Abstract
Breast cancer treatments, such as chemotherapy, radiation, and surgery, often face significant limitations, highlighting the need for more effective and targeted therapies. Here, we investigate the potential of 266 nm laser irradiation of chlorpromazine as a novel approach to develop new antitumoral compounds. We identify six chlorpromazine photocompounds with masses in the range of 178–334 u, along with several dimeric compounds with masses between 566 and 600 u, using an HPLC-MS. In silico approaches assess their pharmacokinetic and pharmacodynamic properties while comparing their toxicity with the parent compound. Molecular docking simulations indicate that some photoproducts have a low estimated free energy of binding to cancer-related targets, suggesting enhanced therapeutic potential compared to chlorpromazine. Additionally, ADME-Tox predictions indicate that these photoproducts may have pharmacokinetic and toxicity profiles similar to chlorpromazine. Overall, this study highlights that laser-generated chlorpromazine photoproducts exhibit enhanced biological activity to breast cancer-related targets compared to chlorpromazine while maintaining a similar ADME-Tox profile.
1. Introduction
The treatments for breast cancer include surgery, chemotherapy, radiotherapy, endocrine therapy, and molecular targeted therapy. Surgery removes cancerous tissue, while chemotherapy, radiotherapy, and targeted therapies aim to destroy cancer cells throughout the body [1]. MCF-7 is the breast cancer cell line that has produced more data of practical knowledge for patient care than any other breast cancer cell line. MCF-7 is an estrogen (ER)-positive and progesterone (PR)-positive breast cancer cell line used in numerous studies, including those focused on anticancer drugs. Steroid hormone receptors, specifically ER, PR, or both (ER/PR), play a crucial role in prognosis [2]. However, the growth of breast cancer cells is controlled not only by ER and PR but also by plasma membrane-associated growth factor receptors. Two particularly important members of this large family are the epidermal growth factor receptor (EGFR), which is activated by the epidermal growth factor (EGF), and the human epidermal growth factor receptor-2 (HER2), both present in MCF-7 cells [3].
Chlorpromazine (CPZ) is a phenothiazine drug introduced in the 1950s, initially to manage allergic reactions. Today, it remains one of the most widely used and affordable antipsychotics for treating schizophrenia. CPZ has been shown to improve symptoms and overall functioning compared to a placebo. Despite its multiple side effects—such as sedation, movement disorders, Parkinsonism, low blood pressure, and significant weight gain—CPZ is included in the World Health Organisation’s Model List of Essential Medicines as one of five key drugs for managing psychotic disorders [4,5]. In this study, we have explored the use of CPZ, after laser irradiation, as a potential targeted breast cancer treatment for the MCF-7 cell line. Phenothiazines are one of the first lead structures in drug discovery history; they have been studied since their synthesis in 1883, with derivatives being used in the treatment of psychiatric disorders since the 1950s [6,7]. However, their use in cancer treatment is a relatively new development, dating back to the early 2000s [8]. Several studies showed the benefits of phenothiazine-derivatives drugs, including CPZ, in various cancer types [9,10,11,12]. Some of them indicate that CPZ has shown anticancer effects, such as inducing apoptosis and inhibiting DNA synthesis [12,13], while others highlighted CPZ’s inhibitory activity on multiple breast cancer cell lines, including MCF-7, TAMR-1, and MDA-MB-231 [14,15,16]. Nonetheless, to the best of our knowledge, the anticancer effect of CPZ has never been clinically tested as a primary treatment. However, we identified two clinical trials repurposing CPZ as an adjuvant therapeutic agent: one in resected stage III colon cancer and another in newly diagnosed glioblastoma [17,18].
Laser irradiation of CPZ using a 266 nm laser, with an average pulse energy of 6.5 mJ/pulse at 10 Hz and a pulse duration of 6 ns, for varying time intervals, results in the generation of a diverse mixture of compounds, forming a unique ‘cocktail’. Laser-irradiated CPZ has proven to have a high antimicrobial activity compared to a non-irradiated one. Regarding the stability of the laser-irradiated CPZ, Pascu et al. concluded that the solutions remain stable for at least three months [19,20,21,22]. Investigations of pseudotumor have demonstrated the enhanced efficacy of CPZ after 20 min of irradiation at a low concentration [23]. Our previous work studied the laser irradiation effect on CPZ prior administration and showed selective cytotoxicity against breast cancer cells (MCF-7). The study concluded that irradiating CPZ before administration enhanced its efficacy compared to the non-irradiated, suggesting a promising area for targeted therapy development [24].
Alexandru et al. have identified and characterised some of the photoproducts that resulted from the laser irradiation of CPZ [25]. However, this study seeks to further identify the photoproducts. For this, the compounds formed during the irradiation were analysed using High-Performance Liquid Chromatography (HPLC) coupled with tandem mass spectrometry (MS) and their MS2 spectra (selected ion fragmentation spectra) were acquired.
Computer-Aided Drug Design (CADD) integrates computational and biological approaches to predict drug–target interactions, pharmacokinetics, and potential toxic or side effects [26]. CADD accelerates the discovery and optimisation of new drug candidates, as well as the repositioning of approved drugs, by using predictive computational models, including those based on artificial intelligence (AI), such as neural networks or deep learning [26,27].
Several drug-like rules, including Lipinsky’s rule of five and Veber’s rule, were applied to the identified photoproducts. These rules serve as guidelines in drug discovery for identifying promising candidates with favourable pharmacokinetic properties [28,29,30]. The Lipinski rule focuses on several physicochemical parameters considered essential for oral bioavailability and pharmacological activity [28]. Similarly, the Veber rule highlights the importance of molecular flexibility and size in optimising oral bioavailability [29].
ADME-Tox is a concept in pharmacology and toxicology that refers to the ADME (Absorption, Distribution, Metabolism, Excretion) of substances within an organism, coupled with their potential toxicological effects (Tox) [31]. To predict the ADME-Tox profile of the identified photoproducts, we chose Deep-PK and ProTox 3.0 [32,33]—the updated versions of ProTox-II and pkCSM—platforms with improved prediction accuracy and broader endpoints relevant to drug-likeness, toxicity, and pharmacokinetics coverage [31,34]. Deep-PK is a deep learning platform that enhances the prediction, analysis, and optimisation of pharmacokinetic (PK) and toxicity properties using Graph Neural Networks and graph-based signatures [31]. ProTox 3.0 is a virtual lab designed to predict the toxicities of small molecules, utilising molecular similarity and machine learning to estimate various toxicity endpoints [34].
This research aims to assess the interaction of the CPZ and its HPLC-MS-identified photoproducts using molecular docking to predict the mechanisms of action as possible targeted anticancer treatments. To achieve this, a comparative analysis will be conducted, aligning phenothiazine derivatives with FDA-approved molecular target drugs for HR-positive or HER2-positive breast cancer types. The drugs under consideration include neratinib (tyrosine kinase inhibitor), as well as letrozole and exemestane (aromatase inhibitors), targeting proteins such as aromatase, FGFR1, estrogen receptor α, EGFR, and HER2 [35].
2. Results and Discussions
2.1. HPLC-MS Analyses
The fragmentation pattern of CPZ (Table S1 Supplementary Materials) has been previously described by Jiménez et al. [36]. Among the MS2 fragments, the ions with the mass/charge rations (m/z) of 86.1 and m/z 58.07 are of particular importance, as they correspond to the N,N-dimethylpropanamine moiety from the drug’s structure. When identifying the irradiation products, conservation of the fragments indicates that the change in structure occurs on the phenothiazine moiety. Promazine and promethazine solutions were analysed with the samples. Chromatograms showing the formation of CPZ photoproducts are displayed in Figures S1–S5 Supplementary Materials. The formation of promazine (C284) was confirmed based on the retention time and the typical fragments: m/z 240.09, m/z 212.06, m/z 199.05, m/z 180.08, m/z 86.1, and m/z 58.07 reported in the literature [25,36,37]. Two photoproducts with m/z 301.14 were recorded (C300ab). The mass difference compared to promazine (∆m = 16 u) suggests the presence of an oxygen atom. Both isomers are characterised by the presence of the m/z 86.1 and m/z 58.07 fragments, suggesting that the structure of the phenothiazine moiety was altered. The mass spectrum recorded for C300a has been associated, by Jiménez et al. [36] and Alexandru et al. [25], with promazine sulfoxide, while the one recorded for C300b was attributed to 2-hydroxypromazine [25,36,37]. Another irradiation product observed was C316, having m/z 317.13, which, when compared with promazine, is consistent with the presence of two additional oxygen atoms in the compound’s structure (∆m = 32 u). This photoproduct was identified as 2-hydroxypromazine sulfoxide based on the recorded mass spectrum (Table S1, Supplementary Materials) [25,36]. In the case of C334, the mass difference compared to CPZ (∆m = 16 u) is consistent with the presence of an oxygen atom. Alexandru et al. [25], Jiménez et al. [36], and Trautwein and Kümmerer [37] have attributed a mass spectrum similar to that recorded for C334 to chlorpromazine sulfoxide. The C178 photoproduct has not been previously reported. The presence of the m/z 86.1 and m/z 58.07 fragments in the compound’s mass spectrum suggests the conservation of the N,N-dimethylpropanamine moiety, while the m/z 77.04 fragment can be associated with the presence of a benzene ring. A proposed formation pathway for C284, C300ab, C316, C335, and C178 is shown in Figure 1. These compounds are generated as a direct or indirect result of laser irradiation of the CPZ solution. The direct mechanism implies the homolytic cleavage of covalent bonds such as C-Cl, resulting in the formation of C284, and even cleavage of the C-S and C-N bonds, which could yield C178 [38,39,40]. The indirect mechanism is strongly related to the photolysis of water molecules upon laser irradiation, which yields hydroxyl radicals [41,42]. These radicals can perform dichlorination [43] of CPZ with the formation of C300b or oxidation of the thioether moiety of either CPZ or C284 to sulfoxide (C334 and C334) [44].
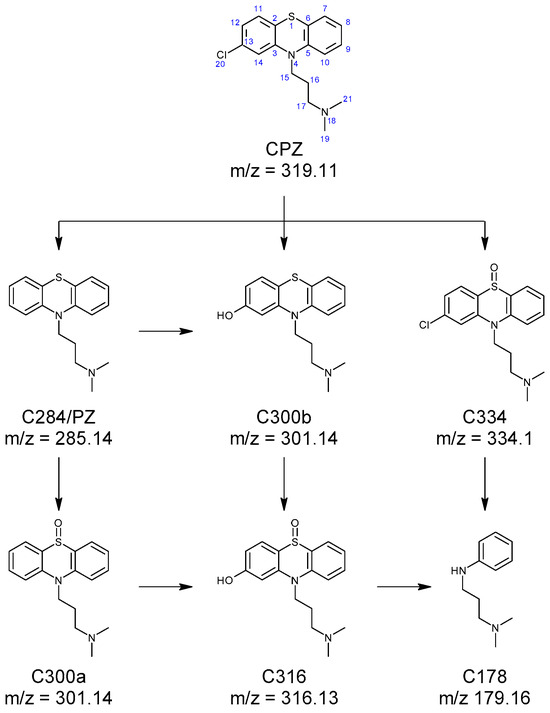
Figure 1.
Formation of C178, C284 (promazine), C300a (promazine sulfoxide), C300b (2-hydroxypromazine), C316 (2-hydroxypromazine sulfoxide), and C334 (chlorpromazine sulfoxide) photoproducts of CPZ.
During the analysis, a few unexpected products were recorded, having m/z in the range 567–601 (C566ab, C582abcde, C598ab, and C600abc). For these compounds, both [M + H]+ and [M + 2H]2+ ions were observed, supporting the idea that they represent dimers of CPZ and/or photoproducts. The absence of an obvious monomer in the MS2 spectra of the compounds further cements the dimer hypothesis. This type of finding has been previously reported in the case of promethazine exposed to UV light [45] and phenothiazine undergoing electrochemical oxidation [46]. All identified dimers retain the m/z 86.1 and m/z 58.07 fragmentation pattern conserved from CPZ, implying the involvement of the phenothiazine moiety in the dimerisation reaction. C566ab have been identified as promazine dimers. Fragmentation of the [M + 2H]2+ shows the simultaneous formation of m/z 482.18 and m/z 86.1, while the m/z 482.18 to m/z 409.09 mass transition (∆m = 73 u) corresponds to the loss of C4H11N from the second N,N-dimethylpropanamine moiety. Thus, for both compounds, the covalent bond is formed between the phenothiazine moieties, at different positions, which correlates with the presence of the m/z 396.08 fragment, corresponding to a phenothiazine dimer radical cation. In the case of C582abcde, the detected m/z matches C566ab, with an additional oxygen atom, suggesting that the compounds comprise C284 covalently bonded to either C300a or C300b. In the spectra of C582a, the presence of the m/z 300.14 and m/z 284.15 fragments correlate to carbon-centred radicals of C284 and C300ab. For the remaining isomers (C582bcde), the m/z 412 (m/z 396 + 16 µ) fragment corresponds to a phenothiazine dimer radical cation where one of the monomers is either a sulfoxide or an alcohol. Thus, the C582abcde isomers can be differentiated based on the position of the oxygen atom and the position of the bond between the monomers. C598ab are consistent with phenothiazine dimers, with two additional oxygen atoms, either as hydroxyl or sulfoxide groups. The fragmentation of the [M + 2H]2+ ion leads to the formation of the m/z 514 and m/z 86.1 ions, accounting for one of the N,N-dimethylpropanamine moieties. For C598a, the m/z 498.23 to m/z 414.09 (∆m = 84 u) accounts for the loss of the second N,N-dimethylpropanamine moiety as C5H10N•. In the case of C598b, the loss of C5H12N• was observed (m/z 514.17 to m/z 428.07, ∆m = 86 u), suggesting that both compounds result from the formation of the covalent bond between the phenothiazine moieties.
The recorded precursor ions for C600abc (m/z 601.22) are consistent with CPZ–promazine dimers. In the case of C600a, this proposed structure is supported by the formation of the m/z 318.12 and m/z 284.13 radical cations upon fragmentation of the [M + 2H]2+ ion. For C600bc, fragmentation of the same ion simultaneously forms m/z 516.14 and m/z 86.1, while the m/z 516.14 to m/z 443.05 is consistent with the loss of C4H11N from the second N,N-dimethylpropanamine moiety, thus showing that the bond between the CPZ and promazine is formed with the participation of the two phenothiazine moieties. Figure 2 and Figure 3 show the abundance of the CPZ photoproducts over the course of laser irradiation (5, 10, 20, 40, 60 min). The relative (Figure 2A) and absolute (Figure 2B) abundance clearly illustrate the time variation in each photoproduct. The reason for this type of representation is that each compound has a different ionisation efficiency depending on its structure [47]. Because of that, the abundance cannot be directly correlated with the concentration in solution. As such, compounds with low ionisation efficiencies would have a low abundance regardless of concentration, thus falsely implying a low concentration in solution. Thus, the abundances of different compounds cannot be directly compared without knowing the ionisation efficiency. In order to bypass this restriction, the relative abundance can be calculated as the measured abundance normalised by the maximum recorded abundance for each compound.
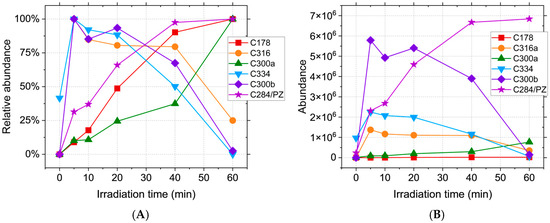
Figure 2.
(A). Relative abundance and (B). abundance of CPZ irradiation products, where relative abundance = recorded abundance/maximum recorded abundance × 100.
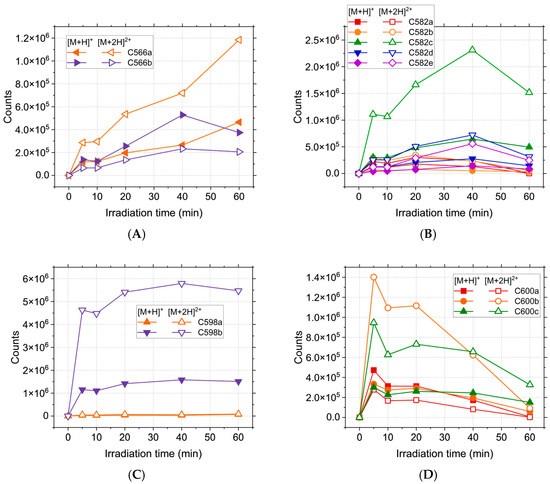
Figure 3.
Abundance of high m/z photoproducts of CPZ irradiation over the course of 60 min: (A) C566ab; (B) C582abcde; (C) C598ab; (D) C600abc.
However, the ionisation energy is likely to have little variation for isomers [48], thus yielding a higher probability of correlation with the actual concentration; as such, Figure 3 shows the likely preferential formation of certain compounds of the isomer sets. In this case, the use of the relative abundance would be detrimental to the reality of the results, falsely implying the equal formation of all compounds, which is likely untrue. The C284 compound is formed continuously during irradiation (Figure 2) and reaches a maximum abundance for the longest tested irradiation time (60 min), as is also the case with C178 and C300a. C300b, C316, and C334 reach a maximum at the beginning of the process (5 min), thus implying that the hydroxyl radical reactions take place predominantly for the shortest irradiation times.
While most of these compounds are not present in the initial solution and thus, to some degree, require laser irradiation in order to be formed, the C334 has a high relative abundance even in the absence of treatment, suggesting that the oxidation of CPZ with the formation of chlorpromazine sulfoxide happens spontaneously in the solution. C566ab are steadily formed during irradiation (Figure 3A) up until the 40 min mark, when C566b suffers a decline while C566a reaches a maximum abundance at 60 min.
Of the C582abcde isomers, C582c is the most abundant, followed by C582d and C582e (Figure 3B), and all three have the maximum concentration at 40 min. Of the two C598ab compounds, C598b appears to be preferentially formed during irradiation. Its abundance shows a sharp increase at the beginning of the process, followed by a plateau, implying a constant formation and degradation of this product. As expected, C600abc reach a maximum abundance at the beginning of the irradiation (5 min), followed by a decrease over the course of the treatment. This behaviour can be attributed to the formation of these compounds from two CPZ molecules, of which one undergoes homolytic cleavage of the C-Cl bond [49,50]. The resulting promazine carbon-centred radical can potentially react with CPZ or other photoproducts in order to either form promazine, 2-hydroxypromazine, or one of the dimers containing a promazine moiety [25,51,52]. While the formation of promazine and most dimeric photoproducts are competing reactions, these dimers can also serve as precursors for C284. Homolytic cleavage of all C566, C582, and C600 isomers can generate C284, thus contributing to the total abundance of promazine displayed in Figure 2. Because the maximum abundance of C284 is reached for the longest treatment time, the compounds formed from it should display the same behaviour. This is the case for C300b, but not C300a. While C284 can form both compounds, it only serves as the main precursor for C300b. C300a can also be generated directly from CPZ, the concentration of which is continuously declining during the process, thus impacting the overall abundance. The mass spectra recorded for the photoproducts do not provide enough data in order to properly distinguish isomers with the same m/z and different retention times. Thus, the possible chemical structures have been randomly distributed between these isomers in order to provide a clear view of the results.
2.2. Drug-like and ADME-Tox Predictions
In this in silico study, we drew and optimised the molecular structures of CPZ, its identified photoproducts, and the clinically used drugs exemestane, letrozole, and neratinib. Table S2 in the Supplementary Materials presents the SMILES codes and the 2D structures of CPZ and its identified photoproducts using the HPLC-MS analyses.
For the identified photoproducts and CPZ, we used SwissADME webserver to predict several drug-likeness filters, including Lipinski’s Rule of Five [53], Veber (Table 1), Ghose, Egan, and Muegge, the bioavailability score, PAINS and Brenk alerts, lead-likeness violations, and synthetic accessibility (Table S3, Supplementary Materials) [54]. The results are presented in Table 1 and in Table S3, Supplementary Materials.

Table 1.
Drug-like predictions of the photoproducts.
The SwissADME results indicate that the CPZ photoproducts C178, C284/PZ, C300, C316, and C334 conform with Lipinski’s Rule, Veber, bioavailability criteria, Ghose, and Egan. However, the compounds C566, C582, C598, and C600 do not adhere to Lipinski’s Rule due to their molecular weights exceeding 500 Daltons and MLOGP values greater than 4.15. This suggests that these compounds are unlikely to be orally active, and alternative methods of administration, like parenteral, should be considered in future studies [55,56]. None of the dimers meet the criteria for Ghose, Egan, or Muegge filters; CPZ and C178 also fail Muegge’s filter. The predicted oral bioavailability indicates that the dimers exhibit significantly lower scores (0.17) compared to CPZ (0.55); therefore, alternative administration routes should be considered. The dimeric compounds, C284/PZ and C300a, trigger one PAINS alert (het_thio_666_A), suggesting a potential risk of assay interference that must be considered in future tests [57]. However, in our previous work, we used several biological assays—including the MTS viability assay, LIVE/DEAD staining, LDH release, ROS and nitric oxide production, and F-actin fluorescence staining—to confirm the selective cytotoxicity of laser-irradiated CPZ against breast cancer cells, despite the PAINS alert [24]. Regarding synthetic accessibility, all dimeric compounds are predicted to be more difficult to synthesise than CPZ.
Our ProTox predictions are presented in Table 2 and show that the photoproducts are similar to CPZ regarding toxicity (Table 2).

Table 2.
Hepatotoxicity (dili), neurotoxicity (neuro), nephrotoxicity (nephro), respiratory toxicity (respi), cardiotoxicity (cardio), hosphoprotein (Tumour Suppressor) p53 (sr_p53) activity, Lethal Dose 50 (LD50), and toxicity class of the photoproducts compared with CPZ.
All the compounds present neurotoxicity and respiratory toxicity; none of them are cardio- or nephrotoxic. Regarding hepatotoxicity, compounds C284, C566, and C600 are predicted to be hepatotoxic according to ProTox 3 predictions, similar to CPZ, while the other photocompounds are predicted to be safe. CPZ and C600 photoproducts are active on the Tumour Suppressor receptor p53. The LD50 of the photoproducts is higher than CPZ, and most of the compounds have a higher or similar toxicity class compared to CPZ (Table 2). To evaluate the ADME-Tox properties of the photoproducts compared with CPZ, we used the Deep-Pk platform. The results show a high similarity between CPZ and its photoproducts, with exceptions in terms of bioavailability, where C582c shows non-bioavailability (Table S4, Supplementary Materials). Regarding the fraction unbound, CPZ has a predicted value of 1.96, lower compared with C566 and C600 compounds. Plasma-protein-binding predictions show that CPZ has a therapeutic index of 44.24, which is lower than 90 and indicates a poor value. Compounds like C566, C582, C589, and C600 present higher therapeutic indexes than CPZ, but still lower than 90. All the photoproducts and CPZ present a high steady-state volume of distribution (VDss) (log VDss > 0.45), indicating that the drug is distributed in tissue rather than plasma. For an anticancer drug, a high VDss is considered an advantage (Table S4, Supplementary Materials). Compounds CPZ, C178, C284/PZ, C300a, C300b, C316, and C334 are inhibitors of Organic Cation Transporter 2 (OCT2), meaning that the photoproducts can interfere with its function and may (i) alter the drug excretion, (ii) affect the pharmacokinetics of other drugs that are substrates of OCT2, (iii) enhance the effectiveness of certain drugs by prolonging their presence in the bloodstream or tissues, and also (iv) have implications for renal function.
All the photoproducts and CPZ are safe in terms of biodegradation. Except for C300b, C316a, and C582e, the photoproducts and CPZ have a high maximum tolerated dose. CPZ and photoproducts C566, C582, C598, and C600 are SR-p53 activators and represent a promising approach in oncology, aiming to leverage the natural cancer-suppressing functions of p53 by reactivating its role in affected cells (Table S4, Supplementary Materials).
2.3. Molecular Docking Approach
The molecular docking results, presented in Table 3, indicate that CPZ photoproducts C178, C284, C300a, C300b, C316, and C334 generally exhibit higher Estimated Free Energy of Binding (EFEB) compared to CPZ when interacting with aromatase, ER, FGFR1, EGFR, HER2, PR, and Beclin 1. However, there are exceptions: C334 interacts with aromatase and FGFR1, and C300a interacts with FGFR1, while C300a/b interacts with Beclin 1, all showing lower EFEB values.

Table 3.
The lowest EFEB (kcal/mol) for each photoproduct in the interaction with molecular targets aromatase, ER, FGFR1, EGFR, HER2, PR, and Beclin 1 was determined after 100 runs using the molecular docking simulation and predicted EFEB. The lowest predicted EFEB values from the identified photoproduct on each target are shown in bold. The overall lowest predicted EFEB value across all compounds on a target is indicated with an underline.
Compounds C566, C582, C598, and C600 demonstrate lower EFEB compared to CPZ when binding to aromatase, FGFR1, EGFR, HER2, PR, and Beclin 1 (Table 3).
A lower EFEB (kcal/mol) indicates a higher probability that the compound will interact with the specific target [58]. According to our predictions, CPZ and most of its identified photoproducts have low predicted binding energies. We compared our results with the medications used in the targeted treatment of breast cancer, exemestane, letrozole, and neratinib, and the results show that photocompounds C566, C582, C598, and C600 have similar or lower EFEB values (Table 3). The lowest EFEB between a photoproduct and a target was obtained when C600b interacts with aromatase (−11.81 kcal/mol), similarly to neratinib. Neratinib and the C600b photoproduct also interact with similar amino acids (aa) (Figure 4). Neratinib forms four conventional hydrogen bond interactions with aromatase aa residues THR310, SER314, PRO429, and CYS437. The C600b compound also binds similarly to the aromatase receptor. Similarly to the results obtained from Hong et al., both neratinib and C600b form favourable interactions with the aa residue THR310 [59].
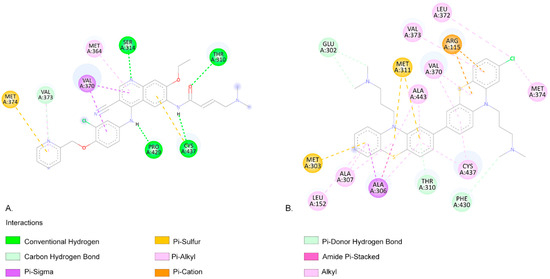
Figure 4.
(A). The two-dimensional representation of the neratinib structure and the aa residues from its binding site when interacting with the aromatase receptor. (B). The two-dimensional structure of C600b and the aa residues from its binding site. Both neratinib and C600b compounds exhibit favourable interactions with the aa residues THR310, VAL370, VAL373, MET374, and CYS437.
When interacting with FGFR1, the CPZ-identified photoproduct C582e has the lowest EFEB (−9.81 kcal/mol) according to our results. Among the clinically used compounds we tested, letrozole has the lowest predicted binding energy (−8.79 kcal/mol). Both compounds have favourable interactions with FGFR1 (Figure 5).
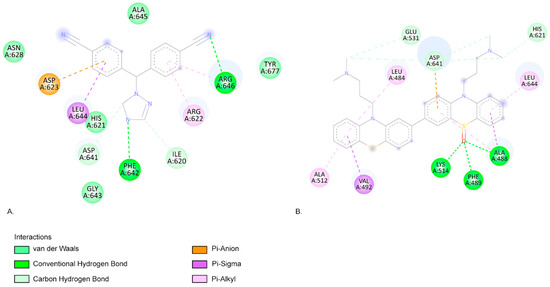
Figure 5.
(A). The two-dimensional representation of the letrozole structure and the aa residues from its binding site when interacting with the FGFR1. (B). The two-dimensional structure of C582e and the aa residues from its binding site. Both letrozole and C582e compounds present favourable interactions with the aa residues ASP641, HIS621, and LEU644.
Letrozole forms two conventional hydrogen bond interactions with aa residues PHE642 and ARG646. C582e forms three hydrogen bonds with aa residues LYS514, ALA488, and PHE489. The LYS514 aa residue is part of the active site of FGFR1 [60]. Pan et al.’s molecular docking simulations also highlight that ASP641 and PHE 642 are key residues for FGFR1 bioactive inhibitors [60]. Similarly, Ravindranathan et al., in their study on new FGFR1 inhibitors, found that the most effective compound, like C582e in our results, forms hydrogen bonds with LYS514 and PHE489 (Figure 5) [61]. Klein et al. also indicate that aa residues LYS514, ASP641, and ALA488 form the FGFR1-binding site [62].
The C566a photoproduct has the lowest binding affinity when interacting with EGFR (−11.07 kcal/mol) (Figure 6) and HER2 (−10.07 kcal/mol) targets, similar to neratinib (−11.04 kcal/mol and −9.41 kcal/mol, respectively (Figure 7)).
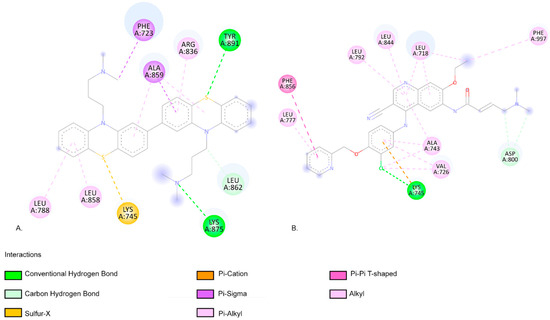
Figure 6.
(A). The two-dimensional representation of the C566a structure and the aa residues from its binding site when interacting with the EGFR. (B). The two-dimensional structure of neratinib and the aa residues from its binding site. Both neratinib and C566a compounds present favourable interactions with the aa residue LYS 45, indicating similar predicted binding sites.
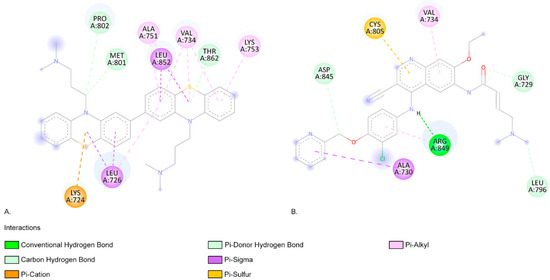
Figure 7.
(A). The two-dimensional representation of the C566a structure and the aa residues from its binding site when interacting with HER2. (B). The two-dimensional structure of neratinib and the aa residues from its binding site. Both neratinib and C566a compounds present favourable interactions with the aa residue VAL 734, indicating similar predicted binding sites.
As shown in Figure 6, neratinib forms a hydrogen bond with the aa residue LYS745 of EGFR. The C566a photoproduct forms two conventional hydrogen bonds with the aa residues LYS875 and TYR891. LYS745 is a highly conserved aa residue across all kinases, and its role in phosphotransfer during catalysis is crucial [63]. Additionally, the C566a photoproduct forms a pi–sigma interaction with PHE723 from the p-loop residue of EGFR (Figure 6). Stover et al. reported that aa residues TYR891 and TYR920 are highly phosphorylated in the breast tumour cell line MCF7 [64].
As shown in Figure 7, the HER2 aa residue and ARG 849 aa residue form a conventional hydrogen bond when interacting with neratinib (Figure 7). The photoproduct C566a exhibits positive binding interactions with multiple aa residues within the HER2-binding pocket, including LEU726, VAL734, ALA751, LYS753, MET801, and LEU852 (Figure 7) [65]. In an in silico screening study, Tung et al. also identified favourable interactions between several alkaloids (such as Sanguinarine, Sorafenib, and Tomatidine, which are potential inhibitors of HER2 in cancer treatment) and the aa residues identified in our molecular docking study (Figure 7) [65]. C566a forms a pi–alkyl interaction with LYS753, a residue that engages in hydrophobic contact with the adenine ring of ATP and plays a role in coordinating the ATP catalytic site. This residue is often targeted by inhibitor drugs to block ATP binding [66,67,68]. Additionally, MET801, located in the hinge region of HER2, is known to interact with ATP or other kinase inhibitors [69,70,71]. C566a forms a carbon–hydrogen bond with MET801 (Figure 7).
In this study, using the LC-MS, we identified the photoproducts generated after laser irradiation, and also, using the molecular docking prediction, we evaluated their possible mechanism of action in cancer therapy. The LC-MS analysis shows that, after irradiation, CPZ forms 18 photocompounds, 5 of which are typical of chlorpromazine degradation under laser irradiation. These included compounds that resulted from chlorpromazine dechlorination (promazine, 2-hydroxypromazine) as well as sulfoxides (promazine sulfoxide, 2-hydroxypromazine sulfoxide, chlorpromazine sulfoxide). Moreover, these compounds reacted either with chlorpromazine or with each other in order to form a series of dimeric photoproducts, which have been identified successfully using the MS2 spectra of the [M + H]+ and [M + 2H]2+ cations. The molecular docking simulations indicate that the dimeric compounds have lower binding EFEB values compared to CPZ and the monomeric photocompounds, suggesting a stronger binding affinity. Dimeric compounds C566a and C600b demonstrate particularly strong interactions.
C566a shows a low EFEB across several key targets, including −11.77 kcal/mol with aromatase, −11.07 kcal/mol with EGFR, and −10.07 kcal/mol (Figure 6A) with HER2, indicating a higher binding affinity compared to CPZ, which has EFEB values of −7.64, −8.42, and −7.56 kcal/mol for the same targets. Similarly, C600b binds strongly to aromatase −11.81 kcal/mol and EGFR −10.26 kcal/mol), surpassing CPZ’s binding efficiency. These photocompounds show a promising therapeutic potential due to their higher binding affinity, suggesting they might be more effective in inhibiting breast cancer-related targets than CPZ itself. The lower binding compared with CPZ affinity in target receptors indicates that a lower dose of compounds may be necessary to achieve the same anticancer effect.
The ADME-Tox profile analysis indicates that some CPZ photocompounds exhibit similar or even improved tolerability compared to CPZ. While CPZ is classified as toxicity class 3, photocompounds C178, C300b, C316a, C566a,b, C582a,b,c, and C598b are classified as toxicity class 4. The CPZ LD50 of 125 mg/kg is lower compared with the predicted LD50 of all the photocompounds; for example, the C178 (LD50 of 560 mg/kg) and C582 variants (LD50 up to 408 mg/kg) show improved safety. Moreover, photocompounds C178, C300a,b, C582a-e, and C598a,b, exhibit no activity in targets like drug-induced liver injury (dili) or p53-signalling-related toxicity (sr_p53), indicating potential safety advantages.
3. Materials and Methods
3.1. Chemicals
Chlorpromazine hydrochloride (CPZ, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was dissolved at a 2 mg/mL concentration in distilled water. The solution was kept at 4 °C and protected from environmental light before and after the irradiation protocol.
3.2. Irradiation Protocol
A 2 mL volume of the CPZ solution was irradiated for 5, 10, 20, 40, or 60 min with a 266 nm pulsed beam emitted by a Nd:YAG laser, Continuum (San Jose, CA, USA), (10 Hz, 6 ns FWHM, 6.5 mJ/pulse, 1.08 MW/pulse) according to the protocol detailed in [25,72]. The 266 nm irradiation was selected to match the main UV absorption band of CPZ [21,24].
3.3. HPLC-MS
The irradiation products of CPZ were investigated using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC Agilent Infinity II 1260 (Agilent, Santa Clara, CA, USA) with an Agilent 6530 Quadrupole-Time of Flight detector (Q-TOF)). The separation of compounds in the samples was achieved on a Zorbax Eclipse Plus RP-C18 column (150 × 3.0 mm, 3.5 µm) at 30 °C using a gradient elution method. The mobile phase comprised water with 0.1% formic acid (A) and acetonitrile (B) and was varied as follows: 0 min–10% B → 10 min—50% B → 11 min—50% B → 11.5 min—10% B → 14 min—10% B (flow rate 0.6 mL/min).
The electrospray ionisation source (Dual AJS ESI) parameters were gas—8 L/min, 300 °C; sheath gas—12 L/min, 350 °C; nebuliser—60 psig; capillary voltage—3500 V; nozzle voltage—1000 V; and fragmentor potential—170 V. The mass spectra (MS and MS2) were acquired in the positive ionisation mode, in the range 50–1000 m/z (mass/charge), for z = 1 and z = 2, and at a scan rate of 2 spectra/s. Two precursors per cycle were selected for MS2 based on abundance, and fragmentation was performed using collision cell potentials of 5, 10, 15, and 20 V. The identification of the molecular formula of each compound was performed based on the isotopic profiles recorded in the Full Scan MS spectra. The mass accuracy was checked for the most abundant monoisotopic ions.
The abundance of the identified photoproducts was attributed to the peak height in the extracted ion chromatograms targeting the corresponding m/z value for each compound. The relative abundance was calculated from the recorded abundances as percentages of the maximum value for each selected m/z value.
3.4. Molecular Modelling
3.4.1. Chemical Structure Retrieval and Drawing
The 2D chemical structures of CPZ and its photoproducts were obtained using the SMILES notation from the PubChem database [73,74]. For the photoproducts, Marvin Sketch software version 21.20.0, 2021, [75] was employed to draw and to generate the SMILES codes of the photoproducts’ chemical structures identified following the laser irradiation of CPZ.
3.4.2. Molecular Optimisation
The 3D optimisation of molecular structures was performed using Marvin Sketch software version 21.20.0, 2021, [75]. The molecules were geometry-optimised using the ‘Clean in 3D → Fine Build’ function in MarvinSketch, which automatically generates conformers for molecular fragments using the Dreiding force field, returning the lowest-energy structure [75]. The optimised molecules were saved in a .mol2 file format.
3.4.3. Preparation for Molecular Docking
For compatibility with the molecular docking simulations software Autodock 4.2.6, the optimised structures were converted to pdbqt format. This conversion was facilitated by the use of Open Babel software, version 3.1.1 [76].
3.5. Drug-Likeness Evaluation of Photoproducts
We applied several screening criteria, including Lipinski and Veber rules, to assess the drug-likeness of the photoproducts using SwissADME webserver [53,54].
3.6. ADME-TOX Predictions
To predict the ADME-Tox profiles of the photoproducts, their SMILES codes were utilised. The analyses were conducted using the Deep-PK platform and ProTox 3.0, which evaluate their pharmacokinetic and toxicological properties [31,34].
3.7. Molecular Docking
Autogrid and Autodock software, versions 4.2.6 [77] were used to predict the mechanism of action of CPZ and its photoproducts, regarding the interaction with the following proteins that are targeted by anticancer medication: human oestrogen receptor alpha (ER) (PDB ID: 6VPF) [78], human androgen receptor (AR) (PDB ID: 1E3G) [79], aromatase (PDB ID: 3S7S) [80], basic fibroblast growth factor receptor 1 (FGFR1) (PDB:3GQL) [81], progesterone receptor (PDB ID: 1SQN) [82], and Beclin 1 (PDB ID: 4DDP) [83].
We have used our previously described molecular docking protocol described in [30,58,84]. The grid parameters were set as described in Table 4.

Table 4.
The grid point spacing, number of grid points, and coordinates of the central grid point of the maps for target proteins used in the molecular docking studies.
4. Conclusions
Laser irradiation at 266 nm changes the CPZ chemical structure, generating 18 photoproducts, including typical degradation products and a series of dimeric compounds. The molecular docking simulations suggest that most of the dimeric photocompounds have lower EFEB values than key targets involved in breast cancer therapy (such as aromatase, ER, FGFR1, EGFR, and HER2) compared to CPZ and its monomeric photocompounds, indicating a potentially more effective cancer treatment at lower doses. The ADME-Tox analysis also suggests improved safety for certain photocompounds, with higher LD50 values and reduced toxicity risks. This highlights that CPZ photoproducts may represent potential candidates for safer, targeted breast cancer therapy. However, future work will focus on the isolation, characterisation, and individual biological tests to assess the identified photoproducts’ anticancer activity.
5. Patents
The photoproducts identified and described in this study, along with their predicted inhibitory activity on breast cancer targets, are protected under the Romanian State Office for Inventions and Trademarks (Patent Application Number: A/00409).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26146668/s1.
Author Contributions
Conceptualisation, A.-M.U., F.B., S.A. and A.S.; methodology, A.-M.U. and F.B.; software, A.-M.U. and F.B.; validation, A.-M.U., F.B., S.A. and A.S.; formal analysis, A.-M.U. and F.B.; investigation, A.-M.U. and F.B.; resources, A.-M.U. and A.S.; data curation, A.-M.U., S.A. and F.B.; writing—original draft preparation, A.-M.U. and F.B.; writing—review and editing, A.-M.U. and A.S.; visualisation, A.-M.U., F.B. and A.S.; supervision, S.A. and A.S.; project administration, A.S.; funding acquisition, A.-M.U. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Institute of Atomic Physics through the project ELI-RO/RDI/2024_022, and by the Romanian Ministry of Education and Research, with the project numbers PN-IV-P2-2.1-TE-2023-1686, and PN-IV-P7.1-PED-2024-1995 within PNCDI IV; the Romanian National Nucleu Programme LAPLAS VII–contract no. 30N/2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to extend our gratitude to our colleague, Ionut Relu Andrei, for his assistance and support throughout this study. His expertise has contributed to the success of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vasileiou, M.; Papageorgiou, S.; Nguyen, N.P. Current Advancements and Future Perspectives of Immunotherapy in Breast Cancer Treatment. Immuno 2023, 3, 195–216. [Google Scholar] [CrossRef]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.S.; Kumar, A.; Vishakha, N.; Behl, T.; Jha, S.K.; Tang, H. Advancements in Clinical Aspects of Targeted Therapy and Immunotherapy in Breast Cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Dudley, K.; Liu, X.; De Haan, S. Chlorpromazine Dose for People with Schizophrenia. Cochrane Database Syst. Rev. 2017, 2017, CD007778. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.E.; Awad, G.A.; Rathbone, J.; Thornley, B.; Soares-Weiser, K. Chlorpromazine versus Placebo for Schizophrenia. Cochrane Database Syst. Rev. 2014, 2015, CD000284. [Google Scholar] [CrossRef]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The Seven Lives of Pharmacology’s First Lead Structure. Drug. Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- First-Generation Antipsychotics: An Introduction—Psychopharmacology Institute. Available online: https://psychopharmacologyinstitute.com/publication/first-generation-antipsychotics-an-introduction-2110 (accessed on 23 June 2021).
- González-González, A.; Vazquez-Jimenez, L.K.; Paz-González, A.D.; Bolognesi, M.L.; Rivera, G. Recent Advances in the Medicinal Chemistry of Phenothiazines. New Anticancer and Antiprotozoal Agents. CMC 2021, 28, 7910–7936. [Google Scholar] [CrossRef]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological Exploitation of the Phenothiazine Antipsychotics to Develop Novel Antitumor Agents–A Drug Repurposing Strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Ekpo, O.E.; Semenya, D.M.; Jardine, A.; Prince, S. Exploitation of a Novel Phenothiazine Derivative for Its Anti-Cancer Activities in Malignant Glioblastoma. Apoptosis 2020, 25, 261–274. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Wu, A.T.H.; Chang, P.M.-H.; Chen, K.-Y.; Yang, C.-N.; Yang, S.-C.; Ho, C.-C.; Chen, C.-C.; Kuo, Y.-L.; Lee, P.-Y.; et al. Trifluoperazine, an Antipsychotic Agent, Inhibits Cancer Stem Cell Growth and Overcomes Drug Resistance of Lung Cancer. Am. J. Respir. Crit. Care Med. 2012, 186, 1180–1188. [Google Scholar] [CrossRef]
- Vlachos, N.; Lampros, M.; Voulgaris, S.; Alexiou, G.A. Repurposing Antipsychotics for Cancer Treatment. Biomedicines 2021, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Kamgar-Dayhoff, P.; Brelidze, T.I. Multifaceted Effect of Chlorpromazine in Cancer: Implications for Cancer Treatment. Oncotarget 2021, 12, 1406–1426. [Google Scholar] [CrossRef]
- Yde, C.W.; Clausen, M.P.; Bennetzen, M.V.; Lykkesfeldt, A.E.; Mouritsen, O.G.; Guerra, B. The Antipsychotic Drug Chlorpromazine Enhances the Cytotoxic Effect of Tamoxifen in Tamoxifen-Sensitive and Tamoxifen-Resistant Human Breast Cancer Cells. Anti-Cancer Drugs 2009, 20, 723–735. [Google Scholar] [CrossRef]
- Yang, C.-E.; Lee, W.-Y.; Cheng, H.-W.; Chung, C.-H.; Mi, F.-L.; Lin, C.-W. The Antipsychotic Chlorpromazine Suppresses YAP Signaling, Stemness Properties, and Drug Resistance in Breast Cancer Cells. Chem.-Biol. Interact. 2019, 302, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Riffell, J.L.; Zimmerman, C.; Khong, A.; McHardy, L.M.; Roberge, M. Effects of Chemical Manipulation of Mitotic Arrest and Slippage on Cancer Cell Survival and Proliferation. Cell Cycle 2009, 8, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Trial of Chlorpromazine Together with Standard of Care in New Diagnosis of Glioblastoma. University of Iowa Clinical Research and Trials. Available online: https://clinicaltrials.uihealthcare.org/studies/phase-i-trial-chlorpromazine-together-standard-care-new-diagnosis-glioblastoma?utm_source=chatgpt.com (accessed on 2 July 2025).
- Control and ChlorproMAZINE 50 MG in Colon Cancer Stage III—Clinical Trials Registry—ICH GCP. Available online: https://ichgcp.net/clinical-trials-registry/NCT05433402?utm_source=chatgpt.com (accessed on 2 July 2025).
- Tozar, T.; Nastasa, V.; Stoicu, A.; Chifiriuc, M.C.; Popa, M.; Kamerzan, C.; Pascu, M.L. In Vitro Antimicrobial Efficacy of Laser Exposed Chlorpromazine against Gram-Positive Bacteria in Planktonic and Biofilm Growth State. Microb. Pathog. 2019, 129, 250–256. [Google Scholar] [CrossRef]
- Tozar, T.; Santos Costa, S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef]
- Andrei, I.R.; Tozar, T.; Dinache, A.; Boni, M.; Nastasa, V.; Pascu, M.L. Chlorpromazine Transformation by Exposure to Ultraviolet Laser Beams in Droplet and Bulk. Eur. J. Pharm. Sci. 2016, 81, 27–35. [Google Scholar] [CrossRef]
- Pascu, M.L.; Danko, B.; Martins, A.; Jedlinszki, N.; Alexandru, T.; Nastasa, V.; Boni, M.; Militaru, A.; Andrei, I.R.; Staicu, A.; et al. Correction: Exposure of Chlorpromazine to 266 Nm Laser Beam Generates New Species with Antibacterial Properties: Contributions to Development of a New Process for Drug Discovery. PLoS ONE 2013, 8, e55767. [Google Scholar] [CrossRef]
- Pirvulescu, R.; Tozar, T.; Stoicu, A.; Pascu, M.L. Application of Optically Modified Medicines in Fighting Pseudotumours. In Laser Optofluidics in Fighting Multiple Drug Resistance; Pascu, M.L., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017; pp. 366–406. ISBN 978-1-68108-498-5. [Google Scholar]
- Udrea, A.M.; Staicu, A.; Smarandache, A.; Andrei, I.R.; Badea, M.A.; Avram, S.; Pascu, M.L.; Pirvulescu, R.A.; Balas, M. Enhancement of Chlorpromazine Efficacy in Breast Cancer Treatment by 266 Nm Laser Irradiation. Sci. Rep. 2024, 14, 30329. [Google Scholar] [CrossRef]
- Alexandru, T.; Staicu, A.; Pascu, A.I.; Radu, E.; Stoicu, A.; Nastasa, V.V.; Dinache, A.C.; Boni, M.; Amaral, L.; Pascu, M.L. Characterization of Mixtures of Compounds Produced in Chlorpromazine Aqueous Solutions by Ultraviolet Laser Irradiation: Their Applications in Antimicrobial Assays. JBO 2014, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gómez, C.; Fonseca-Benítez, A.V.; Guevara-Pulido, J. Design, Synthesis, and in vitro Evaluation of a Carbamazepine Derivative with Antitumor Potential in a Model of Acute Lymphoblastic Leukemia. PLoS ONE 2025, 20, e0319415. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Pagès, J.-M.; Pirvulescu, R.A. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules 2021, 26, 2374. [Google Scholar] [CrossRef]
- Myung, Y.; de Sá, A.G.C.; Ascher, D.B. Deep-PK: Deep Learning for Small Molecule Pharmacokinetic and Toxicity Prediction. Nucleic Acids Res. 2024, 52, W469–W475. [Google Scholar] [CrossRef]
- ProTox-3.0—Prediction of TOXicity of Chemicals. Available online: https://tox.charite.de/protox3/index.php?site=compound_input (accessed on 2 July 2025).
- Deep-PK. Prediction Submission. Available online: https://biosig.lab.uq.edu.au/deeppk/prediction (accessed on 2 July 2025).
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Montazeri Aliabadi, H.; Manda, A.; Sidgal, R.; Chung, C. Targeting Breast Cancer: The Familiar, the Emerging, and the Uncharted Territories. Biomolecules 2023, 13, 1306. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Muñoz, B.E.; Sánchez, M.I.; Pardo, R.; Vega, M.S. Fate of the Drug Chlorpromazine in River Water According to Laboratory Assays. Identification and Evolution over Time of Degradation Products. Sorption to Sediment. Chemosphere 2016, 162, 285–292. [Google Scholar] [CrossRef]
- Trautwein, C.; Kümmerer, K. Degradation of the Tricyclic Antipsychotic Drug Chlorpromazine under Environmental Conditions, Identification of Its Main Aquatic Biotic and Abiotic Transformation Products by LC–MSn and Their Effects on Environmental Bacteria. J. Chromatogr. B 2012, 889–890, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; Tamat, S.R. Photosensitization by Drugs: Photolysis of Some Chlorine-Containing Drugs. J. Pharm. Pharmacol. 1980, 32, 172–177. [Google Scholar] [CrossRef]
- Rosenthal, I.; Ben-Hur, E.; Prager, A.; Riklis, E. Photochemical reactions of chlorpromazine; chemical and biochemical implications. Photochem. Photobiol. 1978, 28, 591–594. [Google Scholar] [CrossRef]
- Shields, D.J.; Chakraborty, M.; Abdelaziz, N.; Duley, A.; Gudmundsdottir, A.D. Review of Laser Flash Photolysis of Organic Molecules (2015–2018). In Photochemistry; Albini, A., Protti, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 47, pp. 70–121. ISBN 978-1-78801-554-7. [Google Scholar]
- Nikogosyan, D.N.; Oraevsky, A.A.; Rupasov, V.I. Two-Photon Ionization and Dissociation of Liquid Water by Powerful Laser UV Radiation. Chem. Phys. 1983, 77, 131–143. [Google Scholar] [CrossRef]
- Stone, D.; Whalley, L.K.; Ingham, T.; Edwards, P.M.; Cryer, D.R.; Brumby, C.A.; Seakins, P.W.; Heard, D.E. Measurement of OH Reactivity by Laser Flash Photolysis Coupled Withlaser-Induced Fluorescence Spectroscopy. Atmos. Meas. Tech. 2016, 9, 2827–2844. [Google Scholar] [CrossRef]
- He, Y.; Wu, J.; Fang, X.; Sonntag, C.V. Hydroxyl-Radical Induced Dechlorination of Pentachlorophenol in Water. In Radiation Technology for Conservation of the Environment, Proceedings of a Symposium, Zakopane, Poland, 8–12 September 1997; International Atomic Energy Agency (IAEA): Vienna, Austria, 1998; pp. 273–280. [Google Scholar]
- Jernigan, C.M.; Fite, C.H.; Vereecken, L.; Berkelhammer, M.B.; Rollins, A.W.; Rickly, P.S.; Novelli, A.; Taraborrelli, D.; Holmes, C.D.; Bertram, T.H. Efficient Production of Carbonyl Sulfide in the Low-NOx Oxidation of Dimethyl Sulfide. Geophys. Res. Lett. 2022, 49, e2021GL096838. [Google Scholar] [CrossRef]
- Chaffman, S.E.; Williams, T.; Miller, J.T.; Davidson, J.T. Identification of an Ultraviolet (UV)-Induced Promethazine Dimer. In Proceedings of the American Academy of Forensic Sciences 71st Annual Scientific Meeting, Baltimore, MD, USA, 18–23 February 2019; p. 267. Available online: https://www.aafs.org/sites/default/files/media/documents/2019%20_Proceedings.pdf (accessed on 2 July 2025).
- Mohamadighader, N.; Nematollahi, D.; Saraei, M. A Comprehensive Study on Electrochemical Oxidation of Phenothiazine in Water-Acetonitrile Mixture: Electrosynthesis of Phenothiazine Dimers. Electrochim. Acta 2022, 425, 140706. [Google Scholar] [CrossRef]
- Leito, I.; Herodes, K.; Huopolainen, M.; Virro, K.; Künnapas, A.; Kruve, A.; Tanner, R. Towards the Electrospray Ionization Mass Spectrometry Ionization Efficiency Scale of Organic Compounds. Rapid Comm. Mass. Spectrom. 2008, 22, 379–384. [Google Scholar] [CrossRef]
- Furuhashi, T.; Weckwerth, W. Isomer Analysis by Mass Spectrometry in Clinical Science. TrAC Trends Anal. Chem. 2023, 159, 116907. [Google Scholar] [CrossRef]
- Davies, A.K.; Navaratnam, S.; Phillips, G.O. Photochemistry of Chlorpromazine [2-Chloro-N-(3-Dimethylaminopropyl)Phenothiazine] in Propan-2-Ol Solution. J. Chem. Soc. Perkin Trans. 1976, 2, 25–29. [Google Scholar] [CrossRef]
- Grimshaw, J.; De Silva, A.P. Photochemistry and Photocyclization of Aryl Halides. Chem. Soc. Rev. 1981, 10, 181. [Google Scholar] [CrossRef]
- Wiley Online Books. Radicals in Organic Synthesis. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527618293 (accessed on 7 March 2024).
- Broeke, L.T.V.D.; Ouijja, E.H.; Bojarski, J.; Henegouwen, G.M.J.B.V. In Vitro photodegradation of chlorpromazine. Photochem. Photobiol. 1994, 59, 140–144. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 2 July 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Wilkinson, B. Drug Discovery beyond the ‘Rule-of-Five’. Curr. Opin. Biotechnol. 2007, 18, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Udrea, A.-M.; Buiu, C.; Staicu, A.; Dabu, A.N.; Avram, S. Photodegradation of Psychotropic Medications: Impact on Efficacy, Safety, and Drug Properties. Comput. Biol. Med. 2025, 191, 110115. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Avram, S.; Nistorescu, S.; Pascu, M.-L.; Romanitan, M.O. Laser Irradiated Phenothiazines: New Potential Treatment for COVID-19 Explored by Molecular Docking. J. Photochem. Photobiol. B Biol. 2020, 211, 111997. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Yuan, Y.; Chen, S. Molecular Characterization of Aromatase. Ann. N. Y. Acad. Sci. 2009, 1155, 112–120. [Google Scholar] [CrossRef]
- Pan, Y.-L.; Liu, Y.-L.; Chen, J.-Z. Computational Simulation Studies on the Binding Selectivity of 1-(1H-Benzimidazol-5-Yl)-5-Aminopyrazoles in Complexes with FGFR1 and FGFR4. Molecules 2018, 23, 767. [Google Scholar] [CrossRef]
- Ravindranathan, K.P.; Mandiyan, V.; Ekkati, A.R.; Bae, J.H.; Schlessinger, J.; Jorgensen, W.L. Discovery of Novel Fibroblast Growth Factor Receptor 1 Kinase Inhibitors by Structure-Based Virtual Screening. J. Med. Chem. 2010, 53, 1662–1672. [Google Scholar] [CrossRef]
- Klein, T.; Tucker, J.; Holdgate, G.A.; Norman, R.A.; Breeze, A.L. FGFR1 Kinase Inhibitors: Close Regioisomers Adopt Divergent Binding Modes and Display Distinct Biophysical Signatures. ACS Med. Chem. Lett. 2014, 5, 166–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spellmon, N.; Li, C.; Yang, Z. Allosterically Targeting EGFR Drug-Resistance Gatekeeper Mutations. J. Thorac. Dis. 2017, 9, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Stover, D.R.; Becker, M.; Liebetanz, J.; Lydon, N.B. Src Phosphorylation of the Epidermal Growth Factor Receptor at Novel Sites Mediates Receptor Interaction with Src and P85α. J. Biol. Chem. 1995, 270, 15591–15597. [Google Scholar] [CrossRef]
- Tung, B.T.; Son, N.N.; Kim, N.B.; Khanh, D.T.H.; Minh, P.H. In Silico Screening of Alkaloids as Potential Inhibitors of HER2 Protein for Breast Cancer Treatment. Vietnam. J. Chem. 2023, 61, 308–317. [Google Scholar] [CrossRef]
- Collier, T.S.; Diraviyam, K.; Monsey, J.; Shen, W.; Sept, D.; Bose, R. Carboxyl Group Footprinting Mass Spectrometry and Molecular Dynamics Identify Key Interactions in the HER2-HER3 Receptor Tyrosine Kinase Interface. J. Biol. Chem. 2013, 288, 25254–25264. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, O.; Ner, M.; Nerín-Fonz, F.; Jiménez-Martínez, Y.; Araripe, D.; Marchal, J.A.; Boulaiz, H.; Gutiérrez-de-Terán, H.; Campos, J.M.; Conejo-García, A. Design, Synthesis, HER2 Inhibition and Anticancer Evaluation of New Substituted 1,5-Dihydro-4,1-Benzoxazepines. J. Enzym. Inhib. Med. Chem. 2021, 36, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, R.; Li, P.; Yang, Y.; Wang, Y.; Mao, H.; Tang, X. Clinical and Structural Insights into the Rare but Oncogenic HER2-Activating Missense Mutations in Non-Small Cell Lung Cancer: A Retrospective ATLAS Cohort Study. Discov. Onc. 2024, 15, 285. [Google Scholar] [CrossRef]
- Zhang, J.; McAndrew, N.P.; Wang, X.; Du, Y.; DiCarlo, B.; Wang, M.; Chen, K.; Yu, W.; Hu, X. Preclinical and Clinical Activity of DZD1516, a Full Blood–Brain Barrier-Penetrant, Highly Selective HER2 Inhibitor. Breast Cancer Res. 2023, 25, 81. [Google Scholar] [CrossRef]
- Chandrika, B.B.; Steephan, M.; Kumar, T.R.S.; Sabu, A.; Haridas, M. Hesperetin and Naringenin Sensitize HER2 Positive Cancer Cells to Death by Serving as HER2 Tyrosine Kinase Inhibitors. Life Sci. 2016, 160, 47–56. [Google Scholar] [CrossRef]
- Sait, K.H.W.; Mashraqi, M.; Khogeer, A.A.; Alzahrani, O.; Anfinan, N.; Sait, H.K.; Almutairi, A.; Alam, Q. Molecular Docking Analysis of HER-2 Inhibitor from the ZINC Database as Anticancer Agents. Bioinformation 2020, 16, 882. [Google Scholar] [CrossRef]
- Pascu, M.L.; Smarandache, A.; Boni, M.; Kristiansen, J.; Nastasa, V.; Andrei, I.R. Spectral Properties of Some Molecular Solutions. Rom. Rep. Phys. 2011, 36, 1267–1284. [Google Scholar]
- PubChem Chlorpromazine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2726 (accessed on 26 June 2025).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- ChemAxon MarvinSketch 21.20.0. 2013. Available online: http://Www.Chemaxon.Com (accessed on 2 July 2025).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Hosfield, D.J.; Weber, S.; Li, N.-S.; Sauvage, M.; Joiner, C.F.; Hancock, G.R.; Sullivan, E.A.; Ndukwe, E.; Han, R.; Cush, S.; et al. Stereospecific Lasofoxifene Derivatives Reveal the Interplay between Estrogen Receptor Alpha Stability and Antagonistic Activity in ESR1 Mutant Breast Cancer Cells. Elife 2022, 11, e72512. [Google Scholar] [CrossRef]
- Matias, P.M.; Donner, P.; Coelho, R.; Thomaz, M.; Peixoto, C.; Macedo, S.; Otto, N.; Joschko, S.; Scholz, P.; Wegg, A.; et al. Structural Evidence for Ligand Specificity in the Binding Domain of the Human Androgen Receptor. J. Biol. Chem. 2000, 275, 26164–26171. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Lo, J.; Morton, D.; Valette, D.; Xi, J.; Griswold, J.; Hubbell, S.; Egbuta, C.; Jiang, W.; An, J.; et al. Novel Aromatase Inhibitors by Structure-Guided Design. J. Med. Chem. 2012, 55, 8464–8476. [Google Scholar] [CrossRef]
- Bae, J.H.; Lew, E.D.; Yuzawa, S.; Tomé, F.; Lax, I.; Schlessinger, J. The Selectivity of Receptor Tyrosine Kinase Signaling Is Controlled by a Secondary SH2 Domain Binding Site. Cell 2009, 138, 514–524. [Google Scholar] [CrossRef]
- Madauss, K.P.; Deng, S.-J.; Austin, R.J.H.; Lambert, M.H.; McLay, I.; Pritchard, J.; Short, S.A.; Stewart, E.L.; Uings, I.J.; Williams, S.P. Progesterone Receptor Ligand Binding Pocket Flexibility: Crystal Structures of the Norethindrone and Mometasone Furoate Complexes. J. Med. Chem. 2004, 47, 3381–3387. [Google Scholar] [CrossRef]
- Huang, W.; Choi, W.; Hu, W.; Mi, N.; Guo, Q.; Ma, M.; Liu, M.; Tian, Y.; Lu, P.; Wang, F.-L.; et al. Crystal Structure and Biochemical Analyses Reveal Beclin 1 as a Novel Membrane Binding Protein. Cell Res. 2012, 22, 473–489. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Staicu, A.; Avram, S. Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking. Pharmaceutics 2022, 14, 2390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).