Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
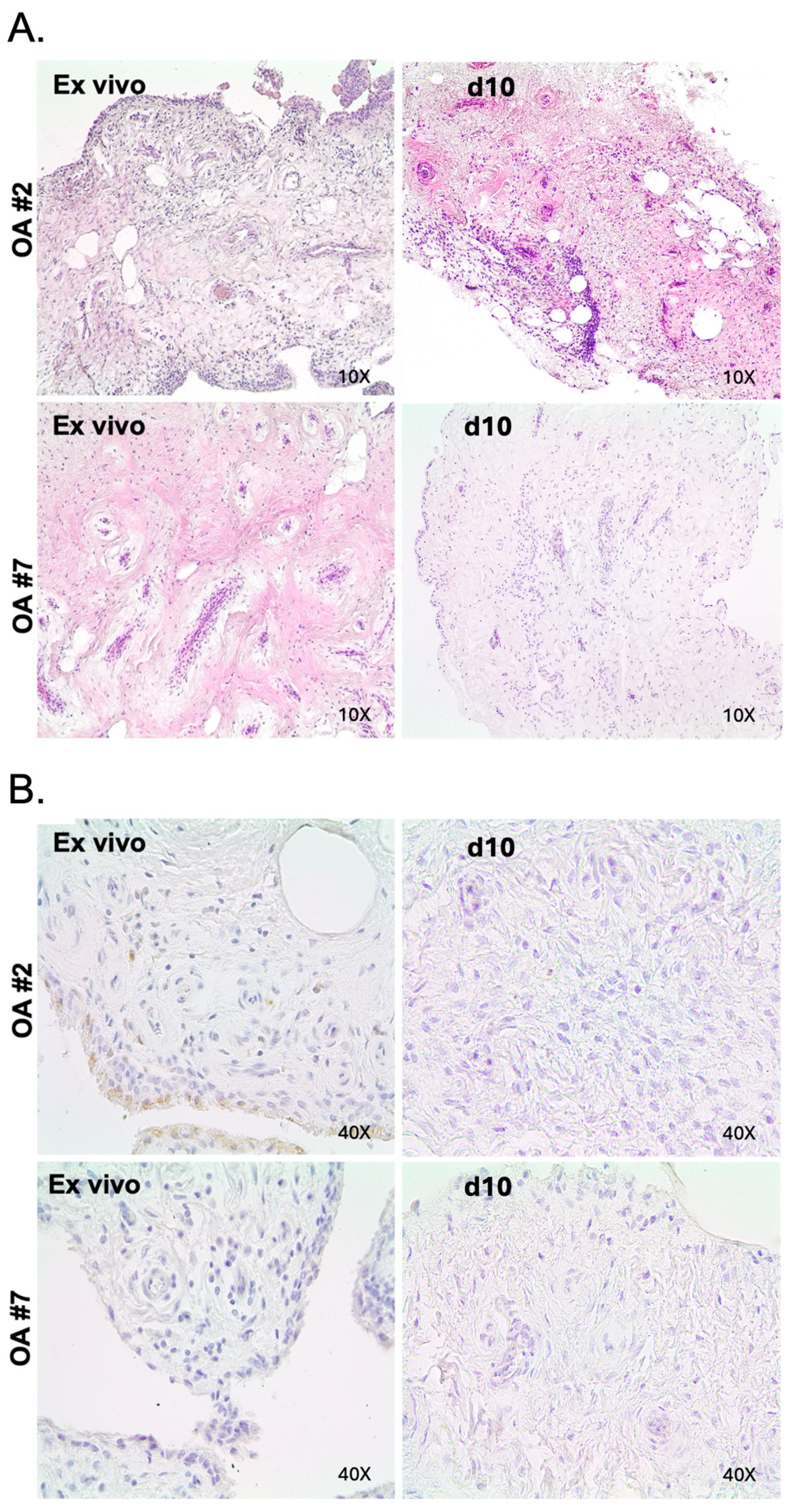
2.2. Maintenance of Histologic Features and Viability in OA in Tissue Explant Culture
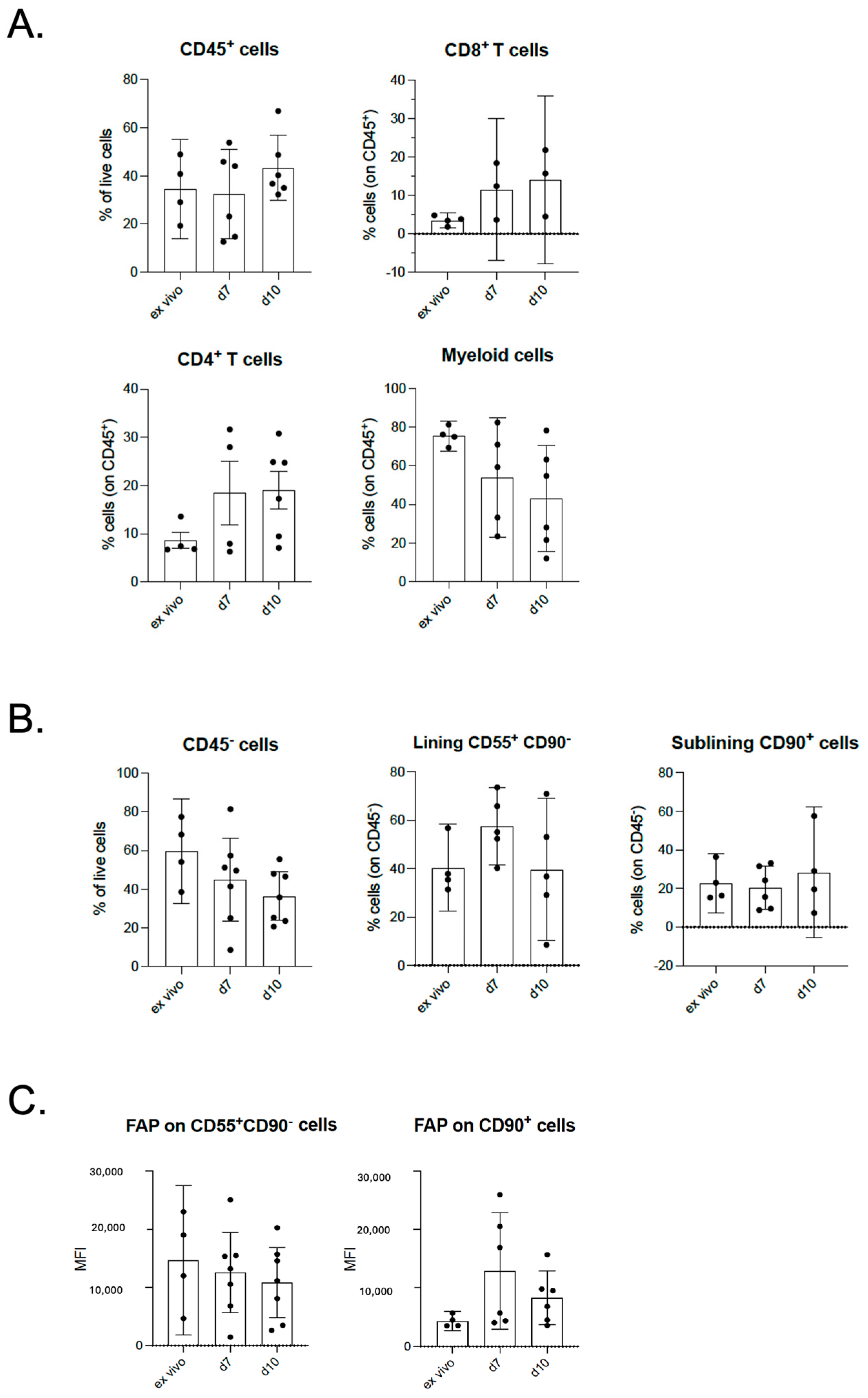
2.3. Maintenance of Cellular Composition in OA in Tissue Explant Culture
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Synovial Explant Culture
4.3. Immunohistochemical Analysis
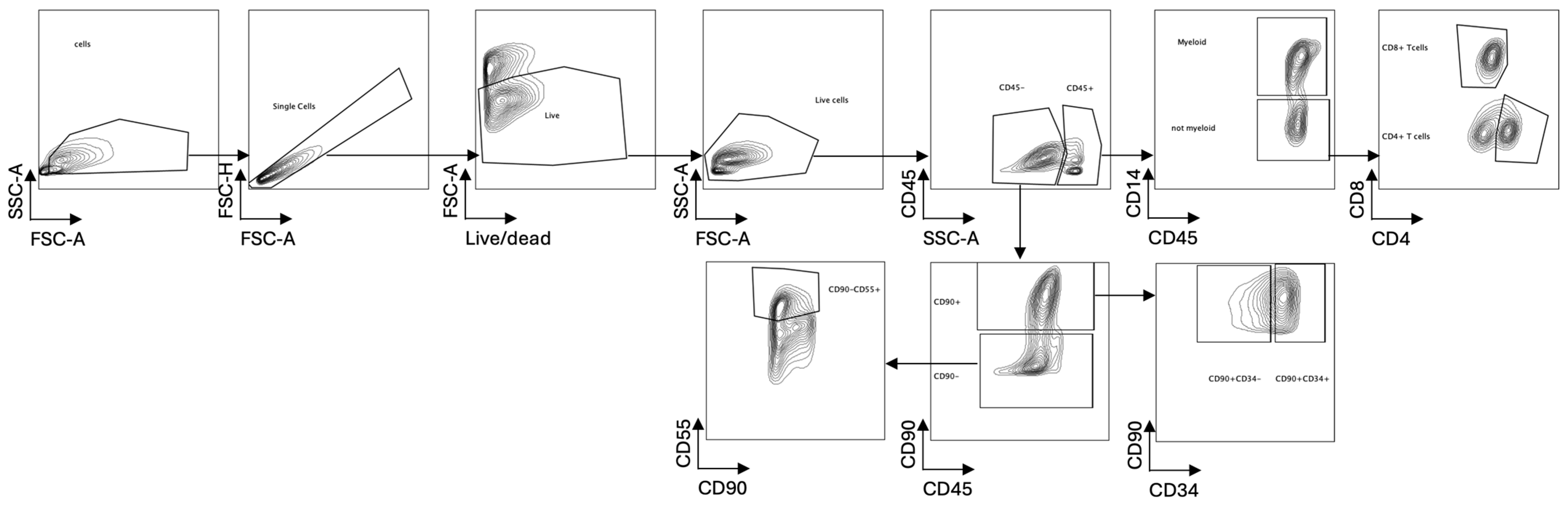
4.4. Flow Cytometric Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| FLS | Fibroblast-like synoviocyte |
| EQ-5D | Questionnaires regarding health-related quality of life |
| HAQ | Health Assessment Questionnaire |
| GH | Global health |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemical |
| KL | Kellgren–Lawrence |
References
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Haque, N.; Huang, J.; Zhai, G. Osteoarthritis year in review 2023: Metabolite and protein biomarkers. Osteoarthr. Cartil. 2023, 31, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Costantini, S.; Grasso, M.; Arciola, C.R.; Dolzani, P. Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression. Int. J. Mol. Sci. 2024, 25, 12082. [Google Scholar] [CrossRef]
- Moulin, D.; Sellam, J.; Berenbaum, F.; Guicheux, J.; Boutet, M.A. The role of the immune system in osteoarthritis: Mechanisms, challenges and future directions. Nat. Rev. Rheumatol. 2025, 21, 221–236. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Silvestri, Y.; Gambari, L.; Cattini, L.; Filardo, G.; Fleury-Cappellesso, S.; Lisignoli, G. From osteoarthritic synovium to synovial-derived cells characterization: Synovial macrophages are key effector cells. Arthritis Res. Ther. 2016, 18, 83. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Trisolino, G.; Goldring, M.B.; Goldring, S.R.; Cigolotti, A.; Pozzuoli, A.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: A coculture study. J. Cell Physiol. 2019, 234, 11176–11187. [Google Scholar] [CrossRef]
- Haltmayer, E.; Ribitsch, I.; Gabner, S.; Rosser, J.; Gueltekin, S.; Peham, J.; Giese, U.; Dolezal, M.; Egerbacher, M.; Jenner, F. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS ONE 2019, 14, e0214709. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Bastiaansen-Jenniskens, Y.M.; Koevoet, W.; Saris, D.B.; Dhert, W.J.; Creemers, L.B.; van Osch, G.J. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage: Establishment and characterization of a long-term cartilage-synovium coculture. Arthritis Rheum. 2011, 63, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.W.Y.; Gomez-Aristizabal, A.; Mahomed, N.; Gandhi, R.; Viswanathan, S. A tool for evaluating novel osteoarthritis therapies using multivariate analyses of human cartilage-synovium explant co-culture. Osteoarthr. Cartil. 2022, 30, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.A.; Sant, S.; Cho, S.K.; Goodman, S.B.; Bunnell, B.A.; Tuan, R.S.; Gold, M.S.; Lin, H. Synovial joint-on-a-chip for modeling arthritis: Progress, pitfalls, and potential. Trends Biotechnol. 2023, 41, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Ultaigh, S.N.; Saber, T.P.; McCormick, J.; Connolly, M.; Dellacasagrande, J.; Keogh, B.; McCormack, W.; Reilly, M.; O’Neill, L.A.; McGuirk, P.; et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res. Ther. 2011, 13, R33. [Google Scholar] [CrossRef]
- Andersen, M.; Boesen, M.; Ellegaard, K.; Christensen, R.; Soderstrom, K.; Soe, N.; Spee, P.; Morch, U.G.; Torp-Pedersen, S.; Bartels, E.M.; et al. Synovial explant inflammatory mediator production corresponds to rheumatoid arthritis imaging hallmarks: A cross-sectional study. Arthritis Res. Ther. 2014, 16, R107. [Google Scholar] [CrossRef]
- Berenbaum, F. Deep phenotyping of osteoarthritis: A step forward. Ann. Rheum. Dis. 2019, 78, 3–5. [Google Scholar] [CrossRef]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Ortolan, A.; Lorenzin, M.; Oliviero, F.; Doria, A.; Scanzello, C.R.; Ramonda, R. Erosive hand osteoarthritis: Latest findings and outlook. Nat. Rev. Rheumatol. 2022, 18, 171–183. [Google Scholar] [CrossRef]
- Baker, K.; Grainger, A.; Niu, J.; Clancy, M.; Guermazi, A.; Crema, M.; Hughes, L.; Buckwalter, J.; Wooley, A.; Nevitt, M.; et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 2010, 69, 1779–1783. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Ohashi, Y.; Fukushima, K.; Okuda, Y.; Suto, A.; Matsui, T.; Kodera, Y.; Sato, M.; Tsukada, A.; Inoue, G.; et al. Fibrocyte Phenotype of ENTPD1+CD55+ Cells and Its Association with Pain in Osteoarthritic Synovium. Int. J. Mol. Sci. 2024, 25, 4085. [Google Scholar] [CrossRef]
- Farah, H.; Wijesinghe, S.N.; Nicholson, T.; Alnajjar, F.; Certo, M.; Alghamdi, A.; Davis, E.T.; Young, S.P.; Mauro, C.; Jones, S.W. Differential Metabotypes in Synovial Fibroblasts and Synovial Fluid in Hip Osteoarthritis Patients Support Inflammatory Responses. Int. J. Mol. Sci. 2022, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Ramonda, R.; Bobacz, K.; Kwok, W.Y.; Elewaut, D.; Huizinga, T.W.J.; Kroon, F.P.B.; Punzi, L.; Smolen, J.S.; Vander Cruyssen, B.; et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): A multicentre, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018, 77, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- de Lange-Brokaar, B.J.; Ioan-Facsinay, A.; van Osch, G.J.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.; Huizinga, T.W.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
| ID | Age | Gender | Joint Replacement |
EQ-5D Pain
(1–5) |
EQ-5D Movement
(1–5) | KL Score (0–4) | HAQ-DI |
GH
0–100 |
|---|---|---|---|---|---|---|---|---|
| 158 | 74 | M | Left knee | 2 | 4 | 4 | 1.125 | 40 |
| 159 | 86 | F | Right knee | 3 | 3 | 3 | 0.375 | 70 |
| 160 | 67 | F | Left hip | 3 | 2 | 3 | 0.125 | 60 |
| 161 | 67 | M | Right knee | 3 | 2 | 4 | 1.125 | 50 |
| 162 | 83 | M | Right hip | 3 | 4 | 3 | 0.625 | 75 |
| 163 | 59 | F | Left knee | 3 | 2 | 4 | 0.75 | 80 |
| 164 | 69 | F | Right knee | 5 | 2 | 4 | 1 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Oria, C.; Cincinelli, G.; Bason, R.; Pisati, F.; Simoncello, F.; Scotti, I.; Giudice, L.; Suardi, I.; Ferrua, P.; Fossati, C.; et al. Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research. Int. J. Mol. Sci. 2025, 26, 6665. https://doi.org/10.3390/ijms26146665
D’Oria C, Cincinelli G, Bason R, Pisati F, Simoncello F, Scotti I, Giudice L, Suardi I, Ferrua P, Fossati C, et al. Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research. International Journal of Molecular Sciences. 2025; 26(14):6665. https://doi.org/10.3390/ijms26146665
Chicago/Turabian StyleD’Oria, Claudia, Gilberto Cincinelli, Ramona Bason, Federica Pisati, Francesca Simoncello, Isabella Scotti, Laura Giudice, Ilaria Suardi, Paolo Ferrua, Chiara Fossati, and et al. 2025. "Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research" International Journal of Molecular Sciences 26, no. 14: 6665. https://doi.org/10.3390/ijms26146665
APA StyleD’Oria, C., Cincinelli, G., Bason, R., Pisati, F., Simoncello, F., Scotti, I., Giudice, L., Suardi, I., Ferrua, P., Fossati, C., Randelli, P. S., Caporali, R., Pagani, M., & Ingegnoli, F. (2025). Patient-Derived Explants of Osteoarthritic Synovium as Ex Vivo Model for Preclinical Research. International Journal of Molecular Sciences, 26(14), 6665. https://doi.org/10.3390/ijms26146665






