Bornscheuer, U.T. Engineering and Evaluation of Thermostable IsPETase variants for PET Degradation. Eng. Life Sci. 2022, 22, 192–203. [Google Scholar] [CrossRef]
- Herzog, K.; Muller, R.-J.; Deckwer, W.-D. Mechanism and Kinetics of the Enzymatic Hydrolysis of Polyester Nanoparticles by Lipases. Polym. Degrad. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Ronkvist, A.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-Catalyzed Hydrolysis of Poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.-J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a Novel Cutinase Homolog with Polyethylene Terephthalate-Degrading Activity from Leaf-Branch Compost by Using a Metagenomic Approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef]
- Carniel, A.; Valoni, E.; Nicomedes, J., Jr.; Gomes, A.; Castro, A. Lipase from Candida antarctica (CALB) and Cutinase from Humicola insolens Act Synergistically for PET Hydrolysis to Terephthalic Acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Barth, M.; Weigl, N.; Lubs, A.; Schulz-Siegmund, M.; Hacker, M.C.; Zimmermann, W. Turbidimetric Analysis of the Enzymatic Hydrolysis of Polyethylene Terephthalate Nanoparticles. J. Mol. Catal. B Enzym. 2014, 103, 72–78. [Google Scholar] [CrossRef]
- De Brito, A.L.C.P.; Mattioni, J.V.; Ramos, G.R.; Nakamura, M.; Toma, H.E. Direct Monitoring of the Enzymatically Sequestering and Degrading of PET Microplastics using Hyperspectral Raman Microscopy. Micron 2024, 187, 103722. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, S.; Soni, M.; Pratap, J.V.; Subramanian, S.; Manickam, N. Enzymatic Degradation of PET by Hydrolase from Brucella intermedia IITR130 and Its Genomic Insights. Biodegradation 2025, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Cavus, A.N.; Cifer, A.B.; Akdogan, K.; Caloglu, B.; Kerimak-Oner, M.N.; Yildirim, D.; Binay, B. A Novel Thermostable PETase from Kibdelosporangium aridum: Heterologous Expression, Immobilization and Poly(ethelene terephthalate) Decomposition Applications. J. Clean. Prod. 2025, 510, 145624. [Google Scholar] [CrossRef]
- Satta, A.; Zampieri, G.; Loprete, G.; Campanaro, S.; Treu, L.; Bergantino, E. Metabolic and Enzymatic Engineering Strategies for Polyethylene Terephthalate Degradation and Valorization. Rev. Environ. Sci. Biotechnol. 2024, 23, 351–383. [Google Scholar] [CrossRef]
- Jia, Y.; Samak, N.A.; Hao, X.; Chen, Z.; Wen, Q.; Xing, J. Hydrophobic Cell Surface Display System of PETase as a Sustainable Biocatalyst for PET Degradation. Front. Microbiol. 2022, 13, 1005480. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Li, W.; Si, C.; Lan, Z.; Liu, Y.; Nakanishi, H.; Li, Z. Development of a Self-Assembled Dual-Enzyme Co-Display Platform on the Surface of the Natural “Chitosan Beads” of Yeast Spores. Int. J. Biol. Macromol. 2025, 286, 138308. [Google Scholar] [CrossRef]
- Seo, H.; Kim, S.; Son, H.F.; Sagong, H.-Y.; Joo, S.; Kim, K.-J. Production of Extracellular PETase from Ideonella sakaiensis using Sec-Dependent Signal Peptide in E. coli. Biochem. Biophys. Res. Commun. 2019, 508, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a Novel Escherichia coli Protein Expression Strain Capable of Correctly Folding Disulfide Bonded Proteins in its Cytoplasm. Microb. Cell Fact. 2012, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Corujo, A.; Uchida, Y.; Saaranen, M.J.; Ruddock, L.W. Escherichia coli Cytoplasmic Expression of Disulfide-Bonded Proteins: Side-by-Side Comparison between Two Competing Strategies. J. Microbiol. Biotechnol. 2024, 34, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine Learning-Aided Engineering of Hydrolases for PET Depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J.R.; et al. Directed Evolution of an Efficient and Thermostable PET Depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Carter, L.M.; MacFarlane, C.E.; Karlock, S.P.; Sen, T.; Kaar, J.L.; Berberich, J.A.; Boock, J.T. Increased Cytoplasmic Expression of PETase Enzymes in E. coli. Microb. Cell Fact. 2024, 23, 319. [Google Scholar] [CrossRef]
- Fohler, L.; Leibetseder, L.; Cserjan-Puschmann, M.; Striedner, G. Manufacturing of the Highly Active Thermophile PETases PHL7 and PHL7mut3 using Escherichia coli. Microb. Cell Fact. 2024, 23, 272. [Google Scholar] [CrossRef]
- Stargardt, P.; Feuchtenhofer, L.; Cserjan-Puschmann, M.; Striedner, G.; Mairhofer, J. Bacteriophage Inspired Growth-Decoupled Recombinant Protein Production in Escherichia coli. ACS Synth. Biol. 2020, 9, 1336–1348. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Z.; Wang, X.; Selvaraj, J.N.; Zhang, G. Genetic Engineering Modification and Fermentation Optimization for Extracellular Production of Recombinant Proteins using Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 1545–1556. [Google Scholar] [CrossRef]
- Cui, L.; Qiu, Y.; Liang, Y.; Du, C.; Dong, W.; Cheng, C.; He, B. Excretory Expression of IsPETase in E. coli by an Enhancer of Signal Peptides and Enhanced PET Hydrolysis. Int. J. Biol. Macromol. 2021, 188, 568–575. [Google Scholar] [CrossRef]
- Fatima, K.; Naqvi, F.; Younas, H. A Review: Molecular Chaperone-Mediated Folding, Unfolding and Disaggregation of Expressed Recombinant Proteins. Cell Biochem. Biophys. 2021, 79, 153–174. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Kaur, J. Strategies for Optimization of Heterologous Protein Expression in E. coli: Roadblocks and Reinforcement. Int. J. Biol. Macromol. 2018, 106, 803–822. [Google Scholar] [CrossRef]
- Niiranen, L.; Espelid, S.; Karlsen, C.R.; Mustonen, M.; Paulsen, S.M.; Heikinheimo, P.; Willassen, N.P. Comparative Expression Study to Increase the Solubility of Cold Adapted Vibrio Proteins in Escherichia coli. Protein Expr. Purif. 2007, 52, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Kang, M.; Kim, M.-J.; Yi, J.; Kang, J.; Bae, J.-H.; Sohn, J.-H.; Sung, B.H. A Novel Protein Fusion Partner, Carbohydrate-Binding Module Family 66, to Enhance Heterologous Protein Expression in Escherichia coli. Microb. Cell Fact. 2021, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, H.; Wang, W.; Xie, B.; Wang, Z.; Lu, J.; Xu, A.; Dong, W.; Zhou, J.; Jiang, M. Boosting Extracellular FastPETase Production in E. coli: A Combined Approach of Cognate Chaperones Co-Expression and Vesicle Nucleating Peptide Tag Fusion. Int. J. Biol. Macromol. 2024, 283, 137857. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.H.; Xu, Z. Microbial Cell-Surface Display. Trends Biotechnol. 2003, 21, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, J.-I.; Park, S.J.; Lee, S.Y.; Park, B.C. Display of Bacterial Lipase on the Escherichia coli Cell Surface by Using FadL as an Anchoring Motif and Use of the Enzyme in Enantioselective Biocatalysis. Appl. Environ. Microbiol. 2004, 70, 5074–5080. [Google Scholar] [CrossRef]
- Yamashita, T.; Matsumoto, T.; Yamada, R.; Ogino, H. Display of PETase on the Cell Surface of Escherichia coli Using the Anchor Protein PgsA. Appl. Biochem. Biotechnol. 2024, 196, 5471–5483. [Google Scholar] [CrossRef]
- Narita, J.; Okano, K.; Tateno, T.; Tanino, T.; Sewaki, T.; Sung, M.-H.; Fukuda, H.; Kondo, A. Display of Active Enzymes on the Cell Surface of Escherichia coli Using PgsA Anchor Protein and Their Application to Bioconversion. Appl. Microbiol. Biotechnol. 2006, 70, 564–572. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.; Ng, I. Simultaneous Release of Recombinant Cellulases Introduced by Coexpressing Colicin E7 Lysis in Escherichia coli. Biotechnol. Bioprocess Eng. 2016, 21, 491–501. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Hu, R.-E.; Hsiang, C.-C.; Ting, W.-W.; Huang, C.-L.; Ng, I.-S. Exploring PETase-Like Enzyme from Shotgun Metagenome and Co-Expressing Colicin E7 in Escherichia coli for Effective PET Degradation. Process Biochem. 2024, 140, 78–87. [Google Scholar] [CrossRef]
- Su, L.; Woodard, R.W.; Chen, J.; Wu, J. Extracellular Location of Thermobifida fusca Cutinase Expressed in Escherichia coli BL21(DE3) without Mediation of a Signal Peptide. Appl. Environ. Microbiol. 2013, 79, 4192–4198. [Google Scholar] [CrossRef]
- Aer, L.; Qin, H.; Wo, P.; Feng, J.; Tang, L. Signal Peptide Independent Secretion of Bifunctional Dual-Hydrolase to Enhance the Bio-Depolymerization of Polyethylene Terephthalate. Bioresour. Technol. 2024, 391, 129884. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Han, L.; Suo, F.; Liu, Z.; Zhou, L.; Zhou, Z. Exploitation of Bacillus subtilis as a Robust Workhorse for Production of Heterologous Proteins and Beyond. World J. Microbiol. Biotechnol. 2018, 34, 145. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Lee, W.; Tran, L.; Wong, S.L. Engineering a Bacillus subtilis Expression-Secretion System with a Strain Deficient in Six Extracellular Proteases. J. Bacteriol. 1991, 173, 4952–4958. [Google Scholar] [CrossRef]
- Wang, N.; Guan, F.; Lv, X.; Han, D.; Zhang, Y.; Wu, N.; Xia, X.; Tian, J. Enhancing Secretion of Polyethylene Terephthalate Hydrolase PETase in Bacillus subtilis WB600 Mediated by the SPamy Signal Peptide. Lett. Appl. Microbiol. 2020, 71, 235–241. [Google Scholar] [CrossRef]
- Huang, X.; Cao, L.; Qin, Z.; Li, S.; Kong, W.; Liu, Y. Tat-Independent Secretion of Polyethylene Terephthalate Hydrolase PETase in Bacillus subtilis 168 Mediated by Its Native Signal Peptide. J. Agric. Food Chem. 2018, 66, 13217–13227. [Google Scholar] [CrossRef]
- Yuan, G.; Wong, S. Isolation and Characterization of Bacillus subtilis groE Regulatory Mutants: Evidence for orf39 in the dnaK Operon as a Repressor Gene in Regulating the Expression of Both groE and dnaK. J. Bacteriol. 1995, 177, 6462–6468. [Google Scholar] [CrossRef]
- Yuan, G.; Wong, S. Regulation of groE Expression in Bacillus subtilis: The Involvement of the Sigma A-Like Promoter and the Roles of the Inverted Repeat Sequence (CIRCE). J. Bacteriol. 1995, 177, 5427–5433. [Google Scholar] [CrossRef][Green Version]
- Xi, X.; Ni, K.; Hao, H.; Shang, Y.; Zhao, B.; Qian, Z. Secretory Expression in Bacillus subtilis and Biochemical Characterization of a Highly Thermostable Polyethylene Terephthalate Hydrolase from Bacterium HR29. Enzyme Microb. Technol. 2021, 143, 109715. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, R.; Xiao, Y.; Wei, Y.; Zhang, H.; Sun, X.; Wang, S.; Cheng, Y.; Wang, X.; Tong, S.; et al. Biodegradation of Highly Crystallized Poly(ethylene terephthalate) Through Cell Surface Codisplay of Bacterial PETase and Hydrophobin. Nat. Commun. 2022, 13, 7138. [Google Scholar] [CrossRef] [PubMed]
- Kosiorowska, K.E.; Moreno, A.D.; Iglesias, R.; Leluk, K.; Mironczuk, A.M. Production of PETase by Engineered Yarrowia lipolytica for Efficient Poly(ethylene terephthalate) Biodegradation. Sci. Total Environ. 2022, 846, 157358. [Google Scholar] [CrossRef] [PubMed]
- Kosiorowska, K.E.; Moreno, A.D.; Iglesias, R.; Biniarz, P.; Mironczuk, A.M. Streamlining Biological Recycling of Poly(ethylene terephthalate) via Pre-Treatment Methods. Int. Biodeterior. Biodegrad. 2024, 193, 105842. [Google Scholar] [CrossRef]
- Cereghino, G.P.; Cregg, J.M. Applications of Yeast in Biotechnology: Protein Production and Genetic Analysis. Curr. Opin. Biotechnol. 1999, 10, 422–427. [Google Scholar] [CrossRef]
- Cereghino, G.P.L.; Cereghino, J.L.; Ilgen, C.; Cregg, J.M. Production of Recombinant Proteins in Fermenter Cultures of the Yeast Pichia pastoris. Curr. Opin. Biotechnol. 2022, 13, 329–332. [Google Scholar] [CrossRef]
- Gemmill, T.R.; Trimble, R.B. Overview of N- and O-Linked Oligosaccharide Structures Found in Various Yeast Species. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 227–237. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Engineering Strategies for Enhanced Production of Protein and Bio-Products in Pichia pastoris: A Review. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef]
- Deng, B.; Yue, Y.; Yang, J.; Yang, M.; Xing, Q.; Peng, H.; Wang, F.; Li, M.; Ma, L.; Zhai, C. Improving the Activity and Thermostability of PETase from Ideonella sakaiensis through Modulating its Post-Translational Glycan Modification. Commun. Biol. 2023, 6, 39. [Google Scholar] [CrossRef]
- Chen, C.-C.; Li, X.; Min, J.; Zeng, Z.; Ning, Z.; He, H.; Long, X.; Niu, D.; Peng, R.; Liu, X.; et al. Complete Decomposition of Poly(ethylene terephthalate) by Crude PET Hydrolytic Enzyme Produced in Pichia pastoris. Chem. Eng. J. 2024, 481, 148418. [Google Scholar] [CrossRef]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef] [PubMed]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.-H.; Linne, U.; Erb, T.; Maier, U.G. Using a Marine Microalga as a Chassis for Polyethylene Terephthalate (PET) Degradation. Microb. Cell Fact. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, S.-B.; Tran, Q.-G.; Cho, D.-H.; Choi, D.-Y.; Lee, Y.J.; Kim, H.-S. Functional Expression of Polyethylene Terephthalate-Degrading Enzyme (PETase) in Green Microalgae. Microb. Cell Fact. 2020, 19, 97. [Google Scholar] [CrossRef]
- Rocco, G.D.; Taunt, H.N.; Berto, M.; Jackson, H.O.; Piccinini, D.; Carletti, A.; Scurani, G.; Braidi, N.; Purton, S. A PETase Enzyme Synthesised in the Chloroplast of the Microalga Chlamydomonas reinhardtii is Active Against Post-Consumer Plastics. Sci. Rep. 2023, 13, 10028. [Google Scholar] [CrossRef]
- Vogtentanz, G.; Collier, K.D.; Bodo, M.; Chang, J.H.; Day, A.G.; Estell, D.A.; Falcon, B.C.; Ganshaw, G.; Jarnagin, A.S.; Kellis, J.T., Jr.; et al. A Bacillus subtilis Fusion Protein System to Produce Soybean Bowman-Birk Protease Inhibitor. Protein Expr. Purif. 2007, 55, 40–52. [Google Scholar] [CrossRef]
- Heinrich, J.; Drewniok, C.; Neugebauer, E.; Kellner, H.; Wiegert, T. The YoaW Signal Peptide Directs Efficient Secretion of Different Heterologous Proteins Fused to a StrepII-SUMO Tag in Bacillus subtilis. Microb. Cell Fact. 2019, 18, 31. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Q.; Liu, Y.; Zeng, C.; Li, Y.; Yan, X. Efficient Secretion of Mussel Adhesion Proteins Using a Chaperone Protein Spy as Fusion Tag in Bacillus subtilis. Biotechnol. J. 2023, 18, 2200582. [Google Scholar] [CrossRef]
- Le, N.T.P.; Phan, T.T.P.; Truong, T.T.T.; Schumann, W.; Nguyen, H.D. N-terminal LysSN-His-Tag Improves the Production of Intracellular Recombinant Protein in Bacillus subtilis. Cell Biochem. Funct. 2023, 41, 823–832. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yang, R. Recent Progress in Bacillus subtilis Spore-Surface Display: Concept, Progress, and Future. Appl. Microbiol. Biotechnol. 2017, 101, 933–949. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, X.; Li, Z.; Ban, X.; Gu, Z.; Tang, X.; Hong, Y.; Cheng, L.; Li, C. Regulation of Cell Membrane Permeability Enhanced the Non-Classical Secretion of γ-Cyclodextrin Glycosyltransferase in Bacillus subtilis. J. Agric. Food Chem. 2022, 70, 16307–16315. [Google Scholar] [CrossRef]
- O’Riordan, N.M.; Juric, V.; O’Neill, S.K.; Roche, A.P.; Young, P.W. A Yeast Modular Cloning (MoClo) Toolkit Expansion for Optimization of Heterologous Protein Secretion and Surface Display in Saccharomyces cerevisiae. ACS Synth. Biol. 2024, 13, 1246–1258. [Google Scholar] [CrossRef]
- Molino, J.V.D.; Saucedo, B.; Kang, K.; Walsh, C.; Diaz, C.J.; Tessman, M.; Mayfield, S. Efficient Secretion of a Plastic Degrading Enzyme from the Green Algae Chlamydomonas reinhardtii. Sci. Rep. 2025, 15, 24690. [Google Scholar] [CrossRef]
- Webster, L.J.; Villa-Gomez, D.; Brown, R.; Clarke, W.; Schenk, P.M. A Synthetic Biology Approach for the Treatment of Pollutants with Microalgae. Front. Bioeng. Biotechnol. 2024, 12, 1379301. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, H.; Hong, H.; Park, J.; Ki, D.; Kim, M.; Kim, H.-J.; Kim, K.-J. Three-Directional Engineering of IsPETase with Enhanced Protein Yield, Activity, and Durability. J. Hazard. Mater. 2023, 459, 132297. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).