1. Introduction
Using secretincan bioactive compounds, Gram-negative bacteria are able to communicate, relieve stress, and attack foreign entities [
1,
2]. Proteins, nucleic acids, and compounds found in the periplasm are packaged into spheres made of their own outer membrane (OM) material. Their cargo, protected during travel by the lipid bilayer, can reach host organisms more reliably than compounds secreted directly to the environment. Proteins displayed on the OM can become a part of outer membrane vesicles (OMVs) and provoke adherence with other organisms [
3]. Manipulation of membrane proteins and OMV cargo can be leveraged to target selected organisms and inject a desired bioactive material [
4,
5].
While the effectiveness of widely used antibiotics is declining, naturally occurring phenomena continue to inspire antibiotic therapies, alternative antibiotic sources, and drug delivery [
1]. Outer membrane vesicles (OMVs) produced by
Pseudomonas aeruginosa exhibit antibacterial properties, attacking other bacteria through encapsulated periplasmic autolysins [
6].
Lysobacter spp. have also been shown to release OMVs with similar bioactive capacities, attributed to compounds within their OMV cargo [
7]. The potential for high OMV production is not limited to these species, as demonstrated by other Gram-negative bacteria like
Escherichia coli [
8]. This presents an opportunity to use
E. coli as a platform for the production of OMVs for drug delivery, the development of novel vaccines [
9], or to emulate an OMV-based antibacterial system.
Several factors affect the production of OMVs. Mechanisms affecting their biogenesis are related to deformations in the OM including: (1) disrupted links between the inner membrane, the peptidoglycan layer, and the outer membrane; (2) accumulation of periplasmic proteins; and (3) curvatures induced by OM proteins [
2,
3,
9,
10]. Kulp et al. suggest that outer membrane vesicle (OMV) production is influenced by both structural characteristics of the periplasm and the metabolic pathways responsible for synthesizing key envelope components, such as membrane proteins, lipopolysaccharides (LPS), and enterobacterial common antigen (ECA) [
11]. Additionally, stress response pathways are reported to affect OMV formation. However, the precise metabolic routes governing OMV biogenesis are not yet fully understood. To address this gap, metabolic network models provide a valuable framework. These models connect genomic data to enzymatic reactions, describing the metabolic states and biological potential of an organism, and can thus help identify conditions or compounds that enhance in vivo OMV production. Further exploration of the effect of gene editing in the metabolic space can increase our understanding of OMV formation and improve its yield.
Being able to produce OMVs in large quantities is an important target. Additionally, the activity and stability of OMVs are important when considering their therapeutic use [
12]. Proteins in the cargo and displayed on the membrane affect their bioactivity [
3]. Toxic LPS in the outer membrane can also affect their medical use [
13]. The OMVs proteome analyses of various Gram-negative bacteria, including
E. coli, have been reported and lipidomic profiles for Klebsiella pneumoniae OMVs [
14,
15,
16,
17] have given an insight into their composition. However, there are currently no lipidomic profiles reported for
E. coli OMVs.
This study involved adapting a genome-scale metabolic network model of Escherichia coli K-12. Subsequently, constraint-based optimization methods were utilized to identify strategies that enhance outer membrane vesicle (OMV) production in E. coli JC8031. Description of the vesicle production was achieved through the model constraints and the objective functions driving the optimization problem. We found four E. coli JC8031 mutant strains with enhanced vesicle production through in silico gene knockouts. To our knowledge, this is the first use of metabolic network models to study OMVs production and the first report on the lipid composition of isolated E. coli OMVs.
In silico modeling was experimentally validated by using a CRISPR-Cas9 platform for in vitro gene deletion. Experimental recovery of OMVs and subsequent lipidomic analysis produced novel results on the lipid profile of E. coli OMVs and the effect of mutations on their lipid composition. The integration of computational and experimental approaches through gene editing to enhance OMVs biogenesis are a step forward for high yield production of OMVs for medical applications.
3. Discussion
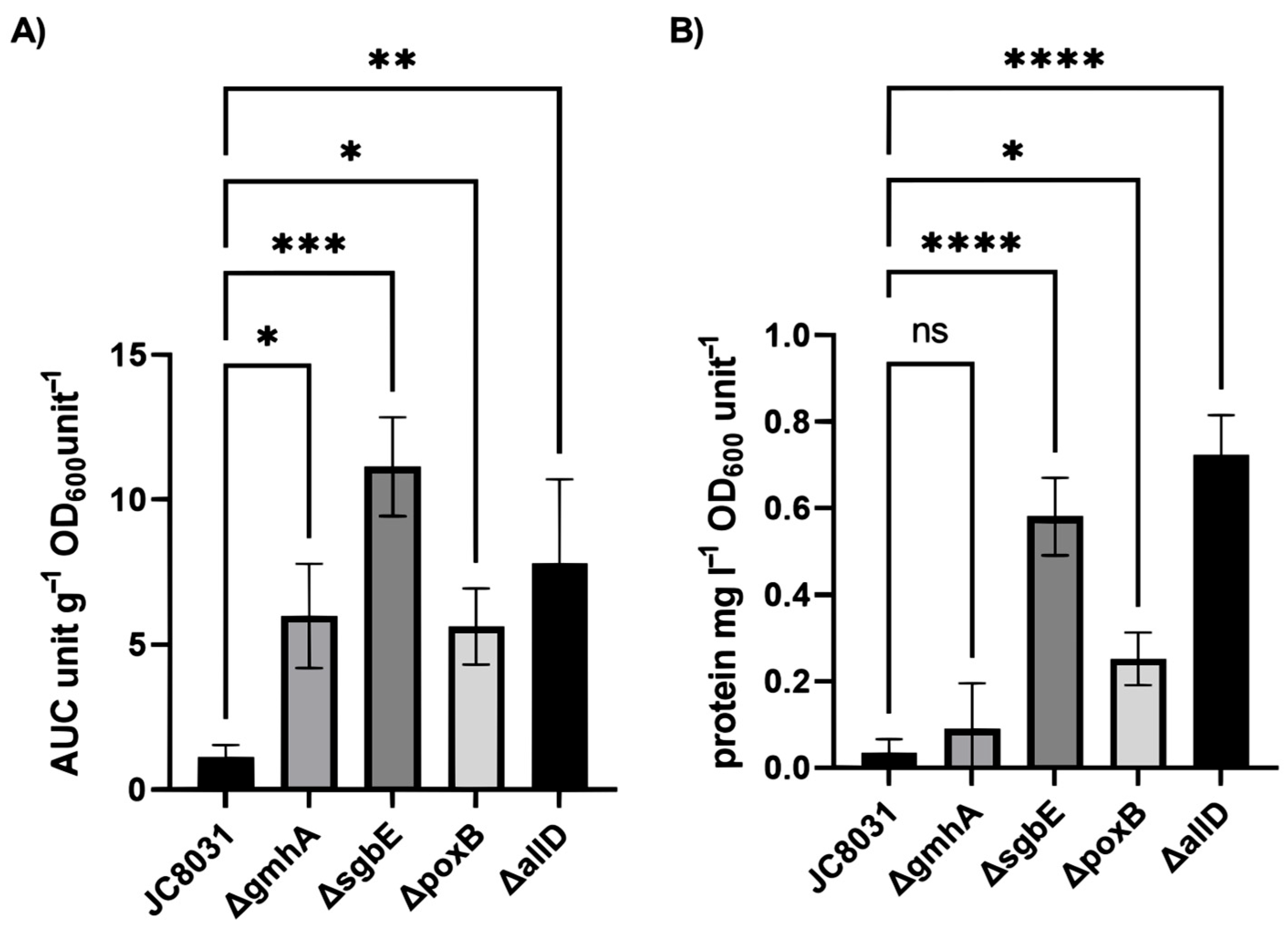
Deletion analysis of the refined model revealed that gene knock-outs enhanced vesicle production, as represented by the objective function. Notably, the gene deletions identified by the OptKnock algorithm were not directly associated with phospholipid metabolism. However, two genes had a direct relation to the outer membrane (poxB, gmhA). poxB encodes for a membrane protein that acts as a pyruvate dehydrogenase. This protein is related to membrane binding and presents interactions with lipids [
27]. gmhA encodes for a phosphoheptose isomerase involved in the lipopolysaccharide biosynthesis and ΔgmhA have shown lipopolysaccharides lacking heptose [
28]. The involvement of these genes with the formation of OM components supports previously proposed mechanisms of vesicle biogenesis through perturbation of membrane proteins, induced curvatures, and membrane integrity [
3]. However, the results show a lack of significance in a ΔgmhA alteration over the protein concentration. The other two deleted genes (allD, sgbE) showed an indirect relation to vesicle production. sgbE encodes for an enzyme from an L-ascorbate utilization operon and is involved in the metabolism of nucleotide and aromatic amino acid precursors [
29]. The allD gene encodes for an enzyme involved in the assimilation of allantoin and allantoin use as a nitrogen source [
30]. These single deletion targets are also indirectly related to vesicle production through the alteration of biomass growth. The use of the Warburg–Christian equation through spectrophotometric methods shows limitation to quantify vesicle production. This method can be complemented with LPS measurements through the Purpald test [
24].
In silico deletion experiments indicated that while the parental strain exhibited a high biomass growth rate, multiple gene deletions did not reduce this rate to a point that would compromise the engineered strain’s utility. Potential discrepancies between the in silico predictions and in vitro outcomes could be observed by experimentally implementing the suggested gene deletions. This implies a challenge in generating multiple subsequent deletions and achieving the expected biomass growth. Here, we found that deletions associated with a reduction in cellular growth also showed a reduced yield for vesicle recovery.
On the other hand, from the industrial point of view, biomass growth was not significantly affected for all mutants while OMVs generation was enhanced up to 10 times in the single deletion mutant strain ΔsgbE, therefore, more attractive when scaling up the process from an economical perspective.
The composition of the produced OMVs is also important when considering their use in therapeutic applications. Previous studies have raised concern on the effect of the recovery method on the size distribution, morphology, and composition of OMVs. However, a comparison of isolation methods has shown that different methods produce a similar effect on vesicle morphology [
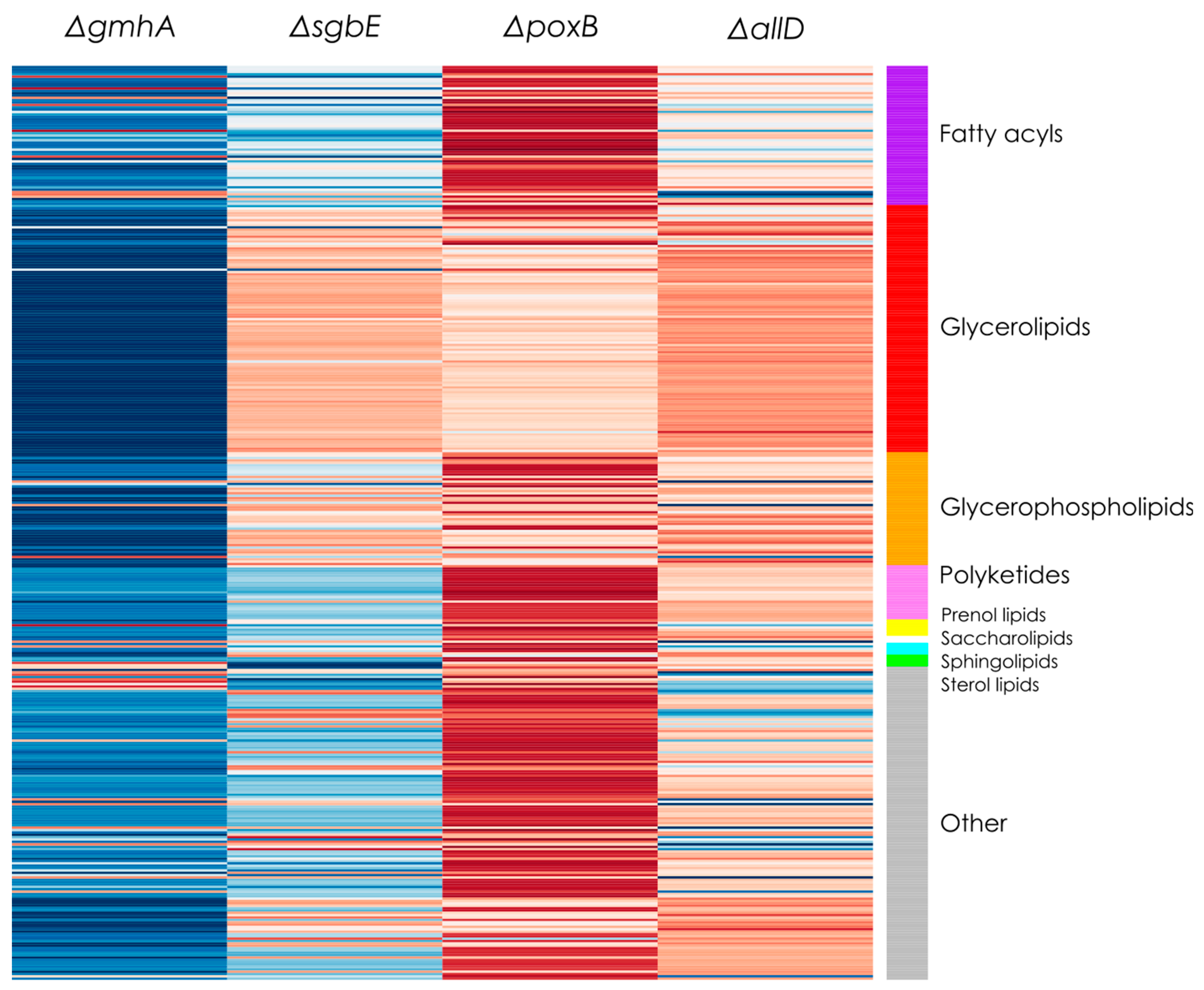
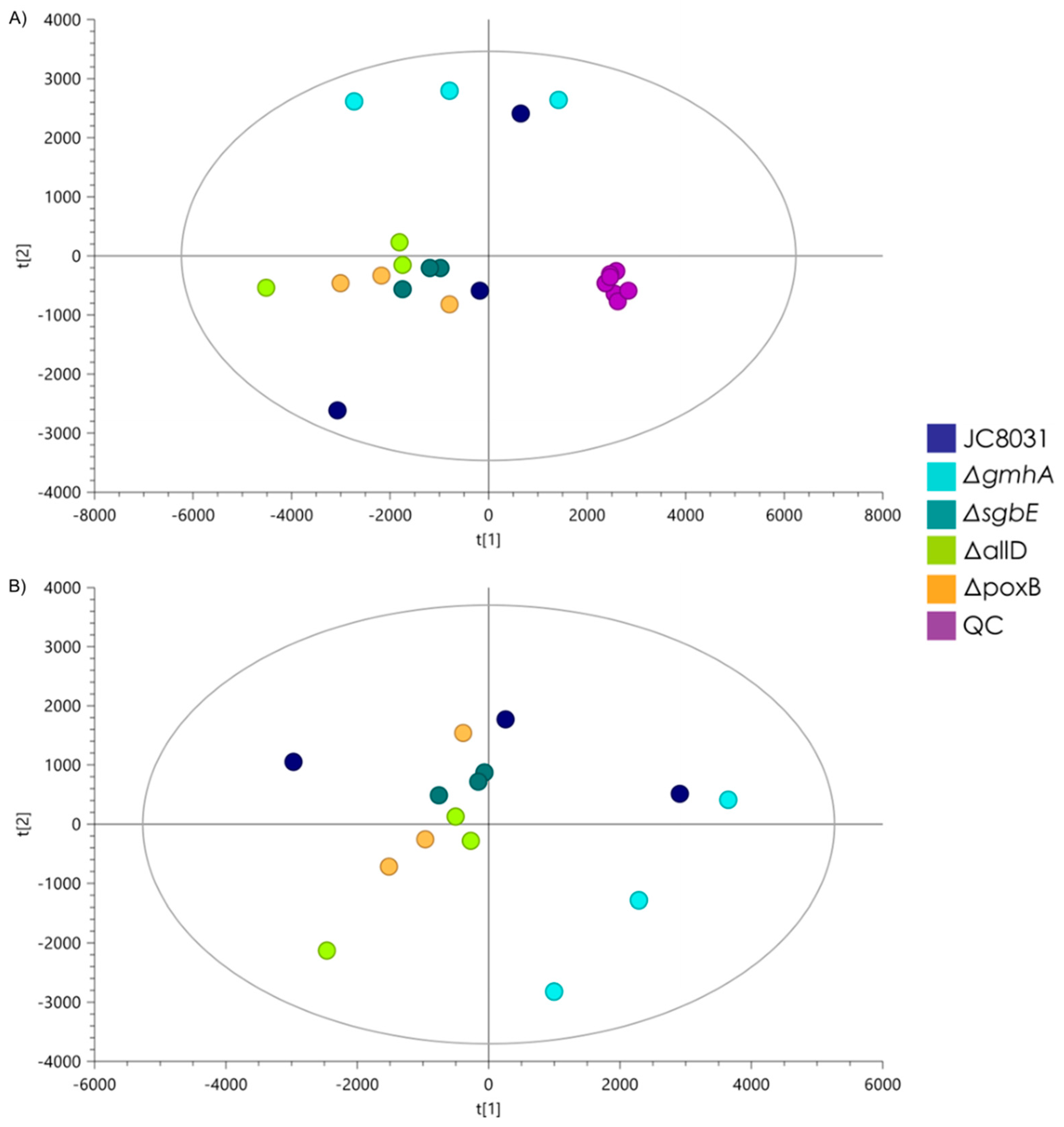
5]. The lipidomic analysis showed no statistically significant change in the composition that separated the single deletion mutants from the JC8031 strain. This gives an insight into the response of general OMVs composition with respect to genome alteration. The lack of change in the lipid profile could be attractive for biomedical applications as it facilitates the study and analysis of structure stability of OMVs as envelopes for the desired cargo and the effect of external factors on their structural integrity.
To the best of our knowledge, the studies reporting OMVs composition are relatively limited, and they are mostly focused on proteomics analysis rather than lipidomics. In addition to the information that can be obtained from searching in academic databases, it is also possible to consult specialized compendiums available online, such as vesiclepedia [
31]. A search in this compendium can also show how information on bacterial OMVs lipidomics is very limited and suggests that the data obtained during this study may be one of few attempts to characterize
E. coli OMVs from the lipidomics point of view (see
Supplementary Materials Table S3).
On the other hand, regardless of the relative lack of information, it is possible to make further analysis of the data if assuming that OMVs lipid composition is similar to the
E. coli outer membrane composition, as suggested by previous studies [
32]. In that case, special attention should be paid to compounds like PGs and PEs, as these are reported to be the prevalent lipid components of the
E. coli outer membrane [
21,
26], particularly for the K12 strain, which is the strain from which the JC8031 strain was derived [
19]. Indeed, PGs and PEs were detected in our OMVs samples, although no significant changes in lipid composition can be inferred from the results when comparing OMVs from the designed single deletion mutants against OMVs from the JC8031 strain [
33]; none of the genes that were deleted during the present study (poxB, sgbE, gmhA, and allD) were reported previously as responsible of causing a significant change in the lipid composition of the bacterium and it might be assumed that they should not cause significant changes in OMVs lipid composition either.
Another important aspect to take into account is the prevalent fatty acyl chains forming part of the OMVs. In our case, the prevalent species are vaccenic acid (18:1) and palmitic acid (16:0) (
Supplementary Materials Table S3), both of them were previously reported as prevalent in
E. coli membranes [
26]. In this last report, it was also stressed that palmitic acid was slightly more prevalent in the outer membrane. Our results show that palmitic acid is indeed slightly more prevalent when analyzed in the PEs context, but a more quantitative analysis is necessary to obtain conclusive data. This kind of analysis will be particularly important considering that OMVs are expected to be relatively rigid, thanks to a higher content of saturated fatty acyl chains [
32].
The lipid profile of
E. coli OMVs presented an unexpected major component in glycerolipids, while expected major components glycerophospholipids were found in second place. While glycerophospholipids are usually present in the outer membrane in large proportions, it is not uncommon for membranes composed of glycerolipids [
34]. The lack of phosphorus in membrane lipids is associated with the depletion of phosphorus in the growth media [
35]. In this case, LB culture media presented a rich nutritious environment for cell growth which provided phosphorous. This implies that the small participation of glycerophospholipids in the
E. coli OMV lipid profile is not due to phosphorus availability. It was not possible to determine if the OMV lipid profile correlates with outer membrane profile in whole
E. coli cells, or if vesicle formation was preferential in regions low in phosphorus or with higher amounts of glycerolipids. Additionally, proteomic studies have found an enhanced carryover of inner membrane to the generated vesicles [
5]. This could have implications for the engineering of membrane proteins for interaction with host cells and could partly account for the unexpected high glycerolipids content in the isolated OMVs. Further work is needed to analyze differences in OMVs from the originating outer membrane and the effect of phosphorus availability on the lipid profile.
Advances in genomics have enabled the identification of an organism’s full gene set. Subsequent genome annotation in
E. coli facilitates the reconstruction of enzymatic reaction networks. These networks represent the potential metabolic states a cell can achieve. However, a significant limitation in metabolic modeling stems from the completeness of genome annotation. Gaps or inaccuracies in annotation can lead to disconnected reactions within the model, thereby altering predicted metabolic flux distributions and potentially causing blocked pathways or preventing in silico growth. To address such limitations in
E. coli, progressively refined metabolic network models have been developed [
18]. These models have expanded in scope, incorporating an increasing number of metabolites and reactions, and extending beyond core metabolism to include secondary metabolism and other cellular functions. Despite these advancements, some reaction discontinuities within the network persist. Model refinement algorithms, such as GrowMatch, facilitate the integration of experimental data to improve the consistency between in silico predictions and biological reality. When applied to the model of a high vesicle-producing
E. coli strain, GrowMatch indicated a reduction in the disparity between model predictions and experimental findings. While this refinement cannot fully rectify all inconsistencies in predicted growth capabilities, it brings the model’s performance closer to available data and observed growth patterns.
The formation of OMVs is driven by structural dynamics of the cell envelope. Certain metabolic pathways contribute to OMV production, primarily by altering phospholipid composition, which can destabilize the envelope and increase vesiculation [
11]. This study centered on the metabolic networks that generate essential OMV building blocks: lipidA and glycerophospholipids, among other cell envelope precursors. Modeling the simultaneous overproduction of these multiple precursors presents a significant challenge due to the dense connectivity within metabolic networks, complicating objective function optimization. This contrasts with studies targeting a single metabolite, where the impact of network modifications is generally easier to trace. To improve the predictive power of the metabolic model, additional information is required as input to further constrain the model and contextualize it to experimental data. Future work should include the improvement of the model through the integration of lipidomic and transcriptomic data. Integration of lipidomic data is challenging as lipids are usually expressed in generic metabolites in metabolic models, and experimental data are not easily associated with the network.
4. Materials and Methods
4.1. Vesicle Recovery and Quantification
A high vesicle yield
E. coli JC8031 strain was selected as the base strain (also referred to as WT strain).
E. coli JC8031 is a tol/pal mutant [
19]. This strain was modeled in silico and used for experimental procedures, vesicle recovery, and gene editing.
E. coli JC8031 was transformed with a GFP production plasmid with an ampicillin resistance cassette for selection.
E. coli harboring a GFP plasmid was cultured in 50 mL of Luria–Bertani (LB) medium supplemented with ampicillin (100 ug/mL final concentration for plasmid selection). Cultures were incubated for 24 h at 37 °C with shaking at 250 rpm to harvest outer membrane vesicles (OMVs) during the late logarithmic growth phase, minimizing cell debris [
11].
Subsequently, the culture was transferred to 50 mL conical tubes and centrifuged at 4500 rpm at 20 °C for 30 min to pellet the cells. A volume of 30 mL of the resulting supernatant was filtered through a 0.45 μm polyamide syringe filter to remove residual cells and larger debris. The clarified filtrate was then ultracentrifuged at 50,000×
g for 3 h at 4 °C to pellet OMVs [
5].
The presence of OMVs was confirmed by observing a translucent pellet exhibiting green fluorescence under blue light. The OMV pellet was resuspended in 1 mL of phosphate-buffered saline (PBS) containing spectinomycin and stored at −80 °C until further analysis. To verify the absence of viable cells, an aliquot of the resuspended OMV solution was plated onto LB agar containing ampicillin (100 µg/mL) and checked for cell growth.
The relative quantity of outer membrane vesicles (OMVs) was estimated based on UV absorption spectroscopy. Absorbance spectra were recorded using a UV spectrophotometer over a wavelength range of 200 nm to 320 nm, with measurements taken at 2 nm intervals. OMV abundance was then determined by calculating the area under the curve (AUC) for the absorbance spectrum within this defined range. Additionally, the protein concentration in each sample was semi-quantitatively estimated using the Warburg–Christian equation using absorbance values at 280 nm for protein content and 260 nm to account for the influence of nucleic acid content [
24]. Vesicle production and protein concentration were normalized to the mass of sample and OD
600nm to account for varying growth rates between sample groups.
4.2. Metabolic Network Model
The
Escherichia coli K-12 genome-scale metabolic model, iML1515, developed by Monk et al. [
18], served as the initial framework for in silico assessment of outer membrane vesicle (OMV) production and identification of optimal culture conditions. Prior to use, this model was curated to confirm the accurate representation of all necessary biomass precursors and a functional biomass growth reaction. Subsequently, the iML1515 model was further adapted to reflect the genetic background of
E. coli strain JC8031 by incorporating its known mutations.
4.3. Model Refinement
Experimental data on outer membrane vesicle (OMV) production levels and growth rates for 3908
E. coli Keio collection knock-out mutants were obtained from Kulp et al. [
11]. Flux balance analysis (FBA) [
36] was employed to simulate the biomass growth rates of 150 of these mutants, which were reported by Kulp et al. [
11] to exhibit a high OMV production phenotype. These FBA simulation results were then compared against the corresponding experimental growth rate data to assess the predictive consistency between our in silico model and the in vitro observations. Subsequently, subsets of this validated dataset were utilized for model-guided strain design using constraint-based optimization methods [
37] to identify targets for enhancing OMV production.
Concordance between in silico growth predictions and experimental observations was categorized into four types: (1) agreement where both predicted and observed growth (Growth/Growth, GG); (2) agreement where both predicted and observed no growth (No Growth/No Growth, NGNG); (3) inconsistency where growth was predicted but not observed experimentally (Growth/No Growth, GNG); and (4) inconsistency where no growth was predicted despite experimental observation of growth (No Growth/Growth, NGG). To reconcile these discrepancies with the Keio collection growth data, the GrowMatch algorithm [
20] was employed. Specifically, GNG inconsistencies were addressed by GrowMatch through the targeted exclusion of reactions from the metabolic model. Conversely, NGG inconsistencies were resolved by incorporating necessary biochemical reactions identified from an external database.
4.4. Model Objective Function Definition
A well-defined objective function is important for accurately simulating the metabolic state associated with outer membrane vesicle production. Optimization of this function alters the in silico metabolic flux distribution, thereby guiding the model towards a biologically meaningful phenotype. Initial attempts to define an OMV-specific objective function using established algorithms based on methodologies by Burgard and Maranas [
38] yielded linear combinations of model reactions. However, these algorithmically derived functions did not align with known OMV biogenesis mechanisms, as they did not prioritize key metabolic steps in precursor synthesis, were not directly linked to OMV composition, and appeared to be over-fitted to the input data. Therefore, the objective function was described according to the expected composition of the
E. coli OM, glycerophospholipids, and lipopolysaccharides [
35] as well as the expectation for an increased phospholipid production to increase OMV formation [
39]. This approach is similar to the constitution of biomass objective functions which are also key to the model [
40].
4.5. Strain Design
To identify gene deletion targets for enhanced OMV production, the calibrated metabolic model incorporating the defined vesiculation objective function was utilized. We employed the constraint-based algorithm OptKnock [
22] to predict beneficial gene knock-outs. OptKnock was configured to identify deletions that maximized the OMV production objective function while simultaneously ensuring a viable biomass production rate, thereby excluding lethal deletion strategies. These simulations were performed on the refined model under conditions mimicking LB medium, with relevant exchange reactions activated to allow metabolite uptake. The OptKnock search was constrained to a maximum of five gene deletions.
4.6. Genome Editing
Target genes were knocked out by complete gene deletion by using CRISPR-Cas9. Guide RNAs were designed for the genes obtained through strain design using the tools from Benchling [
41] and a reference
E. coli genome (NCBI Accession: NC_000913.3). Synthetic cassettes were designed including the N20 from gRNA design (
Table 2) and two segments of 500 bp corresponding to sequence flanking the target gene to provide template availability for homology directed repair of the double-stranded DNA lesion, thereby completely eliminating the targeted gene from the bacterial genome. A system of two plasmids was employed: a plasmid including Cas9 genes and a plasmid for gRNA expression [
42]. All vectors were electroporated.
4.7. Lipidomics
Lipidomic analysis was carried out at MetCore Uniandes (Bogota, Colombia). In total, 40 uL of type I water and 160 uL of HPLC-grade methanol were added to the lyophilized samples. Samples were resuspended in a vortex for two minutes. Then, 400 uL of MTBE was added and samples were vortexed for 60 min. A total of 250 uL of type I water was added and samples were vortexed for two minutes. Samples were centrifuged at 6190 rpm and 25 °C for 10 min. Then, 20 uL of organic phase was transferred to HPLC fixed-insert vials and diluted with 80 uL of MTBE.
Quality control samples were prepared by mixing equal volumes of metabolite extract from each sample. Quality control runs were performed to stabilize the analytic platform. Subsequent quality control runs were employed every five randomized samples.
Lipidomic analysis was implemented in an Agilent Technologies 1260 Liquid Chromatography (Santa Clara, CA, USA) system coupled to a quadrupole time of flight mass analyzer and ionization by electrospray (LC-ESI-QTOF-MS). A total of 1 uL of each sample was injected in a C8 column (InfinityLab Poroshell 120 EC-C8 (150 × 3.0 mm, 2.7 µm)) at 60 °C. A gradient elution was employed composed of 5 mM ammonium formiate in Milli-Q water (Phase A) and 5 mM of ammonium formiate in isopropanol-methanol 15:85 (Phase B) with a constant flux of 0.4 mL/min. Mass spectrometry detection was performed in ESI positive mode in full scan from 100 to 1100 m/z. Mass correction was employed throughout the analysis with two reference masses: m/z 121.509 (C5H4N4) and m/z 922,0098 (C18H18O6N3P3F24).
Lipidomic profiles were obtained by using Agilent Mass Hunter Profinder 10 software employing the Recursive Feature Extraction (RFE) with extraction conditions: 0–31 min and 10,000 counts, positive ion species: (-H, +Cl, +NH4), and no additional mass or species filters. Molecular characteristics found in the solvent blank control were eliminated. Data from alignment, deconvolution, and integration were filtered by calculation of a variation coefficient (VC) of the area in QC samples. Characteristics with CV > 20% were filtered out. Data were normalized based on vesicle sample information: quantity and sample volume.
A selection of statistically significant molecular characteristics was performed through multivariate statistical analysis (MVA) and univariate (UVA). MVA was performed using SIMCA-P + 16.0 software (Umetrics) and UVA was performed by implementing the MetaboAnalyst 4.0 tool (#). Annotation of molecular characteristics was performed using the CEU MASS MEDIATOR tools (#) by batch analysis with the following parameters: tolerance = 10 ppm; databases = LipidMaps; Metabolites = OnlyLipids; input masses mode= m/z masses; ionization mode = positive; adducts = (M + H, M + Cl, M + NH4). Additionally, molecular formulas were generated for statistically significant molecular characteristics by using the Agilent MassHunter Qualitative 10 software with positive ions = (-H, +Cl, +NH4) and elements= (H, C, O, N, P) as parameters.
4.8. Statistical Analysis
Data were analysed using GraphPad Prism version 9.3.1 (GraphPad Software, Boston, MA, USA). A one-way ANOVA was used to estimate the statistical significance of differences within groups. Sidak’s multiple comparison test was used to evaluate statistical differences with wild-type group JC8031. Differences were considered significant at p-values of < 0.05.
5. Conclusions
In this study, we generated four single deletion mutants through in silico metabolic network model strain design and CRISPR-Cas9 genome editing (ΔpoxB, ΔsgbE, ΔgmhA, ΔallD). The four designed strains showed an enhanced vesicle production of up to 10 times that of the wild-type JC8031 strain while maintaining cell growth, while only three displayed a greater protein concentration (ΔpoxB, ΔsgbE, ΔallD). This is a step ahead to obtain OMVs in a large-scale setting and shows that it is plausible to use metabolic network modelling the complex vesicle formation process while targeting single genes.
Lipidomic analysis though LC-ESI-QTOF-MS showed that there were no significant changes in the lipid profile that separated the four mutants and WT in the different groups. The OMV lipid profile remained stable across strains. Recovered E. coli OMVs displayed a lack of phosphorus in their composition as glycerolipids were the majority component while glycerophospholipids were present in a lesser quantity. Knowledge of the E. coli OMV lipid profile and lack of alterations in different single deletion strains is useful to employ OMVs as therapeutic alternatives and drug transport and delivery.
Strain design using metabolic network models encounters difficulties when the target for production is a complex structure, such as OMVs, instead of a single metabolite. Integration of multiple source omic data should be used to improve the model.