2.1. Starting Materials
In the work, four types of PHMS-based materials, namely non-porous (NP_M and NP_D) and porous (P_M and P_D) materials, were functionalized by the moieties originating from Naa. The starting (i.e., subjected to functionalization) materials were obtained by cross-linking of the polymer with a linear, difunctional or cyclic, tetrafunctional vinylsiloxane, M
2Vi or D
4Vi, respectively. The cross-linking process involved hydrosilylation of the vinyl groups in the cross-linker by the Si-H groups of PHMS catalyzed by the Pt complex (the so-called Karstedt catalyst). It was carried out either in the bulk or in water-in-oil high internal phase emulsion (HIPE) to prepare non-porous or porous polyHIPE materials (polyHIPEs), respectively. Hydrosilylation was also applied in the functionalization of the obtained networks. The overall strategy for the preparation of the starting and functionalized materials adopted in the work is schematically depicted in
Figure 1.
As previously mentioned (
Section 1), the studies were primarily aimed at evaluating the impact of the microstructure and the chemical structure of the PHMS-derived materials on their functionalization by Naa. Therefore, both parameters were carefully examined. The microstructure of all the prepared materials was determined using SEM, while their chemical structure was determined by FTIR and
29Si MAS-NMR spectroscopies (
Section 3.6). Additionally, in order to estimate differences in cross-linking degrees of PHMS in the obtained networks, equilibrium swelling measurements were performed (
Section 3.6). Cross-linking degrees seemed to be also an important factor influencing subsequent functionalization by Naa of both non-porous and porous materials. This is because in highly cross-linked networks, some of the Si-H groups, necessary for the reaction, may be inaccessible for Naa molecules, which would hinder functionalization. Moreover, polymer cross-linking degrees could affect the microstructure of the porous materials and thus indirectly influence their functionalization by Naa. The dependence of the microstructure on the polymer cross-linking degree was evident in organic polymer-based polyHIPEs [
36,
37] as well as in polyHIPEs synthesized from poly(methylvinylsiloxane) [
38]. In contrast, the microstructure was not related to the amount of the cross-linker (D
4Vi) applied in the preparation of PHMS-based polyHIPEs [
39]. However, the lowest amount of D
4Vi used in Ref. [
39] (molar ratio of Si-H: Si-Vi groups equal to 1:0.66) was almost quadruple of that employed in the present work (Si-H:Si-Vi groups’ molar ratio equal to 1:0.17,
Section 3.2). Hence, it was of interest to check if the proper polyHIPE microstructure could be developed at such a low amount of a cross-linker as compared to PHMS. Finally, to complete their characterization, skeletal density of all the materials and apparent density of the porous ones were determined (
Section 3.6). Based on skeletal and apparent density, total porosity of the fabricated polyHIPEs was calculated (
Section 3.6).
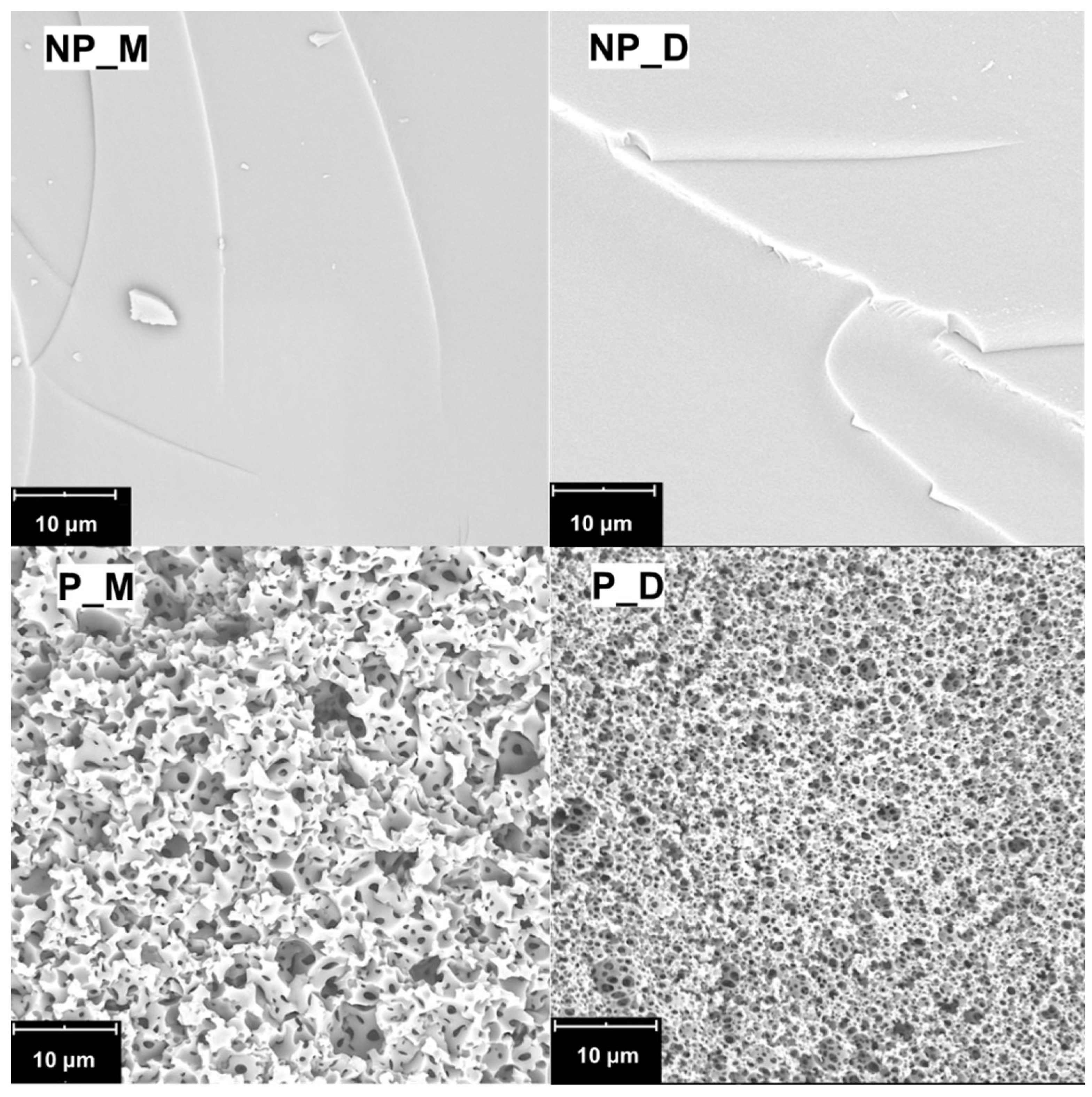
SEM images (
Figure 2) showed that, as expected, the NP_M and NP_D materials, prepared in the bulk, contained no pores and had a smooth surface. The microstructure of the materials synthesized in HIPE (
Figure 2) was typical of that characteristic for polyHIPEs [
40,
41]. Thus, large macropores, often called voids [
40] interconnected by smaller ones, referred to as windows [
40] were visible. Hence, the P_M and P_D materials exhibited open porosity, a feature favorable for applications in which transport of chemicals, nutrients, etc., within a material is required. Differences in the microstructure of the NP_M, NP_D and P_M, P_D materials were also supposed to lead—after modification with biocidal moieties—to various kinds of antibacterials.
According to SEM, voids and windows of higher maximum diameter existed in the P_M than in the P_D sample (
Table 1). Interestingly, voids and windows in P_M and P_D materials were smaller than those in the PHMS-derived polyHIPEs prepared by us previously [
39]. This could be due to lower water content in HIPEs used in the present studies (74 wt%,
Section 3.2) compared to in the previous (82 wt% [
39]) studies. Pore sizes in polyHIPEs are known to decrease as the fraction of water in the emulsion applied for their preparation decreases [
37,
42].
Distribution plots constructed based on quantitative SEM image analysis performed using ImageJ software, version 1,54k (
Section 3.6) showed that the diameters of voids in the P_M were higher than in the P_D polyHIPE, whereas diameters of windows were similar in both materials (
Figure S1,
Table 1). These conclusions were confirmed by a formal statistical Mann–Whitney U-test (
Section 3.6), which gave
p < 0.001 and
p = 0.95 for diameters of voids and windows, respectively (
p is the probability that there is no difference in the analyzed data). As a consequence, the P_M sample was characterized by higher mean and median void diameters while maintaining the same values for the windows as in the P_D sample (
Table 1). Thus, SEM studies pointed to the influence of the structure of the cross-linking agent (linear/cyclic, difunctional/tetrafunctional) on the microstructure of the obtained porous materials.
In addition, SEM results unequivocally proved that a low amount of the cross-linker with respect to PHMS in HIPE did not preclude the formation of the proper polyHIPE microstructure. This confirmed that, as established in our earlier work [
39], in PHMS-based polyHIPEs, this microstructure was developed independently of the reactive groups’ molar ratio in HIPE during their preparation. Since the characteristic macroporous polyHIPE structure reflects the dispersion of internal phase droplets in HIPE around which the polymer network is created [
40,
41], it can be generated only in a stable emulsion. HIPEs whose composition changes upon polymer cross-linking are rather unstable; therefore, network formation has to occur fast before the emulsion collapses. In the present study, a high rate of network formation via hydrosilylation was unlikely due to the very low amount of the vinylsiloxanes used. For this reason, a different cross-linking process must have taken place. It could have involved condensation of Si-OH groups resulting from the hydrolysis of Si-H moieties present in PHMS. Hydrolysis of Si-H groups is catalyzed by metals [
43], including Pt [
44]. Pt catalysts of the hydrosilylation reaction simultaneously catalyze hydrolysis of Si-H groups [
45].
As already stated, spectroscopic (FTIR and
29Si MAS-NMR) investigations were applied to determine the chemical structure of the synthesized networks. In spectra analysis, particular attention was paid to establishing the presence of Si-H groups as they were necessary for further functionalization of the materials carried out by hydrosilylation of Naa (
Figure 1). In view of SEM results, clarifying whether hydrolysis of Si-H to Si-OH groups and condensation of the latter took place in HIPE was an important point in the spectroscopic studies of P_M and P_D materials.
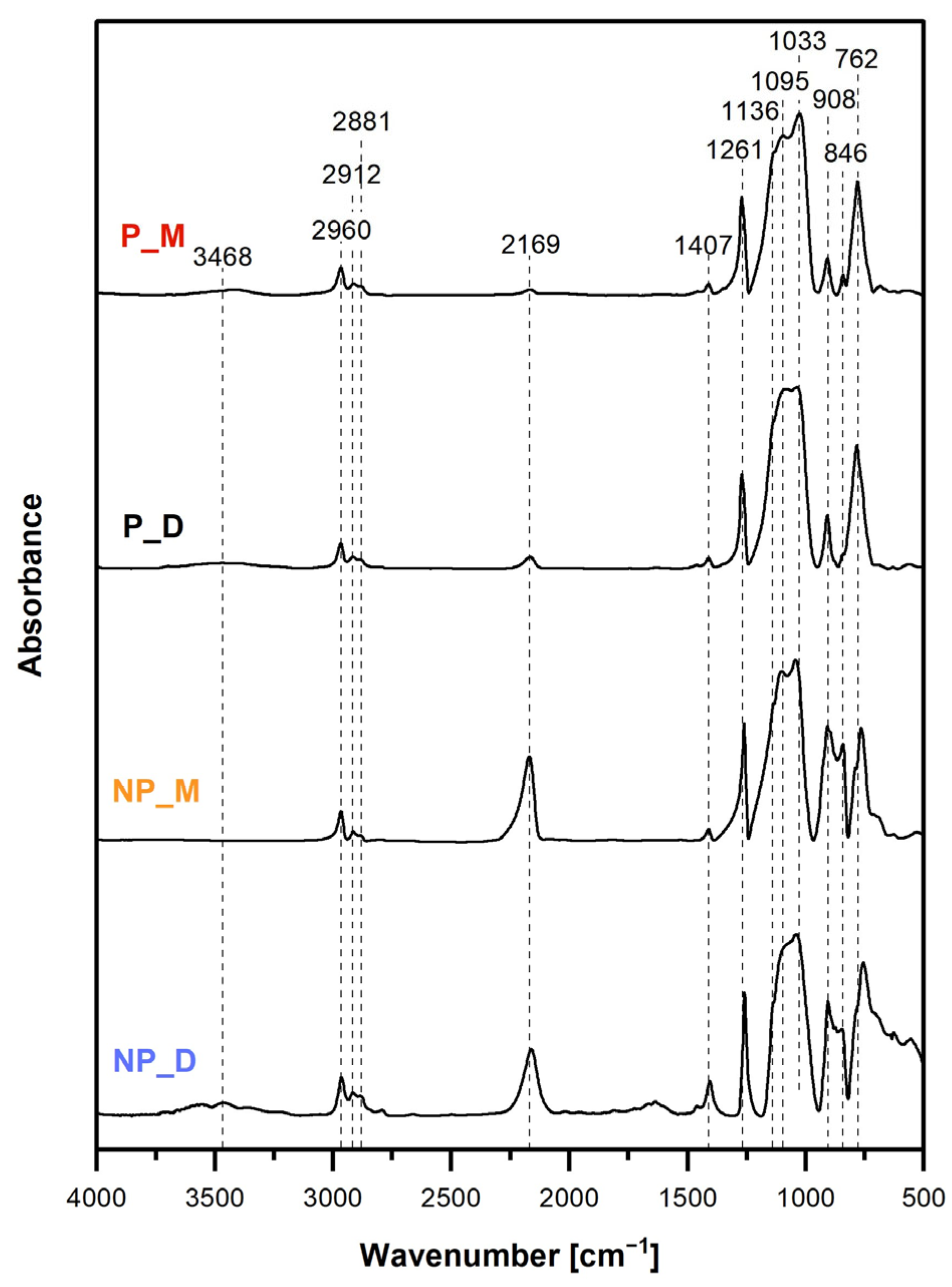
FTIR spectra (
Figure 3) confirmed that all the prepared materials showed the structure expected for the products of M
2Vi or D
4Vi hydrosilylation with PHMS. Thus, they contained Si–O bonds as well as C–H bonds in methyl (Si–CH
3) and ethylene (–CH
2CH
2–) groups. These bonds gave rise to the characteristic bands at 1033 cm
−1 (symmetric stretching vibrations of Si–O–Si [
46]), 762 cm
−1, 1261 cm
−1, 2960 cm
−1 (C–H rocking combined with Si–C stretching, C–H symmetric bending, C–H asymmetric stretching modes, respectively, in Si–CH
3 [
46]), 1136 cm
−1, 2881 cm
−1 and 2912 cm
−1 (C–H bending, C–H symmetric and antisymmetric stretching modes, respectively, in –CH
2CH
2– bridges [
46]).
Moreover, FTIR spectra signified the required existence of Si-H groups in the networks. This was proved by the bands at 908 cm
−1 (Si-H bending vibrations) and 2169 cm
−1 (Si-H stretching vibrations [
46]) seen in the spectra of all the materials (
Figure 3). However, the intensities of these bands in the spectra of the NP_M and NP_D samples were significantly higher than in those of P_M and P_D samples. This indicated higher conversion degrees of Si-H groups occurring in HIPE than in the bulk. Quantitative FTIR spectra analysis in which the ratios of the integral intensities of the band due to the Si–H groups at 2169 cm
−1 and the band ascribed to the C–H bonds in Si–CH
3 groups at 1261 cm
−1 were calculated (
Section 3.6) showed that conversion degrees of Si–H groups for the porous materials were 1.5 times higher than those found for the non-porous ones prepared with the same cross-linker (P_M: 94.8% vs. NP_M: 62.0% and P_D: 88.5% vs. NP_D: 58.3%,
Table 2). Since in all the reactions, the same molar ratio of groups involved in hydrosilylation was used (Si-H:Si-Vi =1:0.17,
Section 3.2), it could be concluded that in HIPEs, Si-H moieties participated in side processes. Hydrolysis was the first process to be considered because of the high water content in the emulsions and because it also occurred in our previous studies [
28,
38,
39].
FTIR spectra of the P_M and P_D materials prepared in HIPEs (
Figure 3) contained a broad band centered at 3468 cm
−1, which could be ascribed to Si–OH groups (O–H stretching mode [
46]). Its low intensities suggested low amounts of Si-OH moieties in these systems, possibly brought about by their fast condensation accompanied by the formation of siloxane bonds in new silsesquioxane (-SiO
3) units (
Figure 1), not detected in the FTIR spectra. The band due to Si-OH bonds was not visible in the spectrum of the NP_M material, but—quite surprisingly—it was distinct in the spectrum of the NP_D sample (
Figure 3). Hence, according to FTIR spectroscopy, hydrolysis of Si-H groups to Si-OH groups proceeded in HIPEs during the preparation of the P_M and P_D materials and also in the bulk when D
4Vi was used as a cross-linker.
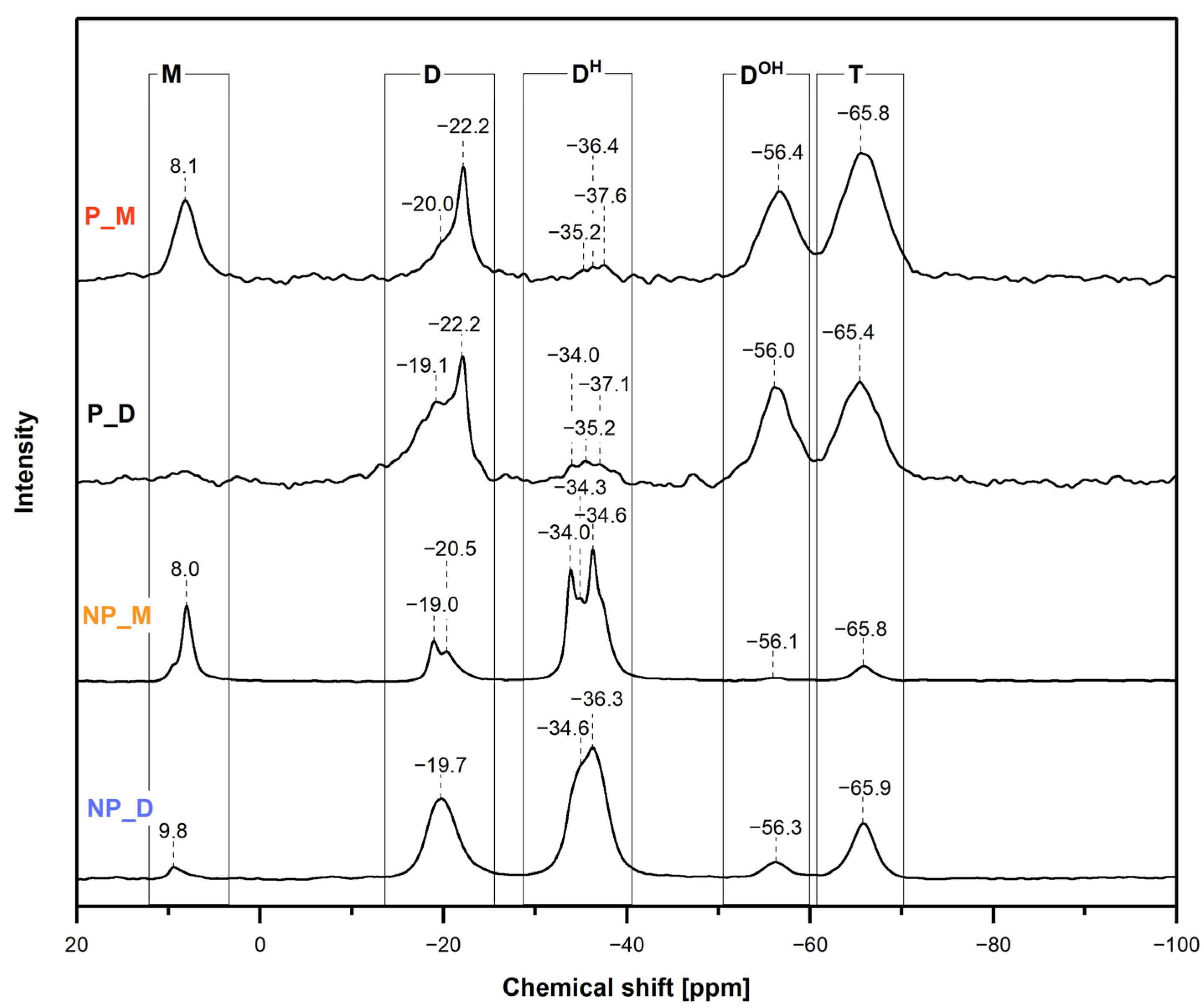
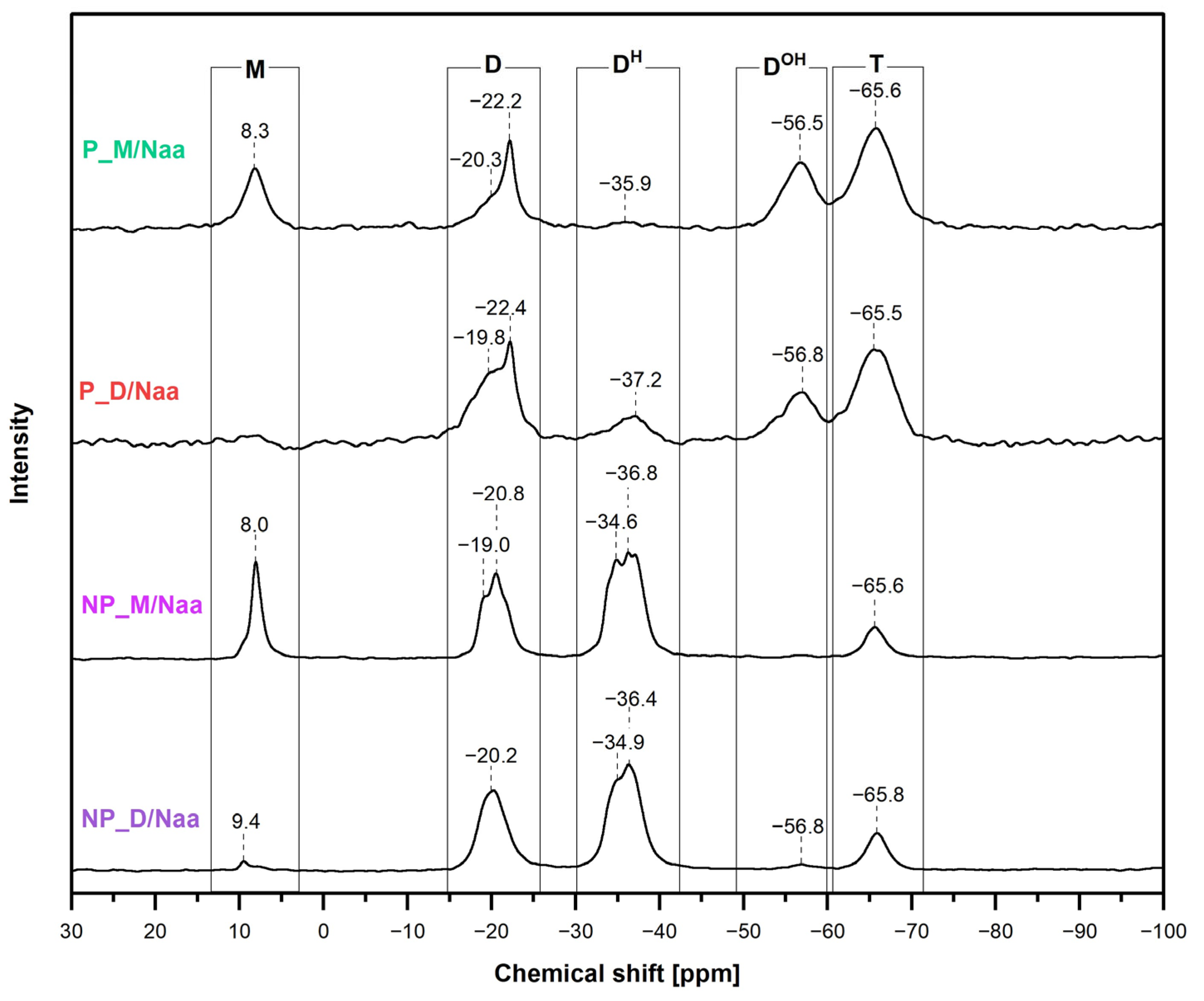
29Si MAS-NMR spectroscopic investigations (
Section 3.6) supported the conclusions drawn from the FTIR spectra and provided some complementary information on the chemical structure of the prepared materials. Thus, the
29Si MAS-NMR (
Figure 4) in line with the FTIR spectra showed that the studied materials were formed by hydrosilylation of the cross-linkers by PHMS and that they contained Si-H and Si-OH groups. In addition,
29Si MAS-NMR corroborated the occurrence of condensation of Si-OH groups, which was not shown by FTIR spectroscopy.
In the
29Si MAS-NMR spectra of the materials obtained using M
2Vi (NP_M and P_M samples), hydrosilylation was manifested by the presence of signals at chemical shift values
δ = 8.0/8.1 ppm and in the range between −19.0 and −22.2 ppm (
Figure 4). The signal at
δ = 8.0/8.1 ppm could be assigned to the [SiO(CH
3)
2(CH
2CH
2)] units resulting from hydrosilylation (
Figure 1) as well as to the [SiO(CH
3)
3] terminal groups in PHMS (
Section 3.1), i.e., to the so-called M units present in siloxane compounds [
47]. However, as could be judged by the shares of this signal in the deconvoluted spectra of the P_M and NP_M materials (
Table 2), the one corresponding to the [SiO(CH
3)
2(CH
2CH
2)] units was its only component. A set of signals in the range between −19.0 and −22.2 ppm in turn originated from the [SiO
2(CH
3)(CH
2CH
2)] or [SiO
2(CH
3)(CH(CH
3))] units in various chemical environments, i.e., the so-called D units of siloxane compounds [
48,
49] formed upon hydrosilylation (
Figure 1). The shares of the M and D signals in the spectra of the NP_M and P_M materials were equal (
Table 2) since hydrosilylation of M
2Vi by PHMS yields an equal number of [SiO
2(CH
3)(CH
2CH
2)]/[SiO
2(CH
3)(CH(CH
3))] and [SiO(CH
3)
2(CH
2CH
2)] units (
Figure 1).
Upon hydrosilylation of D
4Vi by PHMS, exclusively D units ([SiO
2(CH
3)(CH
2CH
2)] or [SiO
2(CH
3)(CH(CH
3))]) were generated (
Figure 1). Their presence in the NP_D and P_D materials was evidenced by broad signals with maxima at
δ = −19.1 or −19.7 and −22.2 ppm in the
29Si MAS-NMR spectra (
Figure 4). Shares of these signals, obtained after spectra deconvolution, were ca. double of those found in the spectra of the networks prepared with M
2Vi (
Table 2). Thus, PHMS hydrosilylation degrees were similar in all the systems (15–18%). It is worth noting that they were close to that expected for the reactive groups’ molar ratio applied in the experiments (Si-H:Si-Vi = 0.17,
Section 3.2).
Signals due to the [SiO
2(CH
3)H] [
47], often referred to as D
H units, were visible at
δ = −34.0–−37.6 ppm in all the recorded
29Si MAS-NMR spectra (
Figure 4). Their shares were markedly higher in the spectra of the non-porous materials than in those of porous materials (
Table 2). This indicated, in agreement with FTIR results, that the NP_M and NP_D samples contained significantly more Si-H groups than the P_M and P_D polyHIPEs. According to
29Si MAS-NMR, the highest fraction of [SiO
2(CH
3)H] units contained the NP_M sample (
Table 2).
Hydrolysis of some Si-H followed by condensation of the resulting Si-OH groups to silsesquioxane groups was demonstrated by the appearance in all the
29Si MAS-NMR spectra of the signals at
δ = −56.0–−56.4 ppm and −65.4–−65.9 ppm (
Figure 4). They could be assigned to the [SiO
2(CH
3)OH], i.e., D
OH units and [SiO
3(CH
3)], called T units, respectively [
47], occurring in the networks (
Figure 1). Shares of these signals in the spectra of the porous materials were higher than in those of the non-porous ones (
Table 2). Thus, as expected, hydrolysis/condensation processes were more pronounced in HIPEs than in the bulk. Hydrolysis in the bulk must have been caused by water contained in the chemicals, which were applied in the experiments without prior purification, or water from the environment (experiments were performed in the flow of Ar, not in a dry box). Out of these two possibilities, the latter seemed more probable as only cross-linkers were not dried before use in the syntheses of the non-porous materials (
Section 3.1). However, cross-linkers did not contain water, which was verified by their FTIR spectra. Nonetheless,
29Si MAS-NMR in concert with FTIR spectroscopy showed a higher hydrolysis degree in the NP_D than in the NP_M sample (
Table 2).
Silsesquioxane (T) units established by
29Si MAS-NMR spectroscopy could constitute additional (with respect to those formed by hydrosilylation) cross-links in the prepared networks. Equilibrium swelling measurements in toluene were performed (
Section 3.6) to explore the effect of the presence of the T groups on cross-link degrees as well as to verify if there were differences in cross-linking degrees in the studied materials.
Experiments revealed that the samples containing more silsesquioxane groups swelled to a lower extent than those of a similar microstructure but with lower T group fractions (P_M vs. P_D and NP_D vs. NP_M materials,
Table 2). Consequently, it could be concluded that the condensation of Si-OH groups contributed to the PHMS cross-linking process and led to the growth in cross-linking degrees in the networks.
It was also found that the porous (P_M and P_D) materials swelled more than the non-porous (NP_M and NP_D) materials (
Table 2). This indicated higher cross-linking degrees in the non-porous networks. It should be noted at this point that swelling of a non-porous polymer network is directly related to its cross-linking level. Estimation of cross-linking levels in polyHIPEs based on equilibrium swelling measurements is not so straightforward because of the possible sorption of the solvent in the pores [
50,
51]. Therefore, to compare cross-linking degrees, polyHIPEs of similar porosity should be examined. In the present studies, P_D material could have absorbed more toluene due to its higher total porosity than that of the P_M polyHIPE (89.4% vs. 80.9%, respectively,
Table 2). However, the difference in porosity of these samples (porosity ratio equal to 1.1) was significantly lower than the difference in their swelling extents (swelling degree ratio: 1.88). Therefore, it could be supposed that various swelling levels of the P_D and P_M networks were mainly attributed to their various cross-linking degrees.
For the non-porous materials, the relationships between cross-linking degrees determined by equilibrium swelling experiments were confirmed by skeletal density measurements (
Section 3.6). As expected, the NP_M sample of a higher cross-linking degree was characterized by a lower skeletal density, while the NP_D sample of a lower cross-linking degree was characterized by a higher skeletal density (
Table 2). In contrast, the relationships between skeletal densities and cross-linking levels of the studied polyHIPEs were not as anticipated. The P_D sample of a lower cross-linking level found by swelling measurements showed higher skeletal density than the P_M one in which the cross-linking density was higher (
Table 2). However, in the case of the P_M and P_D materials, the apparent density was more important as it was connected with their porous structure. Thus, as expected, the P_M polyHIPE exhibited a higher apparent density because of its lower total porosity, whereas the lower apparent density of the P_D polyHIPE could be explained by its higher total porosity (
Table 2).
In summary, studies of the NP_M, NP_D, P_M and P_D materials showed that they differed in their microstructure, contained various amounts of Si-H groups and were characterized by various equilibrium swelling degrees in toluene, a feature related to various cross-linking degrees of the prepared networks but also to the differences in their microstructure.
2.2. Functionalized Materials
In the next part of the work, the non-porous (NP_M, NP_D) and the porous (P_M, P_D) materials were functionalized by Naa. The process involved hydrosilylation of the double carbon–carbon bonds in the allyl groups of Naa molecules by Si-H groups remaining in the networks performed in the presence of Karstedt catalyst (
Section 3.4,
Figure 1). Then, the functionalized materials (NP_M/Naa, NP_D/Naa, P_M/Naa, P_D/Naa) were treated with BnCl or OcBr (
Section 3.5) to obtain the materials containing ammonium groups (NP_M/Naa/BnCl, NP_D/Naa/BnCl, P_M/Naa/BnCl, P_D/Naa/BnCl or NP_M/Naa/OcBr, NP_D/Naa/OcBr, P_M/Naa/OcBr, P_D/Naa/OcBr,
Figure 1).
As found in the studies (
Section 2.1), the starting networks showed different microstructure and equilibrium swelling/cross-linking degrees, which could affect their modification by Naa. Most importantly, however, they differed in the contents of Si-H groups, necessary for the reaction with the amine. Due to hydrolysis, particularly low amounts of Si-H moieties were preserved in the prepared polyHIPEs (P_M and P_D materials). Therefore, after the reactions with Naa, the initial question was whether the modification of the networks had occurred. The answer was provided by elemental (C, H, N) analysis and by FTIR and
29Si-MAS NMR spectroscopies (
Section 3.6).
Elemental analysis showed that all the materials resulting from the reactions with Naa contained nitrogen (
Table 3), which indicated their successful functionalization. However, the amounts of nitrogen in the samples were low and in most cases, they were significantly lower than those calculated based on the stoichiometry of the functionalization process. Expectedly, due to low amounts of Si-H groups remaining in the porous starting networks (
Section 2.1), lower contents of nitrogen were found in the P_M/Naa and P_D/Naa than in the NP_M/Naa and NP_D/Naa samples (
Table 3).
Among both non-porous and porous materials, the amounts of nitrogen were higher for those that swelled to a lower extent (nitrogen contents: P_M/Naa > P_D/Naa, NP_D/Naa > NP_M/Naa,
Table 3; swelling degrees: P_M < P_D, NP_D < NP_M,
Table 2). This, quite surprisingly, implied more efficient functionalization in more cross-linked networks. The possible reason for this phenomenon could be that during the reactions with Naa, performed in toluene (
Section 3.6), the materials were in a swollen state. Possibly, their spatial and high-volume nature in this state limited contact of the reactive groups, thus lowering functionalization yield. The effect of swelling was noticeably stronger in the porous materials. The P_D/Naa sample, prepared from the P_D polyHIPE whose swelling was almost twice that found for the P_M sample (
Table 2), contained three times less nitrogen than the P_M/Naa material (
Table 3). In the case of the non-porous networks, a twofold decrease in the extent of swelling was accompanied by a twofold growth in the nitrogen content (
Table 2 and
Table 3). Since swelling of porous materials was considerably higher than that of the non-porous ones, these findings supported the conclusion on the adverse influence of swelling on the modification by Naa of the cross-linked PHMS studied in the work. Thus, these results suggest that controlling cross-linking density and porosity could be an effective strategy to optimize Naa functionalization efficiency.
Additionally, by elemental analysis, higher contents of carbon in the functionalized non-porous materials compared to porous materials (NP_M/Naa, NP_D/Naa vs. P_M/Naa, P_D/Naa,
Table 3) were determined. This was in line with the higher contents of Naa groups in the former because incorporation of Naa moieties comprising a phenyl ring should lead to increased carbon amounts in the networks.
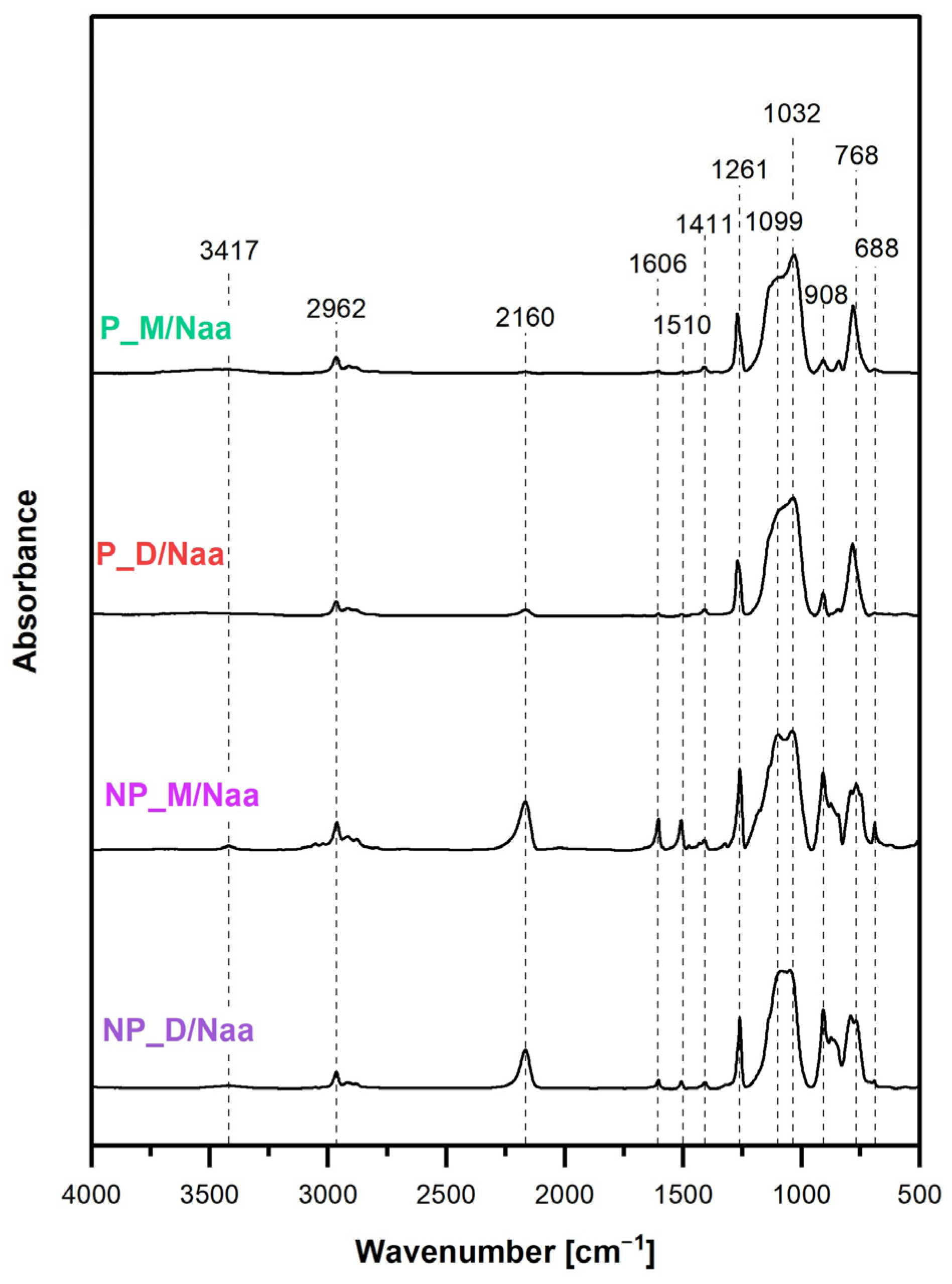
FTIR spectra of the materials treated with Naa (
Figure 5) showed decrease in intensities of the band at 2160 cm
−1, corresponding to stretching vibrations of Si-H groups with respect to those measured before functionalization (
Figure 3). This confirmed that Si-H groups were involved in the reaction. According to quantitative FTIR spectra analysis (
Section 3.6), transformation degrees of Si-H groups were low: 3.5% and 4.3% for the porous materials and 7.2% and 8% for the non-porous ones (
Table 3). Higher transformation degrees of Si-H groups found by FTIR spectroscopy agreed with the higher nitrogen contents in the non-porous samples determined by elemental analysis (
Table 3).
Presence of Naa moieties was demonstrated by weak bands at 3417 cm
−1, 1606 cm
−1 and 1510 cm
−1 in the FTIR spectra of NP_M/Naa and NP_D/Naa samples (
Figure 5). The former band could be unambiguously attributed to N–H stretching vibrations, whereas the band at 1606 cm
−1 was likely the superposition of a weak band due to C = C stretching vibrations of the aromatic ring and an intense band ascribed to N–H deformational vibrations [
46]. These bands were not seen in the spectra of the P_M/Naa and P_D/Naa materials, which could be explained by their low functionalization levels.
Hydrosilylation of Naa by Si-H groups preserved in the studied networks should be accompanied by the formation of new [SiO
2(CH
3)(CH
2CH
2CH
2)]/ [SiO
2(CH
3)(CH(CH
3)CH
2)], i.e., D units in the reaction products (
Figure 1). Simultaneously, the contents of [SiO
2(CH
3)H], i.e., D
H units, should decrease with respect to those in the starting materials. It is worth noting here that even though usually the
anti-Markovnikov product with linear hydrocarbon group predominates in hydrosilylation of double carbon–carbon bonds conducted over Pt catalysts, in many cases, the branched, Markovnikov adduct is formed as well [
45]. Its presence was proved in the products of hydrosilylation of Naa by linear PHMS [
25] and N,N-dimethylallylamine by copolymers containing hydromethylsiloxane and dimethylsiloxane groups [
32] catalyzed by the Karstedt complex. Hence, the generation of [SiO
2(CH
3)(CH(CH
3)CH
2)] units in this work, when the cross-linked PHMS was used in the reaction with Naa, could not be excluded.
Comparison of the
29Si MAS-NMR spectra of the functionalized samples (
Figure 6) with those of the initial ones (
Figure 4) revealed that all expected changes were observed for the P_M/Naa, NP_M/Naa and NP_D/Naa materials (
Table 3). The highest growth in the share of D units and the highest decrease in the D
H ones took place during modification of the NP_M material, which—according to
29Si MAS-NMR spectroscopy—contained the highest amount of Si-H groups (
Table 2). Amounts of D units in the other systems grew to lower extents (1–5%,
Table 3). A small decrease in D
H unit fractions (1–3%,
Table 3) occurred after treatment with Naa of NP_D and P_M networks. Hence,
29Si MAS-NMR spectroscopy confirmed that modification of the studied PHMS networks via hydrosilylation of Naa took place, but its degrees were low. The highest one, indicated by the most significant changes in the spectra, was in the NP_M/Naa sample even though it contained less nitrogen found by elemental analysis than the NP_D/Naa material (
Table 3). This suggested that in the NP_D/Naa network, unreacted amine remained. Possibly, it was trapped in the bulk of the swollen material after the reaction. It should be noted that, as consistently shown by swelling experiments and
29Si MAS-NMR spectra (
Table 2), among the non-porous networks subjected to functionalization, the NP_D was characterized by a higher cross-linking degree than the NP_M one. Therefore, it could have been difficult to remove Naa from the NP_D/Naa sample. Additional experiments would be necessary to find out if the presence of unreacted amine in the functionalized cross-linked PHMS can be avoided. For example, modification by Naa could be performed in the solvents in which the polymer networks do not swell. Alternatively, cross-linking of the previously functionalized linear PHMS could be considered. Such studies were, however, beyond the scope of the present work. They will be our future goals.
Furthermore,
29Si MAS-NMR spectroscopy showed that side reactions proceeded during functionalization of the studied materials as well. The most prominent was condensation of Si-OH groups manifested by the decrease in the shares of the signals due to D
OH units in all the spectra after functionalization with a concomitant increase in the fractions due to T units, evident for the P_D/Naa and NP_M/Naa materials (
Table 3). This is understood since amines, as bases, are catalysts of silanol condensation [
52]. Additionally, a higher decrease in the amount of D
H units than the growth of the amount of D units (
Table 3) pointed to the occurrence of hydrolysis of some Si-H groups in the reaction of NP_M network with Naa. The decrease in the amount of T units (NP_D/Naa material,
Table 3) or the increase in the amount of D
H units (P_D/Naa material,
Table 3) indicated occurrence of other side processes. Overall,
29Si MAS-NMR spectroscopy reflected the complexity of the studied systems in which various chemical transformations took place.
According to SEM (
Figure S2), the microstructure of the starting materials was preserved after functionalization. The surface of the NP_M/Naa and NP_D/Naa samples was smooth and non-porous, while on the surfaces of the P_D/Naa and P_M/Naa samples, macropores, voids and windows were seen.
Modification by Naa caused a small increase in equilibrium swelling in toluene of both non-porous and porous materials cross-linked using M
2Vi (NP_M/Naa vs. NP_M: 1.2-fold, P_M/Naa vs. P_M: 1.3-fold swelling growth,
Table 2 and
Table 3). Swelling of the NP_D/Naa and P_D/Naa samples was the same as before Naa treatment (
Table 2 and
Table 3). This implied that side processes taking place during functionalization involved a small decrease in cross-linking degrees of the NP_M and P_M networks, which did not occur in the NP_D and P_D networks. It should be noted, however, that the growth in total porosity contributed to increased swelling of the P_M/Naa sample. As calculated using skeletal and apparent densities (
Section 3.6), total porosity increased from 80.9% for the P_M (
Table 2) to 92.5% for the P_M/Naa material (
Table 3).
Changes in skeletal density of the non-porous samples upon modification by Naa were small and could be correlated with the changes in their equilibrium swelling. Thus, like swelling, the skeletal density of the NP_M/Naa material (
Table 3) was higher than that of the NP_M one (
Table 2). Similarly, no changes in their swelling led to the practically unchanged skeletal density of the NP_D/Naa (
Table 3) with respect to the NP_D sample (
Table 2).
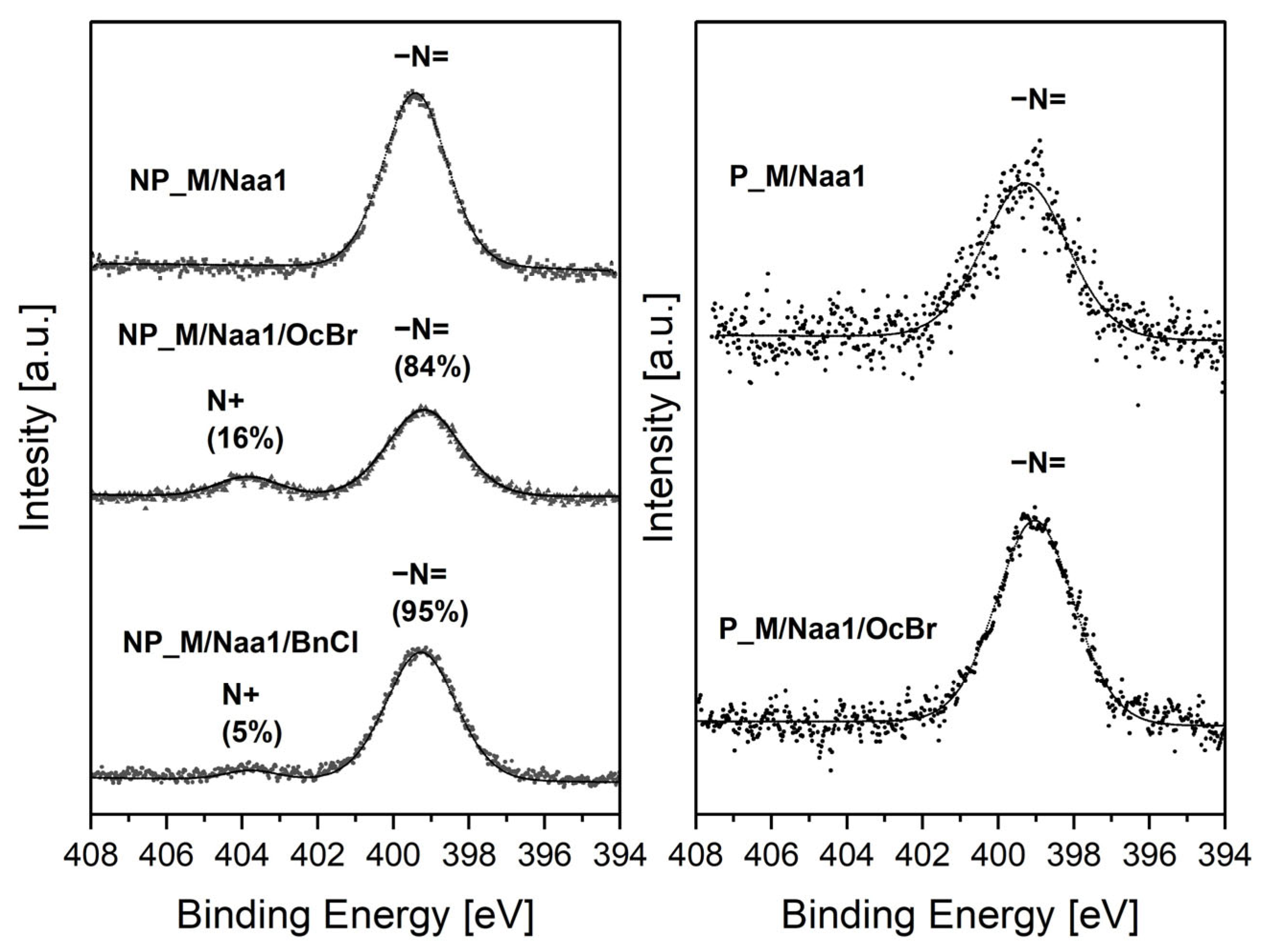
Treatment with BnCl or OcBr (
Section 3.5,
Figure 1) resulted in the conversion of some of the amine groups present in the non-porous materials to ammonium groups, while in the case of the porous networks, this process did not occur or occurred to a very low extent. This was established by N1s XPS spectra of the non-porous and porous samples showing higher functionalization degrees within each group, i.e., NP_M/Naa and P_M/Naa (
Table 3) and products of their reactions with BnCl/OcBr. No changes were detected in the FTIR spectra of the BnCl and OcBr-treated materials (
Figure S3).
The N1s XPS spectra (
Figure 7) of the Naa-functionalized materials contained a single maximum at a binding energy (B.E.) equal to 399.3–399.4 eV assigned to nitrogen atoms in amine groups [
53]. It was more intensive in the spectrum of the NP_M/Naa than in that of the P_M/Naa sample, proving the higher nitrogen content in the former material that was also found by elemental analysis (
Table 3). After treatment with OcBr and BnCl a maximum of low intensity at B.E. = 403.8 eV due to charged nitrogen atoms [
53] appeared in the spectra of the non-porous materials (
Figure 7). This proved that transformation of a part of amino to ammonium groups took place. As could be judged by the shares of the maximum corresponding to the charged nitrogen atoms in the N1s XPS spectra, the process was more efficient when OcBr was used. In contrast, no change was seen in the spectrum of the Naa-functionalized porous sample treated with OcBr (
Figure 7). This implied that amino groups present in this material were either not converted to ammonium ones at all or converted to a low degree, not detected by XPS. It should be mentioned here that incomplete transformation of amino to ammonium groups was also found by N1s XPS for the functionalized polystyrene [
20] and polyurethanes [
24]. In the latter case, conversion degrees were low (18–21%), in line with our work.
In summary, the studies showed that higher functionalization degrees by Naa were obtained for the non-porous PHMS-derived materials in which higher amounts of Si-H groups were present than in polyHIPEs. For the modification process, less swelling of the networks (i.e., higher cross-linking degree and lower porosity) was advantageous. Some of the amino groups incorporated into the non-porous materials were transformed into ammonium ones via the reaction with BnCl and OcBr; the process was more efficient when OcBr was used. Such a reaction did not occur or occurred to a low extent in the case of Naa-functionalized porous networks.
2.3. Antibacterial Properties
Two antibacterial tests were carried out to investigate the antimicrobial properties of porous and non-porous modified PHMS-based materials prepared in the work. The effect of the powdered materials on the bacterial growth kinetics in liquid media was studied and the degree of bacterial reduction by contact with the tested materials was determined (
Section 3.7). The kinetic studies, in which no reference was used, allowed us to compare the antibacterial activity of the modified PHMS-derived samples between themselves. In the bacterial reduction investigations, their activity was related to “undisturbed” bacterial growth proceeding without any substance added. Gram-positive
S. aureus and Gram-negative
E. coli were selected as bacterial models.
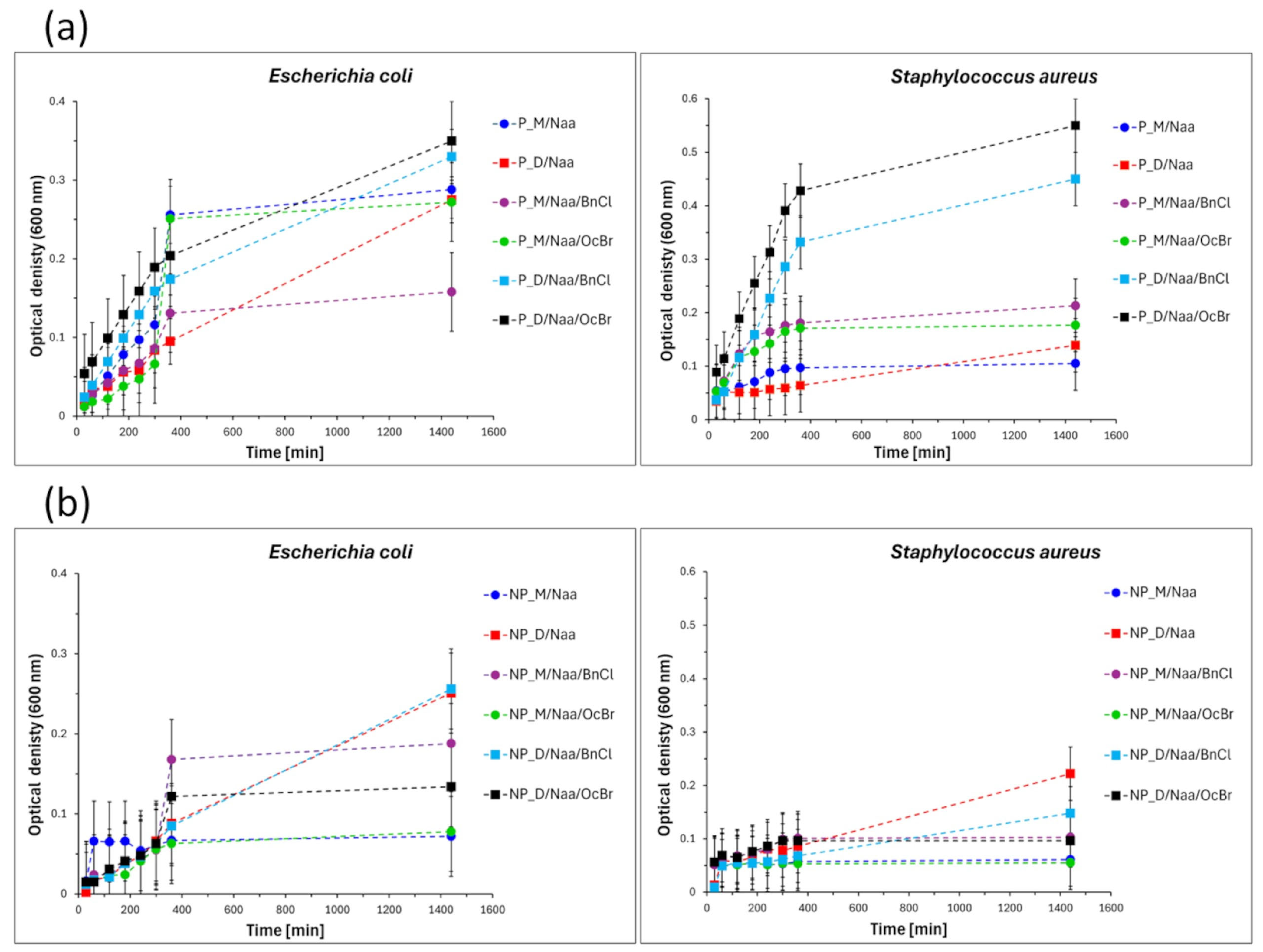
The bacterial growth curves (
Figure 8) showed that some of the tested samples were bacteriostatic, i.e., inhibited the proliferation of bacteria [
54], whereas in the presence of the other ones, this process was unaffected or only slightly affected. Thus, a bacteriostatic effect towards both
S. aureus and
E. coli bacteria was observed for all the modified materials in which M
2Vi was used as a cross-linker (P_M/Naa, P_M/Naa/BnCl, P_M/Naa/OcBr and NP_M/Naa, NP_M/Naa/BnCl, NP_M/Naa/OcBr samples). Most modified networks containing D
4Vi moieties (P_D/Naa, P_D/Naa/BnCl, P_D/Naa/OcBr and NP_D/Naa, NP_D/Naa/BnCl samples) did not exhibit a bacteriostatic effect against either
S. aureus or
E. coli. NP_D/Naa/OcBr was the only exception in this group—it was bacteriostatic towards both bacteria strains studied. These results clearly demonstrated the influence of the cross-linker applied in the preparation of PHMS networks on the antibacterial properties of the modified materials.
Examination of the final optical density values (OD
600) measured in the experiments (
Figure 9) revealed that the strongest bacteriostatic effect (i.e., the lowest OD
600), again towards both
S. aureus and
E. coli, was demonstrated by the NP_M/Naa material (
S. aureus: OD
600 = 0.061,
E. coli: OD
600= 0.072). Treatment with BnCl or OcBr affected the antibacterial activity of the Naa-functionalized materials in different ways. The most spectacular, advantageous influence of OcBr treatment was observed for the NP_D/Naa material. It was inactive towards
S. aureus and
E. coli, but after reaction with OcBr, it inhibited the growth of both types of bacteria (
Figure 8).
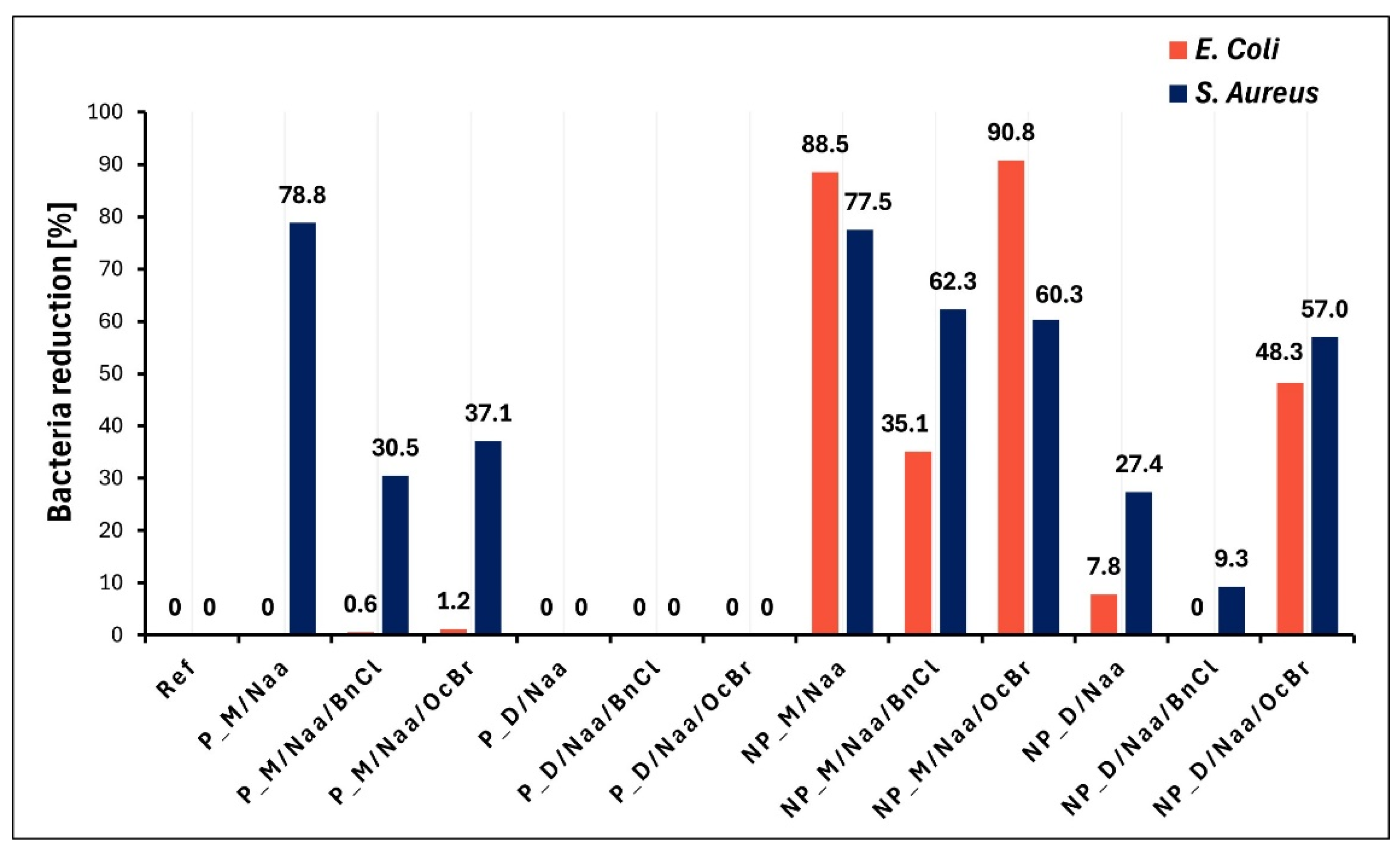
Experiments in which bacterial reduction degrees (R) were determined based on comparison with the reference sample (
Section 3.7) confirmed most of the results of bacterial growth studies. Importantly, among the studied samples, the highest antibacterial effect was exhibited by the NP_M/Naa and NP_M/Naa/OcBr samples against Gram-negative
E. coli (
Figure 9). The high reduction degrees found for these materials (R = 88.5% and 90.8%, respectively,
Figure 9) allowed us to consider them bactericidal towards
E. coli.From the conducted experiments, the antibacterial activity of the materials containing neutral Naa groups was evident even though it is generally accepted that for effective interactions with bacteria, positively charged ammonium groups in amine bactericides are required [
11]. However, it was shown that uncharged,
t-butylamine substituted siloxane copolymers were active by contact against Gram-positive and Gram-negative bacteria [
31]. The operating mechanism was not fully understood; it seemed to be complex as no adsorbed bacteria on the surface of bactericidal polymers were observed by SEM [
31].
Additionally, our antibacterial tests clearly showed that more investigated materials exhibited an effect on the Gram-positive bacteria
S. aureus than on Gram-negative
E. coli (9 vs. 5 samples, respectively,
Figure 9). Higher activity against Gram-positive than Gram-negative bacteria was reported also for other amine/ammonium group-modified siloxane polymers [
29,
31,
32,
33,
34]. In all cases, this difference was explained by structural differences in cell walls of these bacteria. Gram-positive bacterial cells have a loosely packed peptidoglycan layer, while cell walls of Gram-negative bacteria are composed of two membranes (outer and inner) with an additional layer of lipopolysaccharides (LPSs) [
10]. Macromolecular biocides, whose bactericidal effect is due to their disruptive interaction with the bacterial cell wall and/or cytoplasmic membranes [
55], can interact more effectively with Gram-positive bacterial cells. Cell walls of Gram-negative bacteria serve as barriers against external agents, making them less sensitive to environmental factors [
55].
Attempting to rationalize the results of the performed antibacterial studies, the role of three key parameters in which our materials differed, namely functionalization degree by Naa, network cross-linking density and microstructure, should be considered. It should also be taken into account that the tested samples did not swell in aqueous media (verified by swelling experiments in nutrient broth performed in the conditions of antibacterial tests). Therefore, it could be supposed that in the interactions with bacteria, only their surface was involved. This precluded the contribution of free amine trapped in the bulk of the NP_D/Naa sample (
Section 2.2) to the antibacterial activity of this material. Similarly, the reactions with BnCl and OcBr proceeded on the surface of Naa-functionalized materials that did not swell in BnCl and OcBr solutions in acetone applied in the conversion of amino to ammonium groups (
Section 3.5). In this way, the possibility of the formation of low-molecular-weight Naa/BnCl or Naa/OcBr salts (after reaction with free Naa) that could leach to the environment was eliminated. Hence, all the observed antibacterial properties could be attributed to the studied functionalized materials and should be treated as their inherent feature.
The highest activity of the NP_M/Naa sample against
E. coli bacteria (not significantly increased by OcBr treatment) and relatively high activity against
S. aureus bacteria (
Figure 9) could be due to its highest functionalization degree and lowest cross-linking density revealed by
29Si MAS-NMR spectroscopy and swelling experiments (
Table 3, NMR: the lowest share of T units). Bioactivity of functionalized linear, uncross-linked polysiloxanes is believed to be enhanced by the high flexibility of their chains, which allows polymer molecules to adopt conformations that ensure efficient interactions with bacteria cell walls [
30,
34]. In the lightly cross-linked NP_M/Naa network, flexibility of siloxane chains could be partially preserved.
On the other hand, high activity of the P_M/Naa sample towards
S. aureus bacteria (
Figure 9) could be mainly related to the highest cross-linking degree of the P_M network found by
29Si MAS-NMR spectroscopy (
Table 2, the highest share of T units), which meant that functionalization occurred predominantly on the surface of this material. It can be supposed that—even though the functionalization degree of the P_M/Naa material was not high (
Table 3)—biocidal moieties were easily accessible for contact with bacteria, facilitated also by the porous structure of this sample.
Finally, a significant increase in the activity of the NP_D/Naa material treated with OcBr against both investigated bacteria strains (
Figure 9) indicated that in this case, the presence of ammonium groups containing a long alkyl chain was necessary. Presumably, as suggested by moderate R values (
Figure 9), the amount of ammonium groups in the NP_D/Naa/OcBr sample was too low to provide high bioactivity. An antibacterial effect is known to occur after a certain threshold content of ammonium groups in the polymer is attained (e.g., 20% for polyurethanes [
23] and 50% for polymethacrylates [
13] modified by QAS were reported).
In summary, the antibacterial properties of the studied PHMS networks modified by Naa reflected the interplay between their functionalization degrees, cross-linking levels and microstructure. The highest antibacterial activity showed the non-porous material of the highest functionalization degree and the lowest cross-linking degree. Porosity seemed to facilitate access of biocidal moieties to bacteria in the highly cross-linked material of a lower functionalization degree, which resulted in enhanced antibacterial activity.