TGF-β Promotes Endothelial-to-Mesenchymal Transition and Alters Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy
Abstract
1. Introduction
2. Results
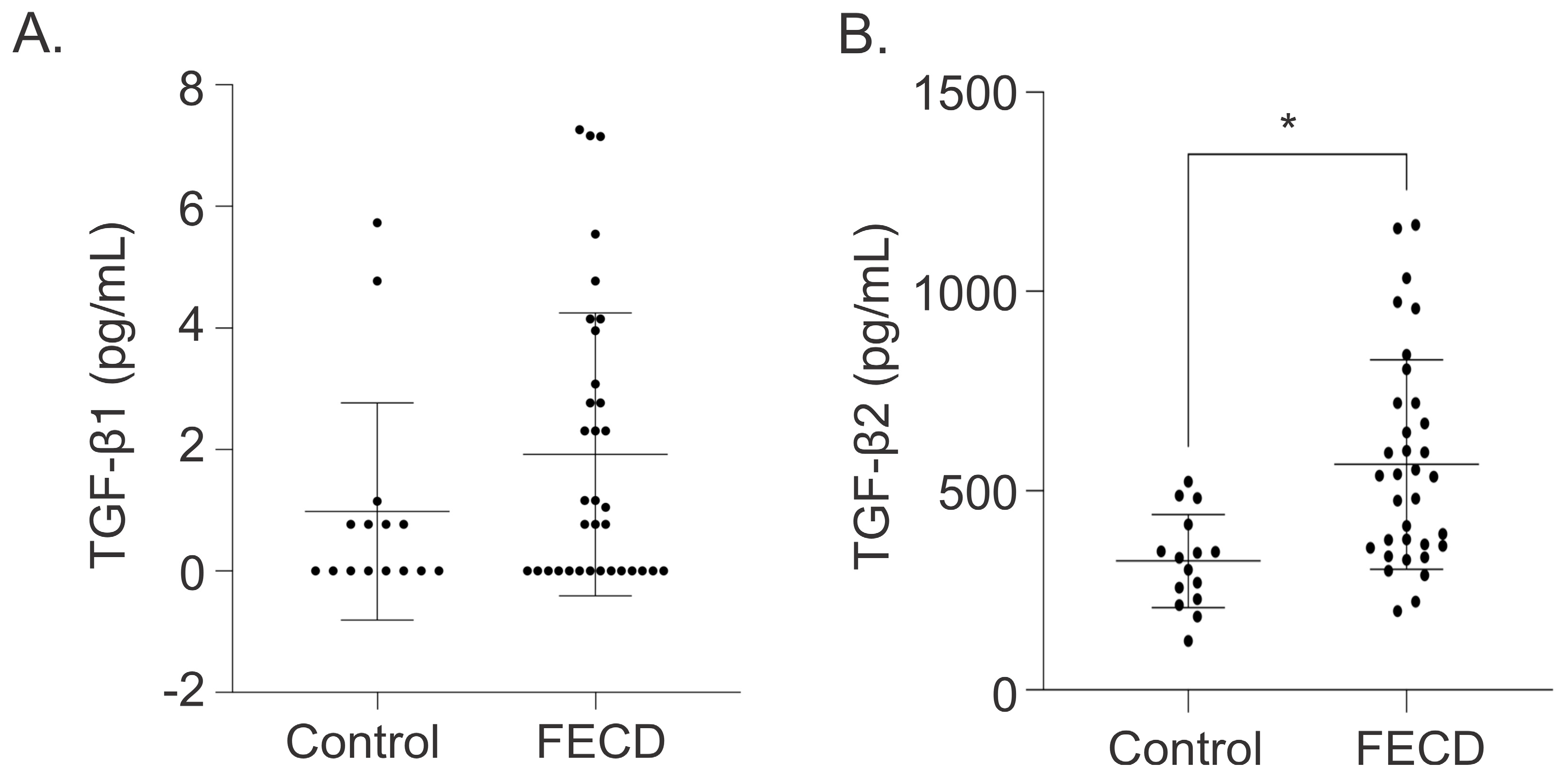
2.1. Elevated Levels of TGF-β2 in Aqueous Humor of Fuchs Endothelial Corneal Dystrophy Patients
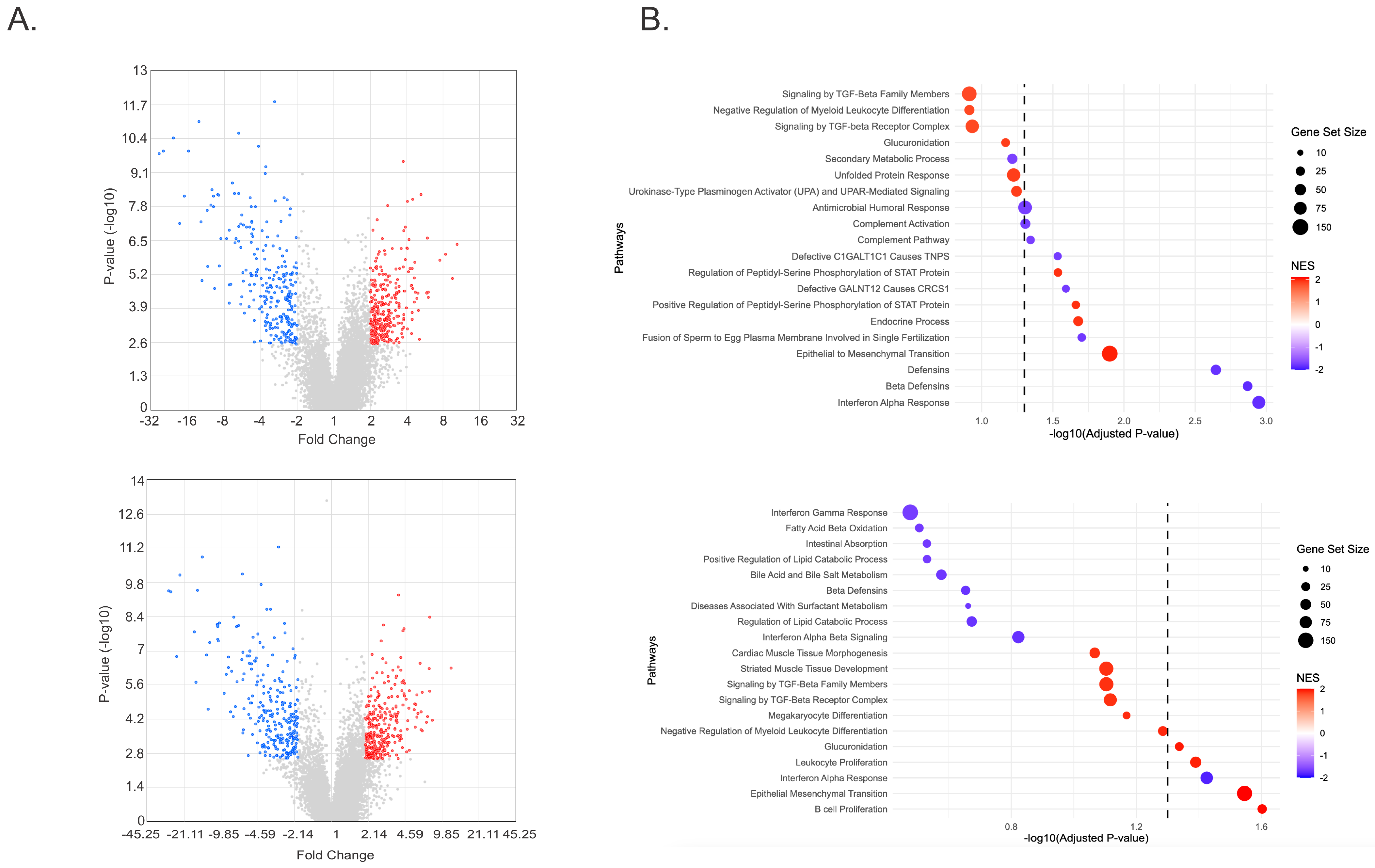
2.2. RNA Sequencing Identifies EMT Pathway Activation in FECD CECs Treated with TGF-β
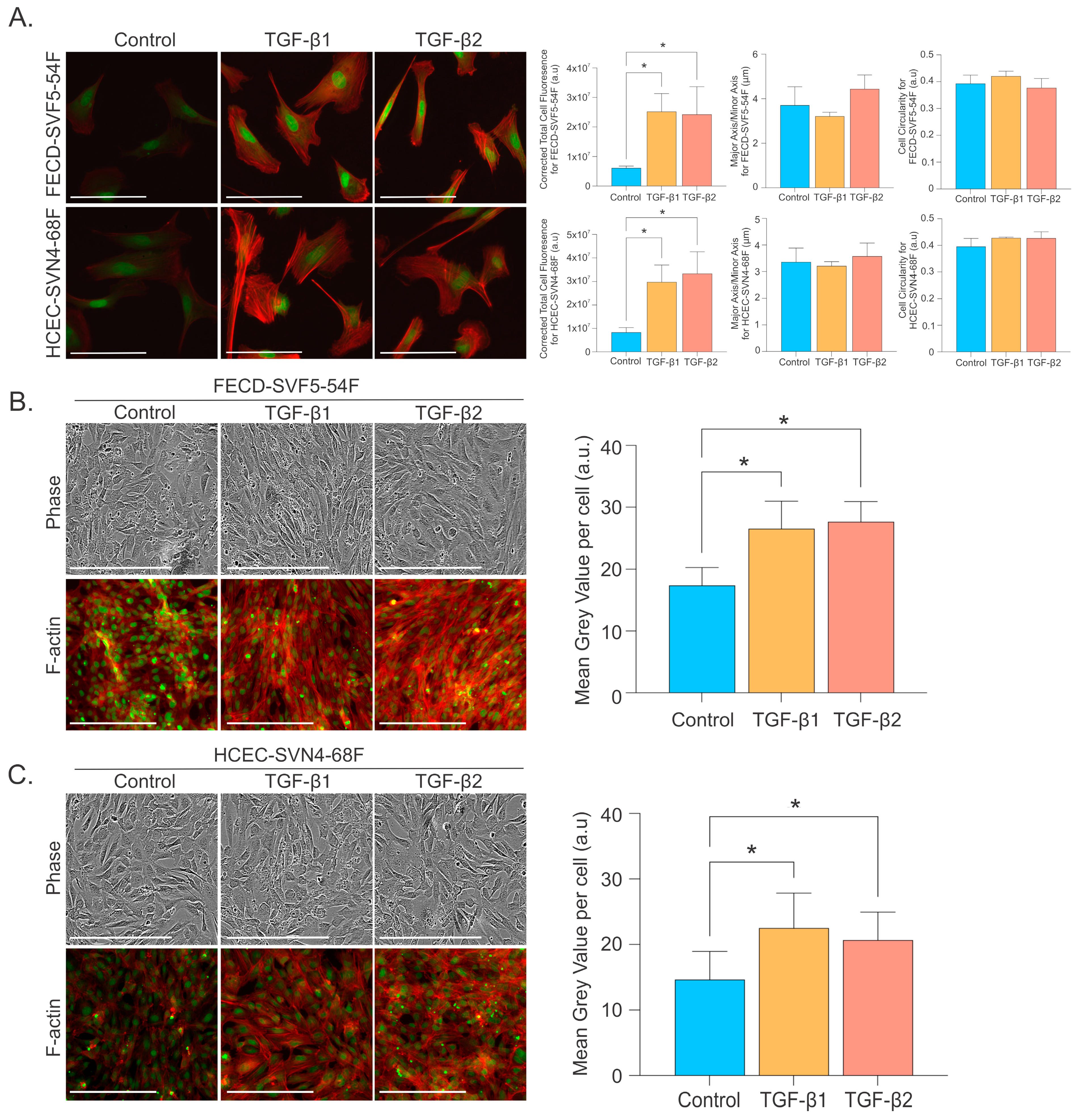
2.3. TGF-β Increases EMT Markers and Alters Morphological Phenotype
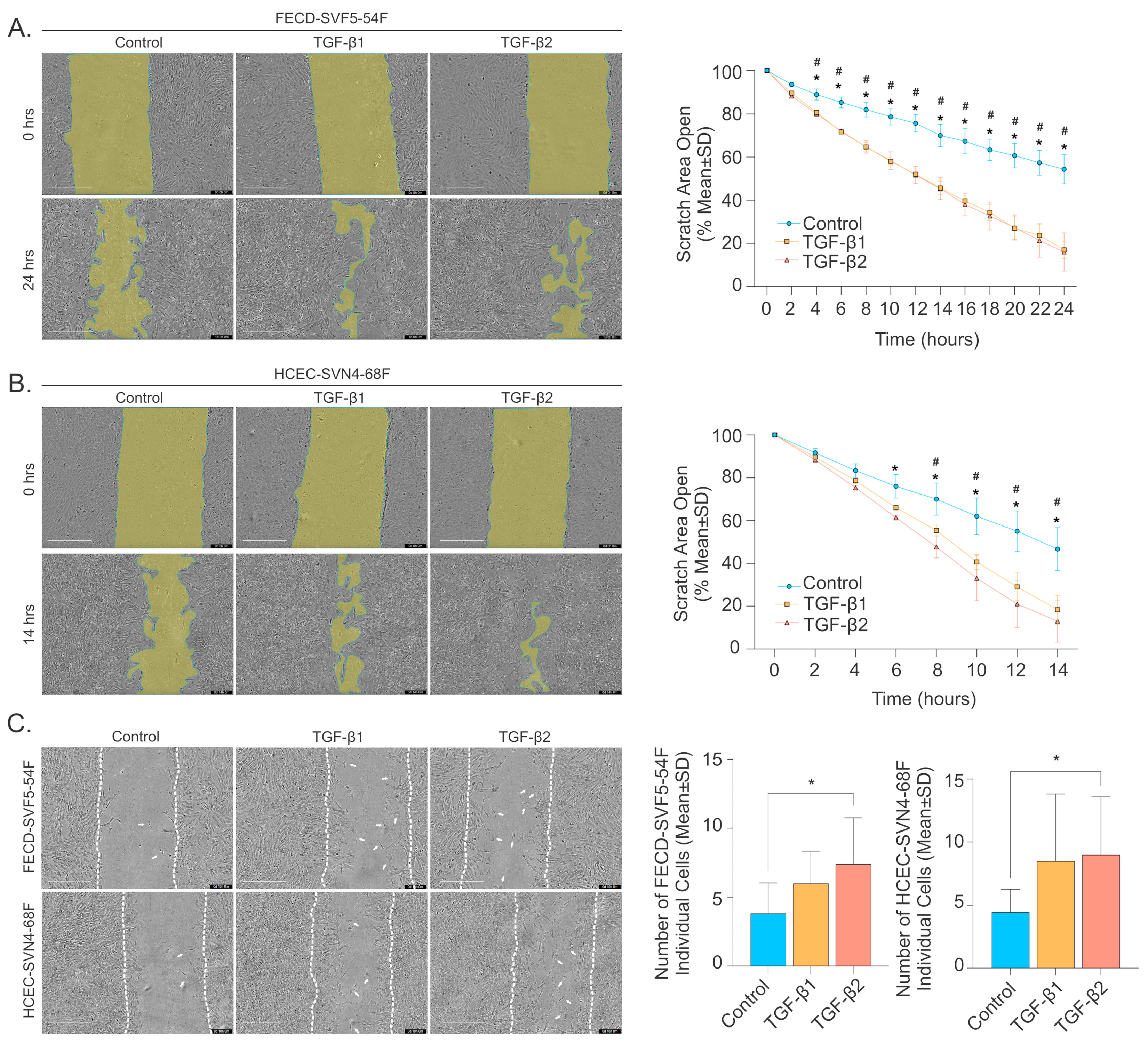
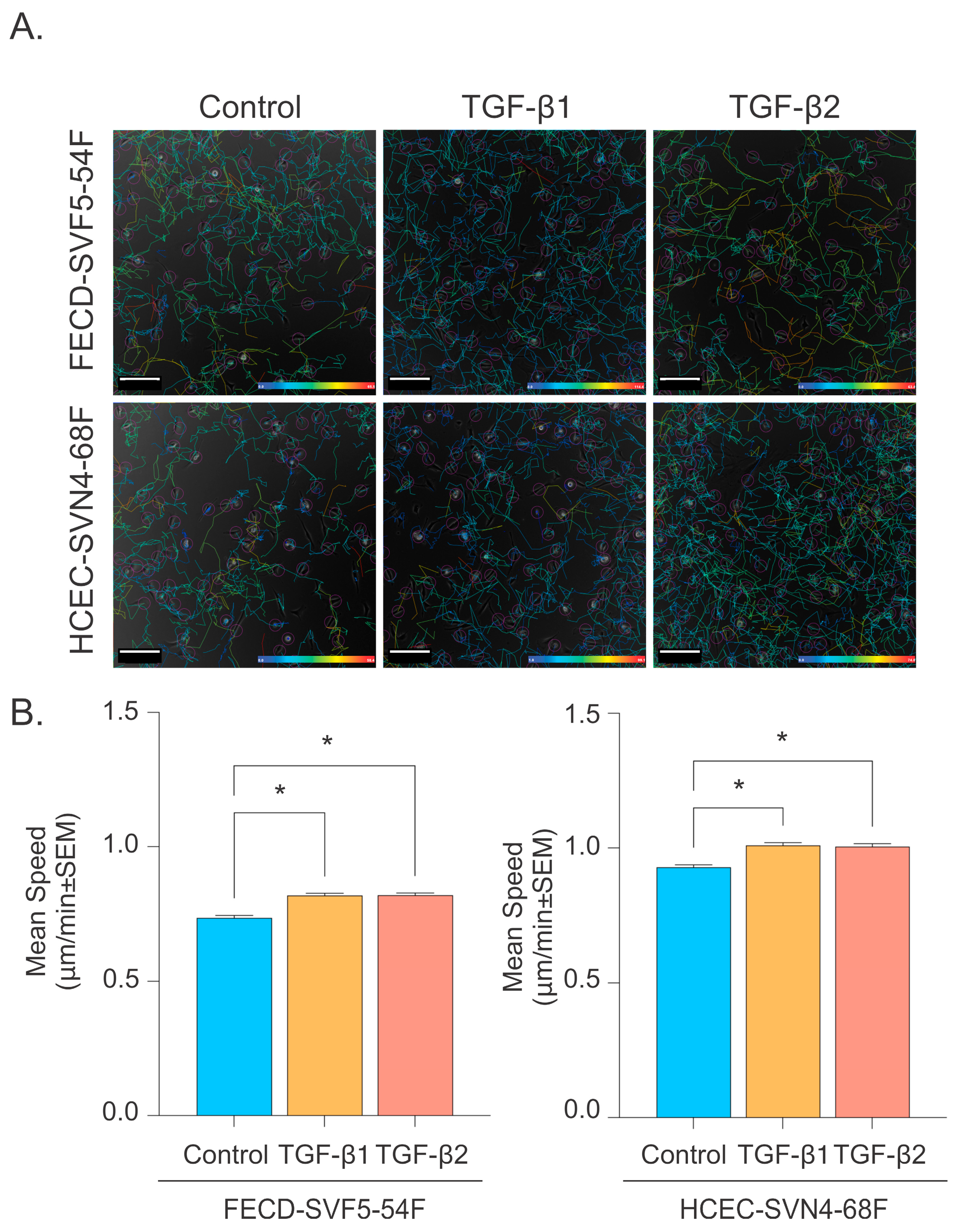
2.4. TGF-β Promotes Corneal Endothelial Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Aqueous Humor Collection
4.3. TGF-β Cytokine Analysis
4.4. RNA Transcriptomics
4.5. Western Blot
4.6. Individual Single-Cell Migration
4.7. Scratch Assay
4.8. F-actin Morphology Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy: The Vicious Cycle of Fuchs Pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial Corneal Dystrophy: Clinical, Genetic, Pathophysiologic, and Therapeutic Aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative Capacity of the Corneal Endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, J.D.; Bowers, A.L.; Margulies, E.H.; Seitzman, G.D.; Kim, S.W.; Saba, S.; Jun, A.S.; Stark, W.J.; Liu, S.H. Serial Analysis of Gene Expression in the Corneal Endothelium of Fuchs’ Dystrophy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 594–599. [Google Scholar] [CrossRef]
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef]
- Van den Bogerd, B.; Zakaria, N.; Adam, B.; Matthyssen, S.; Koppen, C.; Ní Dhubhghaill, S. Corneal Endothelial Cells Over the Past Decade: Are We Missing the Mark(Er)? Transl. Vis. Sci. Technol. 2019, 8, 13. [Google Scholar] [CrossRef]
- Sun, F.; Xi, L.W.Q.; Luu, W.; Enkhbat, M.; Neo, D.; Mehta, J.S.; Peh, G.S.L.; Yim, E.K.F. Preclinical Models for Studying Fuchs Endothelial Corneal Dystrophy. Cells 2025, 14, 505. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Feng, Y.; Li, X.; Lu, Z.; Gu, H.; Li, W.; Hill, L.J.; Ou, S. Evolution of Therapeutic Strategy Based on Oxidant-Antioxidant Balance for Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2024, 34, 247–261. [Google Scholar] [CrossRef]
- Sherrard, E.S. The Corneal Endothelium in Vivo: Its Response to Mild Trauma. Exp. Eye Res. 1976, 22, 347–357. [Google Scholar] [CrossRef]
- Honda, H.; Ogita, Y.; Higuchi, S.; Kani, K. Cell Movements in a Living Mammalian Tissue: Long-term Observation of Individual Cells in Wounded Corneal Endothelia of Cats. J. Morphol. 1982, 174, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Katz, J.I. Pathology of the Corneal Endothelium. Investig. Ophthalmol. Vis. Sci. 1977, 16, 265–268. [Google Scholar]
- Soh, Y.Q.; Peh, G.; George, B.L.; Seah, X.Y.; Primalani, N.K.; Adnan, K.; Mehta, J.S. Predicative Factors for Corneal Endothelial Cell Migration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.; Ní Dhubhghaill, S.; Kocaba, V.; Jager, M.J.; Melles, G.R.J.; Oellerich, S. Early and Late-Onset Cell Migration from Peripheral Corneal Endothelium. PLoS ONE 2023, 18, e0285609. [Google Scholar] [CrossRef]
- Miron, A.; Spinozzi, D.; Bruinsma, M.; Lie, J.T.; Birbal, R.S.; Baydoun, L.; Oellerich, S.; Melles, G.R.J. Asymmetrical Endothelial Cell Migration from in Vitro Quarter-Descemet Membrane Endothelial Keratoplasty Grafts. Acta Ophthalmol. 2018, 96, 828–833. [Google Scholar] [CrossRef]
- Miron, A.; Spinozzi, D.; Dhubhghaill, S.N.; Lie, J.T.; Oellerich, S.; Melles, G.R.J. In Vitro Endothelial Cell Migration from Limbal Edge-Modified Quarter-DMEK Grafts. PLoS ONE 2019, 14, e0225462. [Google Scholar] [CrossRef]
- Kocaba, V.; Katikireddy, K.R.; Gipson, I.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Association of the Gutta-Induced Microenvironment with Corneal Endothelial Cell Behavior and Demise in Fuchs Endothelial Corneal Dystrophy. JAMA Ophthalmol. 2018, 136, 886–892. [Google Scholar] [CrossRef]
- Meekins, L.C.; Rosado-Adames, N.; Maddala, R.; Zhao, J.J.; Rao, P.V.; Afshari, N.A. Corneal Endothelial Cell Migration and Proliferation Enhanced by Rho Kinase (ROCK) Inhibitors in in Vitro and in Vivo Models. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6731–6738. [Google Scholar] [CrossRef]
- Lee, J.G.; Heur, M. Interleukin-1β-Induced Wnt5a Enhances Human Corneal Endothelial Cell Migration through Regulation of Cdc42 and RhoA. Mol. Cell Biol. 2014, 34, 3535–3545. [Google Scholar] [CrossRef]
- Lee, J.G.; Heur, M. Interleukin-1β Enhances Cell Migration through AP-1 and NF-ΚB Pathway Dependent FGF2 Expression in Human Corneal Endothelial Cells Jeong. Biol. Cell 2013, 105, 175–189. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, F.; Yan, C.; Shao, C.; Gu, P.; Fu, Y.; Sun, H.; Fan, X. PTEN Inhibition Accelerates Corneal Endothelial Wound Healing through Increased Endothelial Cell Division and Migration. Investig. Opthalmology Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef] [PubMed]
- Ong Tone, S.; Wylegala, A.; Böhm, M.; Melangath, G.; Deshpande, N.; Jurkunas, U.V. Increased Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy. Ophthalmol. Sci. 2021, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mehta, S.; Patel, K.; Dhupar, N.; Little, N.; Ong Tone, S. Transcription Factor 4 Promotes Increased Corneal Endothelial Cellular Migration by Altering Microtubules in Fuchs Endothelial Corneal Dystrophy. Sci. Rep. 2024, 14, 10276. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-Mesenchymal Transition and Its Implications for Fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Zavadil, J.; Böttinger, E.P. TGF-β and Epithelial-to-Mesenchymal Transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of the Epithelial-Mesenchymal Transition by TGF-β. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef]
- Massagué, J. TGFbeta Signalling in Context. Nat. Rev. Mol. Cell Biol. 2014, 13, 616–630. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.K.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of Transforming Growth Factor Beta in Corneal Function, Biology and Pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [CrossRef]
- Cordeiro, M.F. Beyond Mitomycin: TGF-β and Wound Healing. Prog. Retin. Eye Res. 2002, 21, 75–89. [Google Scholar] [CrossRef]
- Saika, S. TGFβ Pathobiology in the Eye. Lab. Investig. 2006, 86, 106–115. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Santerre, K.; Ghio, S.C.; Proulx, S. TGF-β—Mediated Modulation of Cell—Cell Interactions in Postconfluent Maturing Corneal Endothelial Cells. Cornea 2022, 63, 3. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Hashimoto, K.; Kitahara, M.; Okuda, H.; Ueda, E.; Watanabe, K.; Nakahara, M.; Sato, T.; Kinoshita, S.; Tourtas, T.; et al. Activation of TGF-β Signaling Induces Cell Death via the Unfolded Protein Response in Fuchs Endothelial Corneal Dystrophy. Sci. Rep. 2017, 7, 6801. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Minamiyama, R.; Ho, L.T.Y.; Kay, E.P.; Kawasaki, S.; Tourtas, T.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Young, R.D.; Quantock, A.J.; et al. Involvement of ZEB1 and Snail1 in Excessive Production of Extracellular Matrix in Fuchs Endothelial Corneal Dystrophy. Lab. Investig. 2015, 95, 1291–1304. [Google Scholar] [CrossRef]
- Ogata, F.T.; Verma, S.; Coulson-Thomas, V.J.; Gesteira, T.F. TGF-β-Based Therapies for Treating Ocular Surface Disorders. Cells 2024, 13, 1105. [Google Scholar] [CrossRef]
- Hakim, F.E.; Nagra, A.K.; Dhaliwal, D.K. Descemet Stripping Only: Long-Term Outcomes. Cornea 2024, 43, 994–998. [Google Scholar] [CrossRef]
- Shah, R.D.; Randleman, J.B.; Grossniklaus, H.E. Spontaneous Corneal Clearing after Descemet’s Stripping without Endothelial Replacement. Ophthalmology 2012, 119, 256–260. [Google Scholar] [CrossRef]
- Borkar, D.S.; Veldman, P.; Colby, K.A. Treatment of Fuchs Endothelial Dystrophy by Descemet Stripping without Endothelial Keratoplasty. Cornea 2016, 35, 1267–1273. [Google Scholar] [CrossRef]
- Moloney, G.; Petsoglou, C.; Ball, M.; Kerdraon, Y.; Höllhumer, R.; Spiteri, N.; Beheregaray, S.; Hampson, J.; D’Souza, M.; Devasahayam, R.N. Descemetorhexis without Grafting for Fuchs Endothelial Dystrophy-Supplementation with Topical Ripasudil. Cornea 2017, 36, 642–648. [Google Scholar] [CrossRef]
- Macsai, M.S.; Shiloach, M. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy after Descemet Stripping Only. Cornea 2019, 38, 529–534. [Google Scholar] [CrossRef]
- Matthaei, M.; Gillessen, J.; Muether, P.S.; Hoerster, R.; Bachmann, B.O.; Hueber, A.; Cursiefen, C.; Heindl, L.M. Epithelial-Mesenchymal Transition (EMT)-Related Cytokines in the Aqueous Humor of Phakic and Pseudophakic Fuchs’ Dystrophy Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Brown, C.W.; Matzuk, M.M. Genetic Analysis of the Mammalian Transforming Growth Factor-β Superfamily. Endocr. Rev. 2002, 23, 787–823. [Google Scholar] [CrossRef] [PubMed]
- Chychko, L.; Son, H.S.; Friedrich, M.; Khoramnia, R.; Auffarth, G.U.; Augustin, V.A. Molecular Changes in Aqueous Humor Associated with Inflammation Following Cataract Surgery in Patients with Fuchs’ Endothelial Corneal Dystrophy. Ophthalmol. Ther. 2024, 14, 197–209. [Google Scholar] [CrossRef] [PubMed]
- De Roo, A.K.; Struyf, S.; Foets, B.; van den Oord, J.J. Transforming Growth Factor Beta Switch in Aqueous Humor of Patients with Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 771–772. [Google Scholar] [CrossRef]
- Matthaei, M.; Gillessen, J.; Muether, P.S.; Hoerster, R.; Bachmann, B.O.; Hueber, A.; Cursiefen, C.; Heindl, L.M. Author Response: Transforming Growth Factor Beta Switch in Aqueous Humor of Patients with Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 773. [Google Scholar] [CrossRef]
- Nakagawa, T.; Honda, T.; Inagaki, S.; Yuasa, T.; Tourtas, T.; Schlötzer-Schrehardt, U.; Kruse, F.; Aouimeur, I.; Vaitinadapoule, H.; Travers, G.; et al. Involvement of TGF-β Signaling Pathway-Associated Genes in the Corneal Endothelium of Patients with Fuchs Endothelial Corneal Dystrophy. Exp. Eye Res. 2025, 255, 110334. [Google Scholar] [CrossRef]
- Beaulieu Leclerc, V.; Roy, O.; Santerre, K.; Proulx, S. TGF-Β1 Promotes Cell Barrier Function upon Maturation of Corneal Endothelial Cells. Sci. Rep. 2018, 8, 4438. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, C.; Tian, H.; Xu, J.; Chen, J.; Hu, S.; Li, Q.; Lu, L.; Ou, Q.; Xu, G.-t.; et al. CHIR99021 Balance TGFβ1 Induced Human Corneal Endothelial-to-Mesenchymal Transition to Favor Corneal Endothelial Cell Proliferation. Exp. Eye Res. 2022, 219, 108939. [Google Scholar] [CrossRef]
- Maurizi, E.; Merra, A.; Macaluso, C.; Schiroli, D.; Pellegrini, G. GSK-3 Inhibition Reverts Mesenchymal Transition in Primary Human Corneal Endothelial Cells. Eur. J. Cell Biol. 2023, 102, 151302. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Joyce, N.C.; Meklir, B.; Neufeld, A.H. In Vitro Pharmacologic Separation of Corneal Endothelial Migration and Spreading Responses. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1816–1826. [Google Scholar]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and Molecular Mechanisms in Fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Baratz, K.H.; Tosakulwong, N.; Ryu, E.; Brown, W.L.; Branham, K.; Chen, W.; Tran, K.D.; Schmid-Kubista, K.E.; Heckenlively, J.R.; Swaroop, A.; et al. E2-2 Protein and Fuchs’s Corneal Dystrophy. N. Engl. J. Med. 2010, 363, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Katikireddy, K.R.; White, T.L.; Miyajima, T.; Vasanth, S.; Raoof, D.; Chen, Y.; Price, M.O.; Price, F.W.; Jurkunas, U.V. NQO1 Downregulation Potentiates Menadione-Induced Endothelial-Mesenchymal Transition during Rosette Formation in Fuchs Endothelial Corneal Dystrophy. Free Radic. Biol. Med. 2018, 116, 19–30. [Google Scholar] [CrossRef]
- Afshari, N.A.; Igo, R.P.; Morris, N.J.; Stambolian, D.; Sharma, S.; Pulagam, V.L.; Dunn, S.; Stamler, J.F.; Truitt, B.J.; Rimmler, J.; et al. Genome-Wide Association Study Identifies Three Novel Loci in Fuchs Endothelial Corneal Dystrophy. Nat. Commun. 2017, 8, 14898. [Google Scholar] [CrossRef]
- Biswas, S.; Munier, F.L.; Yardley, J.; Hart-Holden, N.; Perveen, R.; Cousin, P.; Sutphin, J.E.; Noble, B.; Batterbury, M.; Kielty, C.; et al. Missense Mutations in COL8A2, the Gene Encoding the A2 Chain of Type VIII Collagen, Cause Two Forms of Corneal Endothelial Dystrophy. Hum. Mol. Genet. 2001, 10, 2415–2423. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Zaghloul, N.A.; Al-Saif, A.; Davey, L.; Diplas, B.H.; Meadows, D.N.; Eghrari, A.O.; Minear, M.A.; Li, Y.J.; Klintworth, G.K.; et al. Missense Mutations in TCF8 Cause Late-Onset Fuchs Corneal Dystrophy and Interact with FCD4 on Chromosome 9p. Am. J. Hum. Genet. 2010, 86, 45–53. [Google Scholar] [CrossRef]
- Brown, K.A.; Aakre, M.E.; Gorska, A.E.; Price, J.O.; Eltom, S.E.; Pietenpol, J.A.; Moses, H.L. Induction by Transforming Growth Factor-Β1 of Epithelial to Mesenchymal Transition Is a Rare Event in Vitro. Breast Cancer Res. 2004, 6, 215–231. [Google Scholar] [CrossRef]
- Masszi, A.; Di Ciano, C.; Sirokmány, G.; Arthur, W.T.; Rotstein, O.D.; Wang, J.; McCulloch, C.A.G.; Rosivall, L.; Mucsi, I.; Kapus, A. Central Role for Rho in TGF-Β1-Induced α-Smooth Muscle Actin Expression during Epithelial-Mesenchymal Transition. Am. J. Physiol. Renal Physiol. 2003, 284, F911–F924. [Google Scholar] [CrossRef]
- Haynes, J.; Srivastava, J.; Madson, N.; Wittmann, T.; Barber, D.L. Dynamic Actin Remodeling during Epithelial-Mesenchymal Transition Depends on Increased Moesin Expression. Mol. Biol. Cell 2011, 22, 4750–4764. [Google Scholar] [CrossRef]
- Petroll, W.M.; Jester, J.V.; Bean, J.J.; Cavanagh, H.D. Myofibroblast Transformation of Cat Corneal Endothelium by Transforming Growth Factor-Β1, -Β2, and -Β3. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2018–2032. [Google Scholar]
- Joko, T.; Shiraishi, A.; Akune, Y.; Tokumaru, S.; Kobayashi, T.; Miyata, K.; Ohashi, Y. Involvement of P38MAPK in Human Corneal Endothelial Cell Migration Induced by TGF-Β2. Exp. Eye Res. 2013, 108, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, T.; Ikeda, K.; Okada, Y.; Yamanaka, O.; Kitano, A.; Saika, S. Inhibitory Effect of Blocking TGF-β/Smad Signal on Injury-Induced Fibrosis of Corneal Endothelium. Mol. Vis. 2008, 14, 2272–2281. [Google Scholar] [PubMed]
- Nakagawa, H.; Alemi, H.; Wang, S.; Kahale, F.; Blanco, T.; Liu, C.; Yin, J.; Dohlman, T.H.; Dana, R. Descemet Stripping Only Technique for Corneal Endothelial Damage in Mice. Cornea 2023, 42, 470–475. [Google Scholar] [CrossRef]
- Din, N.; Cohen, E.; Popovic, M.; Mimouni, M.; Trinh, T.; Gouvea, L.; Alshaker, S.; Ong Tone, S.; Chan, C.; Slomovic, A.R. Surgical Management of Fuchs Endothelial Corneal Dystrophy: A Treatment Algorithm and Individual Patient Meta-Analysis of Descemet Stripping Only. Cornea 2022, 41, 1188–1195. [Google Scholar] [CrossRef]
- Reimand, J.; Isser, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway Enrichment Analysis and Visualization of Omics Data Using g:Profiler, GSEA, Cytoscape and Enrichment Map. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]

| Characteristics | Control | FECD | p-Value |
|---|---|---|---|
| Total, n (%) | 15 | 34 | |
| DMEK only | - | 4 (11.8) | |
| DMEK+CE+IOL | - | 25 (73.5) | |
| DSO+CE+IOL | - | 5 (14.7) | |
| Age, years (Mean ± SD) [Range] | 73.07 ± 11.50 [55–89] | 67.09 ± 6.40 [52–77] | 0.0238 |
| Sex, n (%) | |||
| Male | 6 (40.0) | 8 (23.5) | |
| Female | 9 (60.0) | 26 (76.5) | |
| TGF-β1, pg/mL (Mean ± SD) [Range] | 0.98 ± 1.79 [0–5.73] | 1.92 ± 2.33 [0–7.26] | 0.1865 |
| TGF-β2, pg/mL (Mean ± SD) [Range] | 323.83 ± 116.74 [123.65–523.71] | 566.43 ± 263.32 [197.96–1167.04] | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Lim, B.; Dhupar, N.; Bhargava, K.; Chen, L.; Moloney, G.; Ong Tone, S. TGF-β Promotes Endothelial-to-Mesenchymal Transition and Alters Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy. Int. J. Mol. Sci. 2025, 26, 6685. https://doi.org/10.3390/ijms26146685
Yan J, Lim B, Dhupar N, Bhargava K, Chen L, Moloney G, Ong Tone S. TGF-β Promotes Endothelial-to-Mesenchymal Transition and Alters Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy. International Journal of Molecular Sciences. 2025; 26(14):6685. https://doi.org/10.3390/ijms26146685
Chicago/Turabian StyleYan, Judy, Brooke Lim, Narisa Dhupar, Kathrine Bhargava, Lina Chen, Greg Moloney, and Stephan Ong Tone. 2025. "TGF-β Promotes Endothelial-to-Mesenchymal Transition and Alters Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy" International Journal of Molecular Sciences 26, no. 14: 6685. https://doi.org/10.3390/ijms26146685
APA StyleYan, J., Lim, B., Dhupar, N., Bhargava, K., Chen, L., Moloney, G., & Ong Tone, S. (2025). TGF-β Promotes Endothelial-to-Mesenchymal Transition and Alters Corneal Endothelial Cell Migration in Fuchs Endothelial Corneal Dystrophy. International Journal of Molecular Sciences, 26(14), 6685. https://doi.org/10.3390/ijms26146685







