Radionuclides Landscape in Prostate Cancer Theranostics
Abstract
1. Introduction
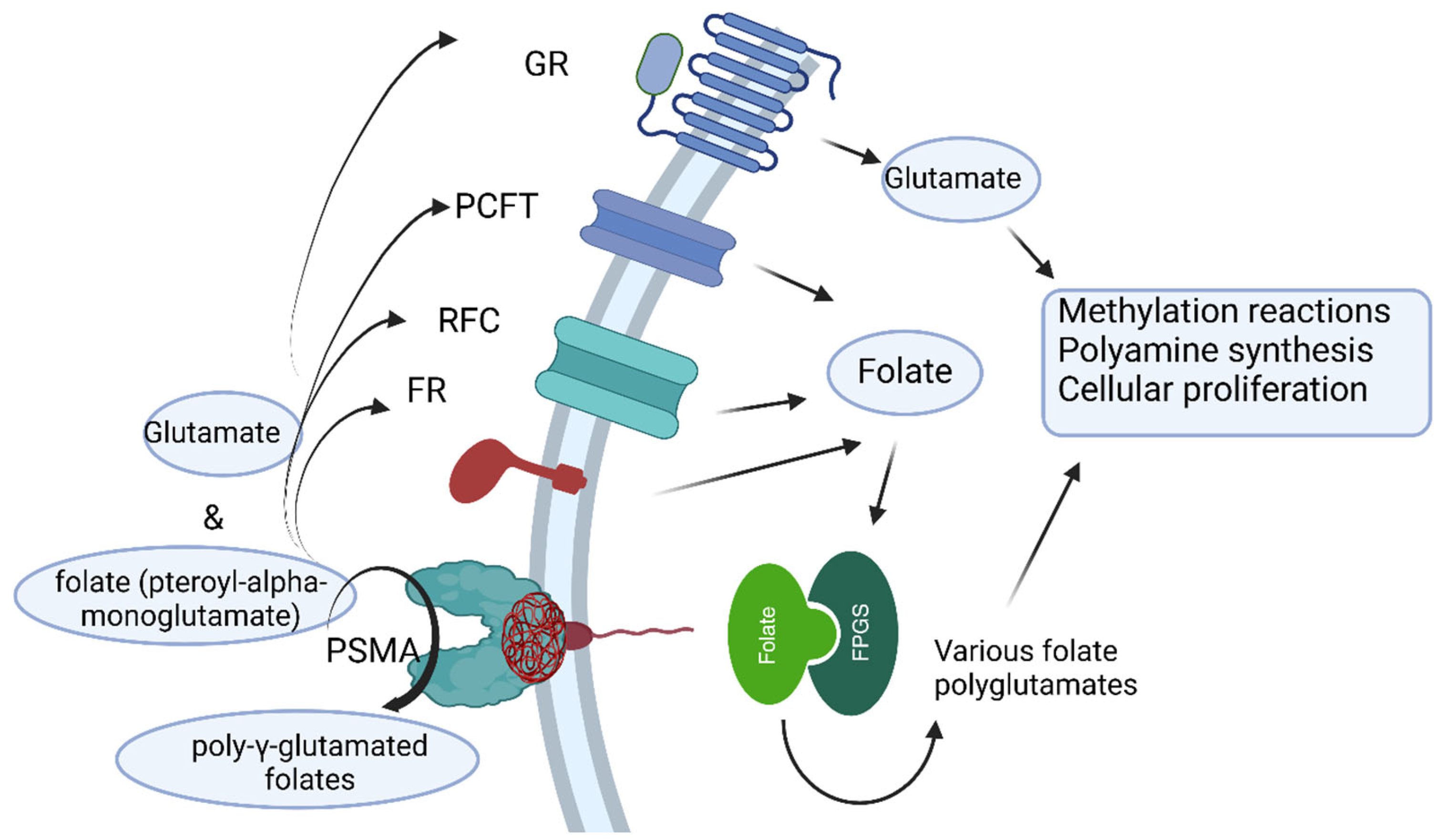
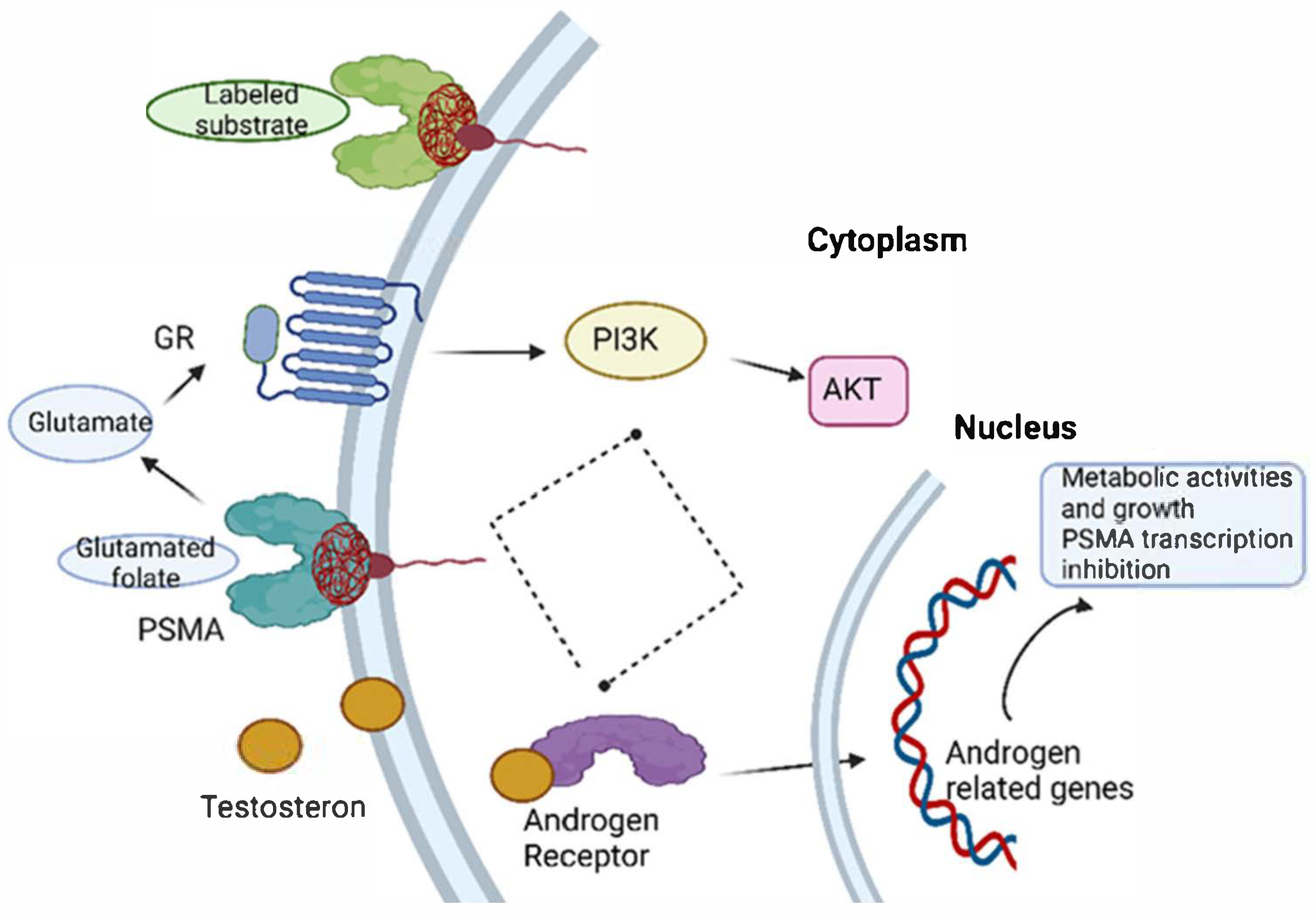
2. Prostate-Specific Membrane Antigen
3. PSMA-Targeted Endoradiotherapy
3.1. Lutetium-177
3.2. Other Isotopes Used in Targeted Radionuclid Therapy of Prostate Cancer
3.3. PSMA-Based Therapy Limitations
| (A) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclide | Decay | t1/2 | ravg | Purpose | A | Status | Year | Brand Name/Agent | Nb. P | Ref. | |
| 18F | β+ | 109.8 min | 2 mm | Imaging | 350 MBq | FDA | 2021 | PYLARIFY/F-18 piflufolastat | ≈400–500 k | [8] | |
| 2023 | POSLUMA/F-18 flotufolastat | ≈5–15 k | [8] | ||||||||
| 61Cu | β+ | 3.33 h | 2 mm | Imaging | - | Phase I/II | Cu-61-NuriPro | ≈50–150 | [85] | ||
| 64Cu | β+ | 12.7 h | 6 mm | Imaging | 350 MBq | Phase III | (-)/Cu-64-SAR-bisPSMA | ≈500–1000 | [31] | ||
| 67Cu | β− | 2.58 d | 2 mm | Therapy | 12 GBq | Phase I/II | (-)/Cu67-SAR-bisPSMA | ≈50–150 | [86] | ||
| 68Ga | β+ | 68 min | 8 mm | Imaging | 300 MBq | FDA | 2020 | LOCAMETZ/Ga-68 gozetotide | ≈10–30 k | [8,9] | |
| 2021 | ILLUCCIX/Ga-68 gozetotide | ≈50–100 k | |||||||||
| 89Zr | β+ | 78.4 h | 2 mm | Imaging | - | Phase I/II | (-)/Zr-89-DFO-huJ591 | ≈200–500 | [64] | ||
| 125I | EC | 59.39 d | <10 µm | Theranostic | 15 kBq | FDA | 1986 | Brachytherapy/No Agent | ≈10–20 k | [68] | |
| 161Tb | β− | 6.89 d | 30 µm | Theranostic | 6.5 GBq | Phase I/II | Tb-161-PSMA-I&T | ≈50–150 k | [87,88] | ||
| 177Lu | β− | 6.65 d | 230 µm | Theranostic | 7.4 GBq | FDA | 2022 | PLUVICTO/Lu-177 vipivotide | ≈15–30 k | [1,9,10] | |
| 2024 | (TBA)/177Lu PSMA-I&T | ≈1–5 k | [89] | ||||||||
| 211At | α | 7.2 h | 70 µm | Theranostic | 72 MBq | Phase I | (-)/At-211-PSMA-5 | ≈20–50 | [52,90] | ||
| 212Pb | β− | 10.6 h | 100 µm | Theranostic | 60 MBq | Phase I/II | (-)/Pb-212-PSMA | <100 | [31,91] | ||
| 213Bi | α, β− | 46.6 min | 80 µm | Therapy | 300 MBq | Preclinical | (-)/Bi-213-DOTATOC 7 | - | [70,71,92] | ||
| 223Ra | α | 11.4 d | 70 µm | Therapy | 4.4 MBq | FDA | 2013 | XOFIGO/Ra-223 dichloride | ≈50–100 k | [93] | |
| 225Ac | α | 9.91 d | 100 µm | Therapy | 9.5 MBq | Phase I/II | (-)/Ac-225-PSMA-617 | ≈700–900 | [49,53] | ||
| 134Ce | Auger | 3.16 d | 500 nm | Imaging | - | Preclinical | (-)/(-) | - | [93,94] | ||
| 134La | β+ | 6.45 min | 1 mm | ||||||||
| (B) | |||||||||||
| Radionuclide | Company | Purpose | Phase | Evaluation | Start Date | Advantages/Disadvantages | Reference | ||||
| 61Cu-NuriProTM | Nuclidium | Imaging | Non-randomized phase 1 trial | Safety and efficacy when compared to an 18F-based, FDA-approved PSMA-targeting radiotracer | 2024 | Advantages: 3.3-hour half-life, greater distribution post-production, easy manufacture, delayed imaging, detection of small metastases. | Public Releases—Nuclidium (https://nuclidium.com/category/public-releases, accessed on 14 June 2025) | ||||
| 61Cu-NODAGA-PSMA I&T | Therapy | Phase I | To be announced | 2025 | To be announced | ||||||
| 64Cu-SAR-bisPSMA | Clarity | Imaging | Phase III non-randomized, single-arm, open-label, multicenter trial | Imaging efficiency for 220 patients with BR; detection of metastases; efficacy will be assessed on both same-day imaging (Day 1, day of administration) and next-day imaging (Day 2, approximately 24 h post-administration) | 2025 | Advantages: fast track designation for PET imaging of prostate-specific membrane, antigen-positive, prostate cancer lesions with suspected metastasis in patients who are candidates for initial definitive therapy. | 64Cu-SAR-bisPSMA in Prostate Cancer and Prostate Cancer Recurrent and Cryotherapy—Clinical Trials Registry—ICH GCP (https://ichgcp.net/clinical-trials-registry/NCT06970847, accessed on 14 June 2025) | ||||
| COBRA | Phase I/II | 52 patients with BR and negative or equivocal standard of care imaging; detection of metastases; efficacy will be assessed on Day 0 and Day 1 (1–4h and 24 ± 6h post-dose) | 2024 | Safe and effective in detecting BR PC patients; in patients with a negative or equivocal SOC scan, it identified lesions in up to 80% of patients and more upon next-day imaging. | Clinical trial information: NCT05249127 (https://www.clinicaltrials.gov/study/NCT05249127, accessed on 14 June 2025) | ||||||
| 89Zr-DFO-huJ591 | imaging | Phase I/II | 50 patients with progressive mCRPC; comparison to whole-body PET/CT scans assessment with bone scintigraphy and cross-sectional imaging | 2015 | Superior targeting of bone lesions relative to CT; soft-tissue lesions imaging less optimal to CT | [95] | |||||
| 161Tb--PSMA-I&T | VIOLET | Imaging/ therapy | Single-center, single-arm, phase I/II trial | 30 patients with progressive, metastatic, castration-resistant prostate cancer | 2024 | Safety dose; dose escalation at three time-point intervals (2–6 h, 18–24 h, and 72–120 h) after the first cycle of 161Tb-PSMA-I&T. | NCT05521412 (https://clinicaltrials.gov/study/NCT05521412/, accessed on 14 June 2025) | ||||
| 211At-PSMA-5 | Imaging/ therapy | First-in-human SPECT/CT image of [211At]PSMA-5 | 15 PC patients | 2024 | Shortest half-life (7.2 h); high therapeutic effect, low abscopal effect, no patient isolation; produced domestically using cyclotron; low renal accumulation at 24h. | SNMMI 2025: First Data of a Novel PSMA-Targeting Radiopharmaceutical Compound SMS-5368: Exploratory and Phase 1/2 Results (https://www.urotoday.com/conference-highlights/snmmi-2025/161575-snmmi-2025-first-data-of-a-novel-psma-targeting-radiopharmaceutical-compound-sms-5368-exploratory-and-phase-1-2-results.html, accessed on 14 June 2025) | |||||
| 212Pb-PSMA | [212Pb]Pb-ADVC001 | Imaging | Prospective, open-label, non-randomized, dose escalation and optimization | Imaging three patients with progressive mCRPC and ECOG 1; PSA levels 0.44, 0.75, 15 µg/L, 3 metastatic lesions | 2024 | Safely administered to mCRPC patients; γ-camera imaging; metastatic targeting, with good biodistribution and clearance. | NCT05720130 (https://www.clinicaltrials.gov/study/NCT05720130, accessed on 14 June 2025) | ||||
| 225Ac--PSMA-617 | Novartis | Therapy | Phase I, open-label dose escalation study for safety | 99 patients enrolled up to 2027 with PSMA-positive prostate cancer who have and have not had prior exposure to [177Lu]Lu-PSMA-617 (177Lu-PSMA-617) or [177Lu]Lu-PSMA I&T (177Lu-PSMA I&T) | 2025 | Study participation of each participant is approximately 18–24 months (12 months from enrollment to end of each treatment (EOT) plus 12 months of long-term follow-up). | NCT04597411 (https://clinicaltrials.gov/study/NCT04597411, accessed on 14 June 2025) | ||||
4. Clinical Trials
5. Recent Advancements in PSMA-Targeted Endotherapeutics
6. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCs | Antibody–Drug Conjugates |
| ADT | Androgen Deprivation Therapy |
| AR | Androgen Receptor |
| ARSIs | Androgen Receptor Signaling Inhibitors |
| BR | Biochemical Recurrence |
| CRPC | Castration-Resistant Prostate Cancer |
| cGA | Glucometabolic Activity |
| CLI | Cerenkov Luminescence Imaging |
| CT | Computer Tomography |
| DDR | DNA Damage Repair |
| DUPA | Dicarboxy Propyl Ureido Pentanedioic Acid |
| EC | Electron Capture |
| FAR | Flexible Autoradiography |
| FDA | Food and Drug Administration |
| FOLR1 | Folate Receptor α |
| FR | Folate Receptor |
| GR | Glutamate Receptor |
| HTRT | Hyperthermia–Radiation Combined Therapy |
| LET | Linear Energy Transfer |
| LDR | Low-dose-rate |
| mCRPC | Metastatic Castration-Resistant Prostate Cancer |
| miRNAs | Micro RNAs |
| NAALDase | N-acetylated Alpha-Linked Acidic Dipeptidase |
| OS | Overall Survival |
| PARPi | Poly-ADP-ribose Polymerase Inhibitors |
| PC | Prostate Cancer |
| PCSCs | Prostate Cancer Stem Cells |
| PET | Positron Emission Tomography |
| PFS | Progression-Free Survival |
| PRMs | Positive Resection Margins |
| PSA | Prostate-Specific Antigen |
| PI3K | Phosphoinositide 3-kinases |
| RFC | Reduced Folate Carrier |
| RT | Radionuclide Therapy |
| SBRT | Stereotactic Body Radiotherapy |
| SGs | Salivary Glands |
References
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Karim-Kos, H.; Coebergh, J.; Byrnes, G.; Antilla, A.; Ferlay, J. Recent trends in incidence of five common cancersin 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Alajati, A.; Kubatka, P.; Giordano, F.; Ritter, M.; Costigliola, V.; Golubnitschaja, O. Prostate cancer treatment costs increase more rapidly than for any other cancer-how to reverse the trend? EPMA J. 2022, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- The Internet Pathology Laboratory for Medical Education. Male Genital Pathology. The University of Utah, Eccles Health Sciences Library. Available online: https://webpath.med.utah.edu/MALEHTML/MALEIDX.html (accessed on 13 May 2009).
- Figueiredo, A.; Costa, L.; Maurício, M.; Figueira, L.; Ramos, R.; Martins-da Silva, C. Nonmetastatic Castration-Resistant Prostate Cancer: Current Challenges and Trends. Clin. Drug Investig. 2022, 42, 631–642. [Google Scholar] [CrossRef]
- Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann. Oncol. 2020, 31, 650–658. [Google Scholar] [CrossRef]
- Posdzich, P.; Darr, C.; Hilser, T.; Wahl, M.; Herrmann, K.; Hadaschik, B.; Grünwald, V. Metastatic Prostate Cancer-A Review of Current Treatment Options and Promising New Approaches. Cancers 2023, 15, 461. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents-Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef]
- Pomykala, K.; Hadaschik, B.; Sartor, O.; Gillessen, S.; Sweeney, C.; Maughan, T.; Hofman, M.; Herrmann, K. Next generation radiotheranostics promoting precision medicine. Ann. Oncol. 2023, 34, 507–519. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; The VISION Investigators. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1100. [Google Scholar] [CrossRef]
- Olivier, T.; Powell, K.; Prasad, V. Lutetium-177-PSMA-617 in Metastatic Castration-resistant Prostate Cancer: Limitations of the VISION Trial. Eur. Urol. 2023, 84, 4–6. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Zimmermann, R.; Abdlkadir, A.S.; Herrmann, K. Radiotheranostics Global Market and Future Developments. Semin. Nucl. Med. 2024, 54, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.M.; Hofferber, R.; Privé, B.M.; de Bakker, M.; Gotthardt, M.; Janssen, M.; de Lange, F.; Muselaers, C.H.; Mehra, N.; Witjes, J.A.; et al. [68Ga]Ga-PSMA-11 PET imaging as a predictor for absorbed doses in organs at risk and small lesions in [177Lu]Lu-PSMA-617 treatment. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Galante, J.R.; Haroon, A.; Wan, S.; Afaq, A.; Payne, H.; Bomanji, J.; Adeleke, S.; Kasivisvanathan, V. The future of PSMA PET and WB MRI as next-generation imaging tools in prostate cancer. Nat. Rev. Urol. 2022, 19, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.; Kroener, R.; Dendl, K.; Kratochwil, C.; Fink, C.; Ristau, J.; Winter, E.; Herfarth, K.; Hatiboglu, G.; Hohenfellner, M.; et al. Detecting and Locating the Site of Local Relapse Using 18F-PSMA-1007 Imaging After Primary Treatment of Prostate Cancer Patients-Potential Impact on PSMA-Guided Radiation Therapy. Mol. Imaging Biol. 2023, 25, 375–383. [Google Scholar] [CrossRef]
- Kamran, S.C.; Vapiwala, N. Approach to Patients with High-Risk Localized Prostate Cancer: Radiation Oncology Perspective. Curr. Treat. Options Oncol. 2024, 25, 84–96. [Google Scholar] [CrossRef]
- Almeida, L.S.; Etchebehere, E.C.S.d.C.; Megías, I.G.; Terán, A.K.C.; Hadaschik, B.; Colletti, P.M.; Herrmann, K.; Giammarile, F.; Bolton, R.C.D. PSMA Radioligand Therapy in Prostate Cancer: Where Are We and Where Are We Heading? Clin. Nucl. Med. 2024, 49, 45–55. [Google Scholar] [CrossRef]
- Giraudet, A.L.; Vinceneux, A.; Pretet, V.; Paquet, E.; Lajusticia, A.S.; Khayi, F.; Badel, J.N.; Boyle, H.; Flechon, A.; Kryza, D. Rationale for Prostate-Specific-Membrane-Antigen-Targeted Radionuclide Theranostic Applied to Metastatic Clear Cell Renal Carcinoma. Pharmaceuticals 2023, 16, 995. [Google Scholar] [CrossRef]
- Wang, J.; Kiess, A. PSMA-targeted therapy for non-prostate cancers. Front. Oncol. 2023, 13, 1220586. [Google Scholar] [CrossRef]
- Ferté, C.; André, F.; Soria, J. Molecular circuits of solid tumors: Prognostic and predictive tools for bedside use. Nat. Rev. Clin.Oncol. 2010, 7, 367–380. [Google Scholar] [CrossRef]
- Perner, S.; Cronauer, M.; Schrader, A.; Klocker, H.; Culig, Z.; Baniahmad, A. Adaptive responses of androgen receptor signaling in castration-resistant prostate cancer. Oncotarget 2015, 6, 35542–35555. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, B.; Guo, C.; Neeb, A.; Paschalis, A.; Sandhu, S.; de Bono, J.S. Prostate-specific Membrane Antigen Biology in Lethal Prostate Cancer and its Therapeutic Implications. Eur. Urol. Focus 2022, 8, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Feng, X. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 11–26. [Google Scholar] [CrossRef]
- Ritawidya, R.; Wongso, H.; Effendi, N.; Pujiyanto, A.; Lestari, W.; Setiawan, H.; Humani, T.S. Lutetium-177-Labeled Prostate-Specific Membrane Antigen-617 for Molecular Imaging and Targeted Radioligand Therapy of Prostate Cancer. Adv. Pharm. Bull. 2023, 13, 701–711. [Google Scholar] [CrossRef]
- Carver, B.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Evans, J.; Malhotra, M.; Cryan, J.; O’Driscoll, C. The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br. J. Pharmacol. 2016, 173, 3041–3079. [Google Scholar] [CrossRef]
- Heck, M.M.; Retz, M.; Tauber, R.; Knorr, K.; Kratochwil, C.; Eiber, M. Radionuklidtherapie des Prostatakarzinoms mittels PSMA-Lutetium. Der Urol. 2016, 56, 32–39. [Google Scholar] [CrossRef]
- Donya, M.; Radford, M.; ElGuindy, A.; Firmin, D.; Yacoub, M.H. Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract. 2014, 2014, 437–448. [Google Scholar] [CrossRef]
- Morgan, K.A.; Rudd, S.E.; Noor, A.; Donnelly, P.S. Theranostic Nuclear Medicine with Gallium-68, Lutetium-177, Copper-64/67, Actinium-225, and Lead-212/203 Radionuclides. Chem. Rev. 2023, 123, 12004–12035. [Google Scholar] [CrossRef]
- Anderson, P.M.; Subbiah, V.; Trucco, M.M. Current and future targeted alpha particle therapies for osteosarcoma: Radium-223, actinium-225, and thorium-227. Front. Med. 2022, 9, 1030094. [Google Scholar] [CrossRef] [PubMed]
- Buatti, J.M.; Rivero, L.R.; Jorgensen, T.J. Radiation-Induced DNA Single-Strand Breaks in Freshly Isolated Human Leukocytes. Radiat. Res. 1992, 132, 200. [Google Scholar] [CrossRef] [PubMed]
- MacManus, D.; Bell, D.; Murphy, A. Linear Energy Transfer. Reference Article. Radiopaedia.org. Available online: https://radiopaedia.org/articles/linear-energy-transfer (accessed on 28 February 2025).
- Rinscheid, A.; Gäble, A.; Wienand, G.; Dierks, A.; Kircher, M.; Günther, T.; Patt, M.; Bundschuh, R.A.; Lapa, C.; Pfob, C.H. Biodistribution and radiation dosimetry of [99mTc]Tc-N4-BTG in patients with biochemical recurrence of prostate cancer. EJNMMI Res. 2024, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef]
- FDA. FDA Expands Pluvicto’s Metastatic Castration-Resistant Prostate Cancer Indication. 28 March 2025. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-pluvictos-metastatic-castration-resistant-prostate-cancer-indication (accessed on 11 April 2025).
- Farolfi, A.; Mei, R.; Ali, S.; Castellucci, P. Theragnostics in prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 333–341. [Google Scholar] [CrossRef]
- Mokoala, K.; Lawal, I.; Lengana, T.; Kgatle, M.; Giesel, F.L.; Vorster, M.; Sathekge, M. PSMA Theranostics: Science and Practice. Cancers 2021, 13, 3904. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.Y.; Rowe, S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 80, 82–94. [Google Scholar] [CrossRef]
- George, S.C.; Samuel, E.J.J. Developments in 177Lu-based radiopharmaceutical therapy and dosimetry. Front. Chem. 2023, 11, 1218670. [Google Scholar] [CrossRef]
- Bakht, M.K.; Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat. Rev. Urol. 2024, 22, 26–45. [Google Scholar] [CrossRef]
- Maharaj, M.; Heslop, L.; Govender, T.; Korowlay, N.; Singh, A.; Choudhary, P.; Sathekge, M. The Outcome and Safety of Re-challenge Lutetium-177 PSMA (177Lu-PSMA) Therapy with Low-Dose Docetaxel as a Radiosensitizer-a Promising Combination in Metastatic Castrate-Resistant Prostate Cancer (mCRPC): A Case Report. Nucl. Med. Mol. Imaging 2021, 55, 136–140. [Google Scholar] [CrossRef]
- Kafka, M.; Horninger, A.; di Santo, G.; Virgolini, I.; Neuwirt, H.; Unterrainer, L.M.; Kunte, S.C.; Deiss, E.; Paffenholz, P.; Heidenreich, A.; et al. Real-world Outcomes and Predictive Biomarkers for 177Lutetium Prostate-specific Membrane AntigenLigand Treatment in Metastatic Castration-resistant Prostate Cancer: A European Association of Urology Young Academic Urologists Prostate Cancer Working Group Multi-institutional Observational Study. Eur. Urol. Oncol. 2024, 7, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Gafita, A.; Telli, T.; Voter, A.; Herrmann, K.; Pomper, M.; Hadaschik, B.; Rowe, S.P.; Fendler, W. Standardized PSMA-PET Imaging of Advanced Prostate Cancer. Semin. Nucl. Med. 2024, 54, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Viscuse, P.; Devitt, M.; Dreicer, R. Clinical Management of Advanced Prostate Cancer: Where Does Radiopharmaceutical Therapy Fit in the Treatment Algorithm? J. Nucl. Med. 2024, 65, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.R. Critical Challenges in Pluvicto Therapy: Incontinent and Anticoagulated Patients. J. Nucl. Med. Technol. 2023, 51, 279–281. [Google Scholar] [CrossRef]
- Almuradova, E.; Seyyar, M.; Arak, H.; Tamer, F.; Kefeli, U.; Koca, S.; Sen, E.; Telli, T.A.; Karatas, F.; Gokmen, I.; et al. The real-world outcomes of Lutetium-177 PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer: Turkish Oncology Group multicenter study. Int. J. Cancer 2024, 154, 692–700. [Google Scholar] [CrossRef]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef]
- Chen, J.; Qi, L.; Tang, Y.; Tang, G.; Gan, Y.; Cai, Y. Current role of prostate-specific membrane antigen-based imaging and radioligand therapy in castration-resistant prostate cancer. Front. Cell Dev. Biol. 2022, 10, 958180. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Scott, A.M. 161Tb-PSMA Unleashed: A Promising New Player in the Theranostics of Prostate Cancer. Nucl. Med. Mol. Imaging 2023, 57, 168–171. [Google Scholar] [CrossRef]
- Mease, R.C.; Kang, C.M.; Kumar, V.; Banerjee, S.R.; Minn, I.; Brummet, M.; Gabrielson, K.L.; Feng, Y.; Park, A.; Kiess, A.P.; et al. An Improved 211At-Labeled Agent for PSMA-Targeted α-Therapy. J. Nucl. Med. 2021, 63, 259–267. [Google Scholar] [CrossRef]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 targeted alpha particle therapy for prostate cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef]
- Ceci, F.; Airò Farulla, L.S.; Bonatto, E.; Evangelista, L.; Aliprandi, M.; Cecchi, L.G.; Mattana, F.; Bertocchi, A.; DE Vincenzo, F.; Perrino, M.; et al. New target therapies in prostate cancer: From radioligand therapy, to PARP-inhibitors and immunotherapy. Q. J. Nucl. Med. Mol. Imaging 2024, 68, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Cloître, M.; Benkhaled, S.; Boughdad, S.; Schaefer, N.; Prior, J.O.; Zeverino, M.; Berthold, D.; Tawadros, T.; Meuwly, J.Y.; Martel, P.; et al. Spatial Distribution of Recurrence and Long-Term Toxicity Following Dose Escalation to the Dominant Intra- 844 Prostatic Nodule for Intermediate-High-Risk Prostate Cancer: Insights from a Phase I/II Study. Cancers 2024, 16, 2097. [Google Scholar] [CrossRef] [PubMed]
- Burgard, C.; Engler, J.; Blickle, A.; Bartholomä, M.; Maus, S.; Schaefer-Schuler, A.; Khreish, F.; Ezziddin, S.; Rosar, F. Change of glucometabolic activity per PSMA expression predicts survival in mCRPC patients non-responding to PSMA radioligand therapy: Introducing a novel dual imaging biomarker. Front. Med. 2024, 10, 1339160. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Gafita, A.; Langbein, T.; Tauber, R.; Seidl, C.; Bruchertseifer, F.; Gschwendt, J.E.; Weber, W.A.; D’Alessandria, C.; Morgenstern, A.; et al. Comparative Analysis of Morphological and Functional Effects of 225Ac- and 177Lu-PSMA Radioligand Therapies (RLTs) on Salivary Glands. Int. J. Mol. Sci. 2023, 24, 16845. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, T.; Zhang, J.; Gao, F.; Yang, L.; Tao, W. The Application of Radiolabeled Targeted Molecular Probes for the Diagnosis and Treatment of Prostate Cancer. Korean J. Radiol. 2023, 24, 574–589. [Google Scholar] [CrossRef]
- Alam, M.R.; Singh, S.B.; Thapaliya, S.; Shrestha, S.; Deo, S.; Khanal, K. A Review of 177Lutetium-PSMA and 225Actinium-PSMAas Emerging Theranostic Agents in Prostate Cancer. Cureus 2022, 14, e29369. [Google Scholar] [CrossRef]
- Akintayo, A.A.; Bilen, M.A.; Abiodun-Ojo, O.A.; Kucuk, O.; Carthon, B.; Chen, Z.; Jani, A.B.; Parent, E.E.; Schuster, D.M. 857 Exploratory study of 18F-fluciclovine pet/ct for response assessment to docetaxel in patients with metastatic castration-resistant 858 prostate cancer. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 218–229. [Google Scholar]
- Cerci, J.J.; Fanti, S.; Lobato, E.E.; Kunikowska, J.; Alonso, O.; Medina, S.; Novruzov, F.; Lengana, T.; Granados, C.; Kumar, R.; et al. Diagnostic Performance and Clinical Impact of 68Ga-PSMA-11 PET/CT Imaging in Early Relapsed Prostate Cancer After Radical Therapy: A Prospective Multicenter Study (IAEA-PSMA Study). J. Nucl. Med. 2022, 63, 240–247. [Google Scholar] [CrossRef]
- Supiot, S.; Vaugier, L.; Pasquier, D.; Buthaud, X.; Magné, N.; Peiffert, D.; Sargos, P.; Crehange, G.; Pommier, P.; Loos, G.; et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur. Urol. 2021, 80, 405–414. [Google Scholar] [CrossRef]
- Lunger, L.; Tauber, R.; Feuerecker, B.; Gschwend, J.E.; Eiber, M.; Heck, M.M. Narrative review: Prostate-specific membrane antigen-radioligand therapy in metastatic castration-resistant prostate cancer. Transl. Androl. Urol. 2021, 10, 3963–3971. [Google Scholar] [CrossRef]
- Dietlein, F.; Kobe, C.; Muñoz Vázquez, S.; Fischer, T.; Endepols, H.; Hohberg, M.; Reifegerst, M.; Neumaier, B.; Schomäcker, K.; Drzezga, A.E.; et al. An 89Zr-Labeled PSMA Tracer for PET/CT Imaging of Prostate Cancer Patients. J. Nucl. Med. 2022, 63, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.D. Radiation Safety Assessment in Prostate Cancer Treatment: A Predictive Approach for I-125 Brachytherapy. Cancers 2024, 16, 1790. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Aziz, U.; Koreshi, Z.U. Radiation Dose Delivered by 125I, 103Pd and 131Cs and Dose Enhancement by Gold Nanoparticle (GNP) Solution in Prostate Brachytherapy: A Comparative Analysis by Monte Carlo Simulation. Iium Eng. J. 2019, 20, 176–187. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Shamis, M.; Yan, W.; Trichter, S.; Sabbas, A.; Goltser, Y.; Christos, P.J.; Brennan, J.S.; Parashar, B.; Nori, D. Role of Isotope Selection in Long-Term Outcomes in Patients With Intermediate-Risk Prostate Cancer Treated With a Combination of External Beam Radiotherapy and Low-Dose-Rate Interstitial Brachytherapy. Urology 2012, 79, 1098–1104. [Google Scholar] [CrossRef]
- Zuber, S.; Weiß, S.; Baaske, D.; Schöpe, M.; Stevens, S.; Bodis, S.; Zwahlen, D.R. Iodine-125 seed brachytherapy for early stage prostate cancer: A single-institution review. Radiat. Oncol. 2015, 10, 49. [Google Scholar] [CrossRef]
- Hirayama, K.; Fukushima, T.; Morita, M.; Oshinomi, K.; Nishimura, K.; Yamatoya, J.; Nakagami, Y.; Toyofuku, K.; Niiya, A.; Kobayashi, R.; et al. Secondary Bladder Cancer After Permanent Iodine-125 Brachytherapy for Prostate Cancer. Showa Univ. J. Med. Sci. 2023, 21, 451–459. [Google Scholar] [CrossRef]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1110. [Google Scholar] [CrossRef]
- Hong, J.H. An Update of Prostate-Specific Membrane Antigen Theranostics in Prostate Cancer. Korean J. Urol. Oncol. 2022, 20, 207–222. [Google Scholar] [CrossRef]
- Sallam, M.; Nguyen, N.T.; Sainsbury, F.; Kimizuka, N.; Muyldermans, S.; Benešová-Schäfer, M. PSMA-targeted radiotheranostics in modern nuclear medicine: Then, now, and what of the future? Theranostics 2024, 14, 3043–3079. [Google Scholar] [CrossRef]
- Lyu, F.; Shang, S.Y.; Gao, X.S.; Ma, M.W.; Xie, M.; Ren, X.Y.; Liu, M.Z.; Chen, J.Y.; Li, S.S.; Huang, L. Uncovering the Secrets of Prostate Cancer’s Radiotherapy Resistance: Advances in Mechanism Research. Biomedicines 2023, 11, 1628. [Google Scholar] [CrossRef]
- Corpetti, M.; Müller, C.; Beltran, H.; de Bono, J.; Theurillat, J.-P. Prostate-Specific Membrane Antigen; Targeted Therapies for Prostate Cancer: Towards Improving Therapeutic Outcome. Eur. Urol. 2024, 85, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Gafita, A.; Murthy, V.; Benz, M.R.; Sonni, I.; Burger, I.A.; Eiber, M.; Emmett, L.; Farolfi, A.; Wolfgang, P.; et al. 177Lu]PSMA Radioligand Therapy: An International Multicenter Retrospective Study. J. Nucl. Med. 2023, 64, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Satapathy, S.; Sharma, A.; Sood, A.; Maheshwari, P.; Gill, H.J.S. Delayed nephrotoxicity after 225Ac-PSMA-617 radioligand therapy. Clin. Nucl. Med. 2022, 47, e466–e467. [Google Scholar] [CrossRef]
- Dhiantravan, N.; Hofman, M.S.; Kumar, A.S.R. Actinium-225 prostate-specific membrane antigen theranostics: Will alpha beat beta? Eur. Urol. 2021, 79, 351–352. [Google Scholar] [CrossRef]
- Rashid, N.S.; Rami, A.; Lang, M.; Stoltenberg, H.; Wolanski, A.; Ritzer, J.; Jacene, H.; Ravi, P. Activity of 177Lu-PSMA-617 in Patients With Advanced Prostate Cancer and Brain Metastases. Clin. Genitourin. Cancer 2025, 23, 102309. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Sayar, E.; Patel, R.A.; Coleman, I.M.; Roudier, M.P.; Zhang, A.; Mustafi, P.; Low, J.-Y.; Hanratty, B.; Ang, L.S.; Bhatia, V.; et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight 2023, 8, e162907. [Google Scholar] [CrossRef]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Tran, H.H.; Yamaguchi, A.; Manning, H.C. Radiotheranostic landscape: A review of clinical and preclinical development. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2685–2709. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, T.B.; Mansi, R.; Del Pozzo, L.; Zanger, S.; Gaonkar, R.H.; McDougall, L.; De Rose, F.; Jaafar-Thiel, L.; Herz, M.; Eiber, M.; et al. 61Cu-PSMA-Targeted PET for Prostate Cancer: From Radiotracer Development to First-in-Human Imaging. J. Nucl. Med. 2024, 65, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2019, 46, 1919–1930. [Google Scholar] [CrossRef]
- Schaefer-Schuler, A.; Burgard, C.; Blickle, A.; Maus, S.; Petrescu, C.; Petto, S.; Bartholomä, M.; Stemler, T.; Ezziddin, S.; Rosar, F. [161Tb]Tb-PSMA-617 radioligand therapy in patients with mCRPC: Preliminary dosimetry results and intra-individual head-to-head comparison to [177Lu]Lu-PSMA-617. Theranostics 2024, 14, 1829–1840. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I and T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Makvandi, M.; Samanta, M.; Martorano, P.; Lee, H.; Gitto, S.B.; Patel, K.; Groff, D.; Pogoriler, J.; Martinez, D.; Riad, A.; et al. Pre-clinical investigation of astatine-211-parthanatine for high-risk neuroblastoma. Commun. Biol. 2022, 5, 1260. [Google Scholar] [CrossRef]
- Griffiths, M.R.; Pattison, D.A.; Latter, M.; Kuan, K.; Taylor, S.; Tieu, W.; Kryza, T.; Meyrick, D.; Lee, B.Q.; Hansen, A.; et al. First-in-Human212Pb-PSMA–Targeted α-Therapy SPECT/CT Imaging in a Patient with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2024, 65, 664. [Google Scholar] [CrossRef]
- Feuerecker, B.; Kratochwil, C.; Ahmadzadehfar, H.; Morgenstern, A.; Eiber, M.; Herrmann, K.; Pomykala, K.L. Clinical Translation of Targeted α−Therapy: An Evolution or a Revolution? J. Nucl. Med. 2023, 64, 685–692. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Bobba, K.; Bidkar, A.; Wadhwa, A.; Meher, N.; Drona, S.; Sorlin, A.; Bidlingmaier, S.; Zhang, L.; Wilson, D.; Chan, E.; et al. Development of CD46 targeted alpha theranostics in prostate cancer using 134Ce/225Ac-Macropa-PEG4-YS5. Theranostics 2024, 14, 1344–1360. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Durack, J.C.; Lyashchenko, S.K.; Cheal, S.M.; Beylergil, V.; Lefkowitz, R.A.; Carrasquillo, J.A.; Martinez, D.F.; Fung, A.M.; et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin. Cancer Res. 2015, 21, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Püllen, L.; Kesch, C.; Krafft, U.; Tschirdewahn, S.; Moraitis, A.; Radtke, J.; Ting, S.; Nader, M.; Wosniack, J.; et al. 18F-PSMA Cerenkov Luminescence and Flexible Autoradiography Imaging in a Prostate Cancer Mouse Model and First Results of a Radical Prostatectomy Feasibility Study in Men. J. Nucl. Med. 2023, 64, 598–604. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health US National Library of Medicine; ClinicalTrials.gov. Cu-64-PSMA-I&T Positron Emission Tomography (PET) Imaging of Metastatic PSMA Positive Lesions in Men with Prostate Cancer. Last Updated 22 March 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05653856 (accessed on 14 April 2025).
- Sandhu, S.; Guo, C.; Hofman, M.S. Radionuclide therapy in prostate cancer: From standalone to combination PSMA theranostics. J. Nucl. Med. 2021, 62, 1660–1668. [Google Scholar] [CrossRef]
- Alati, S.; Singh, R.; Pomper, M.G.; Rowe, S.P.; Sanerjee, S.R. Preclinical Development in Radiopharmaceutical Therapy for Prostate Cancer. Semin. Nucl. Med. 2023, 53, 663–686. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Mdlophane, A.; Maes, A.; Mokoala, K.; et al. mCRPC patients receiving 225Ac-PSMA-617 therapy in post androgen deprivation therapy setting: Response to treatment and survival analysis. J. Nucl. Med. 2022, 63, 1496–1502. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Sohal, I.S.; Iyer, S.G.; Sudarshan, K.; Orellana, E.A.; Ozcan, K.E.; Dos Santos, A.P.; Low, P.S.; Kasinski, A.L. Selective targeting of chemically modified miR-34a to prostate cancer using a small molecule ligand and an endosomal escape agent. Mol. Ther. Nucleic Acids 2024, 35, 102193. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, Y.; Liu, X.; Wu, S.; Wang, M.; Turowski, S.G.; Spernyak, J.A.; Tracz, A.; Abdelaal, A.M.; Sudarshan, K.; et al. Developing Folate-Conjugated miR-34a Therapeutic for Prostate Cancer: Challenges and Promises. Int. J. Mol. Sci. 2024, 25, 2123. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Fendler, W.P.; Morigi, J.J.; Fanti, S. Theranostics in oncology: What radiologists want to know. Eur. J. Radiol. 2021, 142, 109875. [Google Scholar] [CrossRef]
- Kamran, S.C.; Zietman, A.L. Radiation treatment in prostate cancer: Covering the waterfront. BJU Int. 2021, 128, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, C.; Wang, X.; Wu, D. PSMA targeted Therapy: From molecular mechanisms to clinical breakthroughs in castration-resistant prostate cancer. Eur. J. Med. Chem. 2025, 296, 117829. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, M.C.; Patel, R.B.; Anderson, C.J. Combined targeted radiopharmaceutical therapy and immune checkpoint blockade: From preclinical advances to the clinic. J. Nucl. Med. 2022, 63, 1636–1641. [Google Scholar] [CrossRef]
- Kerr, C.P.; Grudzinski, J.J.; Nguyen, T.P.; Hernandez, R.; Weichert, J.P.; Morris, Z.S. Developments in Combining Targeted Radionuclide Therapies and Immunotherapies for Cancer Treatment. Pharmaceutics. 2022, 15, 128. [Google Scholar] [CrossRef]
- Aggarwal, R.; Starzinski, S.; de Kouchkovsky, I.; Koshkin, V.; Bose, R.; Chou, J.; Desai, A.; Kwon, D.; Kaushal, S.; Trihy, L.; et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: An open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023, 24, 1266–1276. [Google Scholar] [CrossRef]
- Inderjeeth, A.J.; Iravani, A.; Subramaniam, S.; Conduit, C.; Sandhu, S. Novel radionuclide therapy combinations in prostate cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231187202. [Google Scholar] [CrossRef]
- Kostos, L.; Buteau, J.P.; Kong, G.; Tran, B.; Haskali, M.B.; Fahey, M.; Crumbaker, M.; Emmett, L.; Hofman, M.S.; Azad, A.A. Clinical Trial Protocol for LuCAB: A Phase I-II Trial Evaluating Cabazitaxel in Combination with [177Lu]Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2025, 66, 572–578. [Google Scholar] [CrossRef]
- Dhiantravan, N.; Emmett, L.; Joshua, A.M.; Pattison, D.A.; Francis, R.J.; Williams, S.; Sandhu, S.; Davis, I.D.; Vela, I.; Neha, N.; et al. Up Front PSMA: A randomized phase 2 study of sequential 177Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naïve prostate cancer (clinical trial protocol). BJU Int. 2021, 128, 331–342. [Google Scholar] [CrossRef]
- Barbieri, C.E.; Bangma, C.H.; Bjartell, A.; Catto, J.W.; Culig, Z.; Grönberg, H.; Luo, J.; Visakorpi, T.; Rubin, M.A. The mutational landscape of prostate cancer. Eur. Urol. 2013, 64, 567–576. [Google Scholar] [CrossRef]
- Zhong, J.; Jang, A.; Garcia, J.; Avril, N.; Li, Q.; Wojtylak, P.; Shore, N.; Tagawa, S.; Barata, P. Chapter Eight—Advances in prostate cancer treatment: Radionuclide therapy for prostate cancer. In Advances in Cancer Research; Fisher, P.B., Tew, K.D., Eds.; Academic Press: New York, NY, USA, 2024; Volume 164, pp. 311–358. [Google Scholar] [CrossRef]
- Mizuno, K.; Beltran, H. Future directions for precision oncology in prostate cancer. Prostate 2022, 82 (Suppl. S1), S86–S96. [Google Scholar] [CrossRef]
- Bauckneht, M.; Ciccarese, C.; Laudicella, R.; Mosillo, C.; D’Amico, F.; Anghelone, A.; Strusi, A.; Beccia, V.; Bracarda, S.; Fornarini, G.; et al. Theranostics revolution in prostate cancer: Basics, clinical applications, open issues and future perspectives. Cancer Treat. Rev. 2024, 124, 102698. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.; Etchebehere, M.; Bogoni, M.; Torricelli, C.; Nogueira-Lima, E.; Deflon, V.M.; Lima, M.; Etchebehere, E. Theranostics Nuclear Medicine in Prostate Cancer. Pharmaceuticals 2024, 17, 1483. [Google Scholar] [CrossRef] [PubMed]
- Andrew, J.; Ezra-Manicum, A.L.; Witika, B.A. Developments in radionanotheranostic strategies for precision diagnosis and treatment of prostate cancer. EJNMMI Radiopharm. Chem. 2024, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Sayegh, N.; Tombal, B.; Hussain, M.; Sweeney, C.J.; Graff, J.N.; Agarwal, N. Metastatic Hormone-Sensitive Prostate Cancer: Toward an Era of Adaptive and Personalized Treatment. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390166. [Google Scholar] [CrossRef]
- Gourdin, T.; Velayati, A. Treatments and challenges in advanced prostate cancer. Curr. Opin. Oncol. 2023, 35, 200–205. [Google Scholar] [CrossRef]
- Neels, O.C.; Kopka, K.; Liolios, C.; Afshar-Oromieh, A. Radiolabeled PSMA Inhibitors. Cancers 2021, 13, 6255. [Google Scholar] [CrossRef]
| Radionuclide | Treatment Response (% of Tumor Decrease) | PSA Levels (% of Serum PSA Decrease) | Disease Recurrence Rate (% of Decrease) | Average Cost of Treatment in Germany | Nb of Treated Patients |
|---|---|---|---|---|---|
| 177Lu | 30–80% | 70% | 40% | €17,000–€28,000 | 15,000–30,000 |
| 225Ac | 50–80% | 90% | 40–60% | €18,000–€29,000 | 700–900 |
| 223Ra | 30–50% | 20–30% | 15–30% | €12,000–€15,000 | 50,000–100,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, M.; Constantin, C.; Hinescu, M.E.; Bleotu, P.G.; Popovici, M.-G.; Zai, M.-I.; Spohr, K.M. Radionuclides Landscape in Prostate Cancer Theranostics. Int. J. Mol. Sci. 2025, 26, 6751. https://doi.org/10.3390/ijms26146751
Neagu M, Constantin C, Hinescu ME, Bleotu PG, Popovici M-G, Zai M-I, Spohr KM. Radionuclides Landscape in Prostate Cancer Theranostics. International Journal of Molecular Sciences. 2025; 26(14):6751. https://doi.org/10.3390/ijms26146751
Chicago/Turabian StyleNeagu, Monica, Carolina Constantin, Mihail Eugen Hinescu, Petrisor Gabriel Bleotu, Mara-Georgiana Popovici, Maria-Iulia Zai, and Klaus Michael Spohr. 2025. "Radionuclides Landscape in Prostate Cancer Theranostics" International Journal of Molecular Sciences 26, no. 14: 6751. https://doi.org/10.3390/ijms26146751
APA StyleNeagu, M., Constantin, C., Hinescu, M. E., Bleotu, P. G., Popovici, M.-G., Zai, M.-I., & Spohr, K. M. (2025). Radionuclides Landscape in Prostate Cancer Theranostics. International Journal of Molecular Sciences, 26(14), 6751. https://doi.org/10.3390/ijms26146751








