Rational Modulation of Liquid–Liquid Phase Separation Offers Novel Ways to Combat Tauopathies
Abstract
1. Introduction
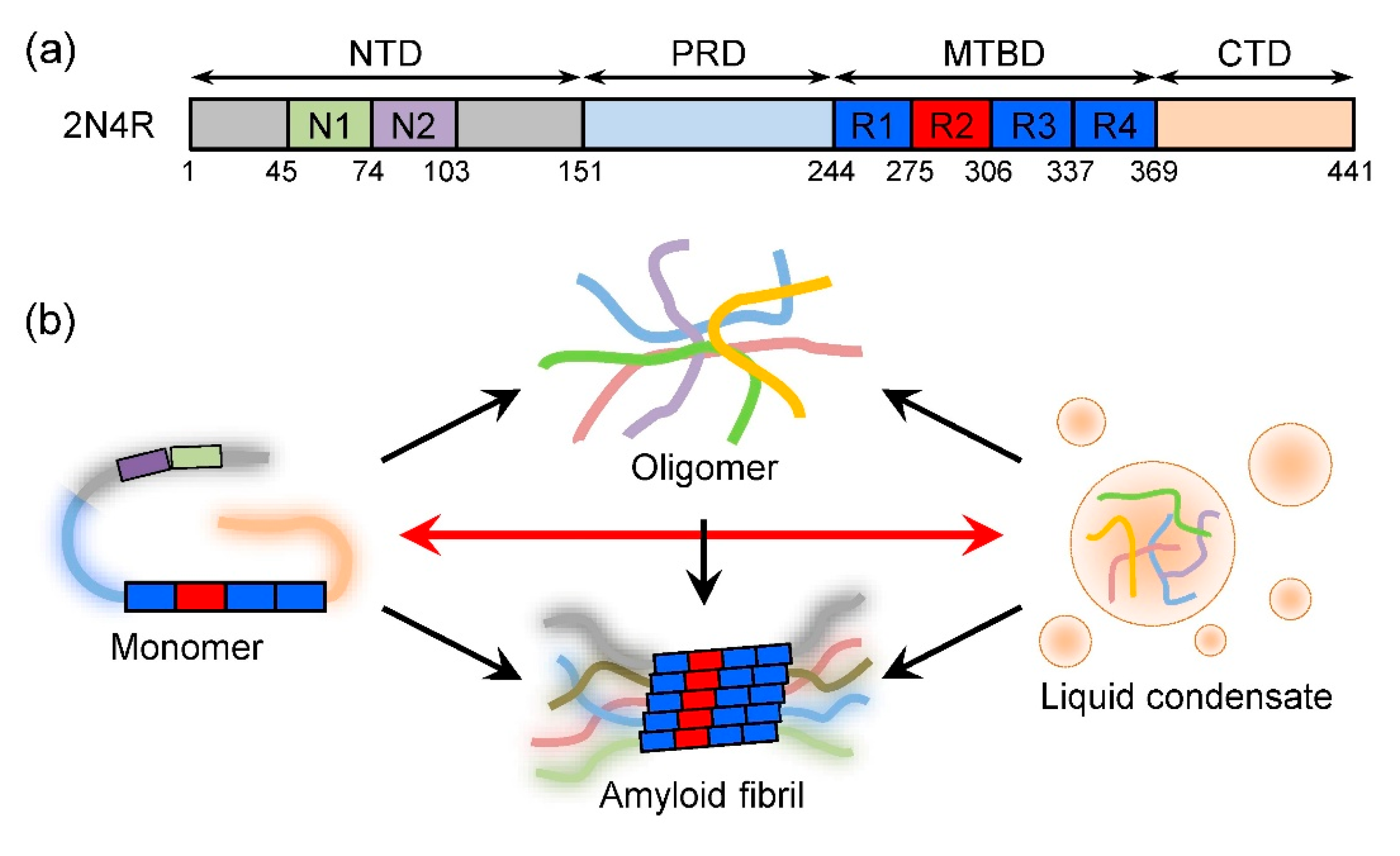
2. Tau Protein and Tauopathies
2.1. Tau Physiology
2.2. Structure of Tau Monomer
2.3. Tau Aggregation and Diseases
3. Liquid Condensates: A New Phase Linked to Tauopathies
3.1. LLPS of Tau
3.2. Physiological Roles of Tau LLPS
3.3. Linking Tau LLPS to Tauopathies
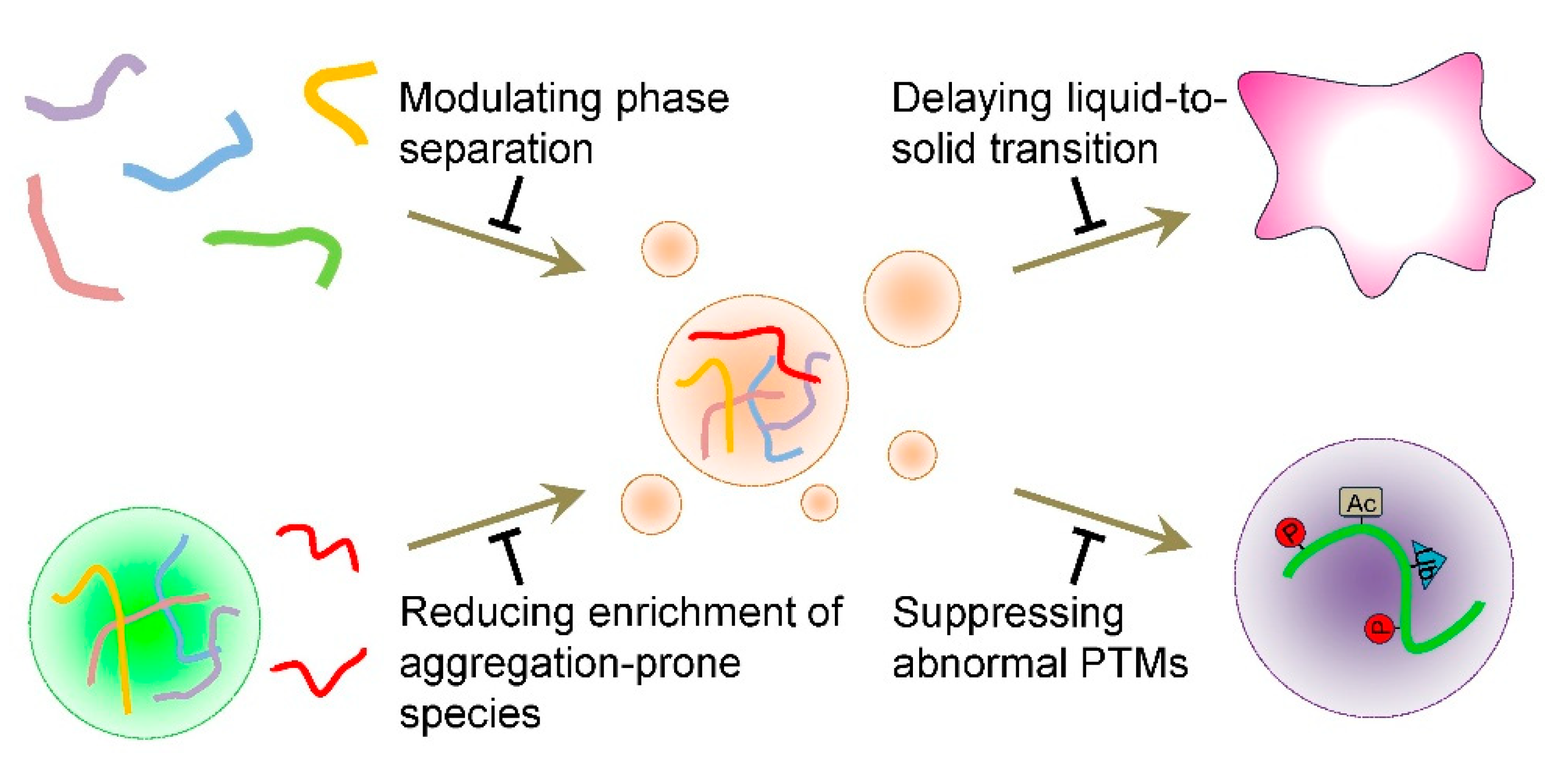
4. Combating Tauopathies in the Context of LLPS
4.1. Modulating Tau Condensate Formation
4.2. Delaying the Liquid-to-Solid Transition of Tau Condensates
4.3. Reducing the Concentration of Aggregation-Prone Species Within Tau Condensates
4.4. Suppressing Abnormal PTMs on Tau Within Condensates
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Venkatramani, A.; Panda, D. Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies. Int. J. Biol. Macromol. 2019, 133, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Parra Bravo, C.; Naguib, S.A.; Gan, L. Cellular and pathological functions of tau. Nat. Rev. Mol. Cell Biol. 2024, 25, 845–864. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein t (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef]
- Wood, J.G.; Mirra, S.S.; Pollock, N.J.; Binder, L.I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (t). Proc. Natl. Acad. Sci. USA 1986, 83, 4040–4043. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, R.; Gu, J.; Tung, Y.C.; Zhou, Y.; Zhou, D.; Wu, R.; Chu, D.; Jin, N.; Deng, K.; et al. Alzheimer’s disease brain contains tau fractions with differential prion-like activities. Acta Neuropathol. Commun. 2021, 9, 28. [Google Scholar] [CrossRef]
- Stancu, I.C.; Vasconcelos, B.; Ris, L.; Wang, P.; Villers, A.; Peeraer, E.; Buist, A.; Terwel, D.; Baatsen, P.; Oyelami, T.; et al. Templated misfolding of tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in tau transgenic mice. Acta Neuropathol. 2015, 129, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Dimou, E.; Katsinelos, T.; Meisl, G.; Tuck, B.J.; Keeling, S.; Smith, A.E.; Hidari, E.; Lam, J.Y.L.; Burke, M.; Lovestam, S.; et al. Super-resolution imaging unveils the self-replication of tau aggregates upon seeding. Cell Rep. 2023, 42, 112725. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zweckstetter, M. Phase separation of the microtubule-associated protein tau. Essays Biochem. 2022, 66, 1013–1021. [Google Scholar] [CrossRef]
- Boyko, S.; Surewicz, W.K. Tau liquid-liquid phase separation in neurodegenerative diseases. Trends Cell Biol. 2022, 32, 611–623. [Google Scholar] [CrossRef]
- Islam, M.; Shen, F.; Regmi, D.; Petersen, K.; Karim, M.R.U.; Du, D. Tau liquid-liquid phase separation: At the crossroads of tau physiology and tauopathy. J. Cell. Physiol. 2024, 239, e30853. [Google Scholar] [CrossRef] [PubMed]
- Ainani, H.; Bouchmaa, N.; Ben Mrid, R.; El Fatimy, R. Liquid-liquid phase separation of protein tau: An emerging process in Alzheimer’s disease pathogenesis. Neurobiol. Dis. 2023, 178, 106011. [Google Scholar] [CrossRef]
- Wegmann, S. Liquid-liquid phase separation of tau protein in neurobiology and pathology. Adv. Exp. Med. Biol. 2019, 1184, 341–357. [Google Scholar] [CrossRef]
- Kosik, K.S.; Han, S. Tau condensates. Adv. Exp. Med. Biol. 2019, 1184, 327–339. [Google Scholar] [CrossRef]
- Hernandez-Vega, A.; Braun, M.; Scharrel, L.; Jahnel, M.; Wegmann, S.; Hyman, B.T.; Alberti, S.; Diez, S.; Hyman, A.A. Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep. 2017, 20, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Lam, A.J.; Tan, T.; Han, J.; Nowakowski, D.W.; Vershinin, M.; Simo, S.; Ori-McKenney, K.M.; McKenney, R.J. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 2019, 21, 1078–1085. [Google Scholar] [CrossRef]
- Siahaan, V.; Krattenmacher, J.; Hyman, A.A.; Diez, S.; Hernandez-Vega, A.; Lansky, Z.; Braun, M. Kinetically distinct phases of tau on microtubules regulate kinesin motors and severing enzymes. Nat. Cell Biol. 2019, 21, 1086–1092. [Google Scholar] [CrossRef]
- Ambadipudi, S.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein tau. Nat. Commun. 2017, 8, 275. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Hamel, C.; Grabinski, T.; Combs, B. Liquid-liquid phase separation induces pathogenic tau conformations in vitro. Nat. Commun. 2020, 11, 2809. [Google Scholar] [CrossRef]
- Wegmann, S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 2018, 37, e98049. [Google Scholar] [CrossRef]
- Boyko, S.; Surewicz, K.; Surewicz, W.K. Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2020, 117, 31882–31890. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, H.; Cai, Q.; Tai, H.C. The enigma of tau protein aggregation: Mechanistic insights and future challenges. Int. J. Mol. Sci. 2024, 25, 4969. [Google Scholar] [CrossRef]
- Su, Q.; Mehta, S.; Zhang, J. Liquid-liquid phase separation: Orchestrating cell signaling through time and space. Mol. Cell 2021, 81, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H. Phase-separated condensates in autophagosome formation and autophagy regulation. J. Mol. Biol. 2025, 168964. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Ma, W.; Yang, B.; Lu, H.; Zhou, F.; Zhang, L. Post-translational modifications in liquid-liquid phase separation: A comprehensive review. Mol. Biomed. 2022, 3, 13. [Google Scholar] [CrossRef]
- Scholl, D.; Deniz, A.A. Conformational freedom and topological confinement of proteins in biomolecular condensates. J. Mol. Biol. 2022, 434, 167348. [Google Scholar] [CrossRef]
- Neve, R.L.; Harris, P.; Kosik, K.S.; Kurnit, D.M.; Donlon, T.A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986, 387, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Potier, M.C.; Ulrich, J.; Crowther, R.A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: Differential expression of tau protein mRNAs in human brain. EMBO J. 1989, 8, 393–399. [Google Scholar] [CrossRef]
- Takuma, H.; Arawaka, S.; Mori, H. Isoforms changes of tau protein during development in various species. Brain Res. Dev. Brain Res. 2003, 142, 121–127. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Chen, S.; Townsend, K.; Goldberg, T.E.; Davies, P.; Conejero-Goldberg, C. MAPT isoforms: Differential transcriptional profiles related to 3R and 4R splice variants. J. Alzheimer’s Dis. 2010, 22, 1313–1329. [Google Scholar] [CrossRef]
- Bachmann, S.; Bell, M.; Klimek, J.; Zempel, H. Differential effects of the six human TAU isoforms: Somatic retention of 2N-TAU and increased microtubule number induced by 4R-TAU. Front. Neurosci. 2021, 15, 643115. [Google Scholar] [CrossRef]
- Kadavath, H.; Cabrales Fontela, Y.; Jaremko, M.; Jaremko, L.; Overkamp, K.; Biernat, J.; Mandelkow, E.; Zweckstetter, M. The binding mode of a tau peptide with tubulin. Angew. Chem. Int. Ed. Engl. 2018, 57, 3246–3250. [Google Scholar] [CrossRef]
- Preuss, U.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. The ‘jaws’ model of tau-microtubule interaction examined in CHO cells. J. Cell Sci. 1997, 110 Pt 6, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.H.; Hejab, N.M.A.; Poepsel, S.; Downing, K.H.; DiMaio, F.; Nogales, E. Near-atomic model of microtubule-tau interactions. Science 2018, 360, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R. Expression of separate isoforms of human tau protein: Correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990, 9, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Tracy, T.E.; Madero-Perez, J.; Swaney, D.L.; Chang, T.S.; Moritz, M.; Konrad, C.; Ward, M.E.; Stevenson, E.; Huttenhain, R.; Kauwe, G.; et al. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell 2022, 185, 712–728. [Google Scholar] [CrossRef]
- Morris, M.; Maeda, S.; Vossel, K.; Mucke, L. The many faces of tau. Neuron 2011, 70, 410–426. [Google Scholar] [CrossRef]
- Papin, S.; Paganetti, P. Emerging evidences for an implication of the neurodegeneration-associated protein tau in cancer. Brain Sci. 2020, 10, 862. [Google Scholar] [CrossRef]
- Alonso, A.D.C.; El Idrissi, A.; Candia, R.; Morozova, V.; Kleiman, F.E. Tau: More than a microtubule-binding protein in neurons. Cytoskeleton 2024, 81, 71–77. [Google Scholar] [CrossRef]
- Liu, C.; Song, X.; Nisbet, R.; Gotz, J. Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions and highlights a putative role for 2N tau in disease. J. Biol. Chem. 2016, 291, 8173–8188. [Google Scholar] [CrossRef] [PubMed]
- Stefanoska, K.; Volkerling, A.; Bertz, J.; Poljak, A.; Ke, Y.D.; Ittner, L.M.; Ittner, A. An N-terminal motif unique to primate tau enables differential protein-protein interactions. J. Biol. Chem. 2018, 293, 3710–3719. [Google Scholar] [CrossRef]
- Kurochkina, N.; Guha, U. SH3 domains: Modules of protein-protein interactions. Biophys. Rev. 2013, 5, 29–39. [Google Scholar] [CrossRef]
- Lau, D.H.; Hogseth, M.; Phillips, E.C.; O’Neill, M.J.; Pooler, A.M.; Noble, W.; Hanger, D.P. Critical residues involved in tau binding to fyn: Implications for tau phosphorylation in Alzheimer’s disease. Acta Neuropathol. Commun. 2016, 4, 49. [Google Scholar] [CrossRef]
- Lee, G.; Newman, S.T.; Gard, D.L.; Band, H.; Panchamoorthy, G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 1998, 111 Pt 21, 3167–3177. [Google Scholar] [CrossRef] [PubMed]
- Sottejeau, Y.; Bretteville, A.; Cantrelle, F.X.; Malmanche, N.; Demiaute, F.; Mendes, T.; Delay, C.; Alves Dos Alves, H.; Flaig, A.; Davies, P.; et al. Tau phosphorylation regulates the interaction between BIN1’s SH3 domain and tau’s proline-rich domain. Acta Neuropathol. Commun. 2015, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, J.; Zhang, B.; Gao, M.; Su, Z.; Huang, Y. The structure and phase of tau: From monomer to amyloid filament. Cell. Mol. Life Sci. 2021, 78, 1873–1886. [Google Scholar] [CrossRef]
- Avila, J.; Jimenez, J.S.; Sayas, C.L.; Bolos, M.; Zabala, J.C.; Rivas, G.; Hernandez, F. Tau structures. Front. Aging Neurosci. 2016, 8, 262. [Google Scholar] [CrossRef]
- Jeganathan, S.; von Bergen, M.; Brutlach, H.; Steinhoff, H.J.; Mandelkow, E. Global hairpin folding of tau in solution. Biochemistry 2006, 45, 2283–2293. [Google Scholar] [CrossRef]
- Mylonas, E.; Hascher, A.; Bernado, P.; Blackledge, M.; Mandelkow, E.; Svergun, D.I. Domain conformation of tau protein studied by solution small-angle X-ray scattering. Biochemistry 2008, 47, 10345–10353. [Google Scholar] [CrossRef]
- Nath, A.; Sammalkorpi, M.; DeWitt, D.C.; Trexler, A.J.; Elbaum-Garfinkle, S.; O’Hern, C.S.; Rhoades, E. The conformational ensembles of a-synuclein and tau: Combining single-molecule FRET and simulations. Biophys. J. 2012, 103, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hong, L.; Krainer, G.; Yao, Q.Q.; Knowles, T.P.J.; Wu, S.; Perrett, S. Conformational expansion of tau in condensates promotes irreversible aggregation. J. Am. Chem. Soc. 2021, 143, 13056–13064. [Google Scholar] [CrossRef] [PubMed]
- Mirbaha, H.; Chen, D.; Morazova, O.A.; Ruff, K.M.; Sharma, A.M.; Liu, X.; Goodarzi, M.; Pappu, R.V.; Colby, D.W.; Mirzaei, H.; et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife 2018, 7, e36584. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Drombosky, K.W.; Hou, Z.; Sari, L.; Kashmer, O.M.; Ryder, B.D.; Perez, V.A.; Woodard, D.R.; Lin, M.M.; Diamond, M.I.; et al. Tau local structure shields an amyloid-forming motif and controls aggregation propensity. Nat. Commun. 2019, 10, 2493. [Google Scholar] [CrossRef]
- Mirbaha, H.; Chen, D.; Mullapudi, V.; Terpack, S.J.; White, C.L., 3rd; Joachimiak, L.A.; Diamond, M.I. Seed-competent tau monomer initiates pathology in a tauopathy mouse model. J. Biol. Chem. 2022, 298, 102163. [Google Scholar] [CrossRef]
- Manger, L.H.; Foote, A.K.; Wood, S.L.; Holden, M.R.; Heylman, K.D.; Margittai, M.; Goldsmith, R.H. Revealing conformational variants of solution-phase intrinsically disordered tau protein at the single-molecule level. Angew. Chem. Int. Ed. Engl. 2017, 56, 15584–15588. [Google Scholar] [CrossRef]
- Foote, A.K.; Manger, L.H.; Holden, M.R.; Margittai, M.; Goldsmith, R.H. Time-resolved multirotational dynamics of single solution-phase tau proteins reveals details of conformational variation. Phys. Chem. Chem. Phys. 2019, 21, 1863–1871. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Goedert, M. Cryo-EM structures of tau filaments from human brain. Essays Biochem. 2021, 65, 949–959. [Google Scholar] [CrossRef]
- Scheres, S.H.W.; Ryskeldi-Falcon, B.; Goedert, M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature 2023, 621, 701–710. [Google Scholar] [CrossRef]
- Mengham, K.; Al-Hilaly, Y.; Oakley, S.; Kasbi, K.; Maina, M.B.; Serpell, L.C. Shapeshifting tau: From intrinsically disordered to paired-helical filaments. Essays Biochem. 2022, 66, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Limorenko, G.; Lashuel, H.A. Revisiting the grammar of tau aggregation and pathology formation: How new insights from brain pathology are shaping how we study and target tauopathies. Chem. Soc. Rev. 2022, 51, 513–565. [Google Scholar] [CrossRef] [PubMed]
- Gotz, J.; Halliday, G.; Nisbet, R.M. Molecular pathogenesis of the tauopathies. Annu. Rev. Pathol. 2019, 14, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Feuillette, S.; Miguel, L.; Frebourg, T.; Campion, D.; Lecourtois, M. Drosophila models of human tauopathies indicate that tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein. J. Neurochem. 2010, 113, 895–903. [Google Scholar] [CrossRef]
- d’Orange, M.; Auregan, G.; Cheramy, D.; Gaudin-Guerif, M.; Lieger, S.; Guillermier, M.; Stimmer, L.; Josephine, C.; Herard, A.S.; Gaillard, M.C.; et al. Potentiating tangle formation reduces acute toxicity of soluble tau species in the rat. Brain 2018, 141, 535–549. [Google Scholar] [CrossRef]
- Watamura, N.; Foiani, M.S.; Bez, S.; Bourdenx, M.; Santambrogio, A.; Frodsham, C.; Camporesi, E.; Brinkmalm, G.; Zetterberg, H.; Patel, S.; et al. In vivo hyperphosphorylation of tau is associated with synaptic loss and behavioral abnormalities in the absence of tau seeds. Nat. Neurosci. 2025, 28, 293–307. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Kopeikina, K.J.; Koffie, R.M.; de Calignon, A.; Hyman, B.T. Are tangles as toxic as they look? J. Mol. Neurosci. 2011, 45, 438–444. [Google Scholar] [CrossRef]
- Bonda, D.J.; Castellani, R.J.; Zhu, X.; Nunomura, A.; Lee, H.G.; Perry, G.; Smith, M.A. A novel perspective on tau in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 639–642. [Google Scholar] [CrossRef]
- Boccalini, C.; Ribaldi, F.; Hristovska, I.; Arnone, A.; Peretti, D.E.; Mu, L.; Scheffler, M.; Perani, D.; Frisoni, G.B.; Garibotto, V. The impact of tau deposition and hypometabolism on cognitive impairment and longitudinal cognitive decline. Alzheimer’s Dement. 2024, 20, 221–233. [Google Scholar] [CrossRef]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.L.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C.; et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Schmitt, F.A.; Kryscio, R.J.; Davis, D.G.; Smith, C.D.; Wekstein, D.R. Neuropathologic substrate of mild cognitive impairment. Arch. Neurol. 2006, 63, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Krammer, C.; Schatzl, H.M.; Vorberg, I. Prion-like propagation of cytosolic protein aggregates: Insights from cell culture models. Prion 2009, 3, 206–212. [Google Scholar] [CrossRef]
- Darricau, M.; Dou, C.; Kinet, R.; Zhu, T.; Zhou, L.; Li, X.; Bedel, A.; Claverol, S.; Tokarski, C.; Katsinelos, T.; et al. Tau seeds from Alzheimer’s disease brains trigger tau spread in macaques while oligomeric-Ab mediates pathology maturation. Alzheimer’s Dement. 2024, 20, 1894–1912. [Google Scholar] [CrossRef]
- Wang, J.; Williams, C.K.; DeTure, M.A.; Magaki, S.D.; Dickson, D.W.; Vinters, H.V.; Seidler, P.M. Tau seeds catalyze fibril-type structures from GFP tau biosensor cells. Structure 2024, 32, 2251–2258. [Google Scholar] [CrossRef]
- Woerman, A.L.; Aoyagi, A.; Patel, S.; Kazmi, S.A.; Lobach, I.; Grinberg, L.T.; McKee, A.C.; Seeley, W.W.; Olson, S.H.; Prusiner, S.B. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc. Natl. Acad. Sci. USA 2016, 113, E8187–E8196. [Google Scholar] [CrossRef]
- Zbinden, A.; Perez-Berlanga, M.; De Rossi, P.; Polymenidou, M. Phase separation and neurodegenerative diseases: A disturbance in the force. Dev. Cell 2020, 55, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Babinchak, W.M.; Surewicz, W.K. Liquid-liquid phase separation and its mechanistic role in pathological protein aggregation. J. Mol. Biol. 2020, 432, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ford, L.K.; Fioriti, L.; McGurk, L.; Zhang, M. Liquid-liquid phase separation in physiology and pathophysiology of the nervous system. J. Neurosci. 2021, 41, 834–844. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Eschmann, N.A.; Zhou, H.; Rauch, J.N.; Hernandez, I.; Guzman, E.; Kosik, K.S.; Han, S. RNA stores tau reversibly in complex coacervates. PLoS Biol. 2017, 15, e2002183. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Wang, X.; Su, Z.; Gao, M.; Huang, Y. Liquid-liquid phase separation of tau: Driving forces, regulation, and biological implications. Neurobiol. Dis. 2023, 183, 106167. [Google Scholar] [CrossRef]
- Rai, S.K.; Savastano, A.; Singh, P.; Mukhopadhyay, S.; Zweckstetter, M. Liquid-liquid phase separation of tau: From molecular biophysics to physiology and disease. Protein Sci. 2021, 30, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular phase separation: From molecular driving forces to macroscopic properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Tompa, P.; Pappu, R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015, 11, 899–904. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Alberti, S. Molecular determinants of condensate composition. Mol. Cell 2025, 85, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Boyko, S.; Qi, X.; Chen, T.H.; Surewicz, K.; Surewicz, W.K. Liquid-liquid phase separation of tau protein: The crucial role of electrostatic interactions. J. Biol. Chem. 2019, 294, 11054–11059. [Google Scholar] [CrossRef]
- Najafi, S.; Lin, Y.; Longhini, A.P.; Zhang, X.; Delaney, K.T.; Kosik, K.S.; Fredrickson, G.H.; Shea, J.E.; Han, S. Liquid-liquid phase separation of tau by self and complex coacervation. Protein Sci. 2021, 30, 1393–1407. [Google Scholar] [CrossRef]
- Abasi, L.S.; Elathram, N.; Movva, M.; Deep, A.; Corbett, K.D.; Debelouchina, G.T. Phosphorylation regulates tau’s phase separation behavior and interactions with chromatin. Commun. Biol. 2024, 7, 251. [Google Scholar] [CrossRef]
- Rai, S.K.; Khanna, R.; Avni, A.; Mukhopadhyay, S. Heterotypic electrostatic interactions control complex phase separation of tau and prion into multiphasic condensates and co-aggregates. Proc. Natl. Acad. Sci. USA 2023, 120, e2216338120. [Google Scholar] [CrossRef]
- Gracia, P.; Polanco, D.; Tarancon-Diez, J.; Serra, I.; Bracci, M.; Oroz, J.; Laurents, D.V.; Garcia, I.; Cremades, N. Molecular mechanism for the synchronized electrostatic coacervation and co-aggregation of alpha-synuclein and tau. Nat. Commun. 2022, 13, 4586. [Google Scholar] [CrossRef]
- Han, Y.; Ye, H.; Li, P.; Zeng, Y.; Yang, J.; Gao, M.; Su, Z.; Huang, Y. In vitro characterization and molecular dynamics simulation reveal mechanism of 14-3-3z regulated phase separation of the tau protein. Int. J. Biol. Macromol. 2022, 208, 1072–1081. [Google Scholar] [CrossRef]
- Moreira, G.G.; Gomes, C.M. Tau liquid-liquid phase separation is modulated by the Ca(2+) -switched chaperone activity of the S100B protein. J. Neurochem. 2023, 166, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ash, P.E.A.; Lei, S.; Shattuck, J.; Boudeau, S.; Carlomagno, Y.; Medalla, M.; Mashimo, B.L.; Socorro, G.; Al-Mohanna, L.F.A.; Jiang, L.; et al. TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau. Proc. Natl. Acad. Sci. USA 2021, 118, e2014188118. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, C.F.; Ibanez de Opakua, A.; Cima-Omori, M.S.; Zweckstetter, M. Determining the physico-chemical composition of biomolecular condensates from spatially-resolved NMR. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218078. [Google Scholar] [CrossRef]
- Majumdar, A.; Dogra, P.; Maity, S.; Mukhopadhyay, S. Liquid-liquid phase separation is driven by large-scale conformational unwinding and fluctuations of intrinsically disordered protein molecules. J. Phys. Chem. Lett. 2019, 10, 3929–3936. [Google Scholar] [CrossRef]
- Zhang, X.; Vigers, M.; McCarty, J.; Rauch, J.N.; Fredrickson, G.H.; Wilson, M.Z.; Shea, J.E.; Han, S.; Kosik, K.S. The proline-rich domain promotes tau liquid-liquid phase separation in cells. J. Cell Biol. 2020, 219, e202006054. [Google Scholar] [CrossRef]
- Hochmair, J.; Exner, C.; Franck, M.; Dominguez-Baquero, A.; Diez, L.; Brognaro, H.; Kraushar, M.L.; Mielke, T.; Radbruch, H.; Kaniyappan, S.; et al. Molecular crowding and RNA synergize to promote phase separation, microtubule interaction, and seeding of tau condensates. EMBO J. 2022, 41, e108882. [Google Scholar] [CrossRef]
- Song, X.; Yang, F.; Yang, T.; Wang, Y.; Ding, M.; Li, L.; Xu, P.; Liu, S.; Dai, M.; Chi, C.; et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat. Cell Biol. 2022, 25, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, A.; Ashtam, A.; Panda, D. EB1 increases the dynamics of tau droplets and inhibits tau aggregation: Implications in tauopathies. ACS Chem. Neurosci. 2024, 15, 1219–1233. [Google Scholar] [CrossRef]
- Siahaan, V.; Tan, R.; Humhalova, T.; Libusova, L.; Lacey, S.E.; Tan, T.; Dacy, M.; Ori-McKenney, K.M.; McKenney, R.J.; Braun, M.; et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat. Chem. Biol. 2022, 18, 1224–1235. [Google Scholar] [CrossRef]
- Longfield, S.F.; Mollazade, M.; Wallis, T.P.; Gormal, R.S.; Joensuu, M.; Wark, J.R.; van Waardenberg, A.J.; Small, C.; Graham, M.E.; Meunier, F.A.; et al. Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles. Nat. Commun. 2023, 14, 7277. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein phase separation: A new phase in cell biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Lyon, A.S.; Peeples, W.B.; Rosen, M.K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 2021, 22, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Pappu, R.V.; Taylor, J.P. Beyond aggregation: Pathological phase transitions in neurodegenerative disease. Science 2020, 370, 56–60. [Google Scholar] [CrossRef]
- Silva, J.L.; Foguel, D.; Ferreira, V.F.; Vieira, T.; Marques, M.A.; Ferretti, G.D.S.; Outeiro, T.F.; Cordeiro, Y.; de Oliveira, G.A.P. Targeting biomolecular condensation and protein aggregation against cancer. Chem. Rev. 2023, 123, 9094–9138. [Google Scholar] [CrossRef]
- Chen, K.; Cao, X. Biomolecular condensates: Phasing in regulated host–pathogen interactions. Trends Immunol. 2025, 46, 29–45. [Google Scholar] [CrossRef]
- Lilek, J.; Ajroud, K.; Feldman, A.Z.; Krishnamachari, S.; Ghourchian, S.; Gefen, T.; Spencer, C.L.; Kawles, A.; Mao, Q.; Tranovich, J.F.; et al. Accumulation of pTau231 at the postsynaptic density in early Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 92, 241–260. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, D.; Savastano, A.; Varga, S.J.; Cima-Omori, M.S.; Becker, S.; Honigmann, A.; Zweckstetter, M. Multivalent Tau/PSD-95 interactions arrest in vitro condensates and clusters mimicking the postsynaptic density. Nat. Commun. 2023, 14, 6839. [Google Scholar] [CrossRef]
- Lucas, L.; Tsoi, P.S.; Ferreon, J.C.; Ferreon, A.C.M. Tau oligomers resist phase separation. Biomolecules 2025, 15, 336. [Google Scholar] [CrossRef]
- Soeda, Y.; Yoshimura, H.; Bannai, H.; Koike, R.; Shiiba, I.; Takashima, A. Intracellular tau fragment droplets serve as seeds for tau fibrils. Structure 2024, 32, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, X.; Tang, Y.; Tan, Y.; Guo, C.; Pan, T.; Zhang, X.; Luo, J.; Wei, G. Pathogenic mutation DK280 promotes hydrophobic interactions involving microtubule-binding domain and enhances liquid-liquid phase separation of tau. Small 2025, 21, e2406429. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fichou, Y.; Longhini, A.P.; Llanes, L.C.; Yin, P.; Bazan, G.C.; Kosik, K.S.; Han, S. Liquid-liquid phase separation of tau driven by hydrophobic interaction facilitates fibrillization of tau. J. Mol. Biol. 2021, 433, 166731. [Google Scholar] [CrossRef]
- Alquezar, C.; Arya, S.; Kao, A.W. Tau post-translational modifications: Dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 2021, 11, 595532. [Google Scholar] [CrossRef]
- Ye, H.; Han, Y.; Li, P.; Su, Z.; Huang, Y. The role of post-translational modifications on the structure and function of tau protein. J. Mol. Neurosci. 2022, 72, 1557–1571. [Google Scholar] [CrossRef]
- Chen, J.; Ma, W.; Yu, J.; Wang, X.; Qian, H.; Li, P.; Ye, H.; Han, Y.; Su, Z.; Gao, M.; et al. (-)-Epigallocatechin-3-gallate, a polyphenol from green tea, regulates the liquid-liquid phase separation of Alzheimer’s-related protein tau. J. Agric. Food Chem. 2023, 71, 1982–1993. [Google Scholar] [CrossRef]
- Powell, W.C.; Nahum, M.; Pankratz, K.; Herlory, M.; Greenwood, J.; Poliyenko, D.; Holland, P.; Jing, R.; Biggerstaff, L.; Stowell, M.H.B.; et al. Post-translational modifications control phase transitions of tau. ACS Cent. Sci. 2024, 10, 2145–2161. [Google Scholar] [CrossRef]
- Ukmar-Godec, T.; Hutten, S.; Grieshop, M.P.; Rezaei-Ghaleh, N.; Cima-Omori, M.S.; Biernat, J.; Mandelkow, E.; Soding, J.; Dormann, D.; Zweckstetter, M. Lysine/RNA-interactions drive and regulate biomolecular condensation. Nat. Commun. 2019, 10, 2909. [Google Scholar] [CrossRef]
- Ferreon, J.C.; Jain, A.; Choi, K.J.; Tsoi, P.S.; MacKenzie, K.R.; Jung, S.Y.; Ferreon, A.C. Acetylation disfavors tau phase separation. Int. J. Mol. Sci. 2018, 19, 1360. [Google Scholar] [CrossRef]
- Trivellato, D.; Floriani, F.; Giorgio Barracchia, C.; Munari, F.; D’Onofrio, M.; Assfalg, M. Site-directed double monoubiquitination of the repeat domain of the amyloid-forming protein tau impairs self-assembly and coacervation. Bioorg. Chem. 2023, 132, 106347. [Google Scholar] [CrossRef]
- Parolini, F.; Tira, R.; Barracchia, C.G.; Munari, F.; Capaldi, S.; D’Onofrio, M.; Assfalg, M. Ubiquitination of Alzheimer’s-related tau protein affects liquid-liquid phase separation in a site- and cofactor-dependent manner. Int. J. Biol. Macromol. 2022, 201, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Allahyartorkaman, M.; Chan, T.H.; Chen, E.H.; Ng, S.T.; Chen, Y.A.; Wen, J.K.; Ho, M.R.; Yen, H.Y.; Kuan, Y.S.; Kuo, M.H.; et al. Phosphorylation-induced self-coacervation versus RNA-assisted complex coacervation of tau proteins. J. Am. Chem. Soc. 2025, 147, 10172–10187. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Xu, L.; Boyko, S.; Surewicz, K.; Surewicz, W.K. Zinc promotes liquid-liquid phase separation of tau protein. J. Biol. Chem. 2020, 295, 5850–5856. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Zhong, T.; Wang, L.Q.; Zhang, N.; Zeng, Y.; Hu, J.Y.; Dang, H.B.; Chen, J.; Liang, Y. Zinc enhances liquid-liquid phase separation of tau protein and aggravates mitochondrial damages in cells. Int. J. Biol. Macromol. 2022, 209, 703–715. [Google Scholar] [CrossRef]
- Yatoui, D.; Tsvetkov, P.O.; La Rocca, R.; Baksheeva, V.E.; Allegro, D.; Breuzard, G.; Ferracci, G.; Byrne, D.; Devred, F. Binding of two zinc ions promotes liquid-liquid phase separation of tau. Int. J. Biol. Macromol. 2022, 223, 1223–1229. [Google Scholar] [CrossRef]
- Mukherjee, S.; Panda, D. Contrasting effects of ferric and ferrous ions on oligomerization and droplet formation of tau: Implications in tauopathies and neurodegeneration. ACS Chem. Neurosci. 2021, 12, 4393–4405. [Google Scholar] [CrossRef]
- Brunello, C.A.; Yan, X.; Huttunen, H.J. Internalized tau sensitizes cells to stress by promoting formation and stability of stress granules. Sci. Rep. 2016, 6, 30498. [Google Scholar] [CrossRef]
- Vanderweyde, T.; Yu, H.; Varnum, M.; Liu-Yesucevitz, L.; Citro, A.; Ikezu, T.; Duff, K.; Wolozin, B. Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies. J. Neurosci. 2012, 32, 8270–8283. [Google Scholar] [CrossRef]
- Apicco, D.J.; Ash, P.E.A.; Maziuk, B.; LeBlang, C.; Medalla, M.; Al Abdullatif, A.; Ferragud, A.; Botelho, E.; Ballance, H.I.; Dhawan, U.; et al. Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat. Neurosci. 2018, 21, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Maziuk, B.F.; Apicco, D.J.; Cruz, A.L.; Jiang, L.; Ash, P.E.A.; da Rocha, E.L.; Zhang, C.; Yu, W.H.; Leszyk, J.; Abisambra, J.F.; et al. RNA binding proteins co-localize with small tau inclusions in tauopathy. Acta Neuropathol. Commun. 2018, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.Y.; Ye, L.Q.; Li, H.L. Molecular interaction of stress granules with tau and autophagy in Alzheimer’s disease. Neurochem. Int. 2022, 157, 105342. [Google Scholar] [CrossRef]
- Advani, V.M.; Ivanov, P. Stress granule subtypes: An emerging link to neurodegeneration. Cell. Mol. Life Sci. 2020, 77, 4827–4845. [Google Scholar] [CrossRef]
- Asadi, M.R.; Sadat Moslehian, M.; Sabaie, H.; Jalaiei, A.; Ghafouri-Fard, S.; Taheri, M.; Rezazadeh, M. Stress granules and neurodegenerative disorders: A scoping review. Front. Aging Neurosci. 2021, 13, 650740. [Google Scholar] [CrossRef]
- VandeVrede, L.; Boxer, A.L.; Polydoro, M. Targeting tau: Clinical trials and novel therapeutic approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef] [PubMed]
- Soeda, Y.; Takashima, A. New insights into drug discovery targeting tau protein. Front. Mol. Neurosci. 2020, 13, 590896. [Google Scholar] [CrossRef]
- Kilgore, H.R.; Young, R.A. Learning the chemical grammar of biomolecular condensates. Nat. Chem. Biol. 2022, 18, 1298–1306. [Google Scholar] [CrossRef]
- Babu, M.; Favretto, F.; Rankovic, M.; Zweckstetter, M. Peptidyl prolyl isomerase A modulates the liquid-liquid phase separation of proline-rich IDPs. J. Am. Chem. Soc. 2022, 144, 16157–16163. [Google Scholar] [CrossRef]
- Tira, R.; Viola, G.; Barracchia, C.G.; Parolini, F.; Munari, F.; Capaldi, S.; Assfalg, M.; D’Onofrio, M. Espresso coffee mitigates the aggregation and condensation of Alzheimer’s associated tau protein. J. Agric. Food Chem. 2023, 71, 11429–11441. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, A.; Mukherjee, S.; Kumari, A.; Panda, D. Shikonin impedes phase separation and aggregation of tau and protects SH-SY5Y cells from the toxic effects of tau oligomers. Int. J. Biol. Macromol. 2022, 204, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Zhong, T.; Chen, Z.X.; Chen, W.; Zhang, N.; Liu, X.L.; Wang, L.Q.; Chen, J.; Liang, Y. Myricetin slows liquid-liquid phase separation of tau and activates ATG5-dependent autophagy to suppress tau toxicity. J. Biol. Chem. 2021, 297, 101222. [Google Scholar] [CrossRef]
- Pradhan, A.; Mishra, S.; Surolia, A.; Panda, D. C1 inhibits liquid-liquid phase separation and oligomerization of tau and protects neuroblastoma cells against toxic tau oligomers. ACS Chem. Neurosci. 2021, 12, 1989–2002. [Google Scholar] [CrossRef]
- Ramesh, M.; Balachandra, C.; Baruah, P.; Govindaraju, T. Cyclic dipeptide-based small molecules modulate zinc-mediated liquid-liquid phase separation of tau. J. Pept. Sci. 2023, 29, e3465. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, J.; Ramirez, L.M.; Gumusdil, E.; Pokhrel, P.; Man, V.H.; Ye, H.; Han, Y.; Liu, Y.; Li, P.; et al. Methylene blue accelerates liquid-to-gel transition of tau condensates impacting tau function and pathology. Nat. Commun. 2023, 14, 5444. [Google Scholar] [CrossRef]
- Prince, P.R.; Hochmair, J.; Brognaro, H.; Gevorgyan, S.; Franck, M.; Schubert, R.; Lorenzen, K.; Yazici, S.; Mandelkow, E.; Wegmann, S.; et al. Initiation and modulation of tau protein phase separation by the drug suramin. Sci. Rep. 2023, 13, 3963. [Google Scholar] [CrossRef]
- Moorthy, H.; Kamala, N.; Ramesh, M.; Govindaraju, T. Biphasic modulation of tau liquid-liquid phase separation by polyphenols. Chem. Commun. 2024, 60, 4334–4337. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, J.; Liu, Y.; Ye, H.; Han, Y.; Li, P.; Gao, M.; Huang, Y. Tannic acid as a biphasic modulator of tau protein liquid-liquid phase separation. Int. J. Biol. Macromol. 2024, 275, 133578. [Google Scholar] [CrossRef]
- Moreira, G.G.; Cantrelle, F.X.; Quezada, A.; Carvalho, F.S.; Cristovao, J.S.; Sengupta, U.; Puangmalai, N.; Carapeto, A.P.; Rodrigues, M.S.; Cardoso, I.; et al. Dynamic interactions and Ca(2+)-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding. Nat. Commun. 2021, 12, 6292. [Google Scholar] [CrossRef]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sakunthala, A.; Gadhe, L.; Poudyal, M.; Sawner, A.S.; Kadu, P.; Maji, S.K. Liquid-liquid phase separation of a-synuclein: A new mechanistic insight for a-synuclein aggregation associated with Parkinson’s disease pathogenesis. J. Mol. Biol. 2023, 435, 167713. [Google Scholar] [CrossRef]
- Berkeley, R.F.; Kashefi, M.; Debelouchina, G.T. Real-time observation of structure and dynamics during the liquid-to-solid transition of FUS LC. Biophys. J. 2021, 120, 1276–1287. [Google Scholar] [CrossRef]
- Xu, B.; Mo, X.; Chen, J.; Yu, H.; Liu, Y. Myricetin inhibits a-synuclein amyloid aggregation by delaying the liquid-to-solid phase transition. Chembiochem 2022, 23, e202200216. [Google Scholar] [CrossRef]
- Li, Y.; Gu, J.; Wang, C.; Hu, J.; Zhang, S.; Liu, C.; Zhang, S.; Fang, Y.; Li, D. Hsp70 exhibits a liquid-liquid phase separation ability and chaperones condensed FUS against amyloid aggregation. iScience 2022, 25, 104356. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Ibanez de Opakua, A.; Purslow, J.A.; Fromm, S.A.; Chatterjee, D.; Zachrdla, M.; Zhuang, S.; Puri, S.; Wolozin, B.; Zweckstetter, M. GSK3b phosphorylation catalyzes the aggregation of tau into Alzheimer’s disease-like filaments. Proc. Natl. Acad. Sci. USA 2024, 121, e2414176121. [Google Scholar] [CrossRef]
- Yabuki, Y.; Matsuo, K.; Komiya, G.; Kudo, K.; Hori, K.; Ikenoshita, S.; Kawata, Y.; Mizobata, T.; Shioda, N. RNA G-quadruplexes and calcium ions synergistically induce tau phase transition in vitro. J. Biol. Chem. 2024, 300, 107971. [Google Scholar] [CrossRef]
- Jonchhe, S.; Pan, W.; Pokhrel, P.; Mao, H. Small molecules modulate liquid-to-solid transitions in phase-separated tau condensates. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113156. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.Q.; Zhong, T.; Liu, X.L.; Zeng, Y.; Qiao, X.; Xie, T.; Chen, Y.; Gao, Y.Y.; Tang, B.; et al. Phase separation and cytotoxicity of tau are modulated by protein disulfide isomerase and s-nitrosylation of this molecular chaperone. J. Mol. Biol. 2020, 432, 2141–2163. [Google Scholar] [CrossRef]
- Chaari, A. Molecular chaperones biochemistry and role in neurodegenerative diseases. Int. J. Biol. Macromol. 2019, 131, 396–411. [Google Scholar] [CrossRef]
- Smith, H.L.; Li, W.; Cheetham, M.E. Molecular chaperones and neuronal proteostasis. Semin. Cell Dev. Biol. 2015, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, Z.; Zhang, S.; Li, Y.; Xia, W.; Wang, C.; Xiang, H.; Liu, Z.; Tan, L.; Fang, Y.; et al. Hsp40 proteins phase separate to chaperone the assembly and maintenance of membraneless organelles. Proc. Natl. Acad. Sci. USA 2020, 117, 31123–31133. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Gu, J.; Tong, Y.; Li, Y.; Gui, X.; Long, H.; Wang, C.; Zhao, C.; Lu, J.; et al. Hsp27 chaperones FUS phase separation under the modulation of stress-induced phosphorylation. Nat. Struct. Mol. Biol. 2020, 27, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Yang, C.C.; Huang, Y.Y.; Chen, Y.A.; Yang, C.W.; Liao, C.Y.; Li, H.; Wu, C.S.; Lin, C.H.; Teng, S.C. The HSP40 family chaperone isoform DNAJB6b prevents neuronal cells from tau aggregation. BMC Biol. 2023, 21, 293. [Google Scholar] [CrossRef]
- Irwin, R.; Faust, O.; Petrovic, I.; Wolf, S.G.; Hofmann, H.; Rosenzweig, R. Hsp40s play complementary roles in the prevention of tau amyloid formation. eLife 2021, 10, e69601. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Dahrendorff, J.; Creodore, S.G.; Dickey, C.A.; Blair, L.J.; Uversky, V.N. Small heat shock protein 22 kDa can modulate the aggregation and liquid-liquid phase separation behavior of tau. Protein Sci. 2021, 30, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.S.; Lipiński, W.P.; Spruijt, E. The role of biomolecular condensates in protein aggregation. Nat. Rev. Chem. 2024, 8, 686–700. [Google Scholar] [CrossRef]
- Siegert, A.; Rankovic, M.; Favretto, F.; Ukmar-Godec, T.; Strohaker, T.; Becker, S.; Zweckstetter, M. Interplay between tau and a-synuclein liquid-liquid phase separation. Protein Sci. 2021, 30, 1326–1336. [Google Scholar] [CrossRef]
- Saini, B.; Mukherjee, T.K. Biomolecular condensates regulate enzymatic activity under a crowded milieu: Synchronization of liquid-liquid phase separation and enzymatic transformation. J. Phys. Chem. B 2023, 127, 180–193. [Google Scholar] [CrossRef]
- O’Flynn, B.G.; Mittag, T. The role of liquid-liquid phase separation in regulating enzyme activity. Curr. Opin. Cell Biol. 2021, 69, 70–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Narlikar, G.J.; Kutateladze, T.G. Enzymatic reactions inside biological condensates. J. Mol. Biol. 2021, 433, 166624. [Google Scholar] [CrossRef]
- Frattini, C.; Promonet, A.; Alghoul, E.; Vidal-Eychenie, S.; Lamarque, M.; Blanchard, M.P.; Urbach, S.; Basbous, J.; Constantinou, A. TopBP1 assembles nuclear condensates to switch on ATR signaling. Mol. Cell 2021, 81, 1231–1245. [Google Scholar] [CrossRef]
- Linhartova, K.; Falginella, F.L.; Matl, M.; Sebesta, M.; Vacha, R.; Stefl, R. Sequence and structural determinants of RNAPII CTD phase-separation and phosphorylation by CDK7. Nat. Commun. 2024, 15, 9163. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Shu, T.; Pantoja, C.F.; Ibanez de Opakua, A.; Zweckstetter, M.; Holt, L.J. Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding. Mol. Cell 2022, 82, 3693–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Lu, T.W.; Stolerman, L.M.; Tenner, B.; Yang, J.R.; Zhang, J.F.; Falcke, M.; Rangamani, P.; Taylor, S.S.; Mehta, S.; et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell 2020, 182, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chuang, Y.; Redding-Ochoa, J.; Zhang, R.; Platero, A.J.; Barrett, A.H.; Troncoso, J.C.; Worley, P.F.; Zhang, W. The autophagy adaptor TRIAD3A promotes tau fibrillation by nested phase separation. Nat. Cell Biol. 2024, 26, 1274–1286. [Google Scholar] [CrossRef]
- Gil-Garcia, M.; Benitez-Mateos, A.I.; Papp, M.; Stoffel, F.; Morelli, C.; Normak, K.; Makasewicz, K.; Faltova, L.; Paradisi, F.; Arosio, P. Local environment in biomolecular condensates modulates enzymatic activity across length scales. Nat. Commun. 2024, 15, 3322. [Google Scholar] [CrossRef]
- Peeples, W.; Rosen, M.K. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 2021, 17, 693–702. [Google Scholar] [CrossRef]
- Harris, R.; Veretnik, S.; Dewan, S.; Baruch Leshem, A.; Lampel, A. Regulation of enzymatic reactions by chemical composition of peptide biomolecular condensates. Commun. Chem. 2024, 7, 90. [Google Scholar] [CrossRef]
- Lin, C.C.; Suen, K.M.; Jeffrey, P.A.; Wieteska, L.; Lidster, J.A.; Bao, P.; Curd, A.P.; Stainthorp, A.; Seiler, C.; Koss, H.; et al. Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Mol. Cell 2022, 82, 1089–1106. [Google Scholar] [CrossRef]
- Lopez-Palacios, T.P.; Andersen, J.L. Kinase regulation by liquid-liquid phase separation. Trends Cell Biol. 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Shen, Y.; Wang, Y.; Zhao, M.; Sun, L. The potential role of ferroptosis in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 80, 907–925. [Google Scholar] [CrossRef]
- Padhi, D.; Baruah, P.; Ramesh, M.; Moorthy, H.; Govindaraju, T. Hybrid molecules synergistically mitigate ferroptosis and amyloid-associated toxicities in Alzheimer’s disease. Redox Biol. 2024, 71, 103119. [Google Scholar] [CrossRef]
- Moorthy, H.; Ramesh, M.; Padhi, D.; Baruah, P.; Govindaraju, T. Polycatechols inhibit ferroptosis and modulate tau liquid-liquid phase separation to mitigate Alzheimer’s disease. Mater. Horiz. 2024, 11, 3082–3089. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, L.; Lin, N.; Gao, M.; Huang, Y. Rational Modulation of Liquid–Liquid Phase Separation Offers Novel Ways to Combat Tauopathies. Int. J. Mol. Sci. 2025, 26, 6709. https://doi.org/10.3390/ijms26146709
Zhang X, Wang L, Lin N, Gao M, Huang Y. Rational Modulation of Liquid–Liquid Phase Separation Offers Novel Ways to Combat Tauopathies. International Journal of Molecular Sciences. 2025; 26(14):6709. https://doi.org/10.3390/ijms26146709
Chicago/Turabian StyleZhang, Xingxing, Lumiao Wang, Nixin Lin, Meng Gao, and Yongqi Huang. 2025. "Rational Modulation of Liquid–Liquid Phase Separation Offers Novel Ways to Combat Tauopathies" International Journal of Molecular Sciences 26, no. 14: 6709. https://doi.org/10.3390/ijms26146709
APA StyleZhang, X., Wang, L., Lin, N., Gao, M., & Huang, Y. (2025). Rational Modulation of Liquid–Liquid Phase Separation Offers Novel Ways to Combat Tauopathies. International Journal of Molecular Sciences, 26(14), 6709. https://doi.org/10.3390/ijms26146709




