Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels
Abstract
1. Introduction
2. Results
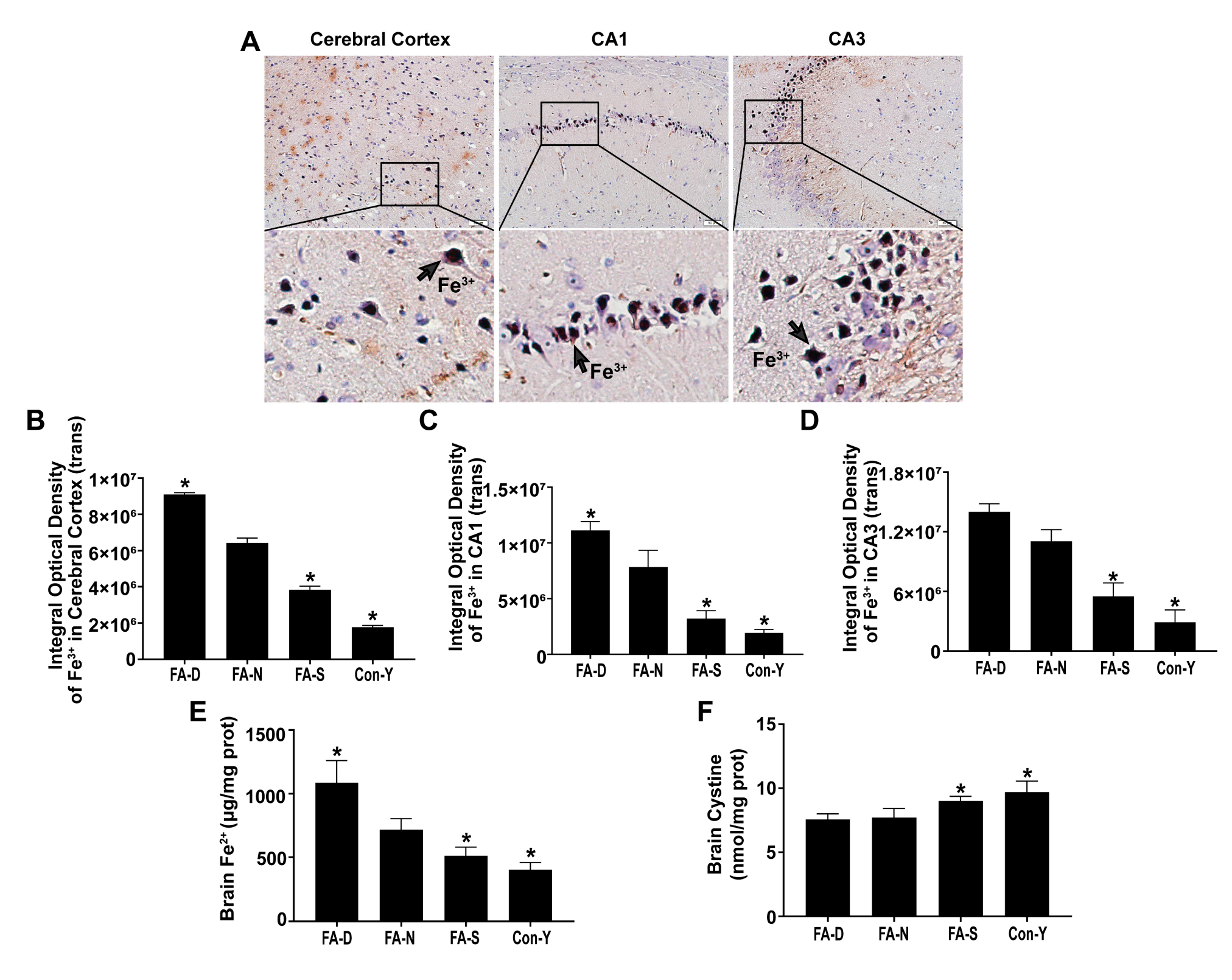
2.1. Folic Acid Supplementation Inhibited Age-Related Brain Iron Deposition and Increased Cystine Levels in Rats
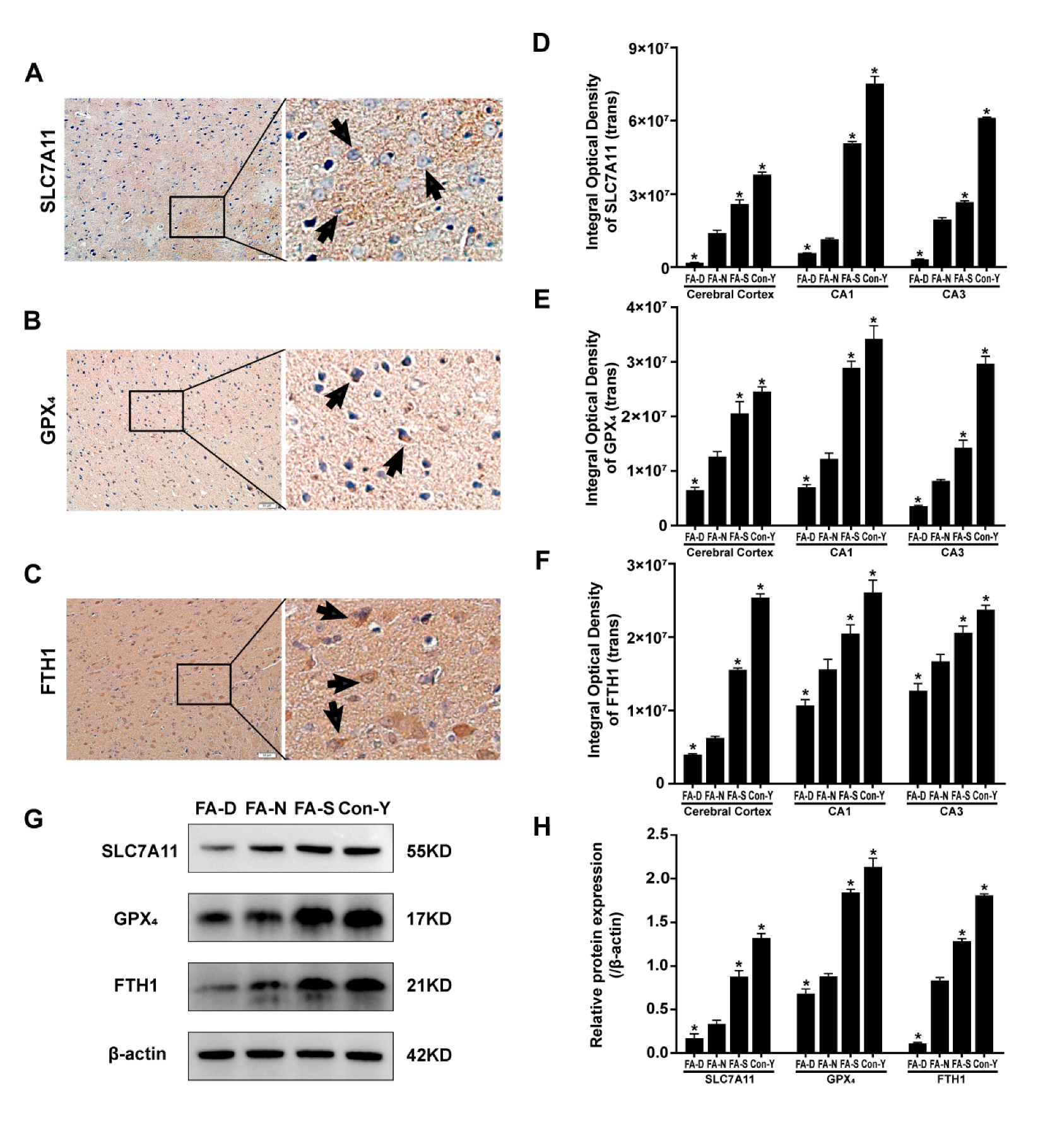
2.2. Folic Acid Supplementation Counteracted the Reduction in the Expression of Ferroptosis-Inhibitory Proteins in the Brains of Aged Rats
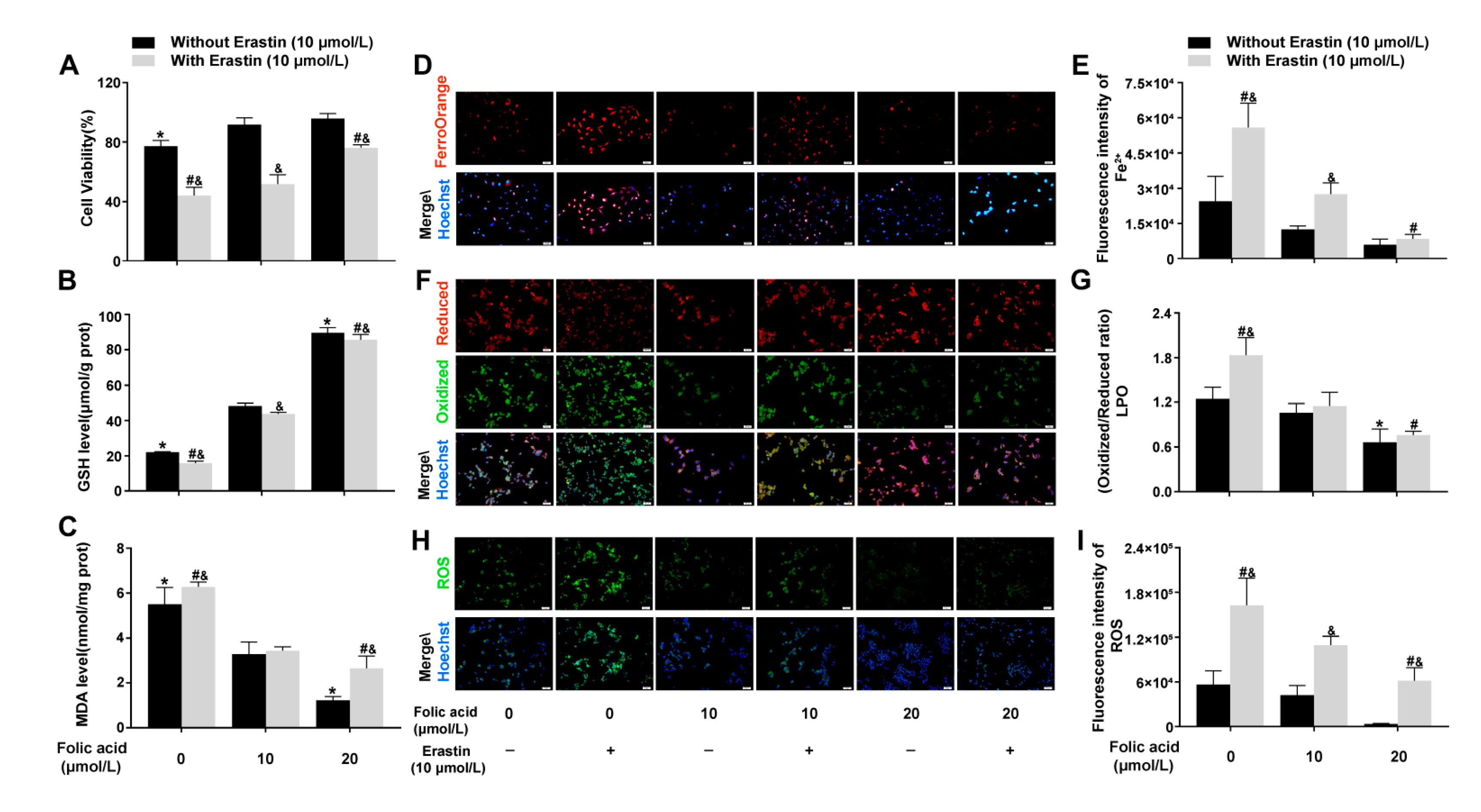
2.3. Folic Acid Supplementation Inhibited Ferroptosis in Erastin-Induced HT-22 Neuronal Cells
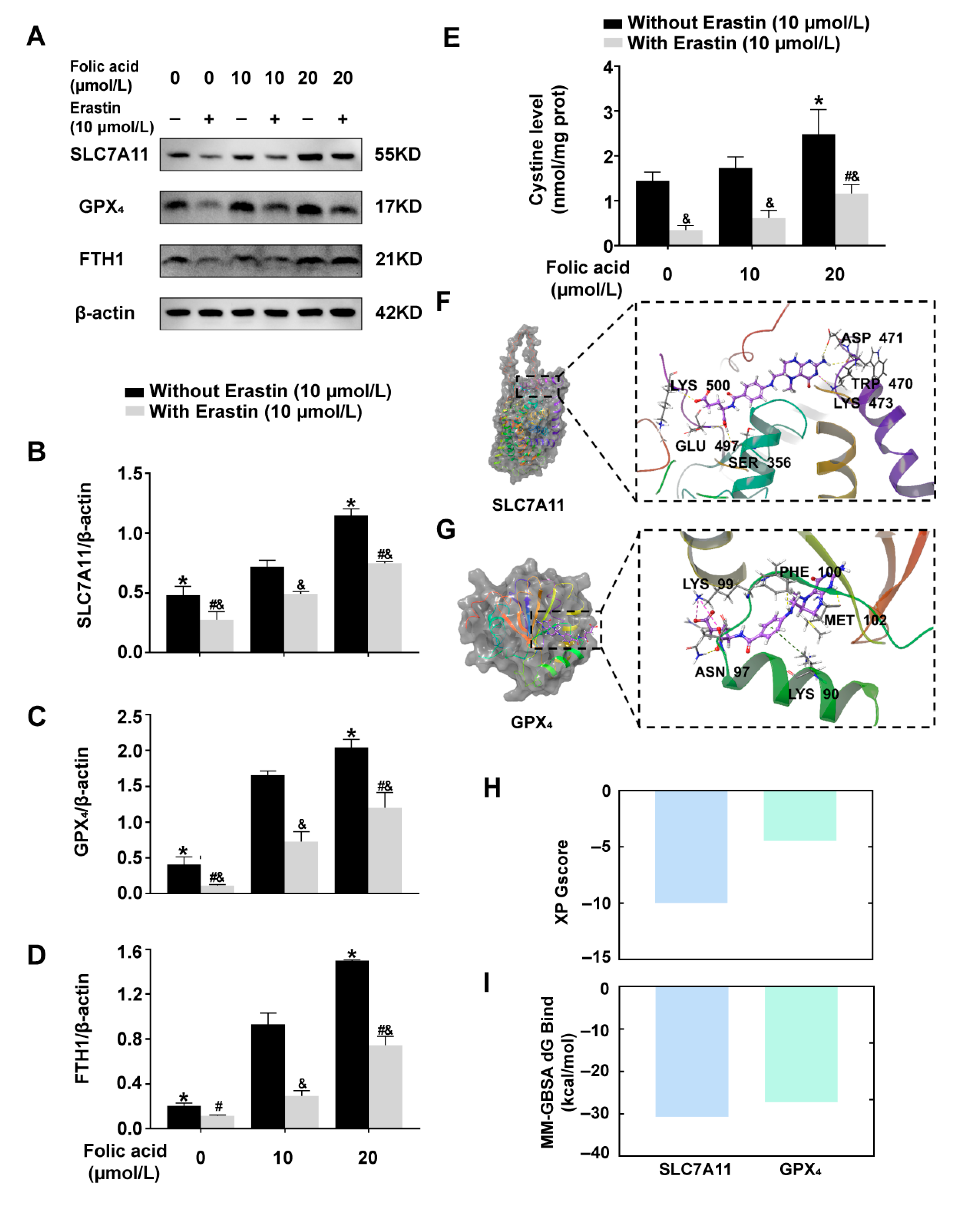
2.4. Folic Acid Supplementation Counteracted the Reduction in the Expression of Ferroptosis-Inhibitory Proteins and Increased Cystine Levels in Erastin-Induced HT-22 Cells
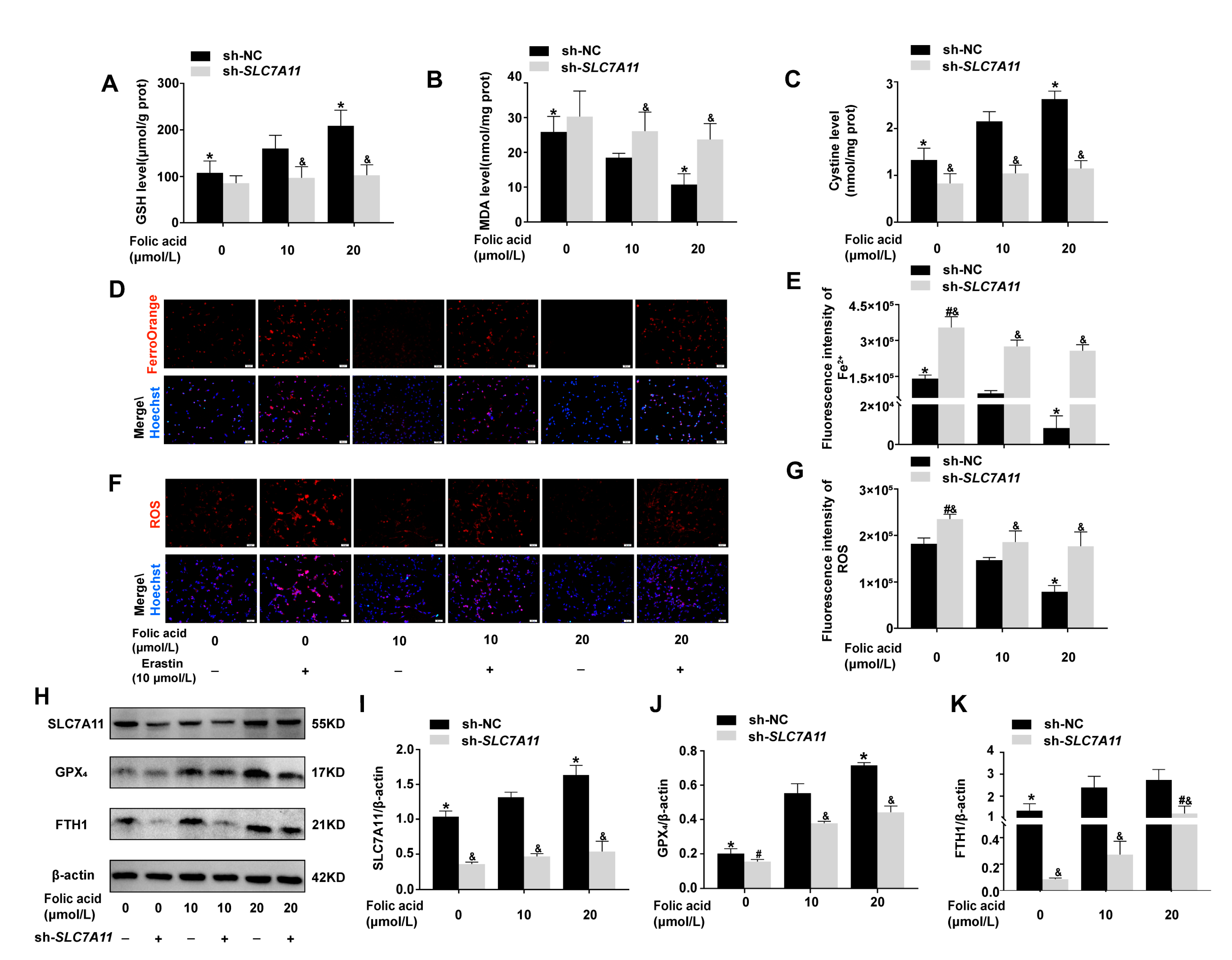
2.5. Folic Acid Inhibited Ferroptosis by Acting on SLC7A11 to Up-Regulate SLC7A11-GSH-GPX4 Pathway and Increase Cystine Levels
3. Discussion
4. Materials and Methods
4.1. Animals and Dietary Treatment
4.2. Cell Culture and Treatment
4.3. Sh-SLC7A11 Lentivirus Transfection and Cell Treatment
4.4. DAB-Enhanced Perls’ Staining
4.5. Brain Tissue Fe2+ Assay
4.6. Cystine Level Assay
4.7. Immunohistochemical Staining
4.8. Western Blot
4.9. Cell Viability Assay
4.10. Intracellular Fe2+, LPO, and ROS Assays
4.11. MDA and GSH Assays
4.12. Molecular Docking
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| DAB | 3,3’-diaminobenzidine |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| ECL | Enhanced chemiluminescence |
| FBS | Fetal bovine serum |
| FTH1 | Ferritin heavy chain 1 |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| GScore | GlideScore |
| Hcy | Homocysteine |
| HHcy | Hyperhomocysteinemia |
| IOD | Integrated optical density |
| LPO | Lipid peroxidation |
| MDA | Malondialdehyde |
| MM-GBSA dG Bind | Molecular mechanics/generalized born surface area f binding free energy |
| MOI | Multiplicity of infection |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PDB | Protein data bank |
| RIPA | Radio immunoprecipitation assay |
| ROS | Reactive oxygen species |
| SD | Sprague Dawley |
| SIRT1 | Silent information regulator sirtuin 1 |
| SLC7A11 | Solute carrier family 7 member 11 |
| System Xc- | Cystine/glutamate antiporter system |
| XP | Extra precision |
| 5-MTHF | 5-methyltetrahydrofolate |
Appendix A
Appendix A.1
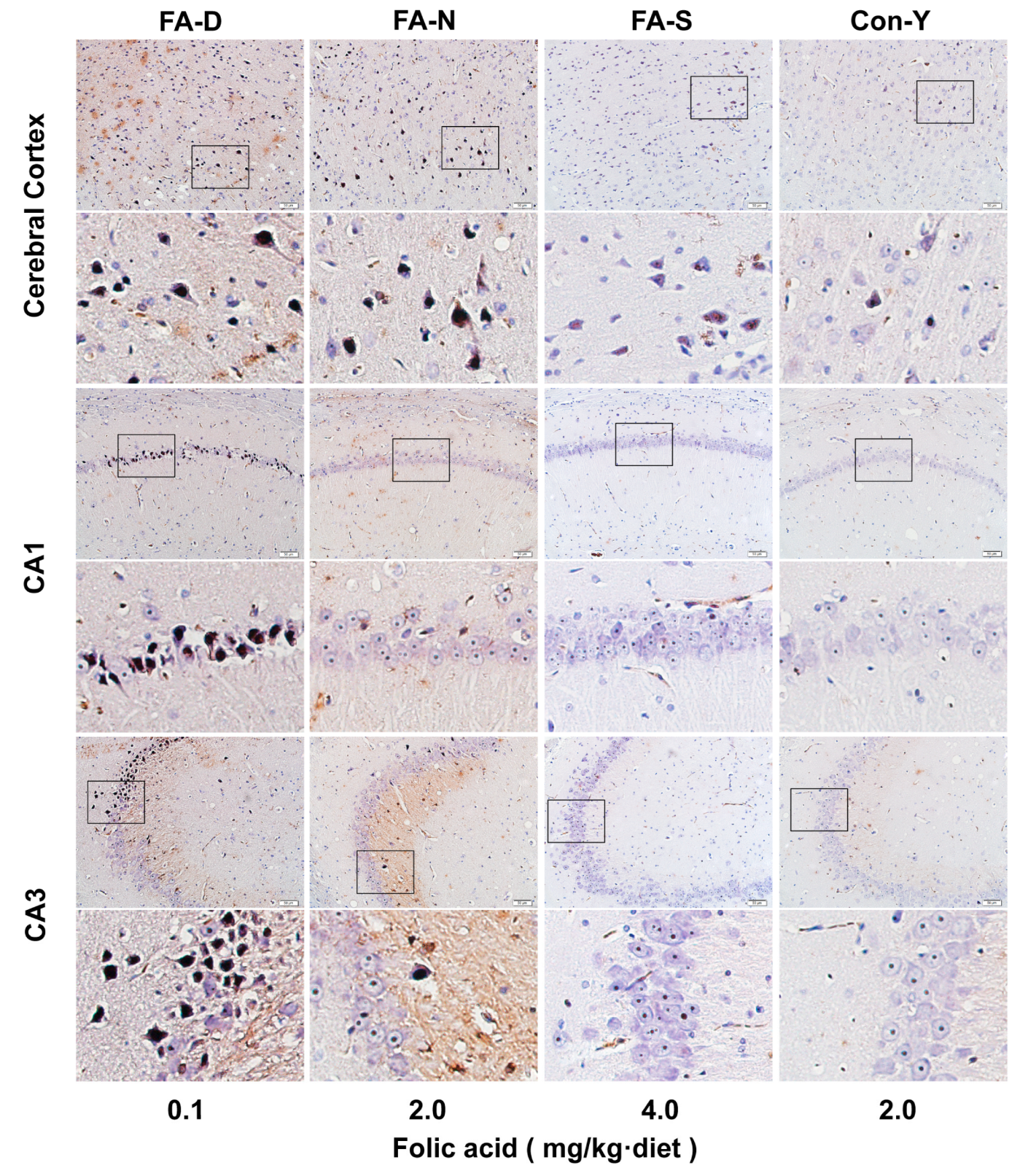
Appendix A.2
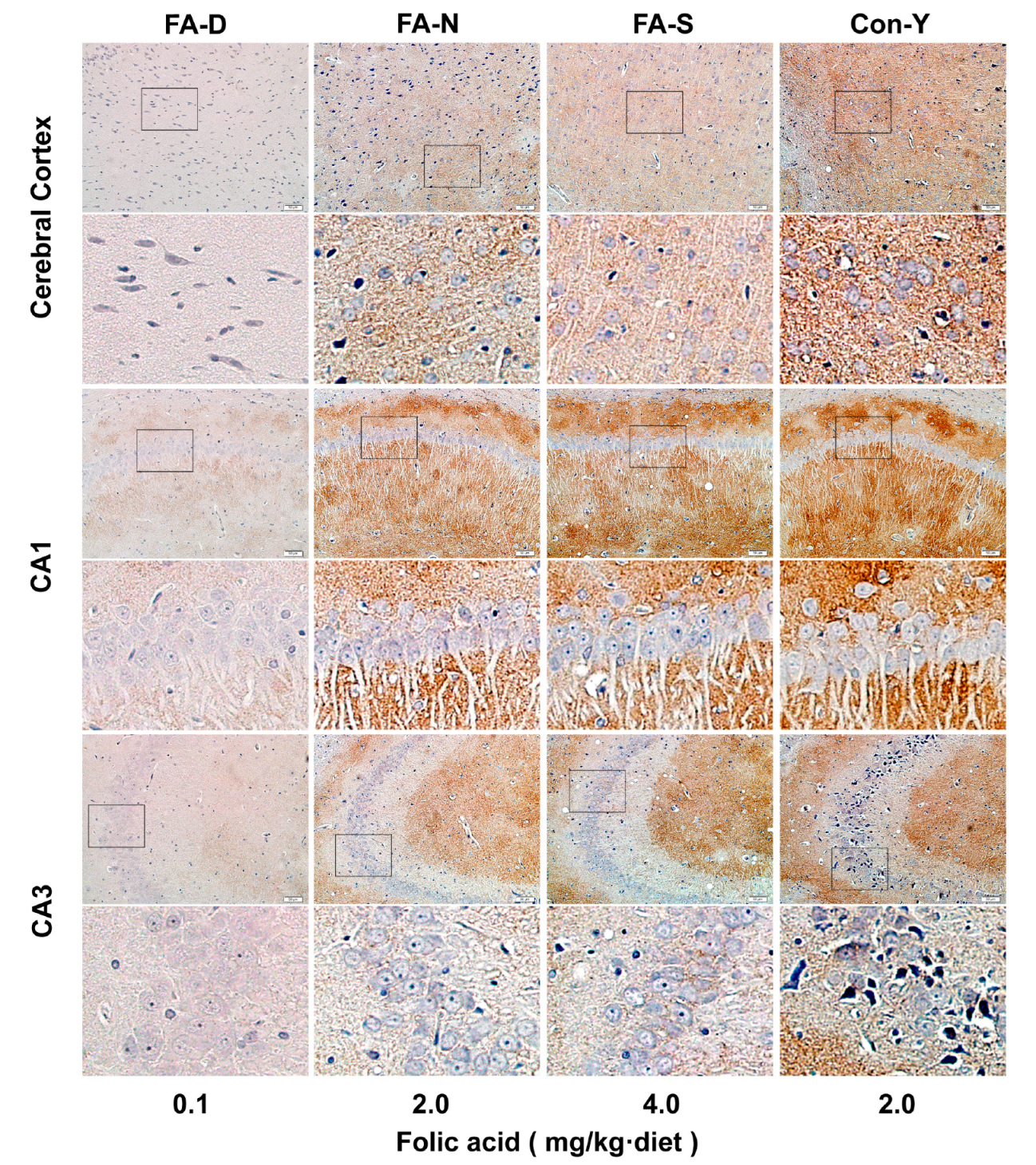
Appendix A.3
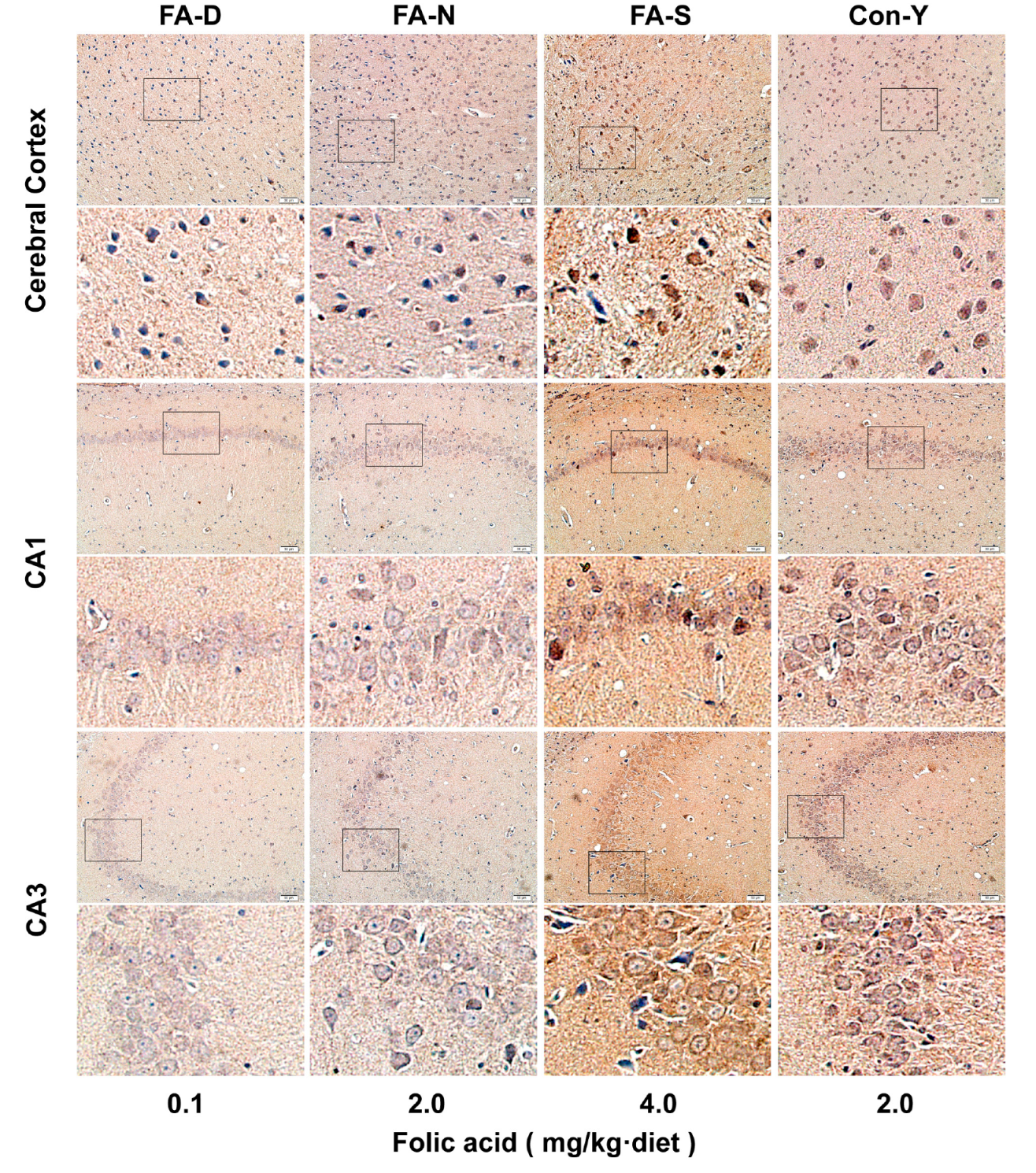
Appendix A.4

Appendix A.5
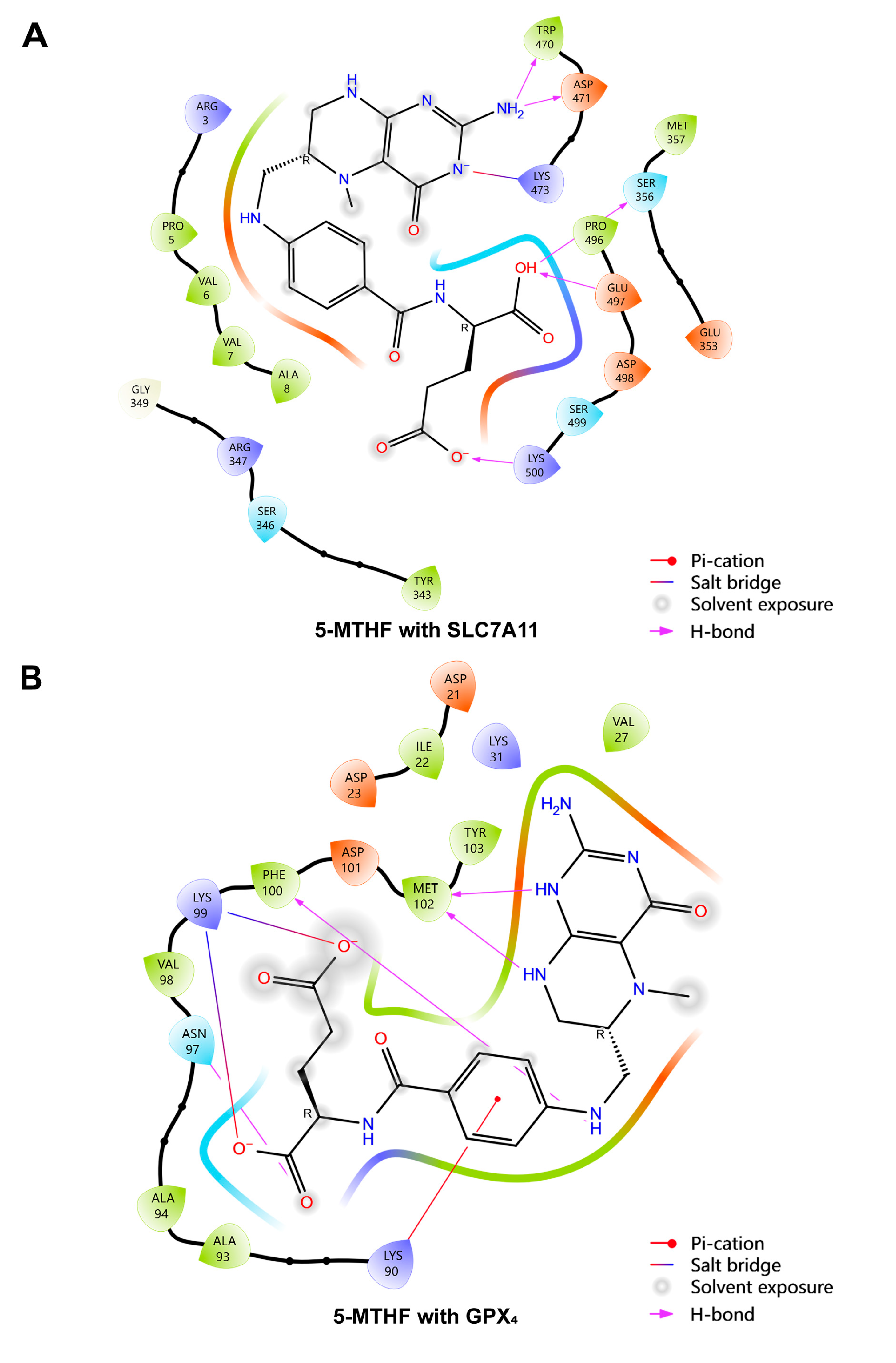
References
- Harper, S. Economic and Social Implications of Aging Societies. Science 2014, 346, 587–591. [Google Scholar] [CrossRef]
- Bloom, D.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; Mckee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic Implications of Population Ageing and Selected Policy Responses. Lancet 2015, 385, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mavaddatiyan, L.; Naeini, S.; Khodabandeh, S.; Hosseini, F.; Skelton, R.P.; Azizi, V.; Talkhabi, M. Exploring the Association between Aging, Ferroptosis, and Common Age-Related Diseases. Arch. Gerontol. Geriatr. 2025, 135, 105877. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, X.; Zhong, H.; Wang, Y. Ferroptosis in Life: To Be or Not to Be. Biomed. Pharmacother. 2023, 159, 114241. [Google Scholar] [CrossRef]
- Trushina, E.; McMurray, C.T. Oxidative Stress and Mitochondrial Dysfunction in Neurodegenerative Diseases. Neuroscience 2007, 145, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Gleitze, S.; Paula-Lima, A.; Núñez, M.T.; Hidalgo, C. The Calcium-Iron Connection in Ferroptosis-Mediated Neuronal Death. Free Radic. Biol. Med. 2021, 175, 28–41. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, Y.; Dong, C.; Wang, Z.; Zhao, J.; Li, Z.; Huang, G.; Li, W. Folic Acid Alleviated Oxidative Stress-Induced Telomere Attrition and Inhibited Apoptosis of Neurocytes in Old Rats. Eur. J. Nutr. 2024, 63, 291–302. [Google Scholar] [CrossRef]
- Chen, T.-F.; Tang, M.-C.; Chou, C.-H.; Chiu, M.-J.; Huang, R.-F.S. Dose-Dependent Folic Acid and Memantine Treatments Promote Synergistic or Additive Protection against aβ(25–35) Peptide-Induced Apoptosis in SH-SY5Y Cells Mediated by Mitochondria Stress-Associated Death Signals. Food Chem. Toxicol. 2013, 62, 538–547. [Google Scholar] [CrossRef]
- Sonkar, S.K.; Kumar, S.; Singh, N.K.; Tandon, R. Hyperhomocysteinemia Induced Locked-in Syndrome in a Young Adult Due to Folic Acid Deficiency. Nutr. Neurosci. 2021, 24, 781–783. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Zhang, X.; Wu, X.; Zhao, Y.; Ren, J. STING-Dependent Induction of Lipid Peroxidation Mediates Intestinal Ischemia-Reperfusion Injury. Free Radic. Biol. Med. 2021, 163, 135–140. [Google Scholar] [CrossRef]
- Liu, L.; Liu, R.; Liu, Y.; Li, G.; Chen, Q.; Liu, X.; Ma, S. Cystine-Glutamate Antiporter xCT as a Therapeutic Target for Cancer. Cell Biochem. Funct. 2021, 39, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-P.; Chen, Y.; Wei, X.; Yu, B.; Xiong, Z.-G.; Lu, C.; Hu, W. Novel Insights into Ferroptosis: Implications for Age-Related Diseases. Theranostics 2020, 10, 11976–11997. [Google Scholar] [CrossRef]
- Ming, T.; Lei, J.; Peng, Y.; Wang, M.; Liang, Y.; Tang, S.; Tao, Q.; Wang, M.; Tang, X.; He, Z.; et al. Curcumin Suppresses Colorectal Cancer by Induction of Ferroptosis via Regulation of P53 and Solute Carrier Family 7 Member 11/Glutathione/Glutathione Peroxidase 4 Signaling Axis. Phytother. Res. 2024, 38, 3954–3972. [Google Scholar] [CrossRef]
- Yan, R.; Xie, E.; Li, Y.; Li, J.; Zhang, Y.; Chi, X.; Hu, X.; Xu, L.; Hou, T.; Stockwell, B.R.; et al. The Structure of Erastin-Bound xCT-4F2hc Complex Reveals Molecular Mechanisms Underlying Erastin-Induced Ferroptosis. Cell Res. 2022, 32, 687–690. [Google Scholar] [CrossRef]
- Stank, A.; Kokh, D.B.; Fuller, J.C.; Wade, R.C. Protein Binding Pocket Dynamics. Acc. Chem. Res. 2016, 49, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Gose, T.; Shafi, T.; Fukuda, Y.; Das, S.; Wang, Y.; Allcock, A.; Gavan McHarg, A.; Lynch, J.; Chen, T.; Tamai, I.; et al. ABCG2 Requires a Single Aromatic Amino Acid to “Clamp” Substrates and Inhibitors into the Binding Pocket. FASEB J. 2020, 34, 4890–4903. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Hernández, P.; Domínguez-Ramírez, L. Role of Cis-Trans Proline Isomerization in the Function of Pathogenic Enterobacterial Periplasmic Binding Proteins. PLoS ONE 2017, 12, e0188935. [Google Scholar] [CrossRef]
- Matsumura, N.; Kinoshita, C.; Aoyama, K. Mechanism of glutathione production in neurons. Nihon Yakurigaku Zasshi 2021, 156, 26–30. [Google Scholar] [CrossRef]
- Alvarez, E.; Ramón, F.; Magán, C.; Díez, E. L-cystine inhibits aspartate-beta-semialdehyde dehydrogenase by covalently binding to the essential 135Cys of the enzyme. Biochim. Biophys. Acta 2004, 1696, 23–29. [Google Scholar] [CrossRef]
- Feng, H.; Yu, J.; Xu, Z.; Sang, Q.; Li, F.; Chen, M.; Chen, Y.; Yu, B.; Zhu, N.; Xia, J.; et al. SLC7A9 Suppression Increases Chemosensitivity by Inducing Ferroptosis via the Inhibition of Cystine Transport in Gastric Cancer. eBioMedicine 2024, 109, 105375. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Xie, Z.; Chen, Y.; Zheng, Z.; Wei, X.; Huang, B.; Shan, Z.; Liu, J.; Fan, S.; et al. Homocysteine Induces Oxidative Stress and Ferroptosis of Nucleus Pulposus via Enhancing Methylation of GPX4. Free Radic. Biol. Med. 2020, 160, 552–565. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Li, W.; Li, Z.; Liu, H.; Gao, Y.; Huang, G. Folic acid modulates VPO1 DNA methylation levels and alleviates oxidative stress-induced apoptosis in vivo and in vitro. Redox Biol. 2018, 19, 81–91. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Zhao, S.; Zhang, X.; Zhang, M.; Xiao, Y.; Wilson, J.X.; Huang, G. Folic Acid Attenuates the Effects of Amyloid β Oligomers on DNA Methylation in Neuronal Cells. Eur. J. Nutr. 2016, 55, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The Role of Nrf2 in Oxidative Stress-Induced Endothelial Injuries. J. Endocrinol. 2015, 225, 83–99. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary folic acid supplementation attenuates maternal high-fat diet-induced fetal intrauterine growth retarded via ameliorating placental inflammation and oxidative stress in rats. Nutrients 2023, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, C.U.; Au-Yeung, K.; Siow, Y.; O, K. 5-methyltetrahydrofolate attenuates oxidative stress and improves kidney function in acute kidney injury through activation of Nrf2 and antioxidant defense. Antioxidants 2022, 11, 1046. [Google Scholar] [CrossRef]
- Du, P.; Zhang, S.; Li, S.; Yang, Y.; Kang, P.; Chen, J.; Gao, T.; Li, C.; Zhang, Q.; Zhang, W. Folic Acid Protects Melanocytes from Oxidative Stress via Activation of Nrf2 and Inhibition of HMGB1. Oxidative Med. Cell. Longev. 2021, 2021, 1608586. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Chen, X.; Linkermann, A.; Jiang, X.; Kang, R.; Kagan, V.E.; Bayir, H.; Yang, W.S.; Garcia-Saez, A.J.; Ioannou, M.S.; et al. A Guideline on the Molecular Ecosystem Regulating Ferroptosis. Nat. Cell Biol. 2024, 26, 1447–1457. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, Y.; Liu, Z.; Liu, J.; Li, Z.; Sun, X.; Zhou, A.; Wu, H. Gualou-Xiebai Herb Pair Ameliorate Atherosclerosis in HFD-Induced ApoE-/- Mice and Inhibit the Ox-LDL-Induced Injury of HUVECs by Regulating the Nrf2-Mediated Ferroptosis. J. Ethnopharmacol. 2024, 326, 117892. [Google Scholar] [CrossRef]
- Zhao, T.; Guo, X.; Sun, Y. Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration. Aging Dis. 2021, 12, 529–551. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.; Zou, M.; Li, L.; Li, Q.; Sun, C.; Xia, W.; Cao, Y.; Wu, L. Folic Acid Improves Abnormal Behavior via Mitigation of Oxidative Stress, Inflammation, and Ferroptosis in the BTBR T+ Tf/J Mouse Model of Autism. J. Nutr. Biochem. 2019, 71, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, M.; Pang, Y.; Li, M.; Ma, W. Folic Acid-Modified Long-Circulating Liposomes Loaded with Sulfasalazine for Targeted Induction of Ferroptosis in Melanoma. ACS Biomater. Sci. Eng. 2024, 10, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, D.; Ruiz-Andrés, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ortega, M.R.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol. JASN 2017, 28, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Di, T.; Wang, W.; Jiang, H. EGCG, GCG, TFDG, or TSA Inhibiting Melanin Synthesis by Downregulating MC1R Expression. IJMS 2023, 24, 11017. [Google Scholar] [CrossRef]
- Dong, X.-N.; Li, M.-T. Inhibitory Effect of Aloperine on Transient Outward Potassium Currents in Rat Cardiac Myocytes. Front. Pharmacol. 2024, 15, 1372973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Wang, Z.; Ren, Q.; Li, Z.; Huang, G.; Li, W. Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels. Int. J. Mol. Sci. 2025, 26, 6669. https://doi.org/10.3390/ijms26146669
Wang Y, Zhang J, Wang Z, Ren Q, Li Z, Huang G, Li W. Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels. International Journal of Molecular Sciences. 2025; 26(14):6669. https://doi.org/10.3390/ijms26146669
Chicago/Turabian StyleWang, Yue, Jingwen Zhang, Zehao Wang, Qinghan Ren, Zhenshu Li, Guowei Huang, and Wen Li. 2025. "Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels" International Journal of Molecular Sciences 26, no. 14: 6669. https://doi.org/10.3390/ijms26146669
APA StyleWang, Y., Zhang, J., Wang, Z., Ren, Q., Li, Z., Huang, G., & Li, W. (2025). Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels. International Journal of Molecular Sciences, 26(14), 6669. https://doi.org/10.3390/ijms26146669





