Multiomics Integration of Parkinson’s Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology
Abstract
1. Introduction
2. Results
2.1. The Integrated Analysis Reveals Specific Changes to the Transcriptional Landscape in PD Patient Samples
2.2. IRE1 Has Higher Activity in Patients with PD
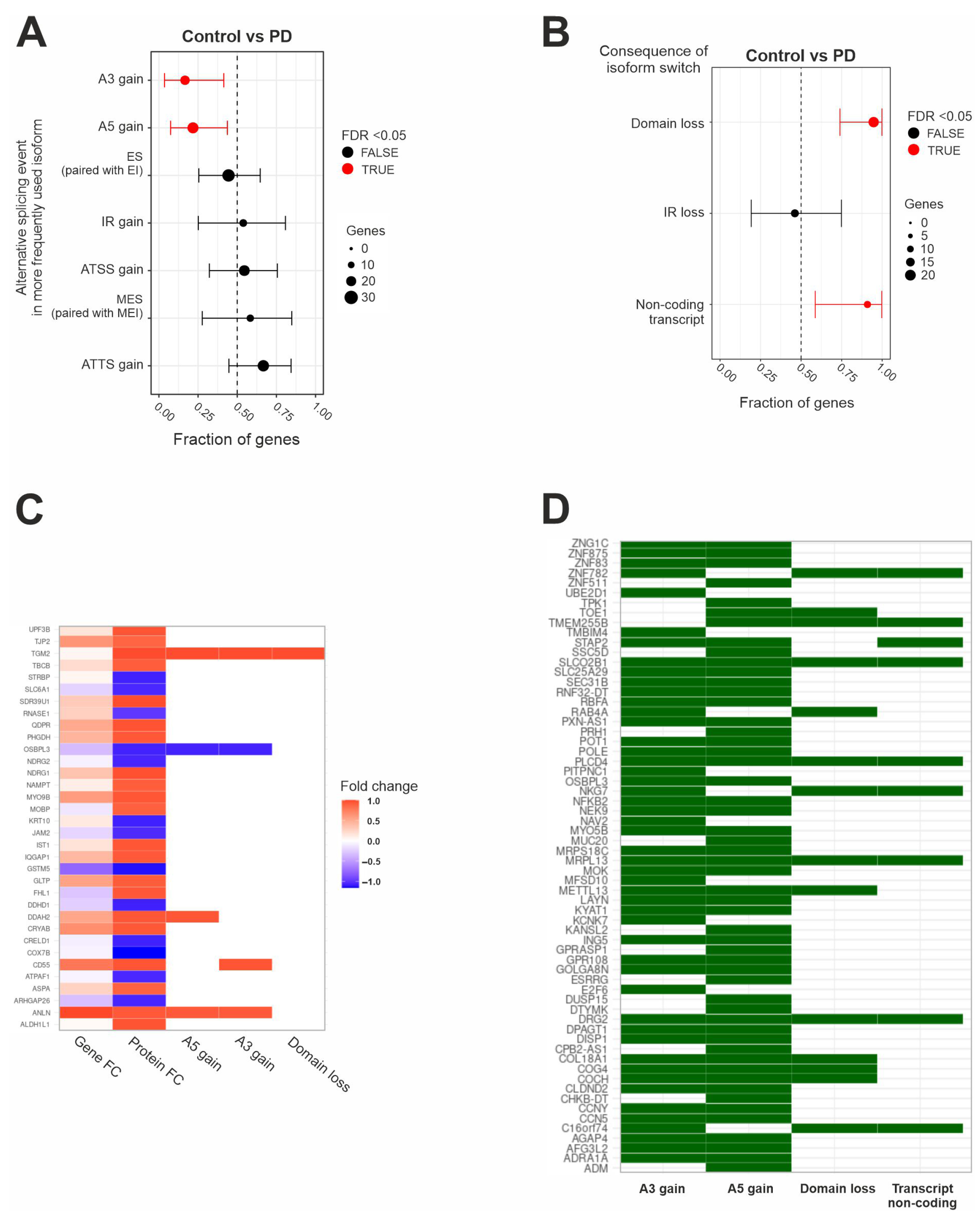
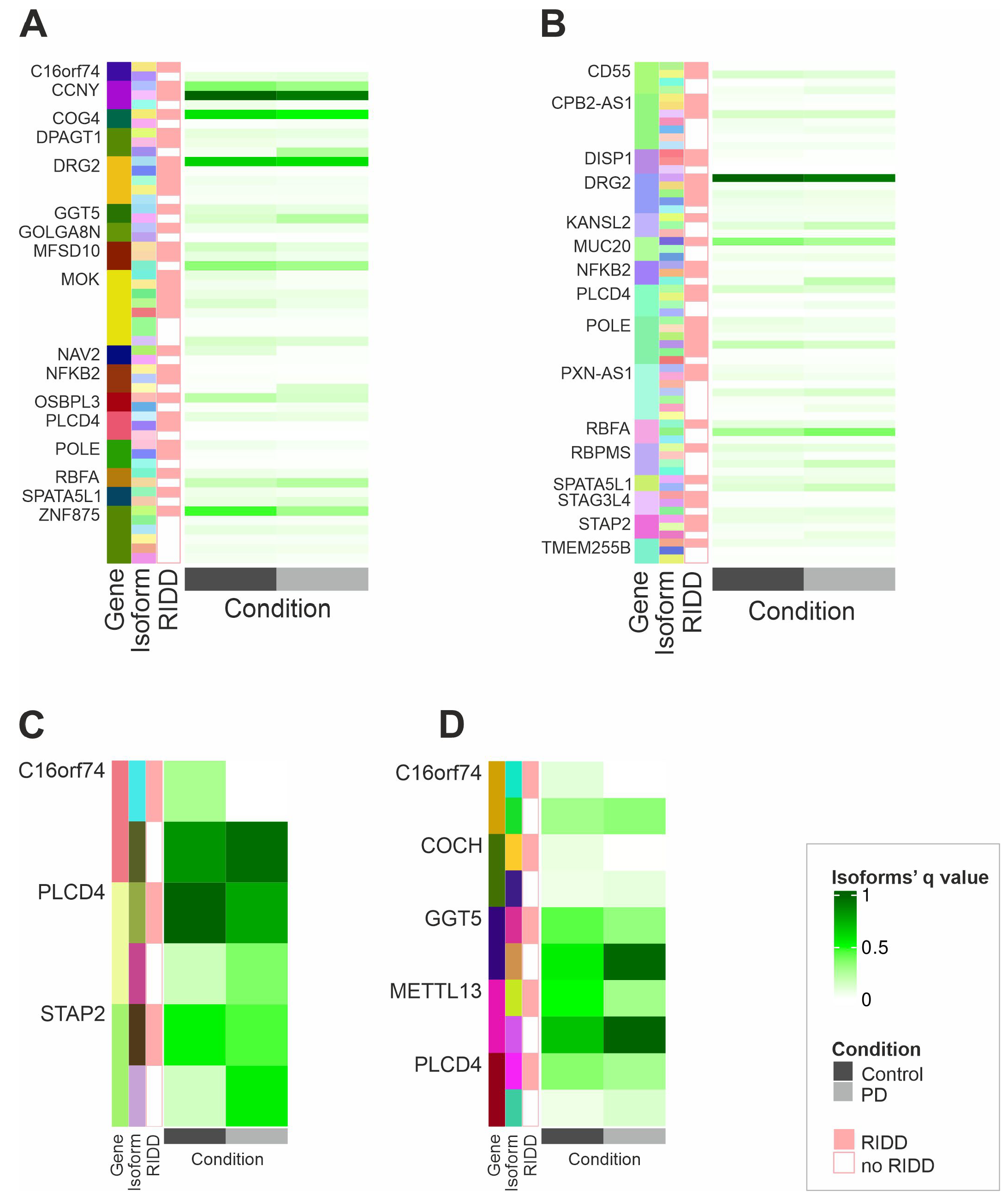
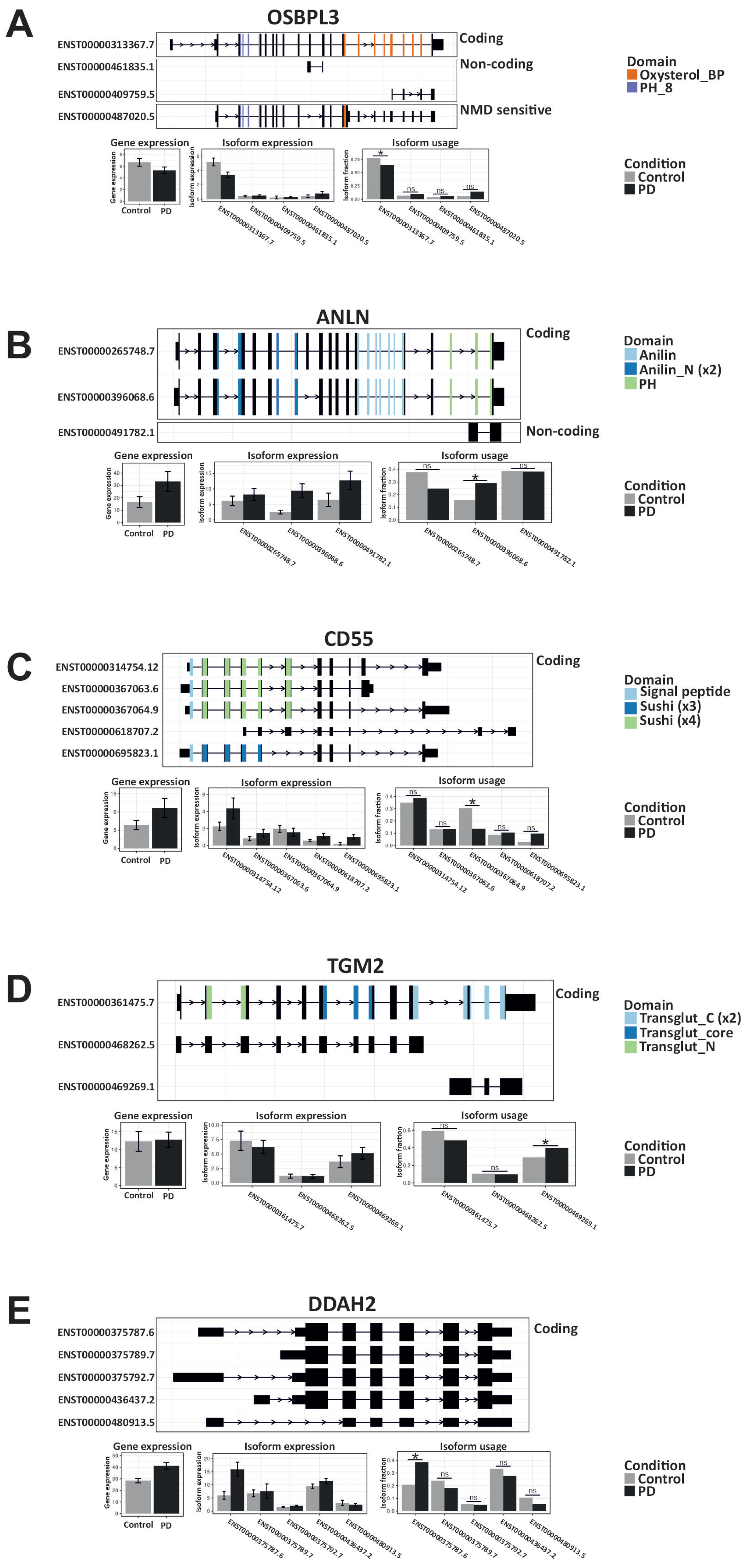
2.3. IRE1’s RIDD Activity and Differential Isoform Switching
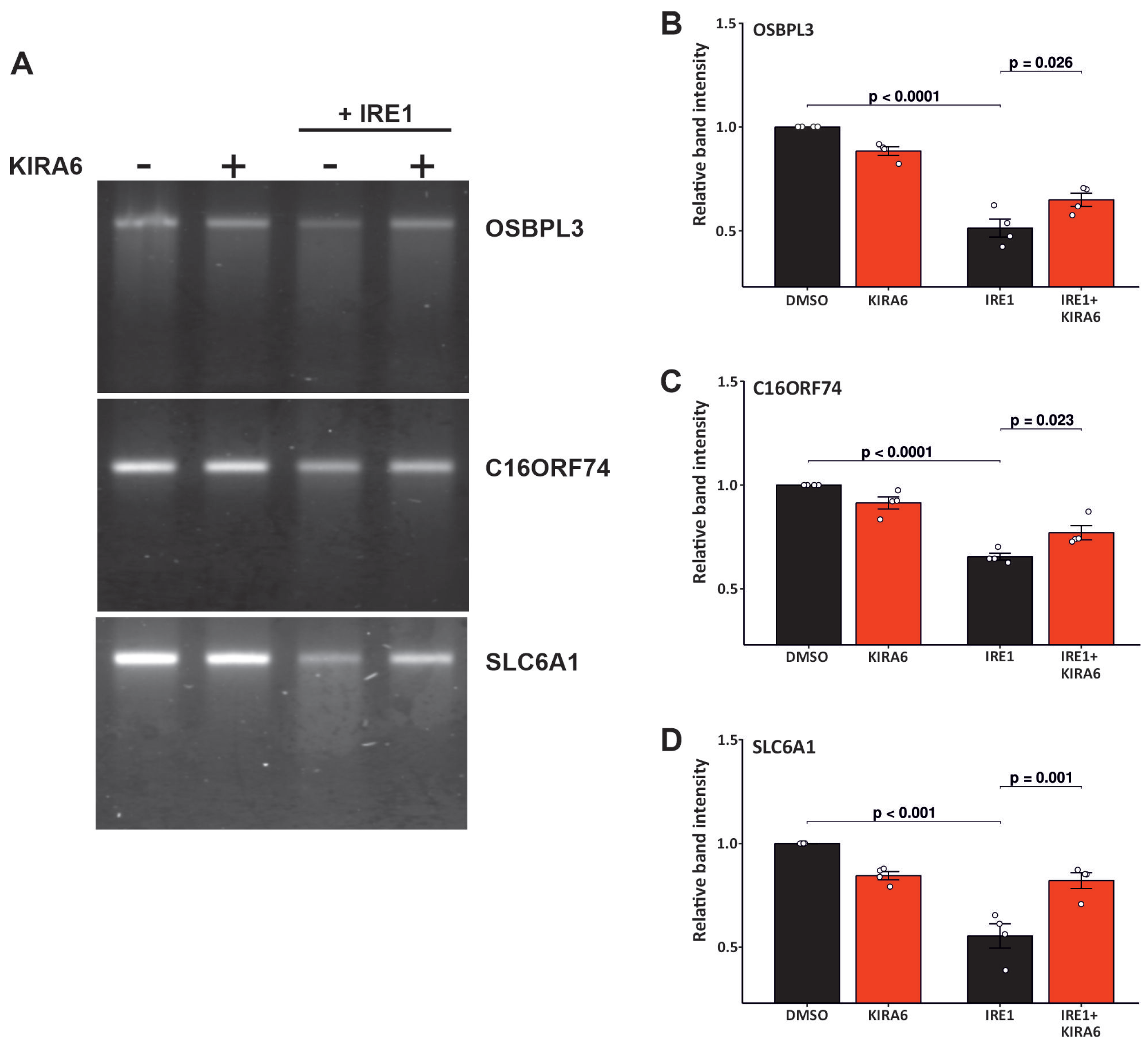
2.4. IRE1 RNAse Activity Leads to the Degradation of OSBPL3, C16orf74, and SLC6A1 mRNAs
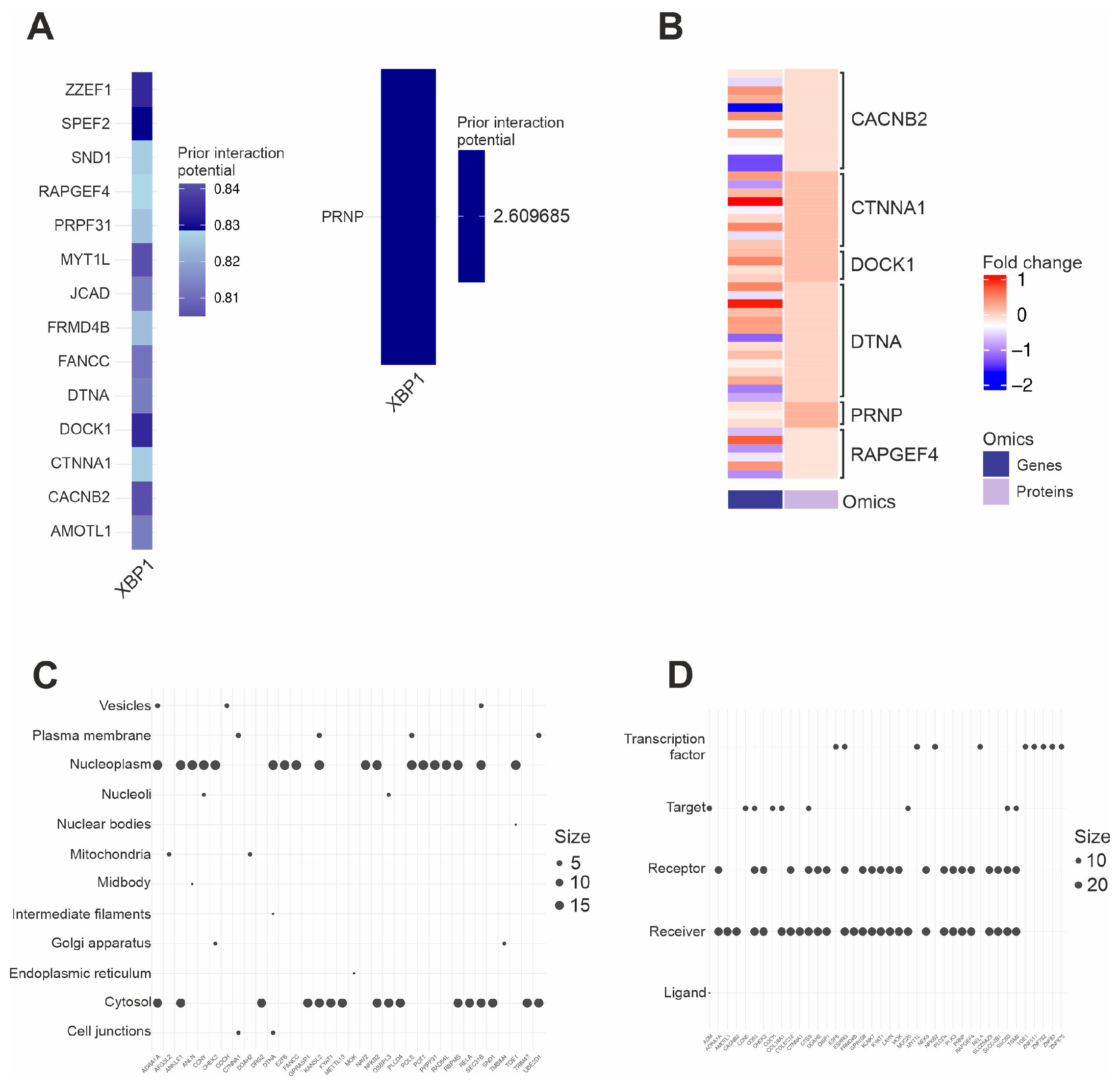
2.5. The Differential Impact of RIDD Activity on Protein Abundance in PD
2.6. Activity of the XBP1 Axis in PD Samples
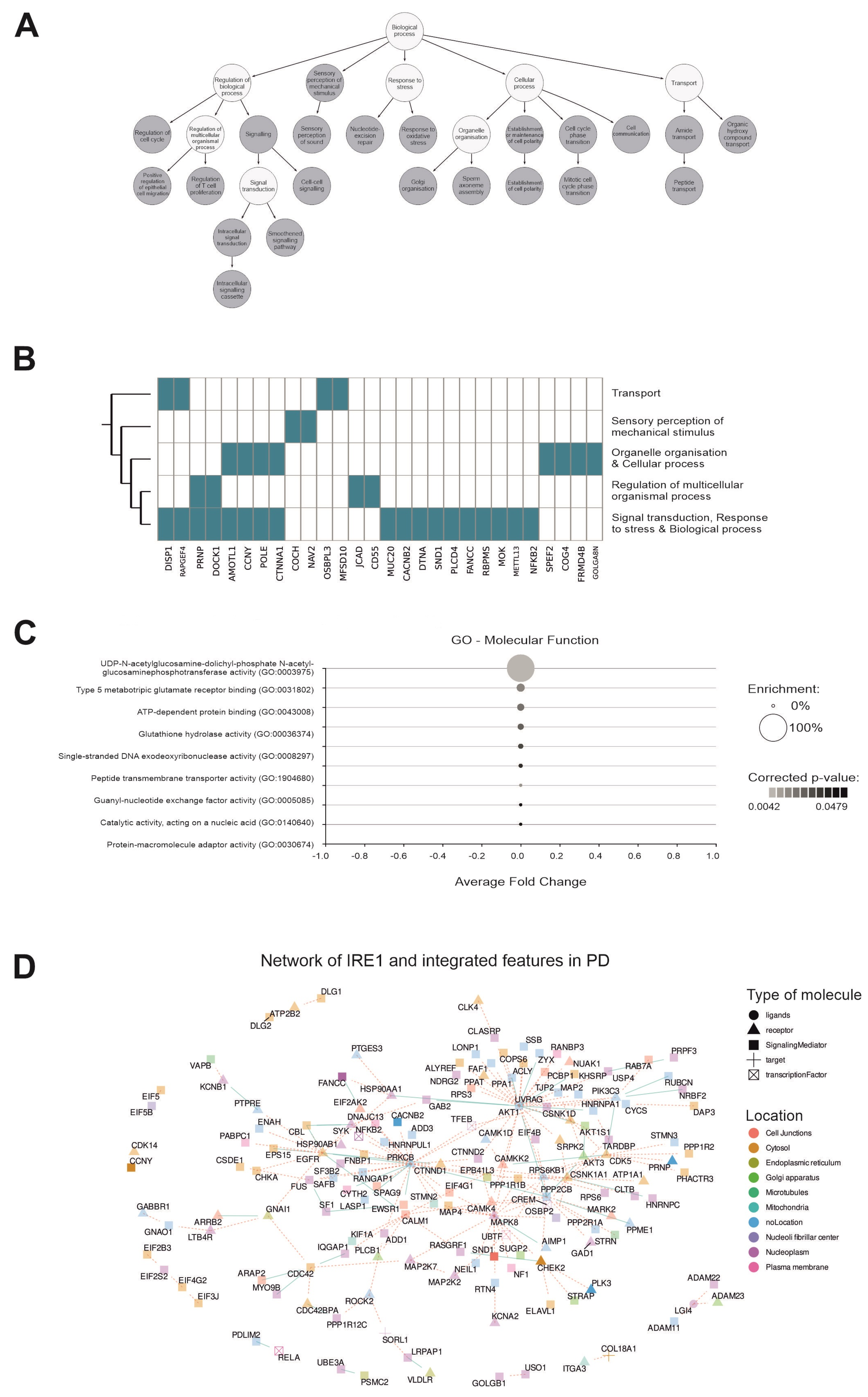
2.7. Subcellular Localization and Molecular Roles of IRE1 Downstream Targets
2.8. The Pathway Enrichment Analysis of IRE1 Downstream Signaling Arms in PD
3. Discussion
4. Materials and Methods
4.1. The Transcriptomics and Proteomics Data
4.2. The Transcriptomics Analyses
4.3. The Proteomics Analysis
4.4. Integration of Omics
4.5. IRE1 Scoring
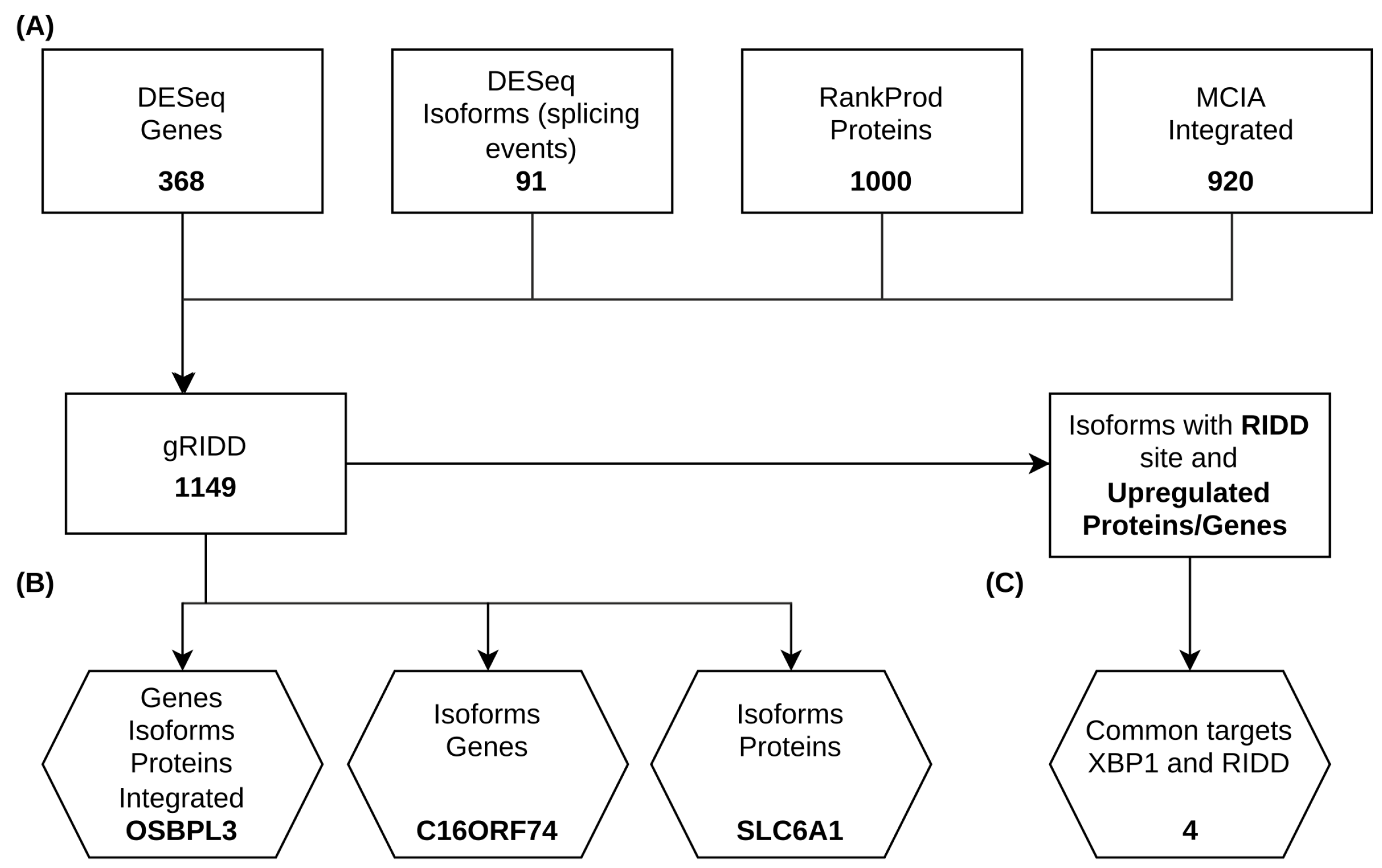
4.6. gRIDD
4.7. Comparison of the Isoforms
4.8. XBP1 Targets
4.9. The In Vitro mRNA Cleavage Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
| 6-OHDA | 6-hydroxydopamine |
| ANLN | Anillin, Actin Binding Protein |
| ATSS | Alternative Transcript Start Site |
| ATTS | Alternative Transcript Termination Site |
| BiP | Binding Immunoglobulin Protein |
| C16orf74 | Chromosome 16 Open Reading Frame 74 |
| CACNB2 | Calcium Voltage-Gated Channel Auxiliary Subunit Beta 2 |
| CCN5 | Cellular Communication Network Factor 5 |
| CD55 | CD55 Molecule (Cromer Blood Group) |
| CHEK2 | Checkpoint Kinase 2 |
| COG4 | Component Of Oligomeric Golgi Complex 4 |
| CPB2-AS1 | CPB2 Antisense RNA 1 |
| CTNNA1 | Catenin Alpha 1 |
| DA | Dopaminergic neurons |
| DDAH2 | DDAH Family Member 2, ADMA-Independent |
| DOCK1 | Dedicator Of Cytokinesis 1 |
| DRG2 | Developmentally Regulated GTP Binding Protein 2 |
| E2F6 | E2F Transcription Factor 6 |
| ER | Endoplasmic Reticulum |
| ES | Exon Skipping |
| GABA | Gamma-Aminobutyric Acid |
| GAT-1 | Gamma-Aminobutyric Acid Transporter 1 |
| GO | Gene Ontology |
| GOLGA8N | Golgin A8 Family Member N |
| IR | Intron Retention |
| IRE1 | Inositol Requiring Enzyme 1 |
| KIRA6 | Kinase-Inhibiting RNase-Attenuator 6 |
| MCIA | Multiple Co-Inertia Analysis |
| MEI | Multiple Exon Inclusion |
| MES | Multiple Exon Skipping |
| METTL13 | Methyltransferase 13, EEF1A N-Terminus And K55 |
| MOI | MultiOmicsIntegrator |
| MPP+ | 1-methyl-4-phenylpyridinium |
| mRNA | Messenger Ribonucleic Acid |
| OSBPL3 | Oxysterol Binding Protein Like 3 |
| PD | Parkinson’s Disease |
| PERK | Protein Kinase (RNA)-like Endoplasmic Reticulum Kinase |
| PLCD4 | Phospholipase C Delta 4 |
| PRNP | Prion Protein (Kanno Blood Group) |
| RIDD | Regulated IRE1-Dependent Decay |
| RNase | Endoribonuclease |
| SLC6A1 | Solute Carrier Family 6 Member 1 |
| STAP2 | Signal Transducing Adaptor Family Member 2 |
| TGM2 | Transglutaminase 2 |
| TJP2 | Tight Junction Protein 2 |
| TPM | Transcripts Per Kilobase Million |
| TH | Tyrosine Hydroxylase |
| UPR | Unfolded Protein Response |
| UTR | Untranslated Regions |
| XBP1 | X-box Binding mRNA 1 |
| XBP1s | Spliced XBP1 |
| XBP1u | Unspliced XBP1 |
References
- Samaila, A.; Hayatudeen, M. Pathophysiology and management of parkinson disease: A review. Bayero J. Pure Appl. Sci. 2019, 11, 238. [Google Scholar] [CrossRef]
- Shahmoradian, S.; Blythe, A.; Genoud, C.; Hench, J.; Moors, T.; Navarro, P.; Castaño-Díez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.; et al. Lewy pathology in parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Shan, J.; Matigian, N.; Todorovic, M.; Cook, A.; Ravishankar, S.; Dong, L.; Neužil, J.; Silburn, P.; Mackay-Sim, A.; et al. Rotenone susceptibility phenotype in olfactory derived patient cells as a model of idiopathic parkinson’s disease. PLoS ONE 2016, 11, e0154544. [Google Scholar] [CrossRef] [PubMed]
- Bélarbi, K.; Cuvelier, E.; Bonte, M.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M. Glycosphingolipids and neuroinflammation in parkinson’s disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef]
- Hoozemans, J.; Van Haastert, E.; Eikelenboom, P.; De Vos, R.; Rozemuller, J.; Scheper, W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007, 354, 707–711. [Google Scholar] [CrossRef]
- Ryu, E.J.; Harding, H.P.; Angelastro, J.M.; Vitolo, O.V.; Ron, D.; Greene, L.A. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J. Neurosci. 2002, 22, 10690–10698. [Google Scholar] [CrossRef]
- Holtz, W.A.; O’Malley, K.L. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J. Biol. Chem. 2003, 278, 19367–19377. [Google Scholar] [CrossRef]
- Maurel, M.; Chevet, E.; Tavernier, J.; Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014, 39, 245–254. [Google Scholar] [CrossRef]
- Cox, J.S.; Shamu, C.E.; Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993, 73, 1197–1206. [Google Scholar] [CrossRef]
- Hollien, J.; Lin, J.H.; Li, H.; Stevens, N.; Walter, P.; Weissman, J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009, 186, 323–331. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Pasat, B.A.; Pilalis, E.; Mnich, K.; Samali, A.; Chatziioannou, A.; Gorman, A.M. MultiOmicsIntegrator—MOI: A nextflow pipeline for integrated omics analyses. Bioinform. Adv. 2024, 4, vbae175. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, A.; Golji, J.; Labadorf, A.T.; Gao, B.; Beach, T.G.; Myers, R.H.; Longo, K.A.; Latourelle, J.C. Integrative analyses of proteomics and RNA transcriptomics implicate mitochondrial processes, protein folding pathways and GWAS loci in Parkinson disease. BMC Med. Genom. 2015, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Erwig, M.S.; Patzig, J.; Steyer, A.M.; Dibaj, P.; Heilmann, M.; Heilmann, I.; Jung, R.B.; Kusch, K.; Möbius, W.; Jahn, O.; et al. Anillin facilitates septin assembly to prevent pathological outfoldings of central nervous system myelin. eLife 2019, 8, e43888. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Paul, K.C.; Bronstein, J.M.; Bordelon, Y.; Horvath, S.; Ritz, B. Parkinson’s disease is associated with DNA methylation levels in human blood and saliva. Genome Med. 2017, 9, 76. [Google Scholar] [CrossRef]
- Yao, H.; Tong, W.; Song, Y.; Li, R.; Xiang, X.; Cheng, W.; Zhou, Y.; He, Y.; Yang, Y.; Liu, Y.; et al. Exercise training upregulates CD55 to suppress complement-mediated synaptic phagocytosis in Parkinson’s disease. J. Neuroinflamm. 2024, 21, 246. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Z.; Li, L.; Zhang, K.; Xu, Y.; Xia, M.; Zhou, J.; Gong, Y.; Chen, J.; Gong, K. ZNF582 overexpression restrains the progression of clear cell renal cell carcinoma by enhancing the binding of TJP2 and ERK2 and inhibiting ERK2 phosphorylation. Cell Death Dis. 2023, 14, 212. [Google Scholar] [CrossRef]
- Wang, C.; He, Y.; He, Y.; Liang, W.; Zhou, C.; Wu, M.; Meng, Z.; Li, W.; Cao, J. Prognostic and biological function value of OSBPL3 in colorectal cancer analyzed by multi-omic data analysis. BMC Gastroenterol. 2023, 23, 270. [Google Scholar] [CrossRef]
- Gorman, A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef]
- Vitting-Seerup, K.; Sandelin, A. The landscape of isoform switches in human cancers. Mol. Cancer Res. 2017, 15, 1206–1220. [Google Scholar] [CrossRef]
- Vitting-Seerup, K.; Sandelin, A. IsoformSwitchAnalyzeR: Analysis of changes in genome-wide patterns of alternative splicing and its functional consequences. Bioinformatics 2019, 35, 4469–4471. [Google Scholar] [CrossRef] [PubMed]
- Le Thomas, A.; Ferri, E.; Marsters, S.; Harnoss, J.M.; Lawrence, D.A.; Zuazo-Gaztelu, I.; Modrusan, Z.; Chan, S.; Solon, M.; Chalouni, C.; et al. Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α. Nat. Commun. 2021, 12, 7310. [Google Scholar] [CrossRef] [PubMed]
- Türei, D.; Valdeolivas, A.; Gul, L.; Palacio-Escat, N.; Klein, M.; Ivanova, O.; Ölbei, M.; Gábor, A.; Theis, F.; Módos, D.; et al. Integrated intra-and intercellular signaling knowledge for multicellular omics analysis. Mol. Syst. Biol. 2021, 17, e9923. [Google Scholar] [CrossRef] [PubMed]
- Belyy, V.; Zuazo-Gaztelu, I.; Alamban, A.; Ashkenazi, A.; Walter, P. Endoplasmic reticulum stress activates human IRE1α through reversible assembly of inactive dimers into small oligomers. eLife 2022, 11, e74342. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 53, S26–S38. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Zeng, L.; Lu, W.; Tang, X.; Zhang, J.; Li, T. Golgi apparatus and neurodegenerative diseases. Int. J. Dev. Neurosci. 2008, 26, 523–534. [Google Scholar] [CrossRef]
- Baba, Y.; Kuroiwa, A.; Uitti, R.J.; Wszolek, Z.K.; Yamada, T. Alterations of T-lymphocyte populations in Parkinson disease. Park. Relat. Disord. 2005, 11, 493–498. [Google Scholar] [CrossRef]
- Koch, S.; Laabs, B.H.; Kasten, M.; Vollstedt, E.J.; Becktepe, J.; Brüggemann, N.; Franke, A.; Krämer, U.M.; Kuhlenbäumer, G.; Lieb, W.; et al. Validity and prognostic value of a polygenic risk score for Parkinson’s disease. Genes 2021, 12, 1859. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E. The role of cannabinoid type 2 receptors in Parkinson’s disease. Biomedicines 2022, 10, 2986. [Google Scholar] [CrossRef]
- Fu, K.A.; Paul, K.C.; Lu, A.T.; Horvath, S.; Keener, A.M.; Bordelon, Y.; Bronstein, J.M.; Ritz, B. DNA methylation-based surrogates of plasma proteins are associated with Parkinson’s disease risk. J. Neurol. Sci. 2021, 431, 120046. [Google Scholar] [CrossRef]
- Lim, H.R.; Vo, M.T.; Kim, D.J.; Lee, U.H.; Yoon, J.H.; Kim, H.J.; Kim, J.; Kim, S.R.; Lee, J.Y.; Yang, C.H.; et al. DRG2 deficient mice exhibit impaired motor behaviors with reduced striatal dopamine release. Int. J. Mol. Sci. 2019, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Gonzalez, C.; Hilgers, V. (Alternative) transcription start sites as regulators of RNA processing. Trends Cell Biol. 2024, 34, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Carew, N.T.; Nelson, A.M.; Liang, Z.; Smith, S.M.; Milcarek, C. Linking endoplasmic reticular stress and alternative splicing. Int. J. Mol. Sci. 2018, 19, 3919. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zhang, H.; Ren, Y.; He, J.; Hao, N. OSBPL3 facilitates endoplasmic reticulum stress and stemness in triple negative breast cancer. Cancer Res. 2024, 84, 1476. [Google Scholar] [CrossRef]
- Hao, N.; Zhou, Y.; Li, Y.; Zhang, H.; Wang, B.; Liu, X.; Ren, Y.; He, J.; Zhou, C.; Tang, X. Clinical value and potential mechanisms of oxysterol-binding protein like 3 (OSBPL3) in human tumors. Front. Mol. Biosci. 2021, 8, 739978. [Google Scholar] [CrossRef]
- Dinh, J.; Yi, D.; Lin, F.; Xue, P.; Holloway, N.D.; Xie, Y.; Ibe, N.U.; Nguyen, H.P.; Viscarra, J.A.; Wang, Y.; et al. The microprotein C16orf74/MICT1 promotes thermogenesis in brown adipose tissue. EMBO J. 2025, 44, 3381–3412. [Google Scholar] [CrossRef]
- Yao, X.R.; Xiao, F.Z.; Xiao, W.T.; Huang, C.Q.; He, J.Y. C16orf74 is a novel prognostic biomarker and associates with immune infiltration in head and neck squamous cell carcinoma. PLoS ONE 2025, 20, e0322701. [Google Scholar] [CrossRef]
- Mermer, F.; Poliquin, S.; Rigsby, K.; Rastogi, A.; Shen, W.; Romero-Morales, A.; Nwosu, G.; McGrath, P.; Demerast, S.; Aoto, J.; et al. Common molecular mechanisms of SLC6A1 variant-mediated neurodevelopmental disorders in astrocytes and neurons. Brain 2021, 144, 2499–2512. [Google Scholar] [CrossRef]
- Xu, Z.; Duan, W.; Yuan, S.; Zhang, X.; You, C.; Yu, J.T.; Wang, J.; Li, J.D.; Deng, S.; Shu, Y. Deep brain stimulation alleviates Parkinsonian motor deficits through desynchronizing GABA release in mice. Nat. Commun. 2025, 16, 3726. [Google Scholar] [CrossRef]
- Tong, Y.; Mukhamejanova, Z.; Zheng, Y.; Wen, T.; Xu, F.; Pang, J. Marine-Derived Xyloketal Compound Ameliorates MPP+-Induced Neuronal Injury through Regulating of the IRE1/XBP1 Signaling Pathway. ACS Chem. Neurosci. 2021, 12, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kou, M.; Deng, Q.; Yu, H.; Mei, J.; Gao, J.; Fu, W.; Ning, B. Acupuncture activates IRE1/XBP1 endoplasmic reticulum stress pathway in Parkinson’s disease model rats. Behav. Brain Res. 2024, 462, 114871. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.R.; Reynolds, R.H.; O’Callaghan, B.; García-Ruiz, S.; Gil-Martínez, A.L.; Botía, J.; Plun-Favreau, H.; Ryten, M. The non-specific lethal complex regulates genes and pathways genetically linked to Parkinson’s disease. Brain 2023, 146, 4974–4987. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Meng, C.; Kuster, B.; Culhane, A.C.; Gholami, A.M. A multivariate approach to the integration of multi-omics datasets. BMC Bioinform. 2014, 15, 162. [Google Scholar] [CrossRef]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for occurrences of a given motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef]
- Sandelin, A.; Alkema, W.; Engström, P.; Wasserman, W.W.; Lenhard, B. JASPAR: An open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004, 32, D91–D94. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasat, B.A.; Moncan, M.; Pilalis, E.; Samali, A.; Chatziioannou, A.; Gorman, A.M. Multiomics Integration of Parkinson’s Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology. Int. J. Mol. Sci. 2025, 26, 6711. https://doi.org/10.3390/ijms26146711
Pasat BA, Moncan M, Pilalis E, Samali A, Chatziioannou A, Gorman AM. Multiomics Integration of Parkinson’s Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology. International Journal of Molecular Sciences. 2025; 26(14):6711. https://doi.org/10.3390/ijms26146711
Chicago/Turabian StylePasat, Bianka Alexandra, Matthieu Moncan, Eleftherios Pilalis, Afshin Samali, Aristotelis Chatziioannou, and Adrienne M. Gorman. 2025. "Multiomics Integration of Parkinson’s Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology" International Journal of Molecular Sciences 26, no. 14: 6711. https://doi.org/10.3390/ijms26146711
APA StylePasat, B. A., Moncan, M., Pilalis, E., Samali, A., Chatziioannou, A., & Gorman, A. M. (2025). Multiomics Integration of Parkinson’s Disease Datasets Reveals Unexpected Roles of IRE1 in Its Pathology. International Journal of Molecular Sciences, 26(14), 6711. https://doi.org/10.3390/ijms26146711








