Functional Autoantibodies Targeting G-Protein-Coupled Receptors and Their Clinical Phenotype in Patients with Long-COVID
Abstract
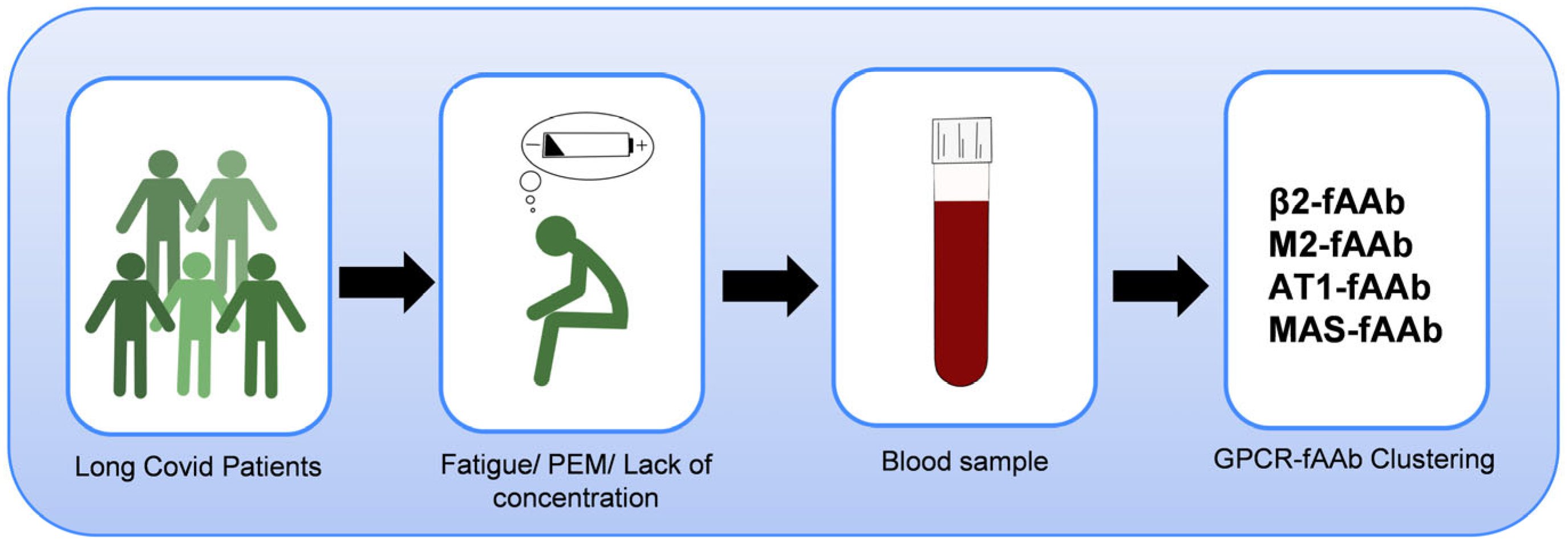
1. Introduction
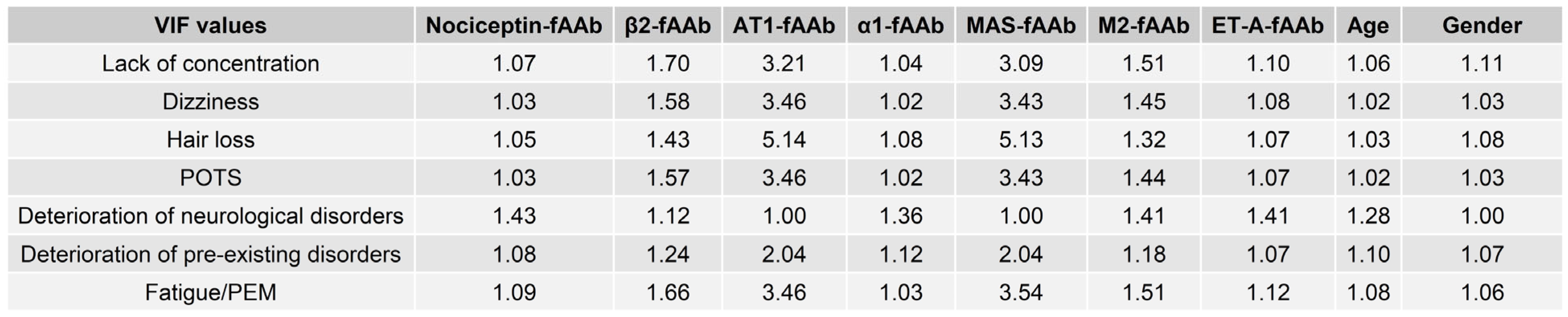
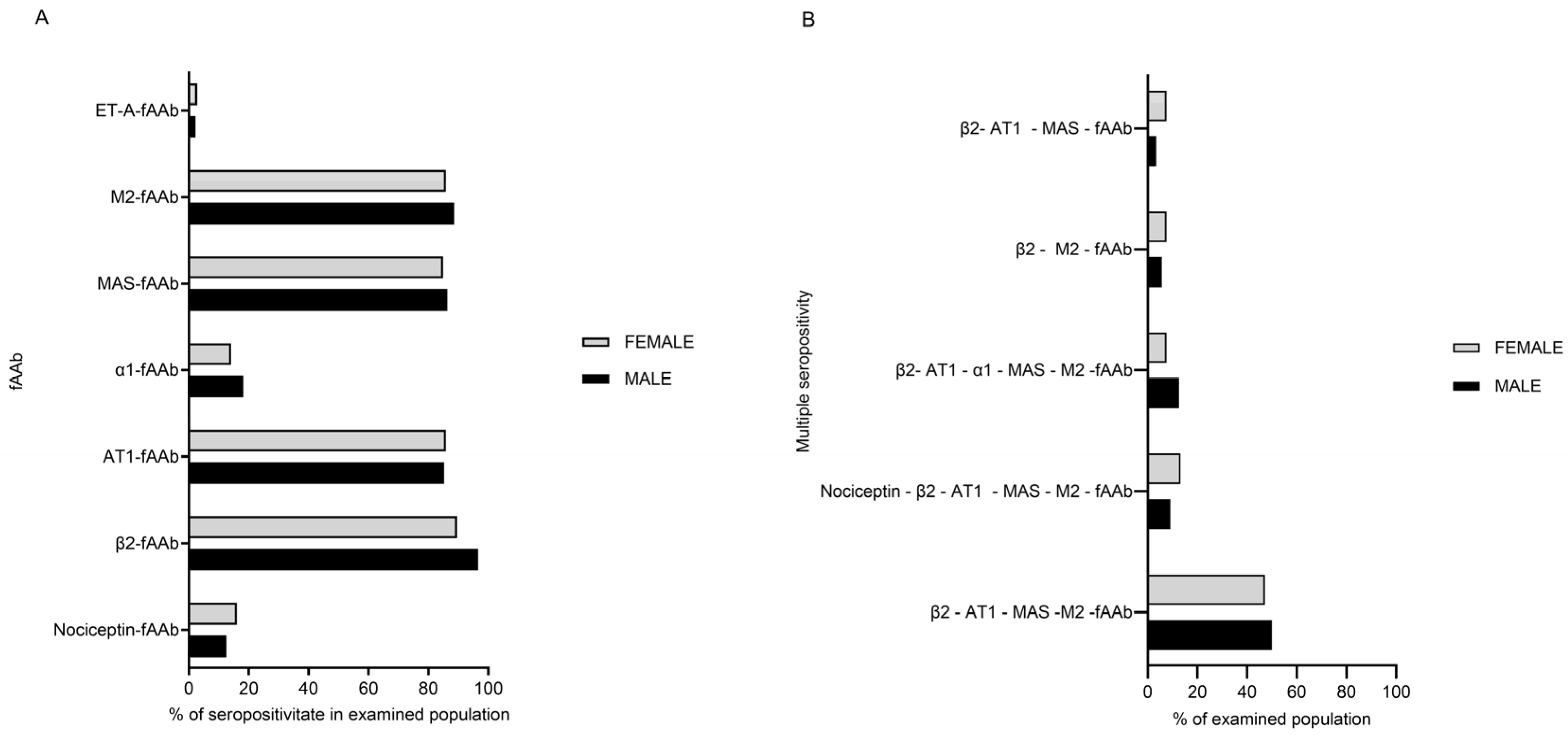
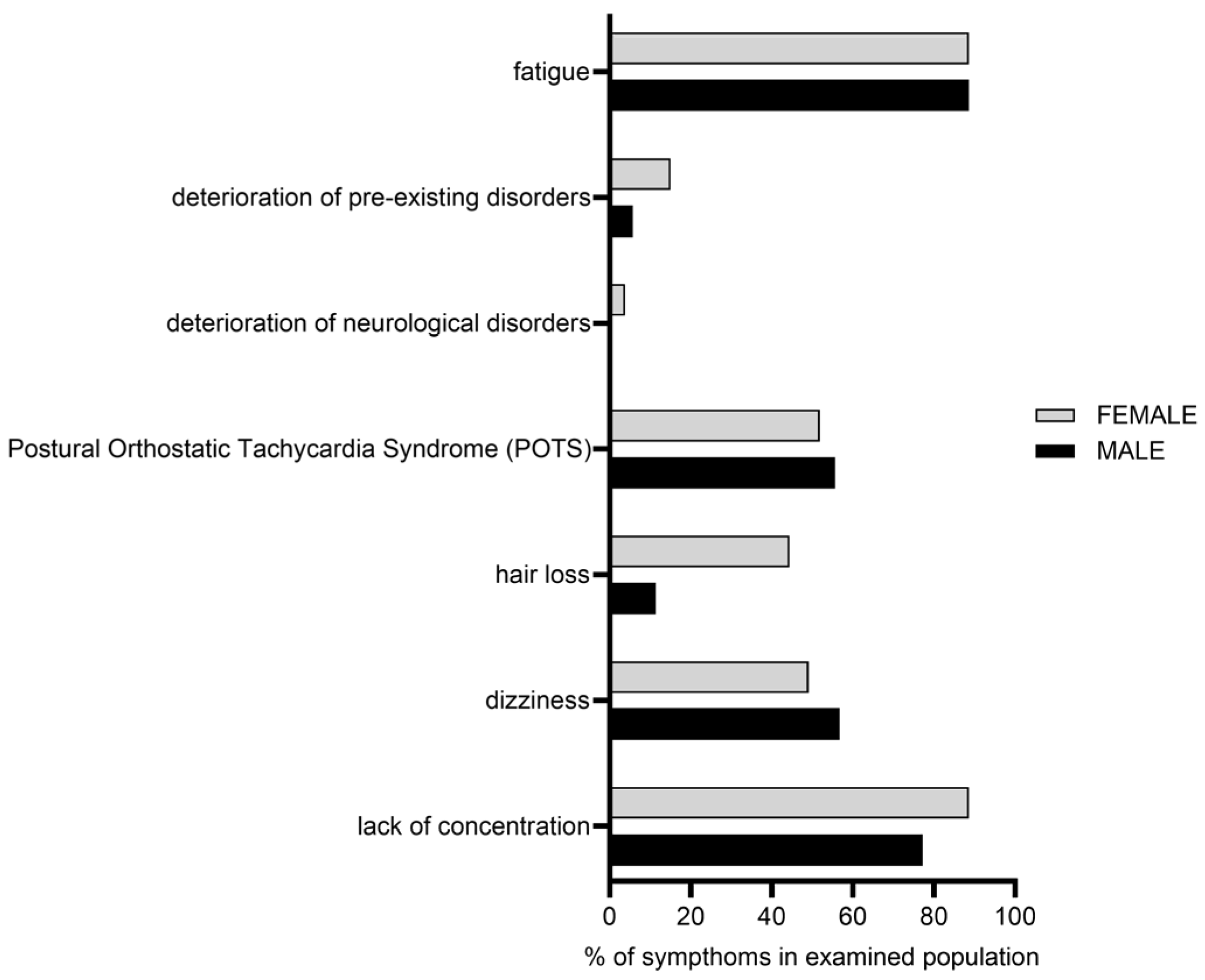
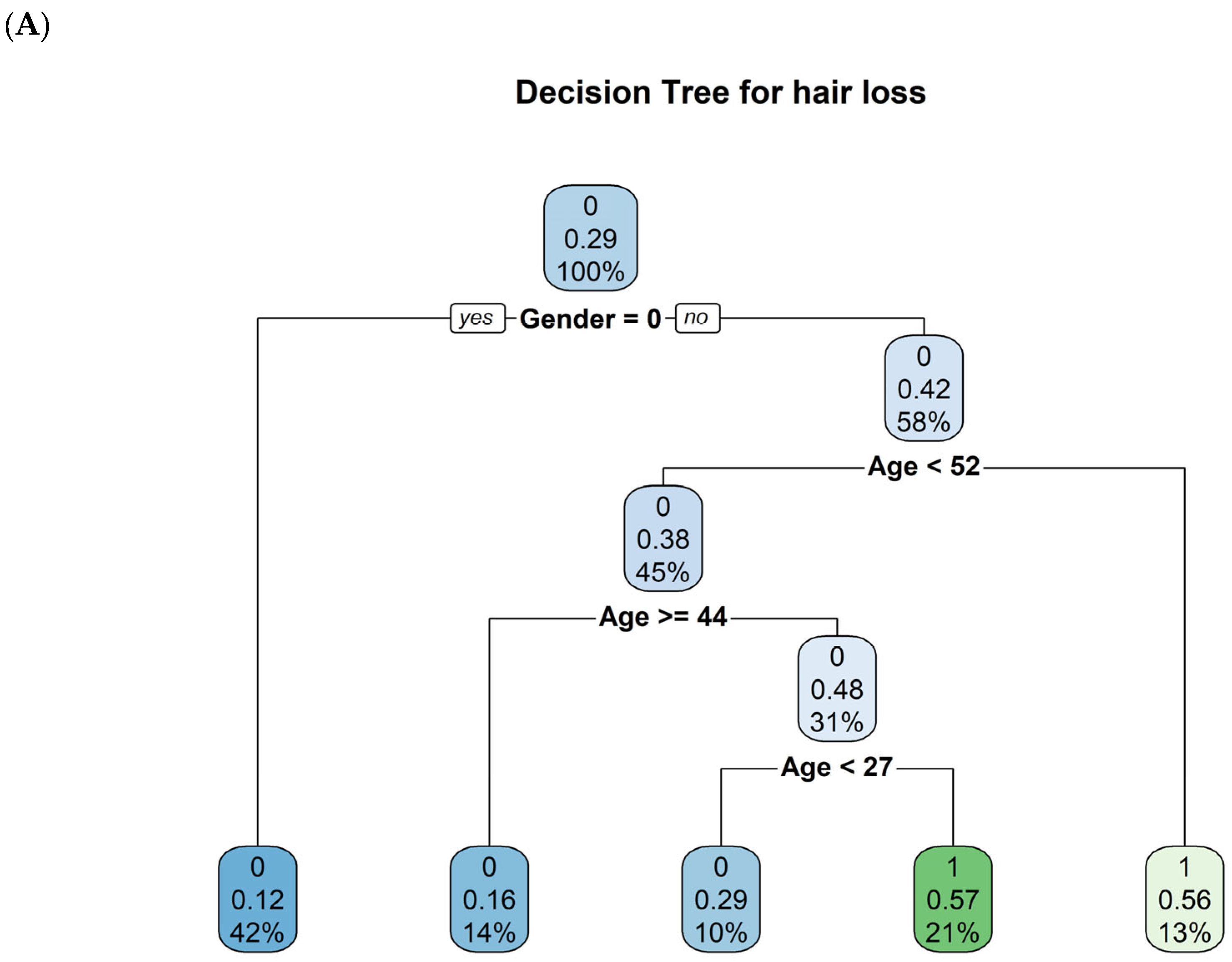
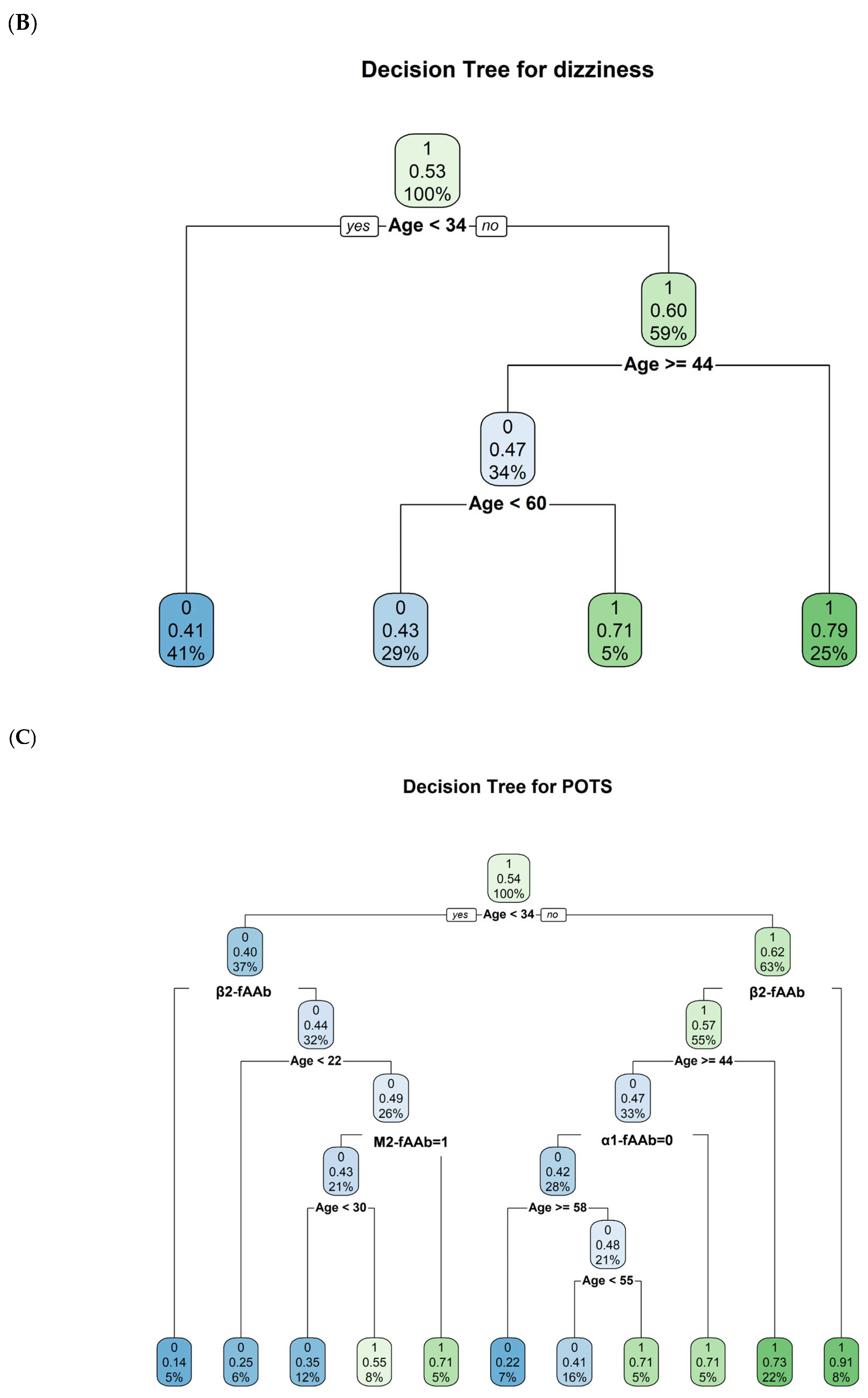
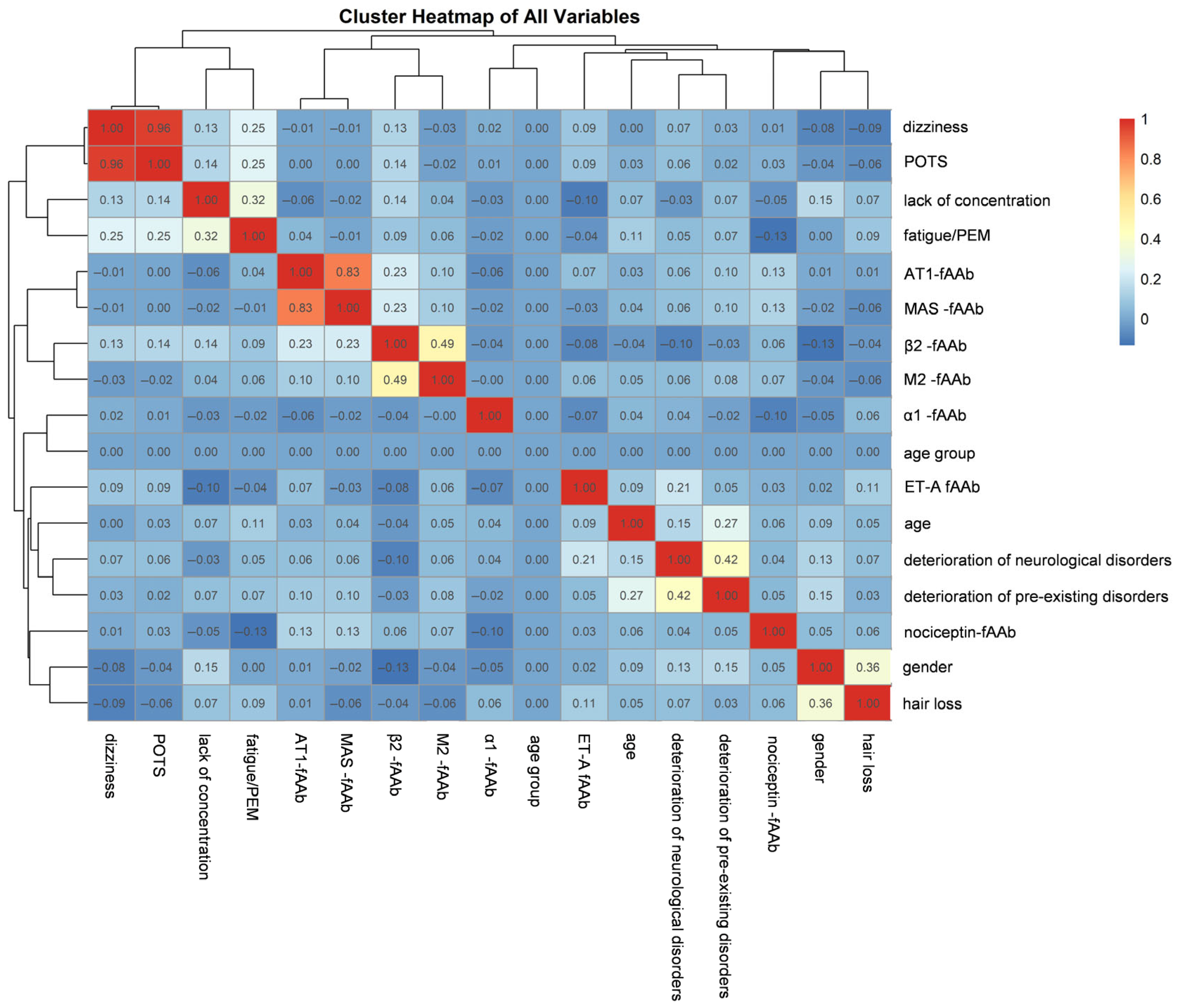
2. Results
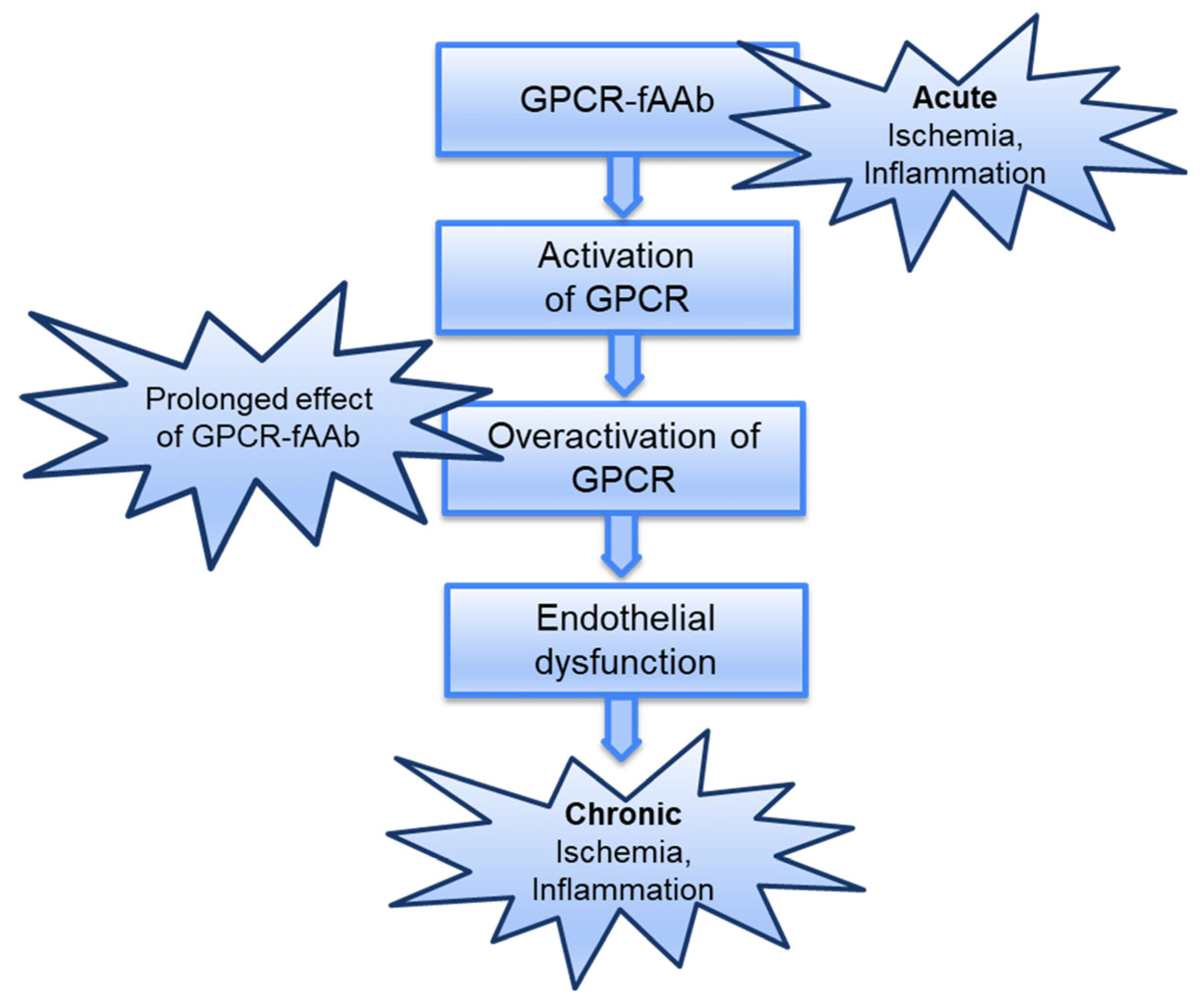
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Measurements of GPCR-fAAb by the Cardiomyocyte (CM)-Bioassay
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCS | Long-COVID Syndrome |
| PCS | Post-COVID Syndrome |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| PEM | Post-exertional malaise |
| POTS | Postural orthostatic tachycardia syndrome |
| AAb | Autoantibodies |
| GPCR-fAAb | G-Protein coupled receptor autoantibodies with functional activity |
| β1-fAAb | Functional autoantibodies targeting the Beta-1 Adrenergic receptor |
| β2-fAAb | Functional autoantibodies targeting the Beta-2 Adrenergic receptor |
| CFS | Chronic fatigue syndrome |
| M2-fAAb | Functional autoantibodies targeting the Muscarinergic M2 receptor |
| AT1-fAAb | Functional autoantibodies targeting the Angiotensin II Type 1 receptor |
| MAS-fAAb | Functional autoantibodies targeting the angiotensin (1–7) Mas receptor |
| α1-fAAb | Functional autoantibodies targeting the Alpha-1 Adrenergic receptor |
| Nociceptin-fAAb | Functional autoantibodies targeting the Nociceptin receptor |
| ETA-fAAb | Functional autoantibodies targeting the Endothelin Type A receptor |
| CI | Confidence Interval |
| VIF | Variance Inflation Factor |
| CM | Cardiomyocyte |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| PARP1 | Poly-ADP-ribose polymerase-1 |
| CRPS | Complex regional pain syndrome |
| AngII | Angiotensin II receptor |
| ACE2 | Angiotensin Converting Enzyme 2 receptor |
| MAS | MAS1 oncogene |
| IgG | Immunoglobulin G antibodies |
| OR | Odds ratio |
| cp | Complexity parameter |
| SD | Standard Deviation |
| M | Mean Deviation |
| FAU | Friedrich-Alexander-Universität Erlangen-Nürnberg |
| 0 | female |
| 1 | male |
| AA4 | Alpha-1 Adrenergic Receptor functional autoantibodies |
| AA6 | Muscarinergic M2 Receptor functional autoantibodes |
| NA | Missing value |
Appendix A
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Sommer, N.; Schmeck, B. Pulmonary manifestations in long COVID. Inn. Med. 2022, 63, 819–829. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Moody, W.E.; Liu, B.; Mahmoud-Elsayed, H.M.; Senior, J.; Lalla, S.S.; Khan-Kheil, A.M.; Brown, S.; Saif, A.; Moss, A.; Bradlow, W.M.; et al. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J. Am. Soc. Echocardiogr. 2021, 34, 562–566. [Google Scholar] [CrossRef]
- Gomez Bravo, R.; Infanti, A.; Billieux, J.; Ritzen, M.; Psy-Long, C.C.; Vogele, C.; Benoy, C. The psychological syndrome associated with Long-COVID: A study protocol. Front. Epidemiol. 2023, 3, 1193369. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- The Writing Committee for the COMEBAC Study Group. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Jennings, G.; Monaghan, A.; Xue, F.; Duggan, E.; Romero-Ortuño, R. Comprehensive Clinical Characterisation of Brain Fog in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 3440. [Google Scholar] [CrossRef]
- Orrù, G.; Bertelloni, D.; Diolaiuti, F.; Mucci, F.; Di Giuseppe, M.; Biella, M.; Gemignani, A.; Ciacchini, R.; Conversano, C. Long-COVID Syndrome? A Study on the Persistence of Neurological, Psychological and Physiological Symptoms. Healthcare 2021, 9, 575. [Google Scholar] [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J.Z. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef]
- El-Rhermoul, F.Z.; Fedorowski, A.; Eardley, P.; Taraborrelli, P.; Panagopoulos, D.; Sutton, R.; Lim, P.B.; Dani, M. Autoimmunity in Long Covid and POTS. Oxf. Open Immunol. 2023, 4, iqad002. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Kanduc, D.; Muller, S.; Makatsariya, A.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am. J. Reprod. Immunol. 2021, 86, e13494. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Schick, M.; Hohberger, B.; Mahajan, A.; Knopf, J.; Schett, G.; Munoz, L.E.; Herrmann, M. Updates on NET formation in health and disease. Semin. Arthritis Rheum. 2019, 49, S43–S48. [Google Scholar] [CrossRef]
- Sotzny, F.; Filgueiras, I.S.; Kedor, C.; Freitag, H.; Wittke, K.; Bauer, S.; Sepulveda, N.; Mathias da Fonseca, D.L.; Baiocchi, G.C.; Marques, A.H.C.; et al. Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in Post COVID Syndrome correlate with symptom severity. Front. Immunol. 2022, 13, 981532. [Google Scholar] [CrossRef]
- Proal, A.D.; Aleman, S.; Bomsel, M.; Brodin, P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; Deeks, S.G.; et al. Targeting the SARS-CoV-2 reservoir in long COVID. Lancet Infect. Dis. 2025, 25, E294–E306. [Google Scholar] [CrossRef]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Furst, J.; Schulze-Rothe, S.; Wallukat, A.; Honicke, A.S.; Muller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Junemann, A.; Hohberger, B.; Rech, J.; Sheriff, A.; Fu, Q.; Schlotzer-Schrehardt, U.; Voll, R.E.; Bartel, S.; Kalbacher, H.; Hoebeke, J.; et al. Agonistic Autoantibodies to the beta2-Adrenergic Receptor Involved in the Pathogenesis of Open-Angle Glaucoma. Front. Immunol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Wallukat, G.; Muller, J.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.J.; Vetter, R.; Salzsieder, E.; Kreutz, R.; Schimke, I. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 2016, 244, 44–47. [Google Scholar] [CrossRef]
- Ferrari, I.; Levin, M.J.; Wallukat, G.; Elies, R.; Lebesgue, D.; Chiale, P.; Elizari, M.; Rosenbaum, M.; Hoebeke, J. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J. Exp. Med. 1995, 182, 59–65. [Google Scholar] [CrossRef]
- Wallukat, G.; Morwinski, M.; Kowal, K.; Forster, A.; Boewer, V.; Wollenberger, A. Autoantibodies against the beta-adrenergic receptor in human myocarditis and dilated cardiomyopathy: Beta-adrenergic agonism without desensitization. Eur. Heart J. 1991, 12 (Suppl. D), 178–181. [Google Scholar] [CrossRef]
- Liao, Y.H.; Wei, Y.M.; Wang, M.; Wang, Z.H.; Yuan, H.T.; Cheng, L.X. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertens. Res. 2002, 25, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Gunning, W.T.; Kvale, H.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Postural Orthostatic Tachycardia Syndrome Is Associated with Elevated G-Protein Coupled Receptor Autoantibodies. J. Am. Heart Assoc. 2019, 8, e013602. [Google Scholar] [CrossRef]
- Wallukat, G.; Pruss, H.; Muller, J.; Schimke, I. Functional autoantibodies in patients with different forms of dementia. PLoS ONE 2018, 13, e0192778. [Google Scholar] [CrossRef]
- Wallukat, G.; Wollenberger, A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed. Biochim. Acta 1987, 46, S634–S639. [Google Scholar]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, O.; et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Szewczykowski, C.; Mardin, C.; Lucio, M.; Wallukat, G.; Hoffmanns, J.; Schröder, T.; Raith, F.; Rogge, L.; Heltmann, F.; Moritz, M.; et al. Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int. J. Mol. Sci. 2022, 23, 7209. [Google Scholar] [CrossRef]
- Kharraziha, I.; Axelsson, J.; Ricci, F.; Di Martino, G.; Persson, M.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome. J. Am. Heart Assoc. 2020, 9, e015989. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Floreani, A.; Leung, P.S.; Gershwin, M.E. Environmental Basis of Autoimmunity. Clin. Rev. Allergy Immunol. 2016, 50, 287–300. [Google Scholar] [CrossRef]
- Wong, F.S.; Wen, L. Innate and adaptive immune responses are highly interconnected at many levels. Curr. Mol. Med. 2009, 9, 1–3. [Google Scholar] [CrossRef]
- Smith, B.R.; Pyle, G.A.; Petersen, V.B.; Hall, R. Interaction of thyroid-stimulating antibodies with the human thyrotrophin receptor. J. Endocrinol. 1977, 75, 401–407. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Cossio, P.M.; Gimeno, M.F.; Gimeno, A.L.; Diez, C.; Laguens, R.P.; Meckert, P.C.; Arana, R.M. Effect of chagasic sera on the rat isolated atrial preparation: Immunological, morphological and function aspects. Cardiovasc. Res. 1976, 10, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Borda, E.; Pascual, J.; Cossio, P.; De La Vega, M.; Arana, R.; Sterin-Borda, L. A circulating IgG in Chagas’ disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin. Exp. Immunol. 1984, 57, 679–686. [Google Scholar] [PubMed]
- Becker, N.P.; Goettel, P.; Mueller, J.; Wallukat, G.; Schimke, I. Functional autoantibody diseases: Basics and treatment related to cardiomyopathies. Front. Biosci. 2019, 24, 48–95. [Google Scholar] [CrossRef]
- Haberland, A.; Holtzhauer, M.; Schlichtiger, A.; Bartel, S.; Schimke, I.; Muller, J.; Dandel, M.; Luppa, P.B.; Wallukat, G. Aptamer BC 007—A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur. J. Pharmacol. 2016, 789, 37–45. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Liles, C.; Khan, M.; Vanderlinde-Wood, M.; Galloway, A.; Zillner, C.; Benbrook, A.; Reim, S.; Collier, D.; et al. Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 2014, 3, e000755. [Google Scholar] [CrossRef]
- Fedorowski, A.; Li, H.; Yu, X.; Koelsch, K.A.; Harris, V.M.; Liles, C.; Murphy, T.A.; Quadri, S.M.S.; Scofield, R.H.; Sutton, R.; et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2017, 19, 1211–1219. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Murphy, T.A.; Nuss, Z.; Liles, J.; Liles, C.; Aston, C.E.; Raj, S.R.; Fedorowski, A.; Kem, D.C. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J. Am. Heart Assoc. 2018, 7, e008351. [Google Scholar] [CrossRef]
- Wallukat, G.; Munoz Saravia, S.G.; Haberland, A.; Bartel, S.; Araujo, R.; Valda, G.; Duchen, D.; Diaz Ramirez, I.; Borges, A.C.; Schimke, I. Distinct patterns of autoantibodies against G-protein-coupled receptors in Chagas’ cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J. Am. Coll. Cardiol. 2010, 55, 463–468. [Google Scholar] [CrossRef]
- Lee, H.C.; Huang, K.T.; Wang, X.L.; Shen, W.K. Autoantibodies and cardiac arrhythmias. Heart Rhythm 2011, 8, 1788–1795. [Google Scholar] [CrossRef]
- Hendrickson, J.E.; Hendrickson, E.T.; Gehrie, E.A.; Sidhu, D.; Wallukat, G.; Schimke, I.; Tormey, C.A. Complex regional pain syndrome and dysautonomia in a 14-year-old girl responsive to therapeutic plasma exchange. J. Clin. Apher. 2016, 31, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Klein-Weigel, P.F.; Bimmler, M.; Hempel, P.; Schopp, S.; Dreusicke, S.; Valerius, J.; Bohlen, A.; Boehnlein, J.M.; Bestler, D.; Funk, S.; et al. G-protein coupled receptor auto-antibodies in thromboangiitis obliterans (Buerger’s disease) and their removal by immunoadsorption. Vasa 2014, 43, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.L.M.; Filgueiras, I.S.; Marques, A.H.C.; Vojdani, E.; Halpert, G.; Ostrinski, Y.; Baiocchi, G.C.; Placa, D.R.; Freire, P.P.; Pour, S.Z.; et al. Severe COVID-19 patients exhibit elevated levels of autoantibodies targeting cardiolipin and platelet glycoprotein with age: A systems biology approach. npj Aging 2023, 9, 21. [Google Scholar] [CrossRef]
- Miranda, M.; Avila, I.; Esparza, J.; Shwartz, Y.; Hsu, Y.C.; Berdeaux, R.; Lowry, W.E. Defining a Role for G-Protein Coupled Receptor/cAMP/CRE-Binding Protein Signaling in Hair Follicle Stem Cell Activation. J. Investig. Dermatol. 2022, 142, 53–64.e3. [Google Scholar] [CrossRef]
- Tammaro, A.; Adebanjo, G.A.R.; Parisella, F.R.; Luzi, F.; Scarabello, A. Hair and nail manifestations of COVID-19. J. Cosmet. Dermatol. 2022, 21, 1339–1346. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat. Commun. 2022, 13, 1220. [Google Scholar] [CrossRef]
- Jiang, Y.; Duffy, F.; Hadlock, J.; Raappana, A.; Styrchak, S.; Beck, I.; Mast, F.D.; Miller, L.R.; Chour, W.; Houck, J.; et al. Angiotensin II receptor I auto-antibodies following SARS-CoV-2 infection. PLoS ONE 2021, 16, e0259902. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Akbari, A.; Hadizadeh, A.; Islampanah, M.; Salavati Nik, E.; Atkin, S.L.; Sahebkar, A. COVID-19, G protein-coupled receptor, and renin-angiotensin system autoantibodies: Systematic review and meta-analysis. Autoimmun. Rev. 2023, 22, 103402. [Google Scholar] [CrossRef]
- Schlick, S.; Lucio, M.; Wallukat, G.; Bartsch, A.; Skornia, A.; Hoffmanns, J.; Szewczykowski, C.; Schröder, T.; Raith, F.; Rogge, L.; et al. Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int. J. Mol. Sci. 2022, 23, 13683. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front. Med. 2021, 8, 754667. [Google Scholar] [CrossRef] [PubMed]
- Dechend, R.; Viedt, C.; Muller, D.N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Virag, L.; Szabo, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef]
- Ganzinelli, S.; Borda, E.; Joensen, L.; Sterin-Borda, L. Chagasic antibodies induce cardiac COX-2/iNOS mRNA expression with PGE2/NO production. Int. J. Cardiol. 2009, 134, 212–223. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Guideri, F.; Acampa, M.; Selvi, E.; Bisogno, S.; Galeazzi, M.; Laghi-Pasini, F. Autoantibody-mediated cardiac arrhythmias: Mechanisms and clinical implications. Basic. Res. Cardiol. 2008, 103, 1–11. [Google Scholar] [CrossRef]
- Wallukat, G.; Schimke, I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin. Immunopathol. 2014, 36, 351–363. [Google Scholar] [CrossRef]
- Haberland, A.; Santos, R.A.; Schimke, I.; Wallukat, G. Are Agonistic Autoantibodies against G-Protein Coupled Receptors Involved in the Development of Long-Term Side Effects of Tumor Chemotherapy? Case Rep. Oncol. 2013, 6, 104–108. [Google Scholar] [CrossRef]
- Wallukat, G.; Wollenberger, A. [Cultivated cardiac muscle cells--a functional test system for the detection of autoantibodies against the beta-adrenergic receptor]. Acta Histochem. Suppl. 1988, 35, 145–149. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, S.; Lucio, M.; Wallukat, G.; Hoffmanns, J.; Schröder, T.; Raith, F.; Szewczykowski, C.; Skornia, A.; Rech, J.; Schottenhamml, J.; et al. Functional Autoantibodies Targeting G-Protein-Coupled Receptors and Their Clinical Phenotype in Patients with Long-COVID. Int. J. Mol. Sci. 2025, 26, 6746. https://doi.org/10.3390/ijms26146746
Hofmann S, Lucio M, Wallukat G, Hoffmanns J, Schröder T, Raith F, Szewczykowski C, Skornia A, Rech J, Schottenhamml J, et al. Functional Autoantibodies Targeting G-Protein-Coupled Receptors and Their Clinical Phenotype in Patients with Long-COVID. International Journal of Molecular Sciences. 2025; 26(14):6746. https://doi.org/10.3390/ijms26146746
Chicago/Turabian StyleHofmann, Sophia, Marianna Lucio, Gerd Wallukat, Jakob Hoffmanns, Thora Schröder, Franziska Raith, Charlotte Szewczykowski, Adam Skornia, Juergen Rech, Julia Schottenhamml, and et al. 2025. "Functional Autoantibodies Targeting G-Protein-Coupled Receptors and Their Clinical Phenotype in Patients with Long-COVID" International Journal of Molecular Sciences 26, no. 14: 6746. https://doi.org/10.3390/ijms26146746
APA StyleHofmann, S., Lucio, M., Wallukat, G., Hoffmanns, J., Schröder, T., Raith, F., Szewczykowski, C., Skornia, A., Rech, J., Schottenhamml, J., Harrer, T., Ganslmayer, M., Mardin, C., Flecks, M., Lakatos, P., & Hohberger, B. (2025). Functional Autoantibodies Targeting G-Protein-Coupled Receptors and Their Clinical Phenotype in Patients with Long-COVID. International Journal of Molecular Sciences, 26(14), 6746. https://doi.org/10.3390/ijms26146746






