Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential
Abstract
1. Introduction
2. Results and Discussions
2.1. HPLC-MS Analyses
2.2. Drug-like and ADME-Tox Predictions
2.3. Molecular Docking Approach
3. Materials and Methods
3.1. Chemicals
3.2. Irradiation Protocol
3.3. HPLC-MS
3.4. Molecular Modelling
3.4.1. Chemical Structure Retrieval and Drawing
3.4.2. Molecular Optimisation
3.4.3. Preparation for Molecular Docking
3.5. Drug-Likeness Evaluation of Photoproducts
3.6. ADME-TOX Predictions
3.7. Molecular Docking
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasileiou, M.; Papageorgiou, S.; Nguyen, N.P. Current Advancements and Future Perspectives of Immunotherapy in Breast Cancer Treatment. Immuno 2023, 3, 195–216. [Google Scholar] [CrossRef]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.S.; Kumar, A.; Vishakha, N.; Behl, T.; Jha, S.K.; Tang, H. Advancements in Clinical Aspects of Targeted Therapy and Immunotherapy in Breast Cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Dudley, K.; Liu, X.; De Haan, S. Chlorpromazine Dose for People with Schizophrenia. Cochrane Database Syst. Rev. 2017, 2017, CD007778. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.E.; Awad, G.A.; Rathbone, J.; Thornley, B.; Soares-Weiser, K. Chlorpromazine versus Placebo for Schizophrenia. Cochrane Database Syst. Rev. 2014, 2015, CD000284. [Google Scholar] [CrossRef]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The Seven Lives of Pharmacology’s First Lead Structure. Drug. Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- First-Generation Antipsychotics: An Introduction—Psychopharmacology Institute. Available online: https://psychopharmacologyinstitute.com/publication/first-generation-antipsychotics-an-introduction-2110 (accessed on 23 June 2021).
- González-González, A.; Vazquez-Jimenez, L.K.; Paz-González, A.D.; Bolognesi, M.L.; Rivera, G. Recent Advances in the Medicinal Chemistry of Phenothiazines. New Anticancer and Antiprotozoal Agents. CMC 2021, 28, 7910–7936. [Google Scholar] [CrossRef]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological Exploitation of the Phenothiazine Antipsychotics to Develop Novel Antitumor Agents–A Drug Repurposing Strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef]
- Omoruyi, S.I.; Ekpo, O.E.; Semenya, D.M.; Jardine, A.; Prince, S. Exploitation of a Novel Phenothiazine Derivative for Its Anti-Cancer Activities in Malignant Glioblastoma. Apoptosis 2020, 25, 261–274. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Wu, A.T.H.; Chang, P.M.-H.; Chen, K.-Y.; Yang, C.-N.; Yang, S.-C.; Ho, C.-C.; Chen, C.-C.; Kuo, Y.-L.; Lee, P.-Y.; et al. Trifluoperazine, an Antipsychotic Agent, Inhibits Cancer Stem Cell Growth and Overcomes Drug Resistance of Lung Cancer. Am. J. Respir. Crit. Care Med. 2012, 186, 1180–1188. [Google Scholar] [CrossRef]
- Vlachos, N.; Lampros, M.; Voulgaris, S.; Alexiou, G.A. Repurposing Antipsychotics for Cancer Treatment. Biomedicines 2021, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Kamgar-Dayhoff, P.; Brelidze, T.I. Multifaceted Effect of Chlorpromazine in Cancer: Implications for Cancer Treatment. Oncotarget 2021, 12, 1406–1426. [Google Scholar] [CrossRef]
- Yde, C.W.; Clausen, M.P.; Bennetzen, M.V.; Lykkesfeldt, A.E.; Mouritsen, O.G.; Guerra, B. The Antipsychotic Drug Chlorpromazine Enhances the Cytotoxic Effect of Tamoxifen in Tamoxifen-Sensitive and Tamoxifen-Resistant Human Breast Cancer Cells. Anti-Cancer Drugs 2009, 20, 723–735. [Google Scholar] [CrossRef]
- Yang, C.-E.; Lee, W.-Y.; Cheng, H.-W.; Chung, C.-H.; Mi, F.-L.; Lin, C.-W. The Antipsychotic Chlorpromazine Suppresses YAP Signaling, Stemness Properties, and Drug Resistance in Breast Cancer Cells. Chem.-Biol. Interact. 2019, 302, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Riffell, J.L.; Zimmerman, C.; Khong, A.; McHardy, L.M.; Roberge, M. Effects of Chemical Manipulation of Mitotic Arrest and Slippage on Cancer Cell Survival and Proliferation. Cell Cycle 2009, 8, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Trial of Chlorpromazine Together with Standard of Care in New Diagnosis of Glioblastoma. University of Iowa Clinical Research and Trials. Available online: https://clinicaltrials.uihealthcare.org/studies/phase-i-trial-chlorpromazine-together-standard-care-new-diagnosis-glioblastoma?utm_source=chatgpt.com (accessed on 2 July 2025).
- Control and ChlorproMAZINE 50 MG in Colon Cancer Stage III—Clinical Trials Registry—ICH GCP. Available online: https://ichgcp.net/clinical-trials-registry/NCT05433402?utm_source=chatgpt.com (accessed on 2 July 2025).
- Tozar, T.; Nastasa, V.; Stoicu, A.; Chifiriuc, M.C.; Popa, M.; Kamerzan, C.; Pascu, M.L. In Vitro Antimicrobial Efficacy of Laser Exposed Chlorpromazine against Gram-Positive Bacteria in Planktonic and Biofilm Growth State. Microb. Pathog. 2019, 129, 250–256. [Google Scholar] [CrossRef]
- Tozar, T.; Santos Costa, S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef]
- Andrei, I.R.; Tozar, T.; Dinache, A.; Boni, M.; Nastasa, V.; Pascu, M.L. Chlorpromazine Transformation by Exposure to Ultraviolet Laser Beams in Droplet and Bulk. Eur. J. Pharm. Sci. 2016, 81, 27–35. [Google Scholar] [CrossRef]
- Pascu, M.L.; Danko, B.; Martins, A.; Jedlinszki, N.; Alexandru, T.; Nastasa, V.; Boni, M.; Militaru, A.; Andrei, I.R.; Staicu, A.; et al. Correction: Exposure of Chlorpromazine to 266 Nm Laser Beam Generates New Species with Antibacterial Properties: Contributions to Development of a New Process for Drug Discovery. PLoS ONE 2013, 8, e55767. [Google Scholar] [CrossRef]
- Pirvulescu, R.; Tozar, T.; Stoicu, A.; Pascu, M.L. Application of Optically Modified Medicines in Fighting Pseudotumours. In Laser Optofluidics in Fighting Multiple Drug Resistance; Pascu, M.L., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017; pp. 366–406. ISBN 978-1-68108-498-5. [Google Scholar]
- Udrea, A.M.; Staicu, A.; Smarandache, A.; Andrei, I.R.; Badea, M.A.; Avram, S.; Pascu, M.L.; Pirvulescu, R.A.; Balas, M. Enhancement of Chlorpromazine Efficacy in Breast Cancer Treatment by 266 Nm Laser Irradiation. Sci. Rep. 2024, 14, 30329. [Google Scholar] [CrossRef]
- Alexandru, T.; Staicu, A.; Pascu, A.I.; Radu, E.; Stoicu, A.; Nastasa, V.V.; Dinache, A.C.; Boni, M.; Amaral, L.; Pascu, M.L. Characterization of Mixtures of Compounds Produced in Chlorpromazine Aqueous Solutions by Ultraviolet Laser Irradiation: Their Applications in Antimicrobial Assays. JBO 2014, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gómez, C.; Fonseca-Benítez, A.V.; Guevara-Pulido, J. Design, Synthesis, and in vitro Evaluation of a Carbamazepine Derivative with Antitumor Potential in a Model of Acute Lymphoblastic Leukemia. PLoS ONE 2025, 20, e0319415. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Pagès, J.-M.; Pirvulescu, R.A. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules 2021, 26, 2374. [Google Scholar] [CrossRef]
- Myung, Y.; de Sá, A.G.C.; Ascher, D.B. Deep-PK: Deep Learning for Small Molecule Pharmacokinetic and Toxicity Prediction. Nucleic Acids Res. 2024, 52, W469–W475. [Google Scholar] [CrossRef]
- ProTox-3.0—Prediction of TOXicity of Chemicals. Available online: https://tox.charite.de/protox3/index.php?site=compound_input (accessed on 2 July 2025).
- Deep-PK. Prediction Submission. Available online: https://biosig.lab.uq.edu.au/deeppk/prediction (accessed on 2 July 2025).
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Montazeri Aliabadi, H.; Manda, A.; Sidgal, R.; Chung, C. Targeting Breast Cancer: The Familiar, the Emerging, and the Uncharted Territories. Biomolecules 2023, 13, 1306. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Muñoz, B.E.; Sánchez, M.I.; Pardo, R.; Vega, M.S. Fate of the Drug Chlorpromazine in River Water According to Laboratory Assays. Identification and Evolution over Time of Degradation Products. Sorption to Sediment. Chemosphere 2016, 162, 285–292. [Google Scholar] [CrossRef]
- Trautwein, C.; Kümmerer, K. Degradation of the Tricyclic Antipsychotic Drug Chlorpromazine under Environmental Conditions, Identification of Its Main Aquatic Biotic and Abiotic Transformation Products by LC–MSn and Their Effects on Environmental Bacteria. J. Chromatogr. B 2012, 889–890, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; Tamat, S.R. Photosensitization by Drugs: Photolysis of Some Chlorine-Containing Drugs. J. Pharm. Pharmacol. 1980, 32, 172–177. [Google Scholar] [CrossRef]
- Rosenthal, I.; Ben-Hur, E.; Prager, A.; Riklis, E. Photochemical reactions of chlorpromazine; chemical and biochemical implications. Photochem. Photobiol. 1978, 28, 591–594. [Google Scholar] [CrossRef]
- Shields, D.J.; Chakraborty, M.; Abdelaziz, N.; Duley, A.; Gudmundsdottir, A.D. Review of Laser Flash Photolysis of Organic Molecules (2015–2018). In Photochemistry; Albini, A., Protti, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 47, pp. 70–121. ISBN 978-1-78801-554-7. [Google Scholar]
- Nikogosyan, D.N.; Oraevsky, A.A.; Rupasov, V.I. Two-Photon Ionization and Dissociation of Liquid Water by Powerful Laser UV Radiation. Chem. Phys. 1983, 77, 131–143. [Google Scholar] [CrossRef]
- Stone, D.; Whalley, L.K.; Ingham, T.; Edwards, P.M.; Cryer, D.R.; Brumby, C.A.; Seakins, P.W.; Heard, D.E. Measurement of OH Reactivity by Laser Flash Photolysis Coupled Withlaser-Induced Fluorescence Spectroscopy. Atmos. Meas. Tech. 2016, 9, 2827–2844. [Google Scholar] [CrossRef]
- He, Y.; Wu, J.; Fang, X.; Sonntag, C.V. Hydroxyl-Radical Induced Dechlorination of Pentachlorophenol in Water. In Radiation Technology for Conservation of the Environment, Proceedings of a Symposium, Zakopane, Poland, 8–12 September 1997; International Atomic Energy Agency (IAEA): Vienna, Austria, 1998; pp. 273–280. [Google Scholar]
- Jernigan, C.M.; Fite, C.H.; Vereecken, L.; Berkelhammer, M.B.; Rollins, A.W.; Rickly, P.S.; Novelli, A.; Taraborrelli, D.; Holmes, C.D.; Bertram, T.H. Efficient Production of Carbonyl Sulfide in the Low-NOx Oxidation of Dimethyl Sulfide. Geophys. Res. Lett. 2022, 49, e2021GL096838. [Google Scholar] [CrossRef]
- Chaffman, S.E.; Williams, T.; Miller, J.T.; Davidson, J.T. Identification of an Ultraviolet (UV)-Induced Promethazine Dimer. In Proceedings of the American Academy of Forensic Sciences 71st Annual Scientific Meeting, Baltimore, MD, USA, 18–23 February 2019; p. 267. Available online: https://www.aafs.org/sites/default/files/media/documents/2019%20_Proceedings.pdf (accessed on 2 July 2025).
- Mohamadighader, N.; Nematollahi, D.; Saraei, M. A Comprehensive Study on Electrochemical Oxidation of Phenothiazine in Water-Acetonitrile Mixture: Electrosynthesis of Phenothiazine Dimers. Electrochim. Acta 2022, 425, 140706. [Google Scholar] [CrossRef]
- Leito, I.; Herodes, K.; Huopolainen, M.; Virro, K.; Künnapas, A.; Kruve, A.; Tanner, R. Towards the Electrospray Ionization Mass Spectrometry Ionization Efficiency Scale of Organic Compounds. Rapid Comm. Mass. Spectrom. 2008, 22, 379–384. [Google Scholar] [CrossRef]
- Furuhashi, T.; Weckwerth, W. Isomer Analysis by Mass Spectrometry in Clinical Science. TrAC Trends Anal. Chem. 2023, 159, 116907. [Google Scholar] [CrossRef]
- Davies, A.K.; Navaratnam, S.; Phillips, G.O. Photochemistry of Chlorpromazine [2-Chloro-N-(3-Dimethylaminopropyl)Phenothiazine] in Propan-2-Ol Solution. J. Chem. Soc. Perkin Trans. 1976, 2, 25–29. [Google Scholar] [CrossRef]
- Grimshaw, J.; De Silva, A.P. Photochemistry and Photocyclization of Aryl Halides. Chem. Soc. Rev. 1981, 10, 181. [Google Scholar] [CrossRef]
- Wiley Online Books. Radicals in Organic Synthesis. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527618293 (accessed on 7 March 2024).
- Broeke, L.T.V.D.; Ouijja, E.H.; Bojarski, J.; Henegouwen, G.M.J.B.V. In Vitro photodegradation of chlorpromazine. Photochem. Photobiol. 1994, 59, 140–144. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 2 July 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Wilkinson, B. Drug Discovery beyond the ‘Rule-of-Five’. Curr. Opin. Biotechnol. 2007, 18, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Udrea, A.-M.; Buiu, C.; Staicu, A.; Dabu, A.N.; Avram, S. Photodegradation of Psychotropic Medications: Impact on Efficacy, Safety, and Drug Properties. Comput. Biol. Med. 2025, 191, 110115. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Avram, S.; Nistorescu, S.; Pascu, M.-L.; Romanitan, M.O. Laser Irradiated Phenothiazines: New Potential Treatment for COVID-19 Explored by Molecular Docking. J. Photochem. Photobiol. B Biol. 2020, 211, 111997. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Yuan, Y.; Chen, S. Molecular Characterization of Aromatase. Ann. N. Y. Acad. Sci. 2009, 1155, 112–120. [Google Scholar] [CrossRef]
- Pan, Y.-L.; Liu, Y.-L.; Chen, J.-Z. Computational Simulation Studies on the Binding Selectivity of 1-(1H-Benzimidazol-5-Yl)-5-Aminopyrazoles in Complexes with FGFR1 and FGFR4. Molecules 2018, 23, 767. [Google Scholar] [CrossRef]
- Ravindranathan, K.P.; Mandiyan, V.; Ekkati, A.R.; Bae, J.H.; Schlessinger, J.; Jorgensen, W.L. Discovery of Novel Fibroblast Growth Factor Receptor 1 Kinase Inhibitors by Structure-Based Virtual Screening. J. Med. Chem. 2010, 53, 1662–1672. [Google Scholar] [CrossRef]
- Klein, T.; Tucker, J.; Holdgate, G.A.; Norman, R.A.; Breeze, A.L. FGFR1 Kinase Inhibitors: Close Regioisomers Adopt Divergent Binding Modes and Display Distinct Biophysical Signatures. ACS Med. Chem. Lett. 2014, 5, 166–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spellmon, N.; Li, C.; Yang, Z. Allosterically Targeting EGFR Drug-Resistance Gatekeeper Mutations. J. Thorac. Dis. 2017, 9, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Stover, D.R.; Becker, M.; Liebetanz, J.; Lydon, N.B. Src Phosphorylation of the Epidermal Growth Factor Receptor at Novel Sites Mediates Receptor Interaction with Src and P85α. J. Biol. Chem. 1995, 270, 15591–15597. [Google Scholar] [CrossRef]
- Tung, B.T.; Son, N.N.; Kim, N.B.; Khanh, D.T.H.; Minh, P.H. In Silico Screening of Alkaloids as Potential Inhibitors of HER2 Protein for Breast Cancer Treatment. Vietnam. J. Chem. 2023, 61, 308–317. [Google Scholar] [CrossRef]
- Collier, T.S.; Diraviyam, K.; Monsey, J.; Shen, W.; Sept, D.; Bose, R. Carboxyl Group Footprinting Mass Spectrometry and Molecular Dynamics Identify Key Interactions in the HER2-HER3 Receptor Tyrosine Kinase Interface. J. Biol. Chem. 2013, 288, 25254–25264. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, O.; Ner, M.; Nerín-Fonz, F.; Jiménez-Martínez, Y.; Araripe, D.; Marchal, J.A.; Boulaiz, H.; Gutiérrez-de-Terán, H.; Campos, J.M.; Conejo-García, A. Design, Synthesis, HER2 Inhibition and Anticancer Evaluation of New Substituted 1,5-Dihydro-4,1-Benzoxazepines. J. Enzym. Inhib. Med. Chem. 2021, 36, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, R.; Li, P.; Yang, Y.; Wang, Y.; Mao, H.; Tang, X. Clinical and Structural Insights into the Rare but Oncogenic HER2-Activating Missense Mutations in Non-Small Cell Lung Cancer: A Retrospective ATLAS Cohort Study. Discov. Onc. 2024, 15, 285. [Google Scholar] [CrossRef]
- Zhang, J.; McAndrew, N.P.; Wang, X.; Du, Y.; DiCarlo, B.; Wang, M.; Chen, K.; Yu, W.; Hu, X. Preclinical and Clinical Activity of DZD1516, a Full Blood–Brain Barrier-Penetrant, Highly Selective HER2 Inhibitor. Breast Cancer Res. 2023, 25, 81. [Google Scholar] [CrossRef]
- Chandrika, B.B.; Steephan, M.; Kumar, T.R.S.; Sabu, A.; Haridas, M. Hesperetin and Naringenin Sensitize HER2 Positive Cancer Cells to Death by Serving as HER2 Tyrosine Kinase Inhibitors. Life Sci. 2016, 160, 47–56. [Google Scholar] [CrossRef]
- Sait, K.H.W.; Mashraqi, M.; Khogeer, A.A.; Alzahrani, O.; Anfinan, N.; Sait, H.K.; Almutairi, A.; Alam, Q. Molecular Docking Analysis of HER-2 Inhibitor from the ZINC Database as Anticancer Agents. Bioinformation 2020, 16, 882. [Google Scholar] [CrossRef]
- Pascu, M.L.; Smarandache, A.; Boni, M.; Kristiansen, J.; Nastasa, V.; Andrei, I.R. Spectral Properties of Some Molecular Solutions. Rom. Rep. Phys. 2011, 36, 1267–1284. [Google Scholar]
- PubChem Chlorpromazine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2726 (accessed on 26 June 2025).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- ChemAxon MarvinSketch 21.20.0. 2013. Available online: http://Www.Chemaxon.Com (accessed on 2 July 2025).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Hosfield, D.J.; Weber, S.; Li, N.-S.; Sauvage, M.; Joiner, C.F.; Hancock, G.R.; Sullivan, E.A.; Ndukwe, E.; Han, R.; Cush, S.; et al. Stereospecific Lasofoxifene Derivatives Reveal the Interplay between Estrogen Receptor Alpha Stability and Antagonistic Activity in ESR1 Mutant Breast Cancer Cells. Elife 2022, 11, e72512. [Google Scholar] [CrossRef]
- Matias, P.M.; Donner, P.; Coelho, R.; Thomaz, M.; Peixoto, C.; Macedo, S.; Otto, N.; Joschko, S.; Scholz, P.; Wegg, A.; et al. Structural Evidence for Ligand Specificity in the Binding Domain of the Human Androgen Receptor. J. Biol. Chem. 2000, 275, 26164–26171. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Lo, J.; Morton, D.; Valette, D.; Xi, J.; Griswold, J.; Hubbell, S.; Egbuta, C.; Jiang, W.; An, J.; et al. Novel Aromatase Inhibitors by Structure-Guided Design. J. Med. Chem. 2012, 55, 8464–8476. [Google Scholar] [CrossRef]
- Bae, J.H.; Lew, E.D.; Yuzawa, S.; Tomé, F.; Lax, I.; Schlessinger, J. The Selectivity of Receptor Tyrosine Kinase Signaling Is Controlled by a Secondary SH2 Domain Binding Site. Cell 2009, 138, 514–524. [Google Scholar] [CrossRef]
- Madauss, K.P.; Deng, S.-J.; Austin, R.J.H.; Lambert, M.H.; McLay, I.; Pritchard, J.; Short, S.A.; Stewart, E.L.; Uings, I.J.; Williams, S.P. Progesterone Receptor Ligand Binding Pocket Flexibility: Crystal Structures of the Norethindrone and Mometasone Furoate Complexes. J. Med. Chem. 2004, 47, 3381–3387. [Google Scholar] [CrossRef]
- Huang, W.; Choi, W.; Hu, W.; Mi, N.; Guo, Q.; Ma, M.; Liu, M.; Tian, Y.; Lu, P.; Wang, F.-L.; et al. Crystal Structure and Biochemical Analyses Reveal Beclin 1 as a Novel Membrane Binding Protein. Cell Res. 2012, 22, 473–489. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Staicu, A.; Avram, S. Target Prediction of 5,10,15,20-Tetrakis(4′-Sulfonatophenyl)-Porphyrin Using Molecular Docking. Pharmaceutics 2022, 14, 2390. [Google Scholar] [CrossRef] [PubMed]
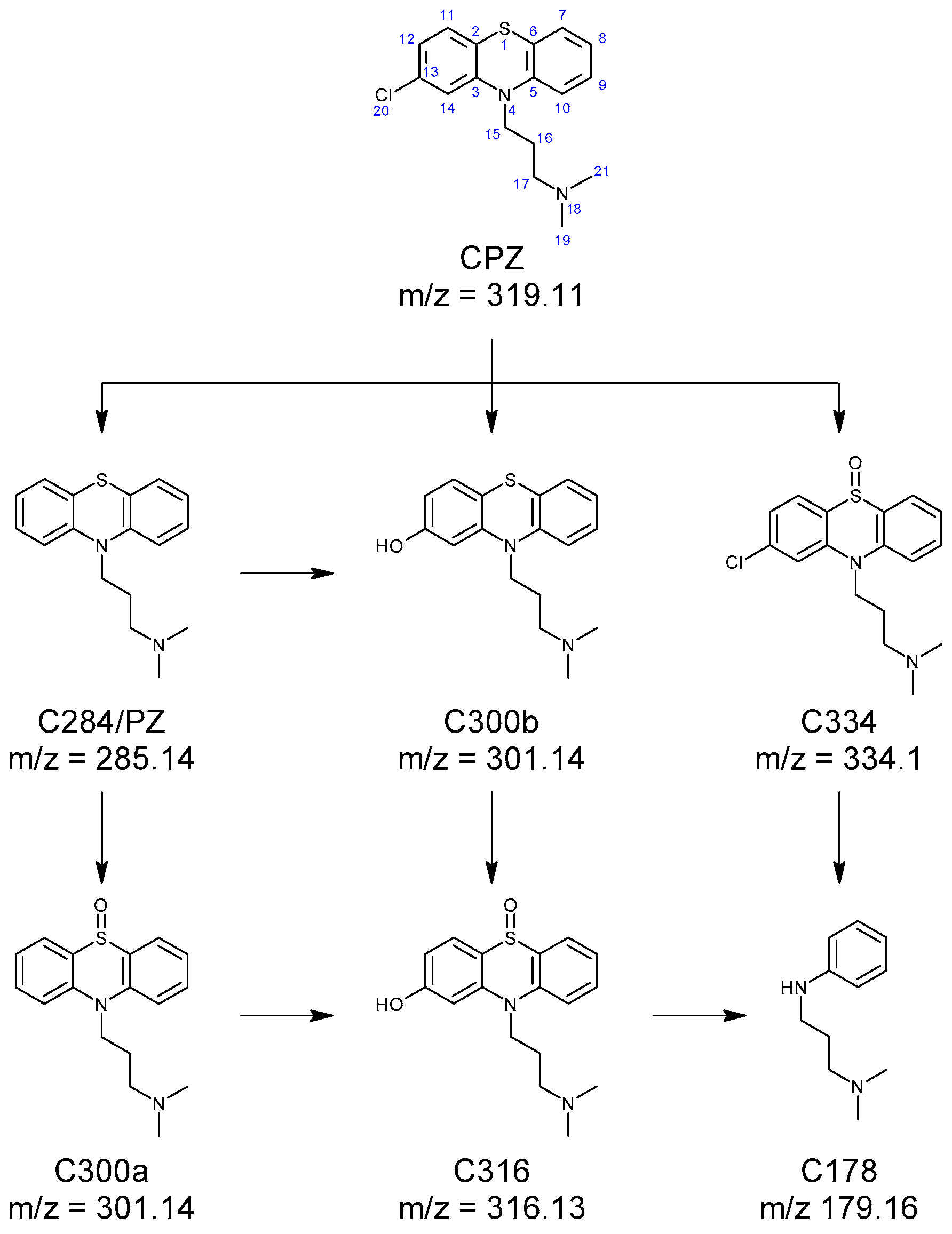
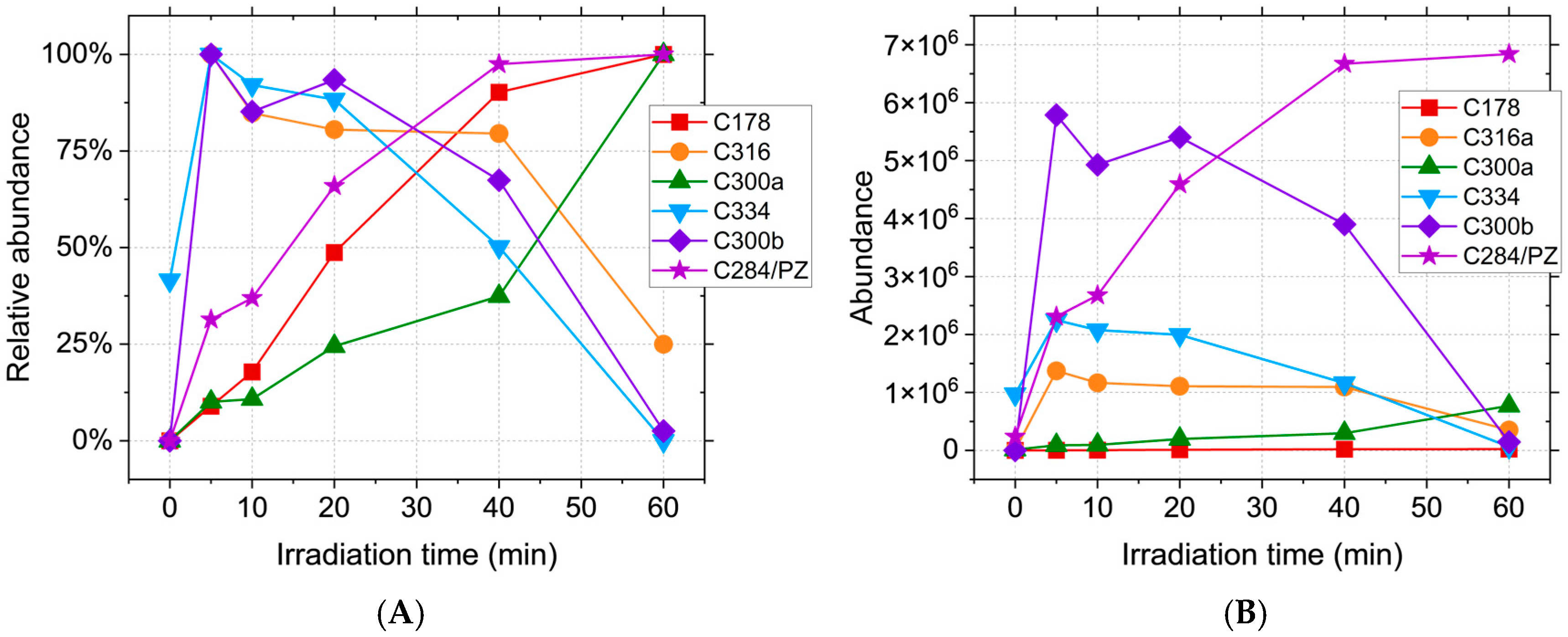
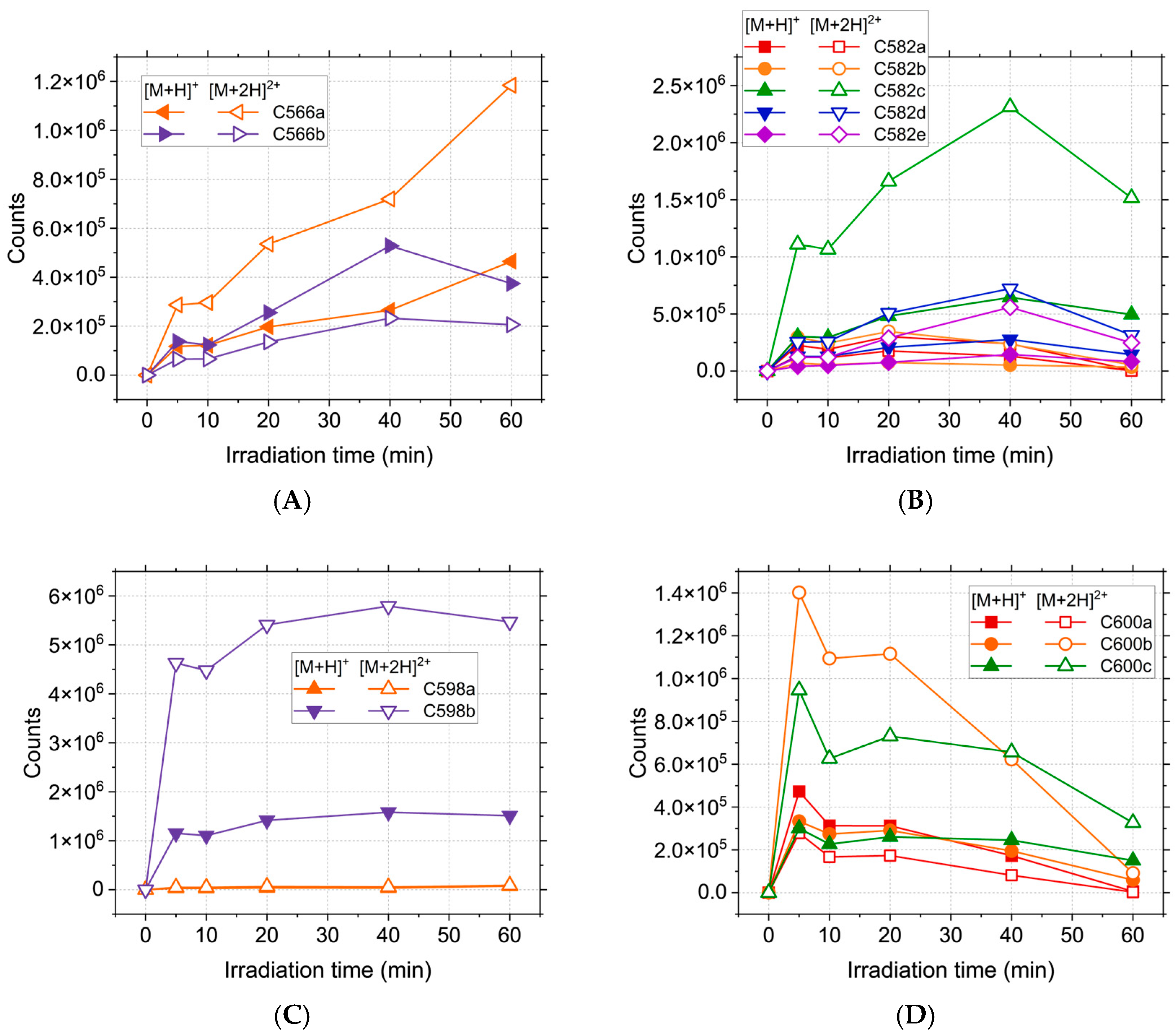
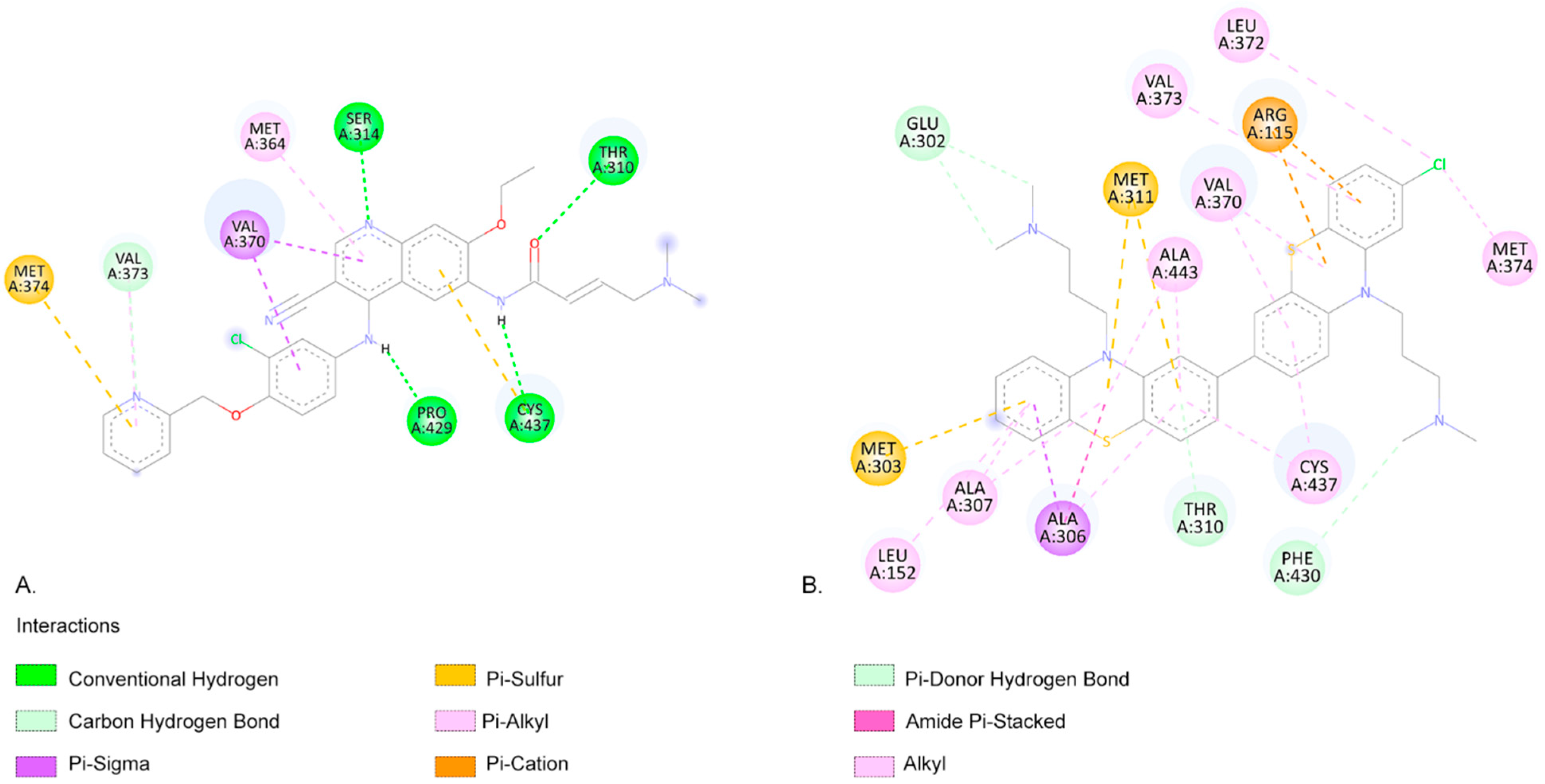
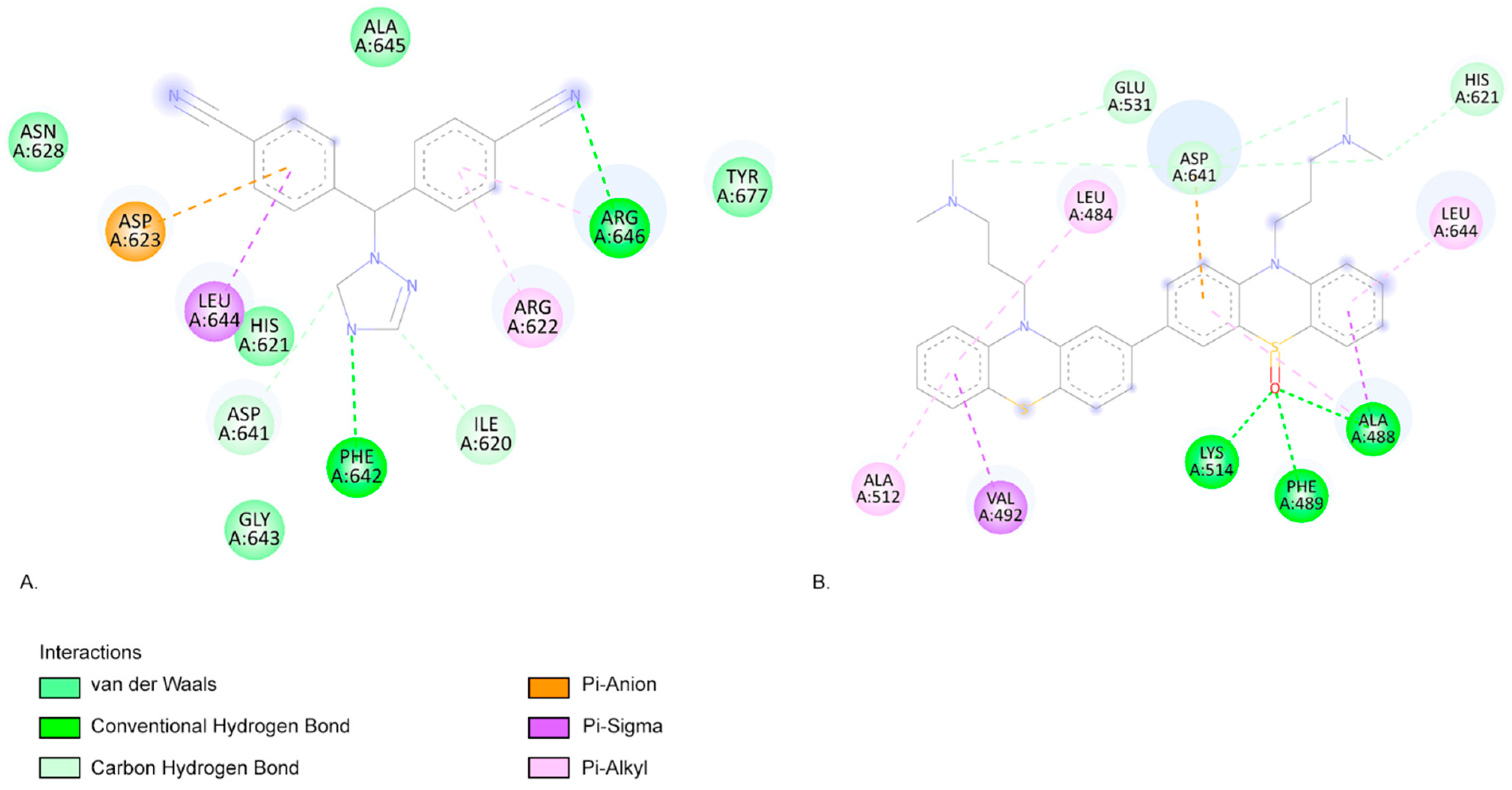
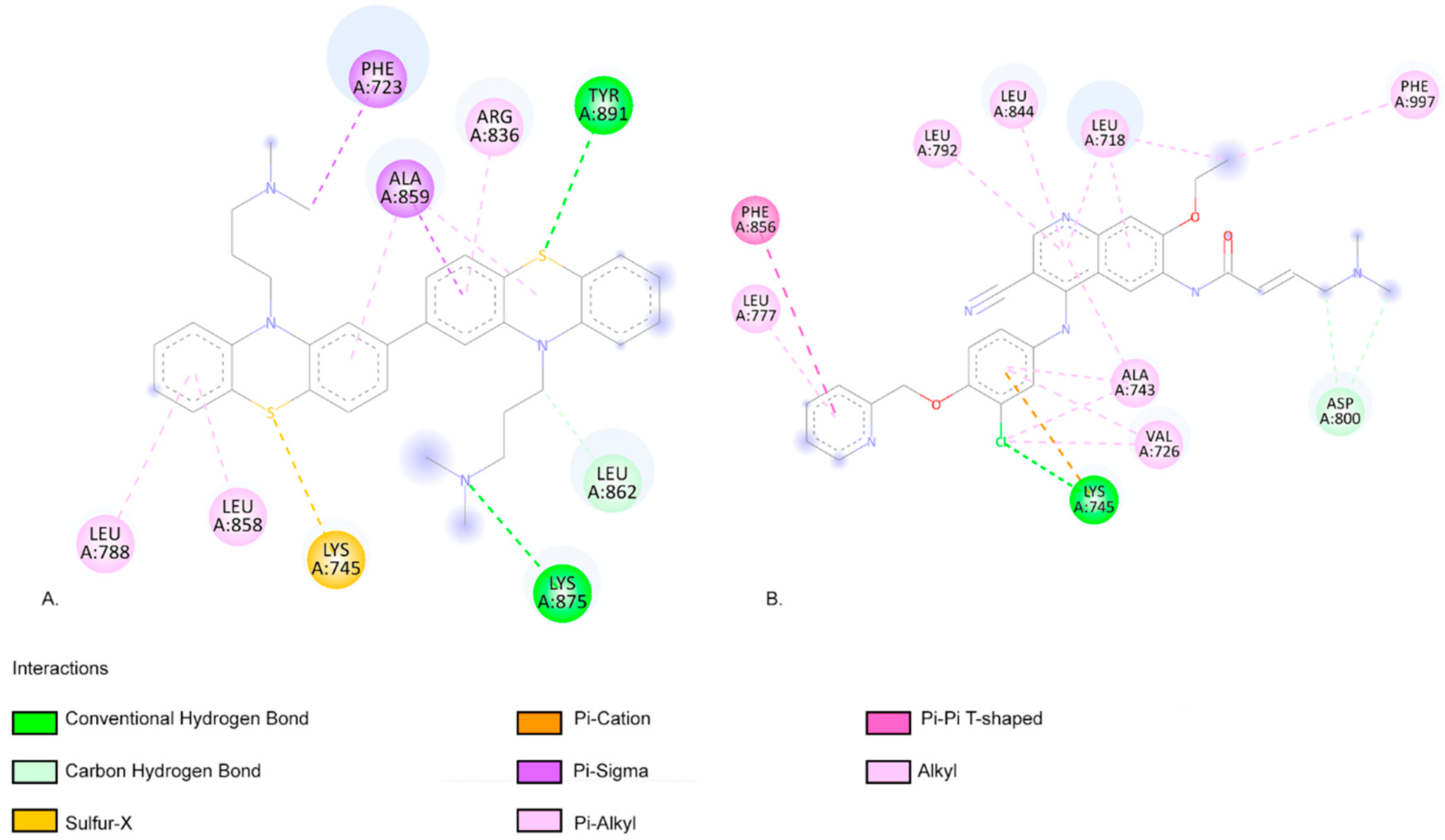
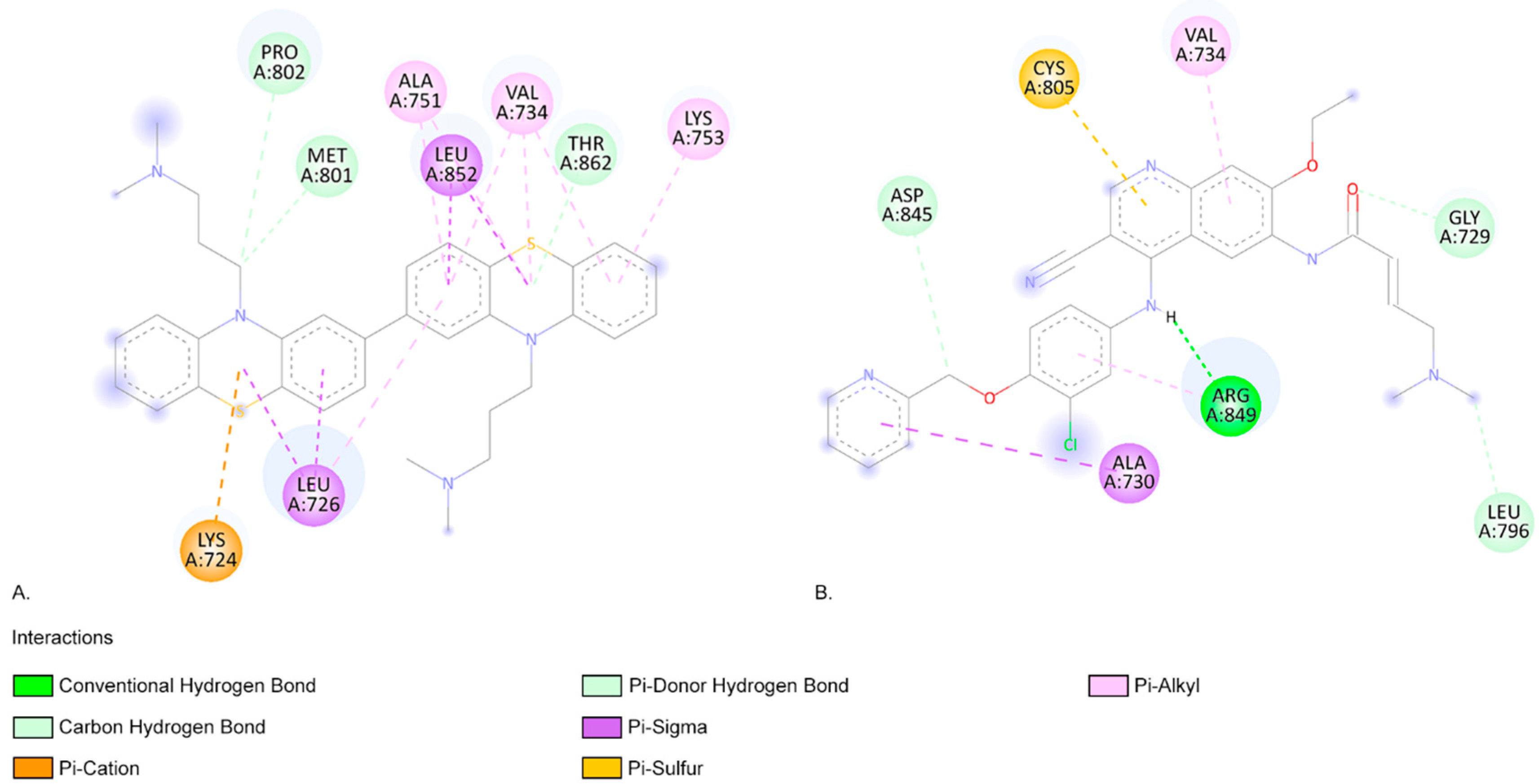
| Compound | Lipinski Rule of Five Validation | Veber Validation |
|---|---|---|
| CPZ | Yes, 1 violation: MLOGP > 4.15 | Yes |
| C178 | Yes | Yes |
| C284/PZ | Yes | Yes |
| C300a | Yes | Yes |
| C300b | Yes | Yes |
| C316a | Yes | Yes |
| C334 | Yes | Yes |
| C566a | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C566b | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C582a | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C582b | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C582c | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C582d | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C582e | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C598a | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C598b | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C600a | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C600b | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| C600c | No; 2 violations: MW > 500, MLOGP > 4.15 | Yes |
| exemestrane | Yes | Yes |
| letrozole | Yes | Yes |
| neratinib | Yes; 1 violation: MW > 500 | No; 1 violation: Rotors > 10 |
| Target | Dili | Neuro | Nephro | Respi | Cardio | sr_p53 | LD50 (mg/kg) | Toxicity Class |
|---|---|---|---|---|---|---|---|---|
| Compound | ||||||||
| CPZ | Active | Active | Inactive | Active | Inactive | Active | 125 | 3 |
| C178 | Inactive | Active | Inactive | Active | Inactive | Inactive | 560 | 4 |
| C284/PZ | Active | Active | Inactive | Active | Inactive | Inactive | 210 | 3 |
| C300a | Inactive | Active | Inactive | Active | Inactive | Inactive | 140 | 3 |
| C300b | Inactive | Active | Inactive | Active | Inactive | Inactive | 408 | 4 |
| C316a | Inactive | Active | Inactive | Active | Inactive | Inactive | 370 | 4 |
| C334 | Inactive | Active | Inactive | Active | Inactive | Inactive | 300 | 3 |
| C566a | Active | Active | Inactive | Active | Inactive | Inactive | 400 | 4 |
| C566b | Active | Active | Inactive | Active | Inactive | Inactive | 400 | 4 |
| C582a | Inactive | Active | Inactive | Active | Inactive | Inactive | 408 | 4 |
| C582b | Inactive | Active | Inactive | Active | Inactive | Inactive | 408 | 4 |
| C582c | Inactive | Active | Inactive | Active | Inactive | Inactive | 408 | 4 |
| C582d | Inactive | Active | Inactive | Active | Inactive | Inactive | 185 | 3 |
| C582e | Inactive | Active | Inactive | Active | Inactive | Inactive | 185 | 3 |
| C598a | Inactive | Active | Inactive | Active | Inactive | Inactive | 185 | 3 |
| C598b | Inactive | Active | Inactive | Active | Inactive | Inactive | 370 | 4 |
| C600a | Active | Active | Inactive | Active | Inactive | Active | 300 | 3 |
| C600b | Active | Active | Inactive | Active | Inactive | Active | 300 | 3 |
| C600c | Active | Active | Inactive | Active | Inactive | Active | 300 | 3 |
| exemestrane | Inactive | Active | Inactive | Active | Inactive | Inactive | 292 | 5 |
| letrozole | Inactive | Active | Inactive | Active | Inactive | Inactive | 1463 | 4 |
| neratinib | Inactive | Active | Active | Active | Inactive | Inactive | 400 | 5 |
| Molecule EFEB | Aromatase kcal/mol | ER kcal/mol | FGFR1 kcal/mol | EGFR kcal/mol | HER2 kcal/mol | PR kcal/mol | Beclin 1 kcal/mol |
|---|---|---|---|---|---|---|---|
| CPZ | −7.64 | −8.49 | −6.74 | −8.42 | −7.56 | −8.69 | −7.41 |
| C178 | −5.63 | −5.27 | −5.13 | −6.29 | −5.86 | −5.42 | −6.39 |
| C284/PZ | −7.18 | −7.96 | −6.31 | −7.61 | −7.45 | −7.90 | −7.22 |
| C300a | −7.20 | −7.94 | −6.97 | −7.29 | −7.21 | −7.80 | −7.46 |
| C300b | −7.10 | −7.71 | −6.50 | −7.98 | −7.45 | −8.19 | −8.17 |
| C316a | −7.08 | −8.29 | −6.74 | −7.41 | −7.53 | −7.94 | −6.95 |
| C334 | −7.69 | −7.79 | −7.18 | −7.58 | −7.55 | −8.28 | −7.39 |
| C566a | −11.77 | −9.00 | −8.32 | −11.07 | −10.07 | −8.62 | −9.74 |
| C566b | −11.17 | −9.26 | −8.63 | −9.65 | −9.95 | −8.90 | −10.18 |
| C582a | −11.50 | −7.92 | −8.96 | −9.91 | −9.94 | −8.21 | −9.86 |
| C582b | −11.27 | −7.64 | −8.85 | −10.15 | −8.88 | −8.24 | −10.07 |
| C582c | −11.18 | −9.05 | −8.20 | −10.78 | −9.41 | −8.74 | −9.38 |
| C582d | −11.29 | −8.30 | −9.19 | −10.07 | −8.68 | −9.55 | −9.33 |
| C582e | −11.47 | −7.17 | −9.81 | −9.53 | −9.92 | −9.04 | −10.22 |
| C598a | −10.99 | −6.62 | −9.40 | −9.35 | −8.17 | −8.92 | −9.25 |
| C598b | −11.13 | −8.25 | −8.38 | −10.27 | −8.15 | −8.36 | −10.09 |
| C600a | −10.90 | −8.16 | −8.04 | −10.39 | −10.04 | −8.41 | −9.40 |
| C600b | −11.81 | −9.03 | −8.63 | −10.26 | −8.97 | −8.45 | −9.03 |
| C600c | −11.48 | −8.48 | −8.63 | −9.56 | −9.28 | −8.99 | −9.91 |
| exemestane | −9.13 | −10.08 | −8.28 | −10.25 | −8.51 | −10.92 | −8.98 |
| letrozole | −8.73 | −9.15 | −8.79 | −8.68 | −8.62 | −8.53 | −7.87 |
| neratinib | −11.83 | −7.80 | −8.65 | −11.04 | −9.41 | −9.16 | −8.31 |
| Target Protein | Grid Point Spacing (Angstroms) | Number of Grid Points (x,y,z) | Coordinates of Central Grid Point of Maps |
|---|---|---|---|
| Aromatase | 0.375 | 102; 126; 112 | 83.659; 50.233; 46.605 |
| HER-2 | 0.375 | 126; 108; 126 | 14.023; 21.179; 31.904 |
| EGFR | 0.375 | 126; 126; 126 | 0.410; 6.290; 18.351 |
| FGFR1 | 0.375 | 82; 82; 126 | 42.690, 1.885, 3.118 |
| Oestrogen receptor Alpha | 0.375 | 98; 78; 126 | 26.173, −4.231, 69.321 |
| Beclin 1 | 0.375 | 112; 98; 98 | 28.891, −3.953, 17.337 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, A.-M.; Bilea, F.; Avram, S.; Staicu, A. Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential. Int. J. Mol. Sci. 2025, 26, 6668. https://doi.org/10.3390/ijms26146668
Udrea A-M, Bilea F, Avram S, Staicu A. Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential. International Journal of Molecular Sciences. 2025; 26(14):6668. https://doi.org/10.3390/ijms26146668
Chicago/Turabian StyleUdrea, Ana-Maria, Florin Bilea, Speranta Avram, and Angela Staicu. 2025. "Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential" International Journal of Molecular Sciences 26, no. 14: 6668. https://doi.org/10.3390/ijms26146668
APA StyleUdrea, A.-M., Bilea, F., Avram, S., & Staicu, A. (2025). Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential. International Journal of Molecular Sciences, 26(14), 6668. https://doi.org/10.3390/ijms26146668






