Butyrate Produced by Gut Microbiota Regulates Atherosclerosis: A Narrative Review of the Latest Findings
Abstract
1. Introduction
2. Degradation of Carbohydrates and Production of Butyrate in the Large Intestinal Tract
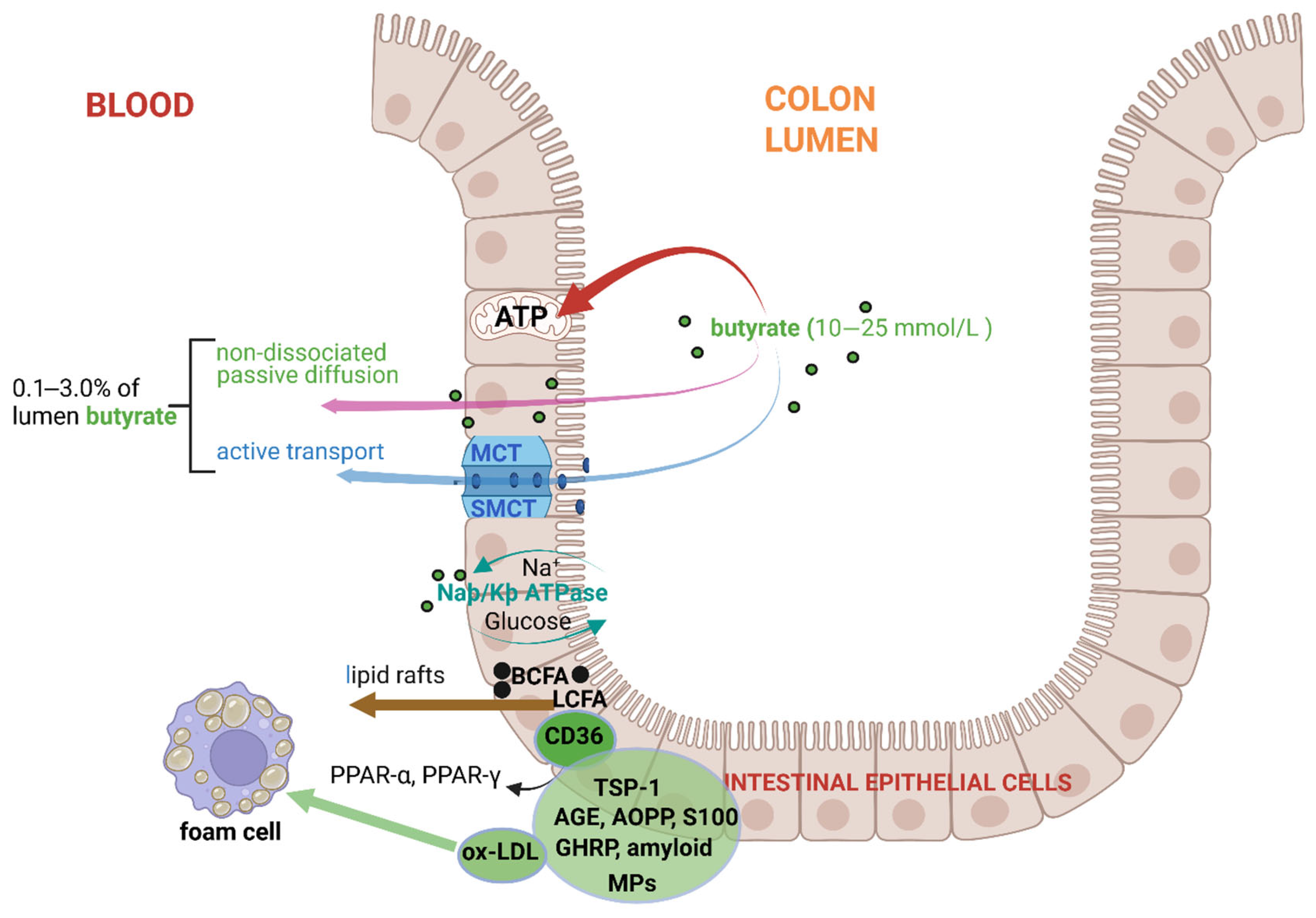
3. Absorption and Uptake of Butyrate by Colonic Intestinal Epithelial Cells (IECs)
4. Transport of Butyrate Across Arterial and Myocardial Endothelia
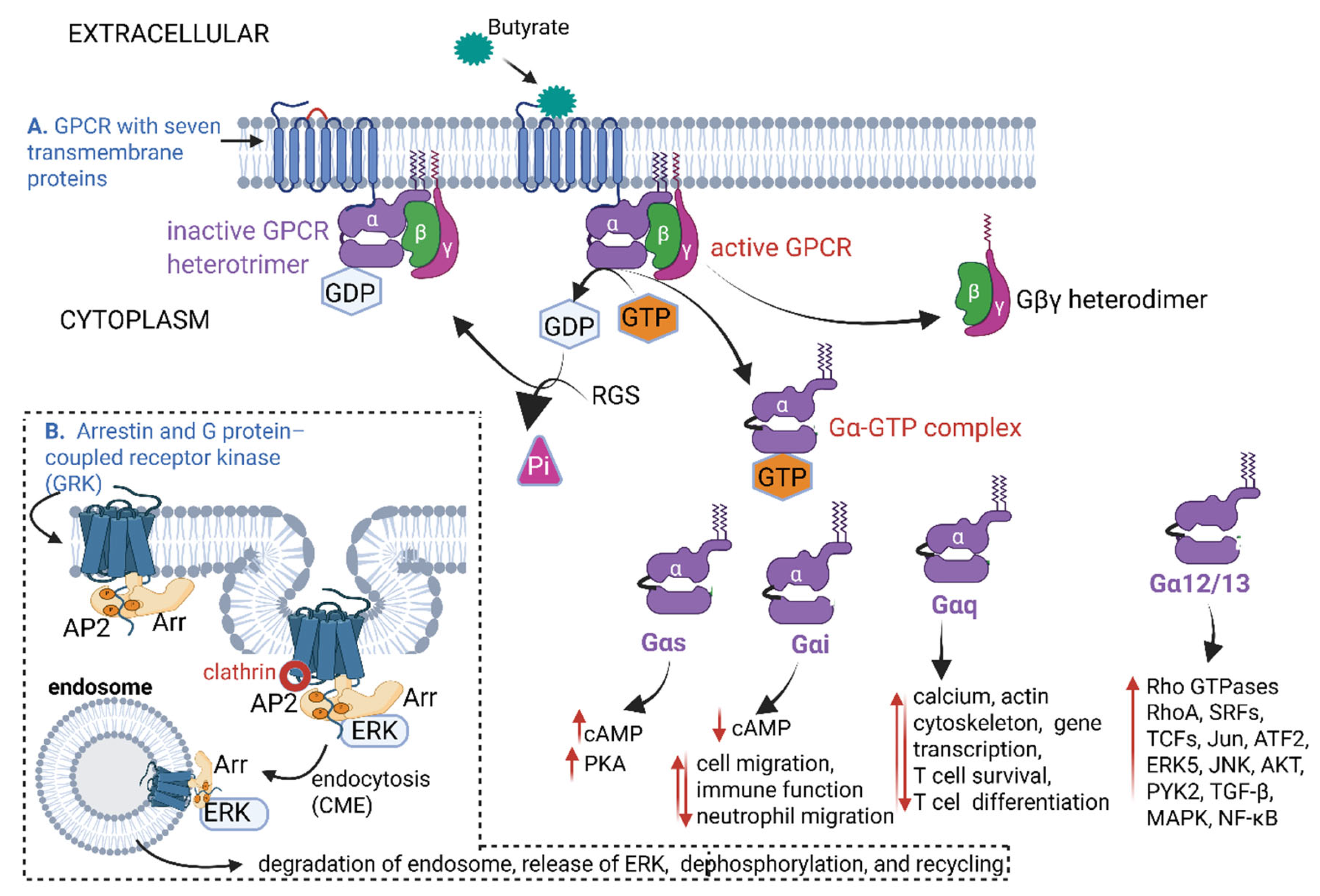
5. Butyrate Interactions with G-Protein-Coupled Receptors (GPCRs)
6. The Role of Nuclear Factor Kappa-B (NF-κB) in Atherosclerosis
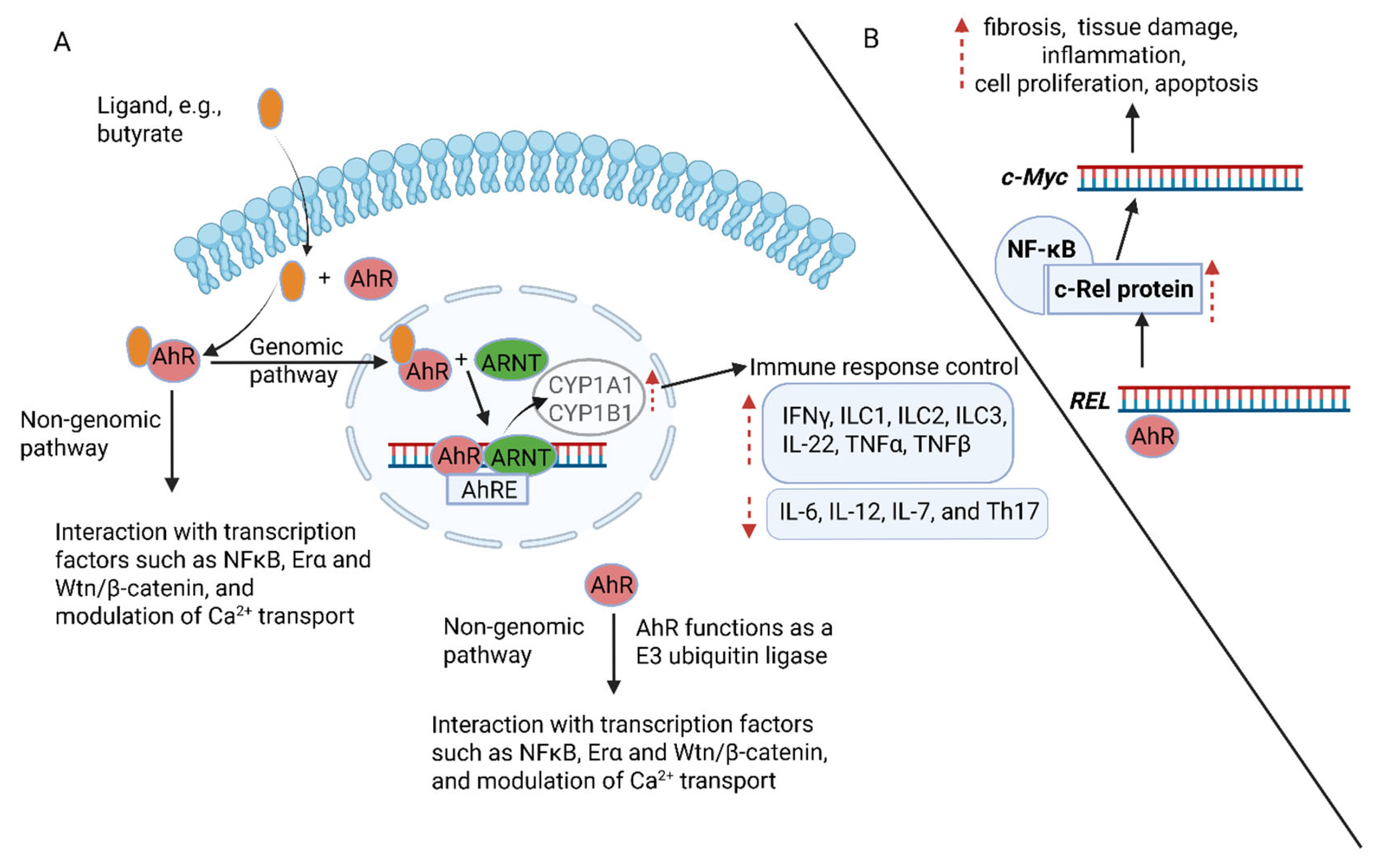
7. Activation of Aryl Hydrocarbon Receptor (AhR) Proteins
8. Restriction of Blood Flow
9. Conclusions
Funding
Conflicts of Interest
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The heart of the world. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Rosendaal, F.R.; Weitz, J.I.; Jaffer, I.H.; Agnelli, G.; Baglin, T.; Mackman, N. Venous thrombosis. Nat. Rev. Dis. Prim. 2015, 1, 15006. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Traghella, I.; Mastorci, F.; Alessia, P.; Pingitore, A.; Vassalle, C. Nontraditional cardiovascular biomarkers and risk factors: Rationale and future perspectives. Biomolecules 2018, 8, 40. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoǧlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M. Circulating isoprostanes: Gatekeepers in the route from oxidative stress to vascular dysfunction. Circ. Res. 2008, 103, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Ramsay, M.; Crowther, N.; Tambo, E.; Agongo, G.; Baloyi, V.; Dikotope, S.; Gómez-Olivé, X.; Jaff, N.; Sorgho, H.; Wagner, R.; et al. H3Africa AWI-Gen Collaborative Centre: A resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob. Health Epidemiol. Genom. 2016, 1, e20. [Google Scholar] [CrossRef]
- Oduaran, O.H.; Tamburini, F.B.; Sahibdeen, V.; Brewster, R.; Gómez-Olivé, F.X.; Kahn, K.; Norris, S.A.; Tollman, S.M.; Twine, R.; Wade, A.N.; et al. Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microb. 2020, 20, 330. [Google Scholar] [CrossRef]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014, 36, 940–949. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2597. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean diet as an antioxidant: The impact on metabolic health and overall wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American gut: An open platform for citizen science microbiome research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef]
- Amiri, P.; Hosseini, S.A.; Ghaffari, S.; Tutunchi, H.; Ghaffari, S.; Mosharkesh, E.; Asghari, S.; Roshanravan, N. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: A comprehensive narrative review. Front. Pharmacol. 2022, 12, 837509. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Belt, S.C.; Bakker, B.M.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N. Beyond butyrate: Microbial fiber metabolism supporting colonic epithelial homeostasis. Trends Microbiol. 2024, 32, 178–189. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Suster, M.S.; Borges, J.I. Short-chain fatty acid receptors and cardiovascular function. Int. J. Mol. Sci. 2022, 23, 3303. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, Z.; Li, Z.; Ling, H.; Song, C. Role and mechanism of action of butyrate in atherosclerotic diseases: A review. J. Appl. Microbiol. 2020, 131, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.C.; Da Silva, J.F.; Navia-Pelaez, J.M.; Leonel, A.J.; Lopes, L.G.; Menezes-Garcia, Z.; Ferreira, A.V.M.; Capettini, L.d.S.A.; Teixeira, L.G.; Lemos, V.S.; et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR-γ in obese Apo E knockout mice. Nutrition 2018, 47, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Oh, H.Y.P.; Visvalingam, V.; Wahli, W. The PPAR-microbiota-metabolic organ trilogy to fine-tune physiology. FASEB J. 2019, 33, 9706–9730. [Google Scholar] [CrossRef]
- Dąbek, J.; Kułach, A.; Gąsior, Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): A new potential therapeutic target in atherosclerosis. Pharmacol. Rep. 2010, 62, 778–783. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The role of KLF2 in the regulation of atherosclerosis development and potential use of KLF2-targeted therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef]
- Gan, J.; Guo, L.; Zhang, X.; Yu, Q.; Yang, Q.; Zhang, Y.; Zeng, W.; Jiang, X.; Guo, M. Anti-inflammatory therapy of atherosclerosis: Focusing on IKKβ. J. Inflamm. 2023, 20, 8. [Google Scholar] [CrossRef]
- Wilson, S.H.; Caplice, N.M.; Simari, R.D.; Holmes, D.R., Jr.; Carlson, P.J.; Lerman, A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis 2000, 148, 23–30. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef]
- Stump, M.; Mukohda, M.; Hu, C.; Sigmund, C.D. PPARγ regulation in hypertension and metabolic syndrome. Curr. Hypertens. Rep. 2015, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.P.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef]
- Ding, Y.; Kang, J.; Liu, S.; Xu, Y.; Shao, B. The protective effects of peroxisome proliferator-activated receptor gamma in cerebral ischemia-reperfusion injury. Front. Neurol. 2020, 11, 588516. [Google Scholar] [CrossRef]
- Brand, K.; Page, S.; Rogler, G.; Bartsch, A.; Brandl, R.; Knuechel, R.; Page, M.; Kaltschmidt, C.; Baeurerle, P.A.; Neumeier, D. Activated transcription factor nuclear factor–kappa B is present in the atherosclerotic lesion. J. Clin. Investig. 1996, 97, 1715–1722. [Google Scholar] [CrossRef]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A necessary but harmful strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef]
- Liu, Y.; Miller, A.R. Ligands to peroxisome proliferator-activated receptors as therapeutic options for metabolic syndrome. Drug Discov. Today Ther. Strateg. 2005, 2, 165–169. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, V.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Corner, G.A.; Augenlicht, L.H. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: Comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000, 60, 4561–4572. [Google Scholar] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Lee, E.-J.; Lee, J.-C.; Kim, W.-K.; Kim, H.-S. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-κB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, M.; Zhang, M.; Xiao, M.; Li, X. Butyrate mitigates TNF-a-induced attachment of monocytes to endothelial cells. J. Bioenerg. Biomembr. 2020, 52, 247–256. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Wijdeveld, M.; Wortelboer, K.; Rampanelli, E.; Levels, J.H.M.; Collard, D.; Cammenga, M.; Nageswaran, V.; Haghikia, A.; Landmesser, U.; et al. Effects of oral butyrate on blood pressure in patients with hypertension: A randomized, placebo-controlled trial. Hypertension 2024, 81, 2124–2136. [Google Scholar] [CrossRef]
- Gee, T.; Farrar, E.; Wang, Y.; Wu, B.; Hsu, K.; Zhou, B.A.; Butcher, J. NFκB (nuclear factor κ-light-chain enhancer of activated B cells) activity regulates cell-type-specific and context-specific susceptibility to calcification in the aortic valve. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 638–655. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- De Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of human salivary glands: Highlights of morphology and developing processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef]
- Barboza, P.S.; Parker, K.L. Carbohydrates: Sugars, fiber and fermentation. In Integrative Wildlife Nutrition; Barboza, P.S., Parker, K.L., Hume, I.D., Eds.; Springer: Berlin, Germany, 2009; pp. 97–118. [Google Scholar]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Layec, S.; Auger, S.; Juste, C.; Henry, C.; Charif, S.; Jaszczyszyn, Y.; Sokol, H.; Beney, L.; Langella, P.; et al. Faecalibacterium duncaniae A2-165 regulates the expression of butyrate synthesis, ferrous iron uptake, and stress-response genes based on acetate consumption. Sci. Rep. 2024, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Igbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Wen, J.; Rawls, J.F. Feeling the burn: Intestinal epithelial cells modify their lipid metabolism in response to bacterial fermentation products. Cell Host Microbe 2020, 27, 314–316. [Google Scholar] [CrossRef]
- Ko, C.-W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, J.W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Schmitt, M.G., Jr.; Soergel, K.H.; Wood, C.M.; Steff, J.J. Absorption of short-chain fatty acids from the human ileum. Am. J. Dig. Dis. 1977, 22, 340–347. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front. Microbiol. 2023, 14, 1244293. [Google Scholar] [CrossRef]
- Saunders, D.R. Absorption of short-chain fatty acids in human stomach and rectum. Nutr. Res. 1991, 11, 841–847. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Mackay, W.; Edwards, C.; Preston, T.; Dodson, B.; Weaver, L. Butyrate production from oligofructose fermentation by the human faecal flora: What is the contribution of extracellular acetate and lactate? Br. J. Nutr. 2006, 96, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.; Brennan, E.A.; Farthing, M.J.; Fairclough, P.D. Acetate uptake by intestinal brush border membrane vesicles. Gut 1991, 32, 383–385. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef]
- Jaskiewicz, J.; Zhao, Y.; Hawes, J.W.; Shimomura, Y.; Crabb, D.W.; Harris, R.A. Catabolism of isobutyrate by colonocytes. Arch. Biochem. Biophys. 1996, 327, 265–270. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; De los Reyes-Gavilán, C.G. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: Associated dietary and anthropometric factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-chain fatty acids—An underexplored class of dairy-derived fatty acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Küsel, K.; Matthies, C. Acetogenic prokaryotes. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 354–420. [Google Scholar]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; De Waard, P.; Plugge, C.M.; De Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, K.C.W.; Van der Zande, M.; Bruyneel, B.; Vervoort, J.J.M.; Ivonne, M.; Rietjens, C.M.; Belzer, C.; Beekmann, K. An in vitro model for microbial fructoselysine degradation shows substantial interindividual differences in metabolic capacities of human fecal slurries. Toxicol. In Vitro 2021, 72, 105078. [Google Scholar] [CrossRef]
- Shetty, S.A.; Kuipers, B.; Atashgahi, S.; Aalvink, S.; Smidt, H.; De Vos, W.M. Inter-species metabolic interactions in an in-vitro minimal human gut microbiome of core bacteria. npj Biofilms Microbiomes 2022, 8, 21. [Google Scholar]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojarvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Pham, V.T.; Lacroix, C.; Braegger, C.P.; Chassard, C. Lactate-utilizing community is associated with gut microbiota dysbiosis in colicky infants. Sci. Rep. 2017, 7, 11176. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Interactions and competition within the microbial community of the human colon: Links between diet and health. Environ. Microbiol. 2007, 9, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate properties in immune-related diseases: Friend or foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986, 261, 4889–4894. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Van Nguyen, C. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 2006, 50, 1140–1149. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The role of dietary advanced glycation end products in metabolic dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Fogliano, V. Dietary advanced glycosylation end-products (dAGEs) and melanoidins formed through the Maillard reaction: Physiological consequences of their intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Zhang, R.; Liu, G.; Xiao, S.; Qiao, X.; Wu, Y.; Gong, Z. Toxicological evaluation of advanced glycation end product Nε-(carboxymethyl)lysine: Acute and subacute oral toxicity studies. Regul. Toxicol. Pharmacol. 2016, 77, 65–74. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Ruas-Madiedo, P.; Kolida, S.; Collins, M.; Rastall, R.; Gibson, G.; De Los Reyes-Gavilán, C.G. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int. J. Food Microbiol. 2009, 135, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, G.; Zhu, L.; Yin, Y.; Zhao, X.; Xiang, C.; Yu, G.; Wang, X. Isolation and characterization of an agaro-oligosaccharide (AO)-hydrolyzing bacterium from the gut microflora of Chinese individuals. PLoS ONE 2024, 9, e91106. [Google Scholar] [CrossRef] [PubMed]
- Ruppin, H.; Bar-Meir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G., Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [CrossRef]
- Rechkemmer, G.; Von Engelhardt, W. Concentration- and pH-dependence of short-chain fatty acid absorption in the proximal and distal colon of guinea pig (Cavia porcellus). Comp. Biochem. Physiol. Part A Mol. Comp. Physiol. 1988, 91, 659–663. [Google Scholar] [CrossRef]
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J. Physiol. 1998, 513, 719–732. [Google Scholar] [CrossRef]
- Miyauchi, S.; Gopal, E.; Fei, Y.-J.; Ganapathy, V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J. Biol. Chem. 2004, 279, 13293–13296. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Gill, R.K.; Saksena, S.; Alrefai, W.A.; Sarwar, Z.; Goldstein, J.L.; Carroll, R.E.; Ramaswamy, K.; Dudeja, P.K. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am. J. Physiol. Cell Physiol. 2005, 289, 846–852. [Google Scholar] [CrossRef]
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Y.; Huang, R.; Wang, F.; Xie, J.; Liu, H.; Wang, Y.; Wang, Y.; Luo, S.; Hu, D. The role of short-chain fatty acids in myocardial ischemia-reperfusion injury. Curr. Nutr. Rep. 2024, 13, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Regen, S.L. The origin of lipid rafts. Biochemistry 2020, 59, 4617–4621. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How important are fatty acids in human health and can they be used in treating diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Hanayama, M.; Yamamoto, Y.; Utsunomiya, H.; Yoshida, O.; Liu, S.; Mogi, M.; Matsuura, B.; Takeshita, E.; Ikeda, Y.; Hiasa, Y. The mechanism of increased intestinal palmitic acid absorption and its impact on hepatic stellate cell activation in nonalcoholic steatohepatitis. Sci. Rep. 2021, 11, 13380. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Ashaq, M.S.; Zhang, S.; Xu, M.; Li, Y.; Zhao, B. The regulatory role of CD36 in hematopoiesis beyond fatty acid uptake. Life Sci. 2024, 339, 122–442. [Google Scholar] [CrossRef]
- Vetter, S.W. Chapter Five: Glycated serum albumin and AGE receptors. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Waltham, MA, USA, 2015; Volume 72, pp. 205–275. [Google Scholar]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Seefeldt, J.M.; Homilius, C.; Hansen, J.; Lassen, T.R.; Jespersen, N.R.; Jensen, R.V.; Boedtkjer, E.; Bøtker, H.E.; Nielsen, R. Short-chain fatty acid butyrate is an inotropic agent with vasorelaxant and cardioprotective properties. J. Am. Heart Assoc. 2024, 13, e033744. [Google Scholar] [CrossRef]
- Wissel, E.F.; Chien, H.Y.; Wei, K.-H.; Lee, Y.-C.; Ullah, K.; Hsieh, P.C.H. Microbial metabolites associated in stool and left ventricle of heart failure patients revealed by meta-analysis. Sci. Rep. 2025, 15, 14576. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.T.; Dyck, J.R.B. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim. Biophys. Acta 2016, 1861, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Byrne, N.J.; Kim, T.T.; Levasseur, J.; Masson, G.; Boisvenue, J.J.; Febbraio, M.; Dyck, J.R.B. Cardiomyocyte-specific ablation of CD36 accelerates the progression from compensated cardiac hypertrophy to heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H552–H560. [Google Scholar] [CrossRef]
- Abumrad, N.A.; Goldberg, I.J. CD36 actions in the heart: Lipids, calcium, inflammation, repair and more? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1442–1449. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Pyrkowska, A.; Duda, M.K.; Bednarski, T.; Maczewski, M.; Langfort, J.; Dobrzyn, A. Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1348–E1358. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef]
- Ohgami, N.; Nagai, R.; Ikemoto, M.; Arai, H.; Kuniyasu, A.; Horiuchi, S.; Nakayama, H. Cd36, a member of the class b scavenger receptor family, as a receptor for advanced glycation end products. J. Biol. Chem. 2001, 276, 3195–3202. [Google Scholar] [CrossRef]
- Meng, J.; Sakata, N.; Takebayashi, S.; Asano, T.; Futata, T.; Araki, N.; Horiuchi, S. Advanced glycation end products of the Maillard reaction in aortic pepsin-insoluble and pepsin-soluble collagen from diabetic rats. Diabetes 1996, 45, 1037–1043. [Google Scholar] [CrossRef]
- Mori, T.; Takahashi, K.; Higashi, T.; Takeya, M.; Kume, S.; Kawabe, Y.; Kodama, T.; Horiuchi, S. Localization of advanced glycation end products of Maillard reaction in bovine tissues and their endocytosis by macrophage scavenger receptors. Exp. Mol. Pathol. 1995, 63, 135–152. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Kaur, G.; Verma, S.K.; Singh, D.; Singh, N.K. Role of G-proteins and GPCRs in cardiovascular pathologies. Bioengineering 2023, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.R.; Hauser, A.S.; Vedel, L.; Strachan, R.T.; Huang, X.-P.; Gavin, A.C.; Shah, S.D.; Nayak, A.P.; Haugaard-Kedström, L.M.; Penn, R.B.; et al. Discovery of human signaling systems: Pairing peptides to G protein-coupled receptors. Cell 2019, 179, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.M.; Mai, T.L.; Chen, C.M. Visualizing the GPCR network: Classification and evolution. Sci. Rep. 2017, 7, 15495. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7. [Google Scholar] [CrossRef]
- Birch, C.A.; Molinar-Inglis, O.; Trejo, J. Subcellular hot spots of GPCR signaling promote vascular inflammation. Curr. Opin. Endocr. Metab. Res. 2021, 16, 37–42. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharm. 2013, 13, 935–940. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, M.-T.; Han, J.-H. GPR41 and GPR43: From development to metabolic regulation. Biomed. Pharmacother. 2024, 175, 116735. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Kuhnert, F.; Mancuso, M.R.; Shamloo, A.; Wang, H.T.; Choksi, V.; Florek, M.; Su, H.; Fruttiger, M.; Young, W.L.; Heilshorn, S.C.; et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 2010, 330, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; Spohr, T.C.L.d.S.; Do Amaral, R.F.; Da Fonseca, A.C.C.; Garcia, C.; Mendes, F.d.A.; Freitas, C.; DosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Sig. Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Kots, A.Y.; Gumanova, N.G.; Akhmedzhanov, N.M.; Varentsov, S.I.; Gerasimova, C.I.; Bulargina, T.V.; Shakhov, Y.A. The GTP-binding regulatory proteins, Gs and Gi, are altered in erythrocyte membranes of patients with ischemic heart disease resulting from coronary atherosclerosis. Arterioscler. Thromb. 1993, 13, 1244–1251. [Google Scholar] [CrossRef]
- Tuteja, N. Signaling through G protein coupled receptors. Plant Signal. Behav. 2009, 4, 942–947. [Google Scholar] [CrossRef]
- Kee, T.R.; Khan, S.A.; Neidhart, M.B.; Masters, B.M.; Zhao, V.K.; Kim, Y.K.; Kyle, C.; McGill, P.; Woo, J.-A.A. The multifaceted functions of β-arrestins and their therapeutic potential in neurodegenerative diseases. Exp. Mol. Med. 2024, 56, 129–141. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Min, J.K.; Kim, J.H.; Yamagishi, A.; Mochizuki, N.; Kwon, J.Y.; Park, Y.W.; Kim, Y.M.; Kwon, Y.G. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal. 2006, 18, 994–1005. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, L.; Peng, Y. The structural basis of the G protein-coupled receptor and ion channel axis. Curr. Res. Struct. Biol. 2025, 9, 100165. [Google Scholar] [CrossRef]
- Weis, W.I.; Kobilka, B.K. The molecular basis of G protein–coupled receptor activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef]
- Oakley, R.H.; Laporte, S.A.; Holt, J.A.; Caron, M.G.; Barak, L.S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000, 275, 17201–17210. [Google Scholar] [CrossRef]
- Sanni, S.J.; Hansen, J.T.; Bonde, M.M.; Speerschneider, T.; Christensen, G.L.; Munk, S.; Gammeltoft, S.; Hansen, J.L. ß-arrestin 1 and 2 stabilize the angiotensin II type I receptor in distinct high-affinity conformations. Br. J. Pharmacol. 2010, 161, 150–161. [Google Scholar] [CrossRef]
- Jones, K.T.; Echeverry, M.; Mosser, V.A.; Gates, A.; Jackson, D.A. Agonist mediated internalization of M2 mAChR is ß-arrestin-dependent. J. Mol. Signal. 2006, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Haider, R.S.; Matthees, E.S.F.; Drube, J.; Reichel, M.; Zabel, U.; Inoue, A.; Chevigné, A.; Krasel, C.; Deupi, X.; Hoffmann, C. ß-arrestin1 and 2 exhibit distinct phosphorylation-dependent conformations when coupling to the same GPCR in living cells. Nat. Commun. 2022, 13, 5638. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yu, Y.R.; Badea, C.T.; Kovacs, J.J.; Xiong, X.; Comhair, S.; Piantadosi, C.A.; Rajagopal, S. Vascular endothelial growth factor receptor 3 regulates endothelial function through ß-arrestin 1. Circulation 2019, 139, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Alamanda, V.; Singh, S.; Lawrence, N.J.; Chellappan, S.P. Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity. Biochem. Biophys. Res. Commun. 2012, 418, 56–61. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Liu, M.-Z.; Zhu, D.-N.; Yan, H.; Ke, P.; Wei, W.; Han, T.; Liu, C. Depletion of β-arrestin-1 in macrophages enhances atherosclerosis in ApoE−/− mice. Int. Immunopharmacol. 2023, 125, 111085. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Pulliam, S.R.; Pellom, S.T.; Shanker, A.; Adunyah, S.E. Butyrate regulates the expression of inflammatory and chemotactic cytokines in human acute leukemic cells during apoptosis. Cytokine 2016, 84, 74–87. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, W.L.; Xu, A.M. Cholesterol metabolism in tumor microenvironment: Cancer hallmarks and therapeutic opportunities. Int. J. Biol. Sci. 2024, 20, 2044–2071. [Google Scholar] [CrossRef]
- Tang, J.; Zhan, M.N.; Yin, Q.Q.; Zhou, C.X.; Wang, C.L.; Wo, L.L.; He, M.; Chen, G.Q.; Zhao, Q. Impaired p65 degradation by decreased chaperone-mediated autophagy activity facilitates epithelial-to-mesenchymal transition. Oncogenesis 2017, 6, e387. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Sig. Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Klymkowsky, M.W. Unexpected functional redundancy between Twist and Slug (Snail2) and their feedback regulation of NF-κB via Nodal and Cerberus. Dev. Biol. 2009, 331, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, Z.; Bischoff, J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [Google Scholar] [CrossRef]
- Jackson, A.O.; Regine, M.A.; Subrata, C.; Long, S. Molecular mechanisms and genetic regulation in atherosclerosis. Int. J. Cardiol. Heart Vasc. 2018, 21, 36–44. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiroi, T.; Ishii, M.; Hagiwara, T.; Wajima, T.; Miyazaki, A.; Kiuchi, Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through activation of the Jak2 tyrosine kinase pathway in vascular endothelial cells. Int. J. Biochem. Cell Biol. 2008, 40, 755–765. [Google Scholar] [CrossRef]
- Hernandez-Navarro, I.; Botana, L.; Diez-Mata, J.; Tesoro, L.; Jimenez-Guirado, B.; Gonzalez-Cucharero, C.; Alcharani, N.; Zamorano, J.L.; Saura, M.; Zaragoza, C. Replicative endothelial cell senescence may lead to endothelial dysfunction by increasing the BH2/BH4 ratio induced by oxidative stress, reducing BH4 availability, and decreasing the expression of eNOS. Int. J. Mol. Sci. 2024, 25, 9890. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Wang, W.; Diamond, S.L. Does elevated nitric oxide production enhance the release of prostacyclin from shear stressed aortic endothelial cells? Biochem. Biophys. Res. Commun. 1997, 233, 748–751. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef]
- Pan, S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid. Redox Signal. 2009, 11, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.M.; Leszczynska-Piziak, J.; Abramson, S.B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Investig. 1992, 90, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Qin, L.; Baeyens, N. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef] [PubMed]
- Bouly, M.; Bourguignon, M.P.; Roesch, S.; Rigouin, P.; Gosgnach, W.; Bossard, E.; Royere, E.; Diguet, N.; Sansilvestri-Morel, P.; Bonnin, A.; et al. Aging increases circulating BH2 without modifying BH4 levels and impairs peripheral vascular function in healthy adults. Transl. Res. 2021, 238, 36–48. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Gao, D.; Wan, L.; Inuzuka, H.; Berg, A.H.; Tseng, A.; Zhai, B.; Shaik, S.; Bennett, E.; Tron, A.E.; Gasser, J.A.; et al. Rictor forms a complex with cullin-1 to promote SGK1 ubiquitination and destruction. Mol. Cell 2010, 39, 797–808. [Google Scholar] [CrossRef]
- Rudic, R.D.; Shesely, E.G.; Maeda, N.; Smithies, O.; Segal, S.S.; Sessa, W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Investig. 1998, 101, 731–736. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Deby-Dupont, G.P.; Reginster, J.Y.; Ayache, N.; Pujol, J.P.; Henrotin, Y.E. Regulation by reactive oxygen species of interleukin-1beta, nitric oxide and prostaglandin E(2) production by human chondrocytes. Osteoarthr. Cartil. 2002, 10, 547–555. [Google Scholar] [CrossRef]
- Salvemini, D.; Misko, T.P.; Masferrer, J.L.; Seibert, K.; Currie, M.G.; Needleman, P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. USA 1993, 90, 7240–7244. [Google Scholar] [CrossRef] [PubMed]
- Minuz, P.; Barrow, S.E.; Cockcroft, J.R.; Ritter, J.M. Effects of non-steroidal anti-inflammatory drugs on prostacyclin and thromboxane biosynthesis in patients with mild essential hypertension. Br. J. Clin. Pharmacol. 1990, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Walshe, T.E.; Ferguson, G.; Connell, P.; O’Brien, C.; Cahill, P.A. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro coculture model of the retinal vasculature. Investig. Ophthalmol. Vis. Sci. 2005, 46, 375–382. [Google Scholar] [CrossRef]
- Tiefenbacher, C.P. Tetrahydrobiopterin: A critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2484–H2488. [Google Scholar] [CrossRef]
- Crabtree, C.P.; Hale, A.B.; Channon, K.M. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic. Biol. Med. 2011, 50, 1639–1646. [Google Scholar] [CrossRef]
- Carreau, A.; Kieda, C.; Grillon, C. Nitric oxide modulates the expression of endothelial cell adhesion molecules involved in angiogenesis and leukocyte recruitment. Exp. Cell Res. 2011, 317, 29–41. [Google Scholar] [CrossRef]
- Yan, Y.; Thakur, M.; Van der Vorst, E.P.C.; Weber, C.; Döring, Y. Targeting the chemokine network in atherosclerosis. Atherosclerosis 2021, 330, 95–106. [Google Scholar] [CrossRef]
- Koenig, S.N.; Mohler, P.J. The evolving role of ankyrin-B in cardiovascular disease. Heart Rhythm 2017, 14, 1884–1889. [Google Scholar] [CrossRef]
- Conway, D.E.; Sakurai, Y.; Weiss, D.; Vega, J.D.; Taylor, W.R.; Jo, H.; Eskin, S.G.; Marcus, C.B.; McIntire, L.V. Expression of CYP1A1 and CYP1B1 in human endothelial cells: Regulation by fluid shear stress. Cardiovasc. Res. 2009, 81, 669–677. [Google Scholar] [CrossRef]
- Bock, K.W. Human AHR functions in vascular tissue: Pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem. Pharmacol. 2019, 159, 116–120. [Google Scholar] [CrossRef]
- Dabir, P.; Marinic, T.E.; Krukovets, I.; Stenina, O.I. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ. Res. 2008, 102, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Duarte-Silva, M.; De Barros Cardoso, C.R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Yamamoto, N.; Kodoi, R.; Fukuda, I.; Yoshida, K.; Ashida, H. Antagonistic and agonistic effects of indigoids on the transformation of an aryl hydrocarbon receptor. Arch. Biochem. Biophys. 2008, 470, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, P.; Martin, M.V.; McCormick, W.A.; Nguyen, L.P.; Glover, E.; Bradfield, C.A. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch. Biochem. Biophys. 2004, 423, 309–316. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Sun, R.; Zhou, S.; Li, M.; Feng, M.; Xie, Y. Dual character of flavonoids in attenuating and aggravating ischemia-reperfusion-induced myocardial injury. Exp. Ther. Med. 2017, 14, 1307–1314. [Google Scholar] [CrossRef]
- Zhu, K.; Meng, Q.; Zhang, Z.; Yi, T.; He, Y.; Zheng, J.; Lei, W. Aryl hydrocarbon receptor pathway: Role, regulation and intervention in atherosclerosis therapy (Review). Mol. Med. Rep. 2019, 20, 4763–4773. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Zhu, J.; Van den Eynde, B.J. AHR and tryptophan metabolism: A collaborative dynamics of immune regulation. Genes Immun. 2024, 25, 170–171. [Google Scholar] [CrossRef]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nádvorník, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes 2022, 14, 2105637. [Google Scholar] [CrossRef]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional communication between the aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism. npj Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Aggerbeck, M.; Aggerbeck, L.; Coumoul, X. The aryl hydrocarbon receptor system. Drug Metabol. Drug Interact. 2012, 27, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl hydrocarbon receptor in oxidative stress as a double agent and its biological and therapeutic significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef]
- Thiel, G.; Backes, T.M.; Guethlein, L.A.; Rössler, O.G. Chromatin-embedded reporter genes: Quantification of stimulus-induced gene transcription. Gene 2021, 787, 145645. [Google Scholar] [CrossRef]
- Hunter, J.E.; Leslie, J.; Perkins, N.D. c-Rel and its many roles in cancer: An old story with new twists. Br. J. Cancer 2016, 114, 1–6. [Google Scholar] [CrossRef]
- Aung, H.H.; Lame, M.W.; Gohil, K.; He, G.; Denison, M.S.; Rutledge, J.C.; Wilson, D.W. Comparative gene responses to collected ambient particles in vitro: Endothelial responses. Physiol. Genom. 2011, 43, 917–929. [Google Scholar] [CrossRef]
- Yisireyili, M.; Saito, S.; Abudureyimu, S.; Adelibieke, Y.; Ng, H.Y.; Nishijima, F.; Takeshita, K.; Murohara, T.; Niwa, T. Indoxyl sulfate-induced activation of (Pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS ONE 2014, 9, e109268. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.; Haarmann-Stemmann, T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010, 391, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Serna, E.; Cespedes, C.; Vina, J. Anti-aging physiological roles of aryl hydrocarbon receptor and its dietary regulators. Int. J. Mol. Sci. 2020, 22, 374. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ojeda, S.; Suarez, A.; Valls, A.; Verdú, D.; Pereda, J.; Ortiz-Zapater, E.; Carretero, J.; Mauricio, M.D.; Serna, E. The role of aryl hydrocarbon receptor in the endothelium: A systematic review. Int. J. Mol. Sci. 2023, 24, 13537. [Google Scholar] [CrossRef]
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef]
- Tabas, I.; Lichtman, A.H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Yu, X.-H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.-K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef]
- Guerrini, V.; Gennaro, M.L. Foam cells: One size doesn’t fit all. Trends Immunol. 2019, 40, 1163–1179. [Google Scholar] [CrossRef]
- Kobielarz, M.; Kozuń, M.; Gąsior-Głogowska, M.; Chwiłkowska, A. Mechanical and structural properties of different types of human aortic atherosclerotic plaques. J. Mech. Behav. Biomed. Mater. 2020, 109, 103837. [Google Scholar] [CrossRef]
- Vilaplana-Carnerero, C.; Giner-Soriano, M.; Dominguez, À.; Morros, R.; Pericas, C.; Álamo-Junquera, D.; Toledo, D.; Gallego, C.; Redondo, A.; Grau, M. Atherosclerosis, cardiovascular disease, and COVID-19: A narrative review. Biomedicines 2023, 11, 1206. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, S.H.; Buschmann, I.R.; Jost, M.M.; Hoefer, I.E.; Grundmann, S.; Andert, J.P.; Ulusans, S.; Bode, C.; Piek, J.J.; van Royen, N. Differential effects of MCP-1 and leptin on collateral flow and arteriogenesis. Cardiovasc. Res. 2004, 64, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Zabroski, I.O.; Nugent, M.A. Lipid raft association stabilizes VEGF receptor 2 in endothelial cells. Int. J. Mol. Sci. 2021, 22, 798. [Google Scholar] [CrossRef]
- Capozzi, A.; Manganelli, V.; Riitano, G.; Caissutti, D.; Longo, A.; Garofalo, T.; Sorice, M.; Misasi, R. Advances in the pathophysiology of thrombosis in antiphospholipid syndrome: Molecular mechanisms and signaling through lipid rafts. J. Clin. Med. 2023, 12, 891. [Google Scholar] [CrossRef]
- Schecter, A.D.; Spirn, B.; Rossikhina, M.; Giesen, P.L.; Bogdanov, V.; Fallon, J.T.; Fisher, E.A.; Schnapp, L.M.; Nemerson, Y.; Taubman, M.B. Release of active tissue factor by human arterial smooth muscle cells. Circ. Res. 2000, 87, 126–132. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Zhang, X.; Du, Y.; Chen, G.; Xiang, P.; Ling, W.; Wang, D. Terpene lactucopicrin limits macrophage foam cell formation by a reduction of lectin-like oxidized low-density lipoprotein receptor-1 in lipid rafts. Mol. Nutr. Food Res. 2022, 66, 2100905. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule–1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Chigaev, A.; Wu, Y.; Williams, D.B.; Smagley, Y.; Sklar, L.A. Discovery of very late antigen-4 (VLA-4, α4β1 integrin) allosteric antagonists. J. Biol. Chem. 2011, 286, 5455–5463. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, N.; Cheng, X. Regulatory T cells in chronic heart failure. Front. Immunol. 2021, 12, 732794. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Johnson-Tidey, R.R.; McGregor, J.L.; Taylor, P.R.; Poston, R.N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am. J. Pathol. 1994, 144, 952–961. [Google Scholar] [PubMed]
- Polgar, J.; Matuskova, J.; Wagner, D.D. The P-selectin, tissue factor, coagulation triad. J. Thromb. Haemost. 2005, 3, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Escopy, S.; Chaikof, E.L. Targeting the P-selectin/PSGL-1 pathway: Discovery of disease-modifying therapeutics for disorders of thromboinflammation. Blood VTH 2024, 1, 100015. [Google Scholar] [CrossRef]
- Paez, A.; Méndez-Cruz, A.R.; Varela, E.; Rodriguez, E.; Guevara, J.; Flores-Romo, L.; Montaño, L.F.; Massó, F.A. HUVECs from newborns with a strong family history of myocardial infarction overexpress adhesion molecules and react abnormally to stimulating agents. Clin. Exp. Immunol. 2005, 141, 449–458. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy induces pyroptosis and atherosclerosis via the lipid raft-mediated NOX-ROS-NLRP3 inflammasome pathway in apoE−/− mice. Cells 2022, 11, 2438. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Cui, Y.; Chi, J.; Hao, H.; Hill, M.A.; Liu, Z. ROS in atherosclerosis: What we know? In Oxidative Stress in Cardiovascular-Metabolic Diseases; Eid, A.H., Kobeissy, F., El-Yazbi, A.F., Eds.; Springer: Cham, Switzerland, 2024; pp. 141–161. [Google Scholar]
- Channon, K.M. Oxidative stress and coronary plaque stability. J. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1751–1752. [Google Scholar] [CrossRef][Green Version]
- Das Neves, M.F.; Batuca, J.R.; Alves, J.D. The role of high-density lipoprotein in the regulation of the immune response: Implications for atherosclerosis and autoimmunity. Immunology 2021, 164, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Hu, L.; Yang, C.; Nemat, A.; Xian, G.; Zhang, J.; Zeng, Q. Optimal antithrombotic therapy for patients with STEMI undergoing PCI at high risk of bleeding. Curr. Atheroscler. Rep. 2019, 21, 22. [Google Scholar] [CrossRef]
- Sieg, S.F.; Bazdar, D.A.; Zidar, D.; Freeman, M.; Lederman, M.M.; Funderburg, N.T. Highly oxidized low-density lipoprotein mediates activation of monocytes but does not confer interleukin-1β secretion nor interleukin-15 transpresentation function. Immunology 2020, 159, 221–230. [Google Scholar] [CrossRef]
- Lian, S.; Zhai, X.; Wang, X.; Zhu, H.; Zhang, S.; Wang, W.; Wang, Z.; Huang, J. Elevated expression of growth-regulated oncogene-alpha in tumor and stromal cells predicts unfavorable prognosis in pancreatic cancer. Medicine 2016, 95, e4328. [Google Scholar] [CrossRef]
- Engelhardt, E.; Toksoy, A.; Goebeler, M.; Debus, S.; Bröcker, E.B.; Gillitzer, R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol. 1998, 153, 1849–1860. [Google Scholar] [CrossRef]
- Yeap, W.; Wong, K.; Shimasaki, N.; Teo, E.C.Y.; Quek, J.K.S.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, H.; Wang, J.; Liu, Q.; Wu, C.; Chen, K. Aberrant expression of FcγRIIIA (CD16) contributes to the development of atherosclerosis. Gene 2012, 498, 91–95. [Google Scholar] [CrossRef]
- Yip, D.; Ahmad, A.; Karapetis, C.S.; Hawkins, C.A.; Harper, P.G. Matrix metalloproteinase inhibitors: Applications in oncology. Investig. New Drugs 1999, 17, 387–399. [Google Scholar] [CrossRef]
- Fields, G.B. The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.M.T. Butyrate Produced by Gut Microbiota Regulates Atherosclerosis: A Narrative Review of the Latest Findings. Int. J. Mol. Sci. 2025, 26, 6744. https://doi.org/10.3390/ijms26146744
Dicks LMT. Butyrate Produced by Gut Microbiota Regulates Atherosclerosis: A Narrative Review of the Latest Findings. International Journal of Molecular Sciences. 2025; 26(14):6744. https://doi.org/10.3390/ijms26146744
Chicago/Turabian StyleDicks, Leon M. T. 2025. "Butyrate Produced by Gut Microbiota Regulates Atherosclerosis: A Narrative Review of the Latest Findings" International Journal of Molecular Sciences 26, no. 14: 6744. https://doi.org/10.3390/ijms26146744
APA StyleDicks, L. M. T. (2025). Butyrate Produced by Gut Microbiota Regulates Atherosclerosis: A Narrative Review of the Latest Findings. International Journal of Molecular Sciences, 26(14), 6744. https://doi.org/10.3390/ijms26146744





