ROMP and Vinyl Polynorbornenes with Vanadium(III) and Nickel(II) diNHC Complexes
Abstract
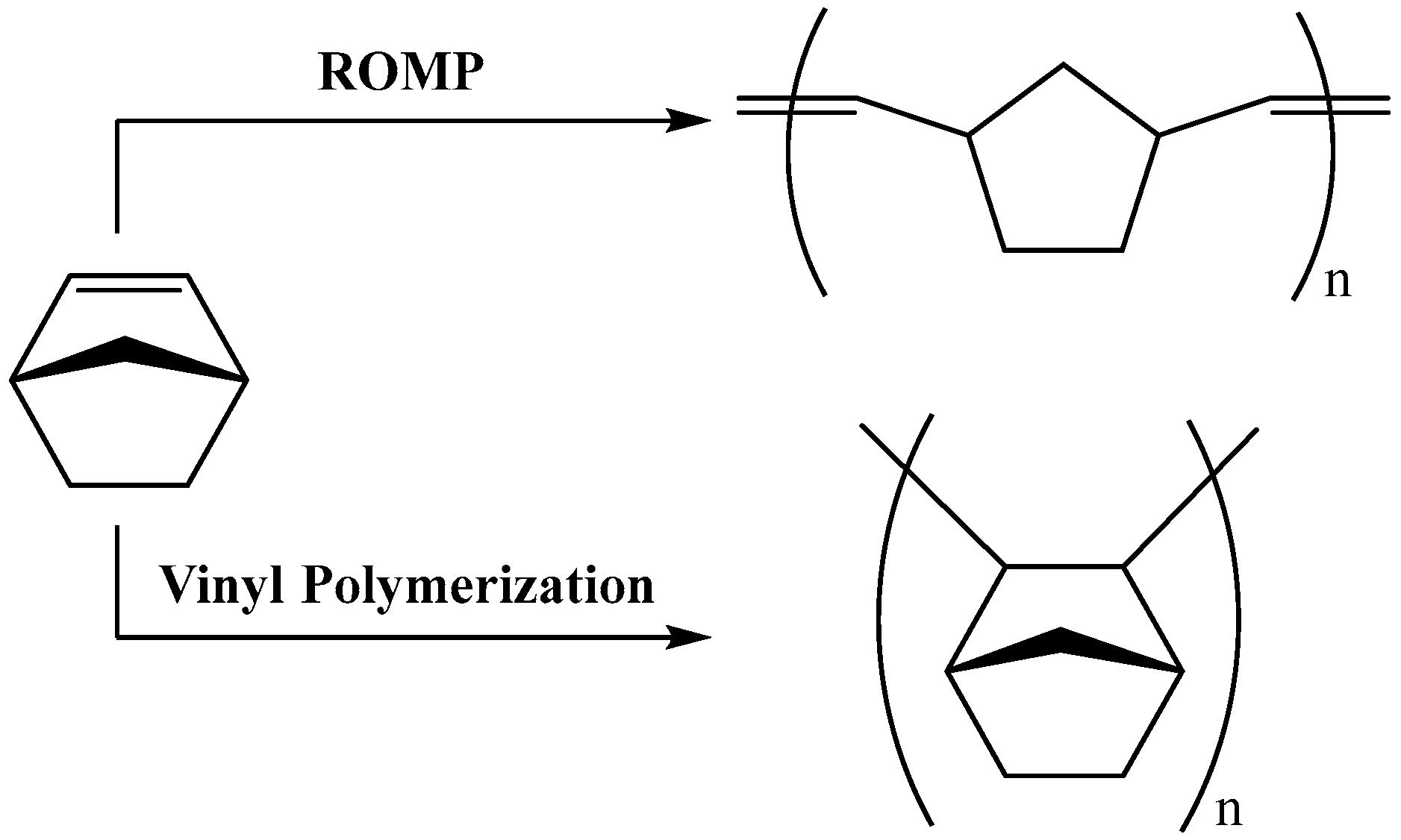
1. Introduction
2. Results
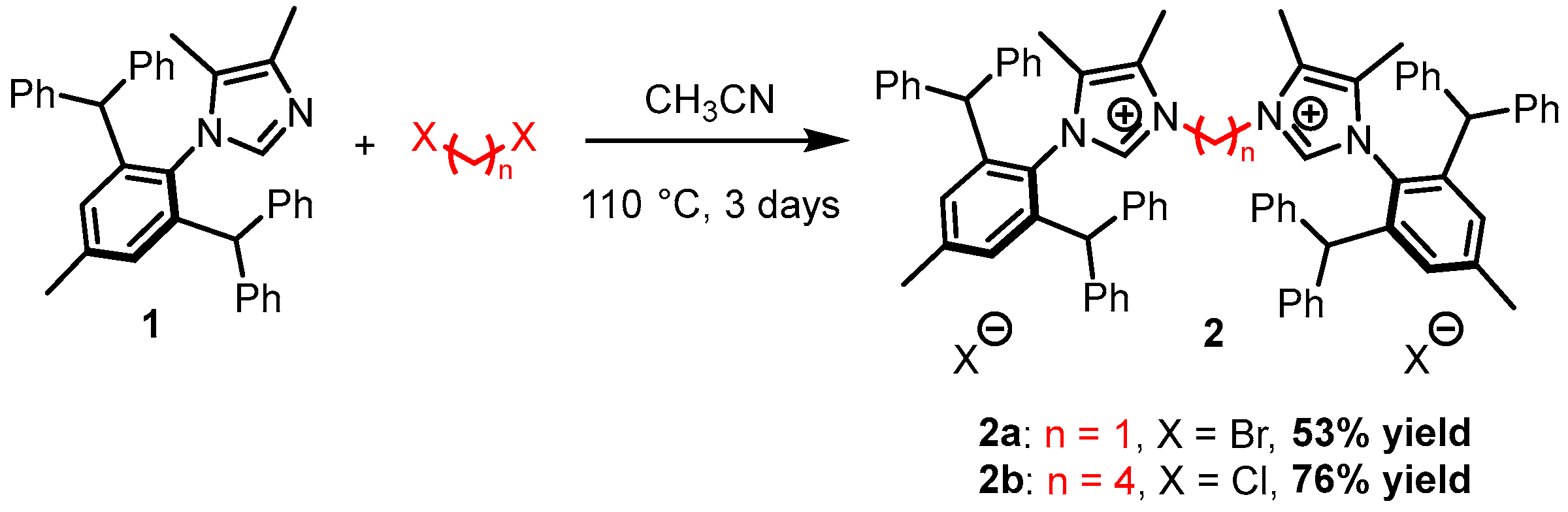
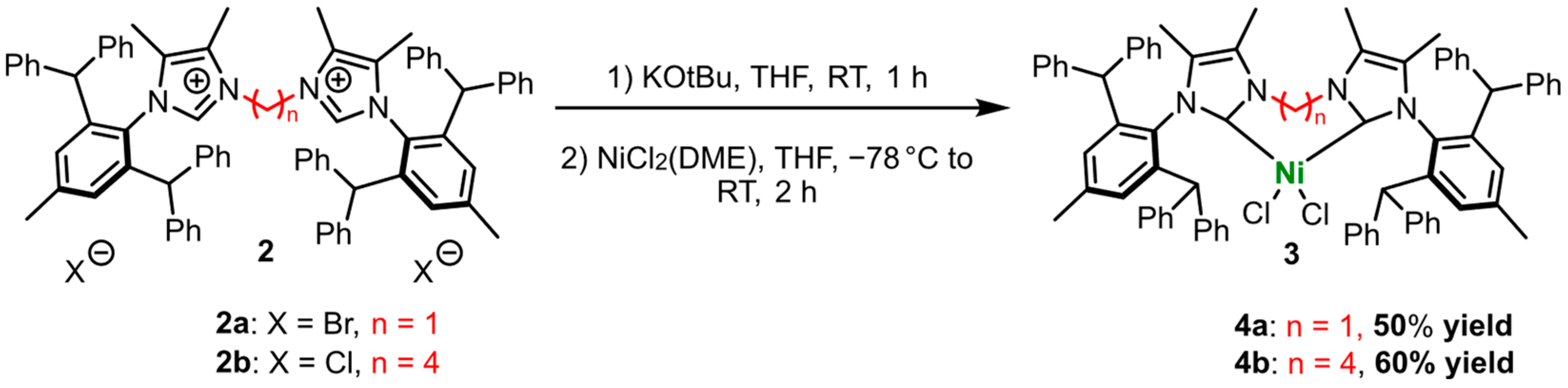
2.1. Synthesis of the diNHC Pre-Ligands
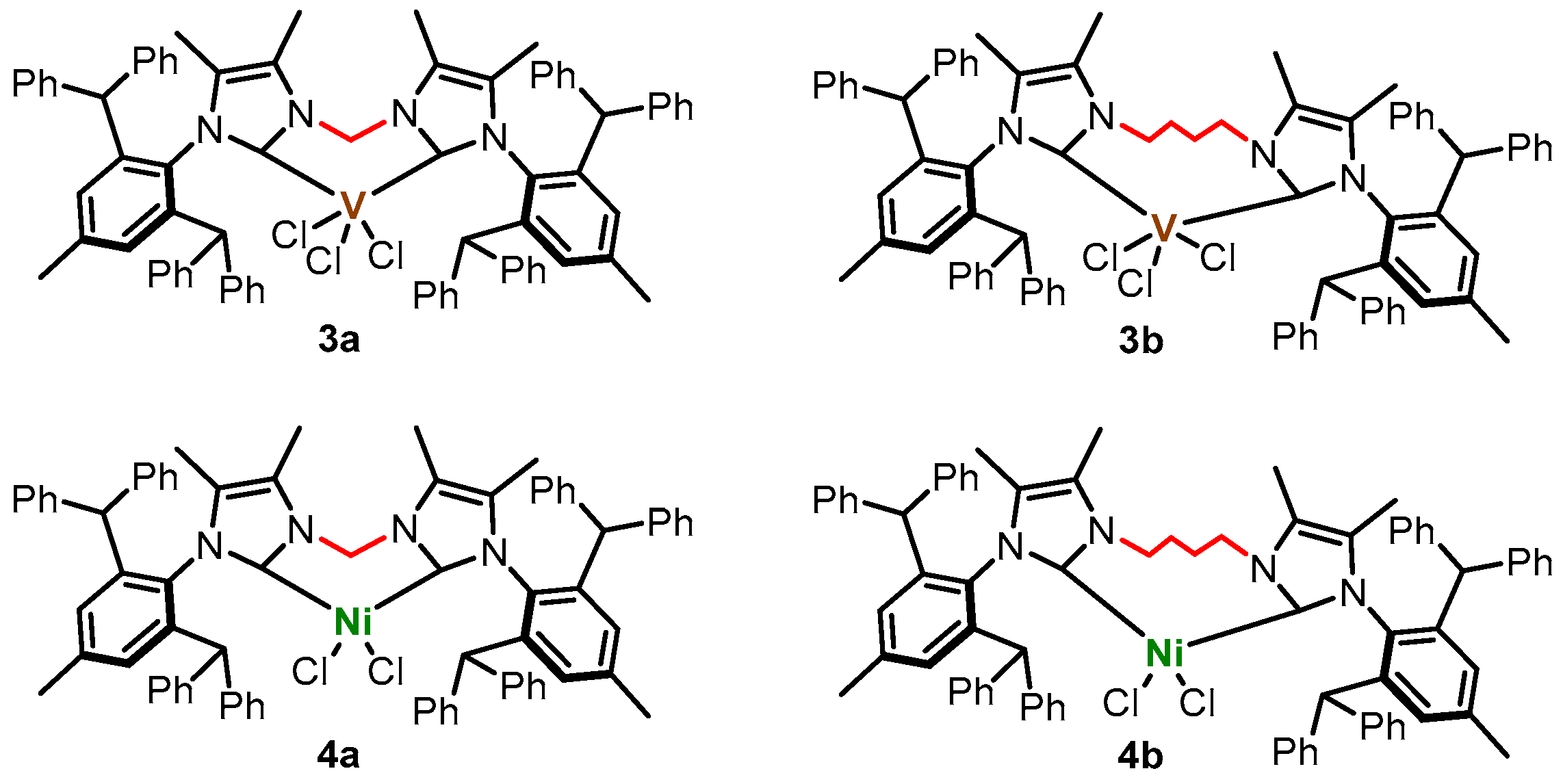
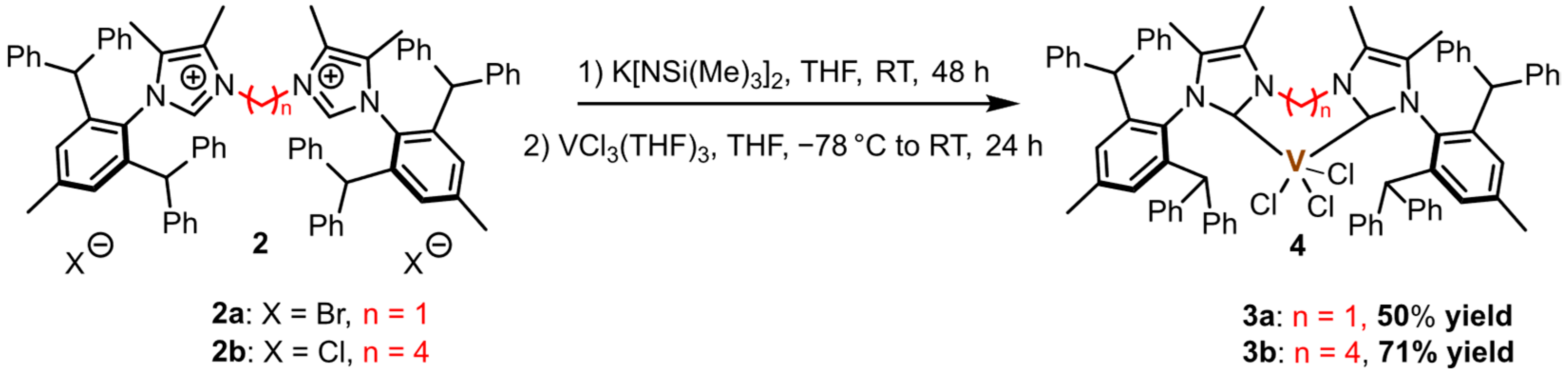
2.2. Synthesis of Vanadium(III) Complexes
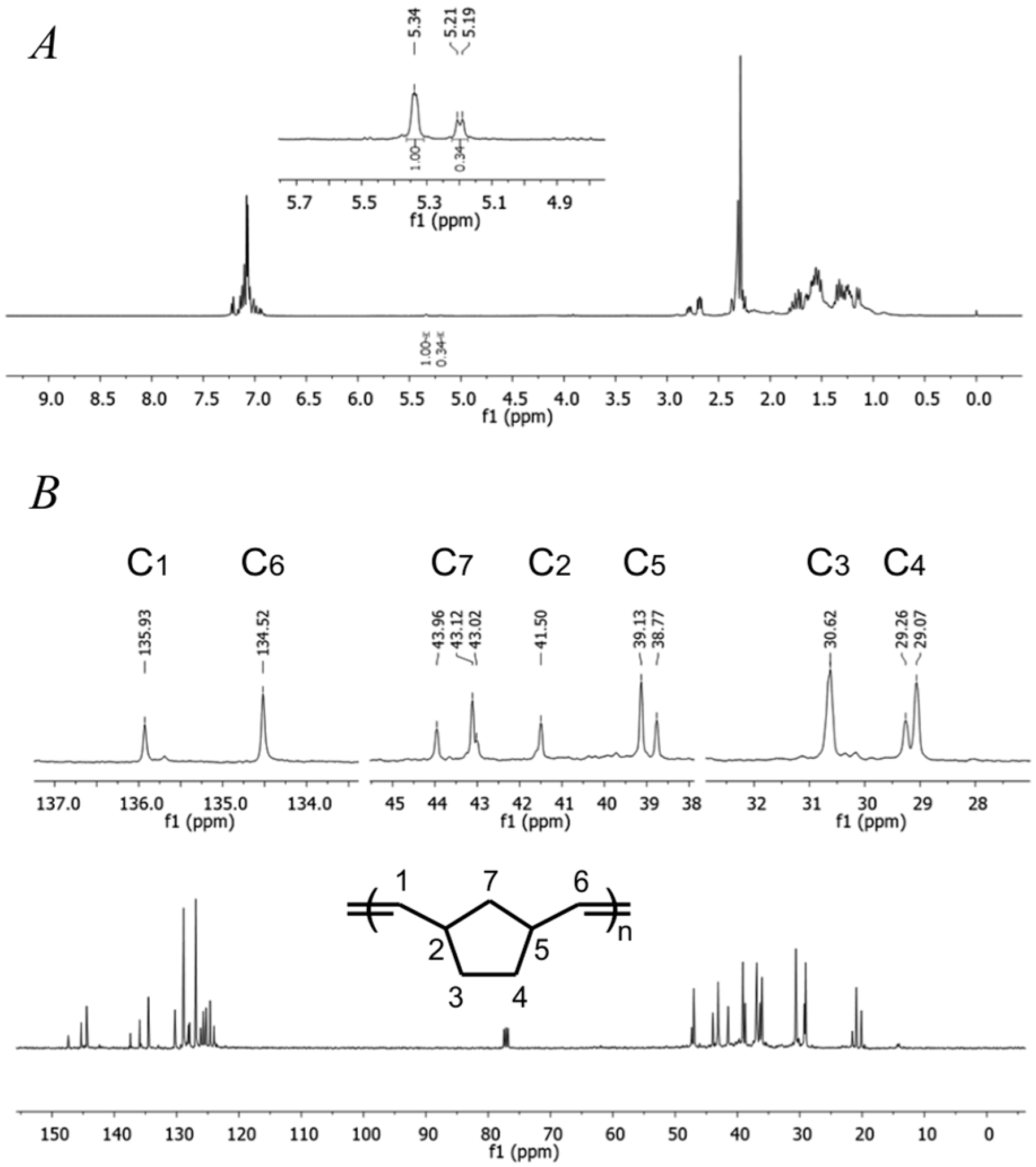
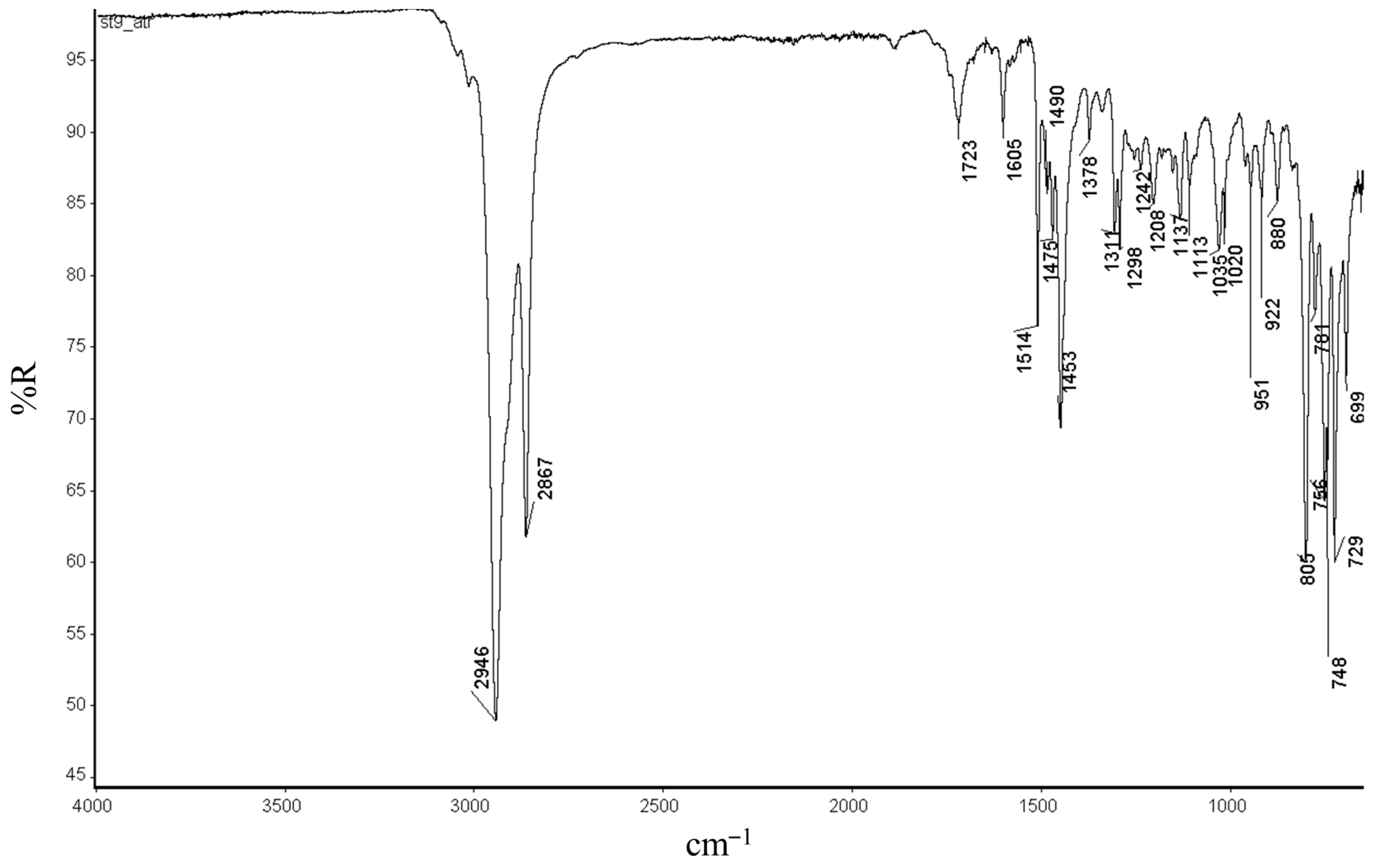
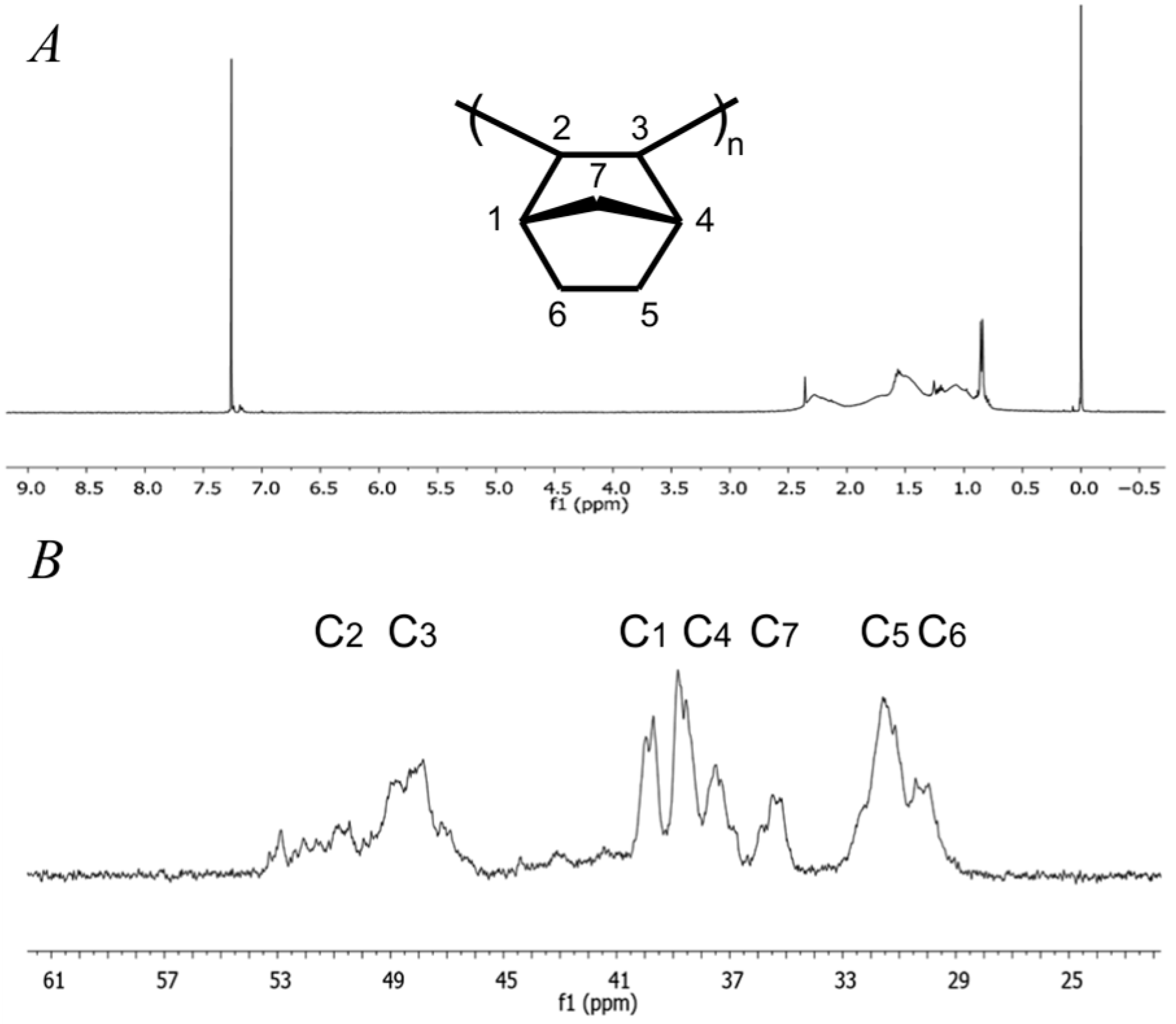
2.3. Norbornene Polymerization Catalyzed by Vanadium(III) Complexes
2.4. Synthesis of Nickel(II) Complexes
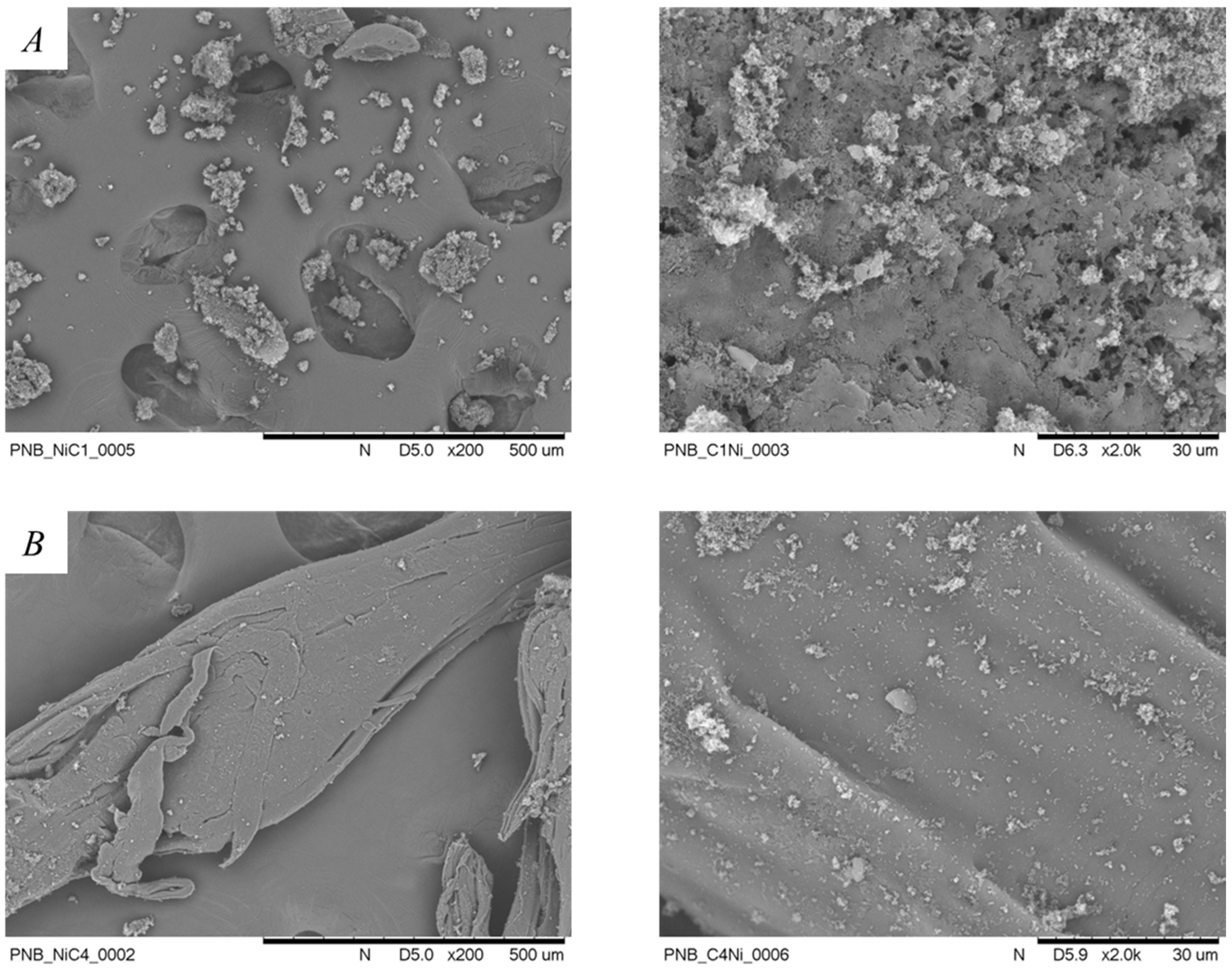
2.5. Norbornene Polymerization Catalyzed by Nickel(II) Complexes
3. Materials and Methods
3.1. Synthesis
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, K.H.; Twieg, R.J.; Ravikiran, R.; Rhodes, L.F.; Shick, R.A.; Yankelevich, D.; Knoesen, A. Synthesis and nonlinear-optical properties of vinyl-addition poly(norbornene)s. Macromolecules 2004, 37, 5163–5178. [Google Scholar] [CrossRef]
- Blank, F.; Janiak, C. Metal Catalysts for the Vinyl/addition Polymerization of Norbornene. Coord. Chem. Rev. 2009, 253, 827–861. [Google Scholar] [CrossRef]
- Wang, X.; Jeong, Y.L.; Love, C.; Stretz, H.A.; Stein, G.E.; Long, B.K. Design, synthesis, and characterization of vinyl-addition polynorbornenes with tunable thermal properties. Polym. Chem. 2021, 12, 5831–5841. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Johnson, L.K.; Grubbs, R.H.; Ziller, J.W. Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media. J. Am. Chem. Soc. 1992, 114, 3974–3975. [Google Scholar] [CrossRef]
- Flores, I.D.R.; van Koten, G. Ring-opening metathesis polymerization of norbornene catalyzed by a Ru(II)-vinylidene complex. Tetrahedron Lett. 1999, 40, 1401–1404. [Google Scholar]
- Nomura, K.; Sagara, A.; Imanishi, Y. Olefin polymerization and ring-opening metathesis polymerization of norbornene by (arylimido)(aryloxo)vanadium(V) complexes of the type VX2(NAr)(OAr’). Remarkable effect of aluminum cocatalyst for the coordination and insertion and ring-opening metathesis polymerization. Macromolecules 2002, 35, 1583–1590. [Google Scholar]
- Nakayama, Y.; Katsuda, K.; Yasuda, H. Ring-opening metathesis polymerization of norbornene with catecholato complexes of tungsten(VI) as effective catalyst precursors. Polym. J. 2003, 35, 896–900. [Google Scholar] [CrossRef]
- Nomura, K.; Atsumi, T.; Fujiki, M.; Yamada, J. Efficient ring-opening metathesis polymerization of norbornene by vanadium-alkylidenes generated in situ from V(NAr)Cl2(L) (L: Ketimide, aryloxo). J. Mol. Catal. A Chem. 2007, 275, 1–8. [Google Scholar] [CrossRef]
- Autenrieth, B.; Schrock, R.R. Stereospecific Ring-opening metathesis polymerization (ROMP) of norbornene and tetracyclododecene by Mo and W initiators. Macromolecules 2015, 48, 2493–2503. [Google Scholar] [CrossRef]
- Chaimongkolkunasin, S.; Hou, X.; Nomura, K. Ring opening metathesis polymerization of norbornene and tetracyclododecene with cyclooctene by using (arylimido)vanadium(V)–alkylidene Catalyst. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3067–3074. [Google Scholar] [CrossRef]
- Wang, X.; Dai, L.; Jie, S.; Bu, Z.; Li, B. Telechelic carboxyl-terminated polynorbornenes and copolymers via chain-transfer ring-opening metathesis polymerization. Chem. Sel. 2020, 5, 8512–8517. [Google Scholar]
- Cruz, T.R.; Masson, G.H.C.; Amorim, K.A.E.; Machado, A.E.H.; Goi, B.E.; Carvalho, V.P., Jr. Ru/Pd Complex and its monometallic fragments as catalysts for norbornene polymerization via ROMP and addition. Catalysts 2022, 12, 1111. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Z.; Wang, Z.; Song, H.; Li, Q.; Li, H.; Hu, Y. Vanadium (III) catalysts with bulky bis-NHCs ligands for ethylene-norbornene (co)polymerization. Appl. Catal. A Gen. 2023, 661, 1–7. [Google Scholar] [CrossRef]
- Janiak, C.; Lassahn, P.G. Metal catalysts for the vinyl polymerization of norbornene. J. Mol. Catal. A Chem. 2001, 166, 193–209. [Google Scholar] [CrossRef]
- Janiak, C.; Lassahn, P.G. The Vinyl homopolymerization of norbornene. Macromol. Rapid Commun. 2001, 22, 479–492. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Sonzogni, M.; Losio, S.; Forlini, F.; Locatelli, P.; Tritto, I.; Licchelli, M. Vinylic polymerization of norbornene by late transition metal-based catalysis. Macromol. Chem. Phys. 2001, 202, 2052–2058. [Google Scholar] [CrossRef]
- Patil, A.O.; Zushma, S.; Stibrany, R.T.; Rucker, S.P.; Wheeler, L.M. Vinyl-type polymerization of norbornene by nickel(II) bisbenzimidazole catalysts. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2095–2106. [Google Scholar] [CrossRef]
- Barnes, D.A.; Benedikt, G.M.; Goodall, B.L.; Huang, S.S.; Kalamarides, H.A.; Lenhard, S.; McIntosh, L.H.; Selvy, K.T.; Shick, R.A.; Rhodes, L.F. Addition polymerization of norbornene-type monomers using neutral nickel complexes containing fluorinated aryl ligands. Macromolecules 2003, 36, 2623–2632. [Google Scholar] [CrossRef]
- He, X.; Wu, Q. Polymerization of norbornene using bis(β-ketoamino)nickel(II)/MAO catalytic systems. J. Appl. Polym. Sci. 2006, 101, 4172–4180. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Wu, Q. Vinyl polymerization of norbornene by nickel(II) complexes bearing β-diketiminate ligands. Appl. Organometal. Chem. 2007, 21, 965–969. [Google Scholar] [CrossRef]
- Ma, R.; Hou, Y.; Gao, J.; Bao, F. Recent Progress in the vinylic polymerization and copolymerization of norbornene catalyzed by transition metal catalysts. Polym. Rev. 2009, 49, 249–287. [Google Scholar] [CrossRef]
- Arduengo, A.J. Looking for stable carbenes: The difficulty in starting anew. Acc. Chem. Res. 1999, 32, 913–921. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes; Wiley: Hoboken, NJ, USA, 2014; pp. 1–24. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gonzalez, S. N-Heterocyclic Carbenes; Royal Society of Chemistry: London, UK, 2016; pp. 534–566. [Google Scholar]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar] [CrossRef]
- Herrmann, W.A. N-Heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in late transition metal catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Cazin, C.S.J. N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Springer: Dordrecht, The Netherlands, 2011; Volume 32, pp. 1–22. [Google Scholar]
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; Wiley: Hoboken, NJ, USA, 2017; pp. 17–51. [Google Scholar]
- Peris, E. Smart N-heterocyclic carbene ligands in catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903. [Google Scholar] [CrossRef]
- Halikowska-Tarasek, K.; Ochędzan-Siodłak, W.; Dziuk, B.; Szostak, R.; Szostak, M.; Bisz, E. IPr*diNHC: Sterically adaptable dinuclear N-heterocyclic carbenes. Inorg. Chem. 2025, 64, 7851–7857. [Google Scholar] [CrossRef]
- Beligny, S.; Blechert, S. N-Heterocyclic Carbene–Ruthenium Complexes in Olefin Metathesis; Wiley: Hoboken, NJ, USA, 2006; pp. 1–25. [Google Scholar]
- Despagnet-Ayoub, E.; Ritter, T. N-Heterocyclic carbenes as ligands for olefin metathesis catalysts. Top. Organomet. Chem. 2007, 21, 193–218. [Google Scholar]
- Colacino, E.; Martinez, J.; Lamaty, F. Preparation of NHC–ruthenium complexes and their catalytic activity in metathesis reaction. Coord. Chem. Rev. 2007, 251, 726–764. [Google Scholar] [CrossRef]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ren, H.; Xu, S.; Song, H.; Liu, B.; Wang, B. Synthesis, structures, and norbornene polymerization behavior of bis(aryloxide-N-heterocyclic carbene) palladium complexes. Organometallics 2009, 28, 5934–5940. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhu, Y. Nickel(II) complexes bearing pyrazolylimine ligand: Synthesis, structure, and catalytic properties for vinyl-type polymerization of norbornene. Appl. Organomet. Chem. 2010, 24, 308–313. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Kong, Y.; Cheng, M.; Ren, H.; Xu, S.; Song, H.; Yang, M.; Liu, B.; Wang, B. Synthesis, structures, and norbornene polymerization behavior of bis(aryloxide-N-heterocyclic carbene) nickel complexes. Organometallics 2011, 30, 1677–1681. [Google Scholar] [CrossRef]
- Berding, J.; Lutz, M.; Spek, A.L.; Bouwman, E. Nickel N-heterocyclic carbene complexes in the vinyl polymerization of norbornene. Appl. Organometal. Chem. 2011, 25, 76–81. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, S.; Li, Z.; Wang, Q.; Weng, L. Direct synthesis of cis-dihalido-bis(NHC) complex of nickel(II) and catalytic application in olefin addition polymerization: Effect of halogen co-ligands and density functional theory study. Dalton Trans. 2013, 42, 12020–12030. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Grisi, F. Novel olefin metathesis ruthenium catalysts bearing backbone-substituted unsymmetrical NHC ligands. Organometallics 2014, 33, 5932–5935. [Google Scholar] [CrossRef]
- Yoon, J.S.; Cena, N.; Markarian, C.; Schrod, Y. Olefin metathesis catalysts bearing hemilabile NHC ligands: Effect of remote torsional strain on activity. J. Catal. 2023, 421, 376–383. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, L.; Wu, S.; Shi, X.; Han, Y. Applications of metal N-heterocyclic carbene complexes in olefin polymerizations. Inorg. Chem. Front. 2024, 11, 6246–6274. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Elser, I.; Buchmeiser, M.R.; Nomura, K. Vanadium(V) arylimido alkylidene N-heterocyclic carbene alkyl and perhalophenoxy alkylidenes for the cis, syndiospecific ring opening metathesis polymerization of norbornene. Organometallics 2021, 40, 2017–2022. [Google Scholar] [CrossRef]
- Manzini, S.; Urbina-Blanco, C.A.; Slawin, A.M.Z.; Nolan, S.P. Effect of ligand bulk in ruthenium-catalyzed olefin metathesis: IPr* vs. IPr. Organometallics 2012, 31, 6514–6517. [Google Scholar] [CrossRef]
- Izquierdo, F.; Manzinia, S.; Nolan, S.P. The use of the sterically demanding IPr* and related ligands in catalysis. Chem. Commun. 2014, 50, 14926–14937. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Li, G.; Flach, C.; Mendelsohn, R.; Lalancette, R.; Szostak, R.; Szostak, M. IPr#—Highly hindered, broadly applicable N-heterocyclic carbenes. Chem. Sci. 2021, 12, 10583–10589. [Google Scholar]
- Huffer, A.; Jeffery, B.; Waller, B.J.; Danopoulos, A.A. Synthesis of bis N-heterocyclic carbenes, derivatives and metal complexes. C. R. Chim. 2013, 16, 557–565. [Google Scholar] [CrossRef]
- Kreisel, K.A.; Yap, G.P.A.; Theopold, K.H. A Chelating N-heterocyclic carbene ligand in organochromium chemistry. Organometallics 2006, 25, 4670–4679. [Google Scholar] [CrossRef]
- Horrer, G.; Krummenacher, I.; Mann, S.; Braunschweig, H.; Radius, U. N-Heterocyclic carbene and cyclic (alkyl)(amino)carbene complexes of vanadium(III) and vanadium(V). Dalton Trans. 2022, 51, 11054. [Google Scholar] [CrossRef]
- Collins, S.; Hasan, G.; Joshi, A.; McIndoe, J.S.; Linnolahti, M. Are methylaluminoxane activators sheets? ChemPhysChem 2021, 14, 1326–1335. [Google Scholar] [CrossRef]
- Collins, S.; Joshi, A.; Linnolahti, M. Formation and structure of hydrolytic methylaluminoxane activators. Chem. A Eur. J. 2021, 27, 15460–15471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.-J.; Lai, J.-Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Mater. Today Bio 2022, 13, 100183. [Google Scholar] [CrossRef]
- Dresch, L.C.; Araujo, B.B.D.; Casagrande, O.D.L., Jr.; Stieler, R. A novel class of nickel(II) complexes containing selenium-based bidentate ligands applied in ethylene oligomerization. RSC Adv. 2016, 6, 104338. [Google Scholar] [CrossRef]
- Thapa, R.; Kilyanek, S.M. Synthesis and structural characterization of nickel(II) complexes of 20-membered macrocyclic rings bearing chelating bis(N-heterocyclic carbene) ligands. J. Organomet. Chem. 2019, 901, 120937. [Google Scholar] [CrossRef]
- Wojnar, M.K.; Ziller, J.W.; Heyduk, A.F. Two-electron mixed valency in a heterotrimetallic nickel–vanadium–nickel complex. Inorg. Chem. 2023, 62, 1405–1413. [Google Scholar] [CrossRef]
- Gimeno, I.; Luis, F.; Marcuello, C.; Pallarés, M.C.; Lostao, A.; de Ory, M.C.; Gomez, A.; Granados, D.; Tejedor, I.; Natividad, E.; et al. Localized Nanoscale Formation of Vanadyl Porphyrin 2D MOF Nanosheets and Their Optimal Coupling to Lumped Element Superconducting Resonators. J. Phys. Chem. C 2025, 129, 973–982. [Google Scholar] [CrossRef]

| Catalyst | Cocatalyst | Yield [g] | Activity × 10−3 [gPNB/molMt/h] | Mw [kDa] | Mw/Mn |
|---|---|---|---|---|---|
| 3a | AlEt2Cl, ETA | 1.76 | 293.3 | 2.03 | 1.3 |
| 3b | AlEt2Cl, ETA | 1.86 | 310.0 | 2.10 | 1.3 |
| 4a | MMAO | 0.55 | 91.7 | 73.14 | 2.4 |
| 4b | MMAO | 0.39 | 65.0 | 97.23 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halikowska-Tarasek, K.; Bisz, E.; Siodłak, D.; Dziuk, B.; Ochędzan-Siodłak, W. ROMP and Vinyl Polynorbornenes with Vanadium(III) and Nickel(II) diNHC Complexes. Int. J. Mol. Sci. 2025, 26, 6691. https://doi.org/10.3390/ijms26146691
Halikowska-Tarasek K, Bisz E, Siodłak D, Dziuk B, Ochędzan-Siodłak W. ROMP and Vinyl Polynorbornenes with Vanadium(III) and Nickel(II) diNHC Complexes. International Journal of Molecular Sciences. 2025; 26(14):6691. https://doi.org/10.3390/ijms26146691
Chicago/Turabian StyleHalikowska-Tarasek, Katarzyna, Elwira Bisz, Dawid Siodłak, Błażej Dziuk, and Wioletta Ochędzan-Siodłak. 2025. "ROMP and Vinyl Polynorbornenes with Vanadium(III) and Nickel(II) diNHC Complexes" International Journal of Molecular Sciences 26, no. 14: 6691. https://doi.org/10.3390/ijms26146691
APA StyleHalikowska-Tarasek, K., Bisz, E., Siodłak, D., Dziuk, B., & Ochędzan-Siodłak, W. (2025). ROMP and Vinyl Polynorbornenes with Vanadium(III) and Nickel(II) diNHC Complexes. International Journal of Molecular Sciences, 26(14), 6691. https://doi.org/10.3390/ijms26146691






