Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation
Abstract
1. Introduction
2. Results
2.1. Heart Assessment at Necropsy
2.2. Effect of Imatinib and BGP-15-Induced Changes on PARP1 Concentration in the Heart
2.3. Effect of Imatinib and BGP-15-Induced Changes on the Expressions of NF-κB/p65 and Nrf2 in the Heart
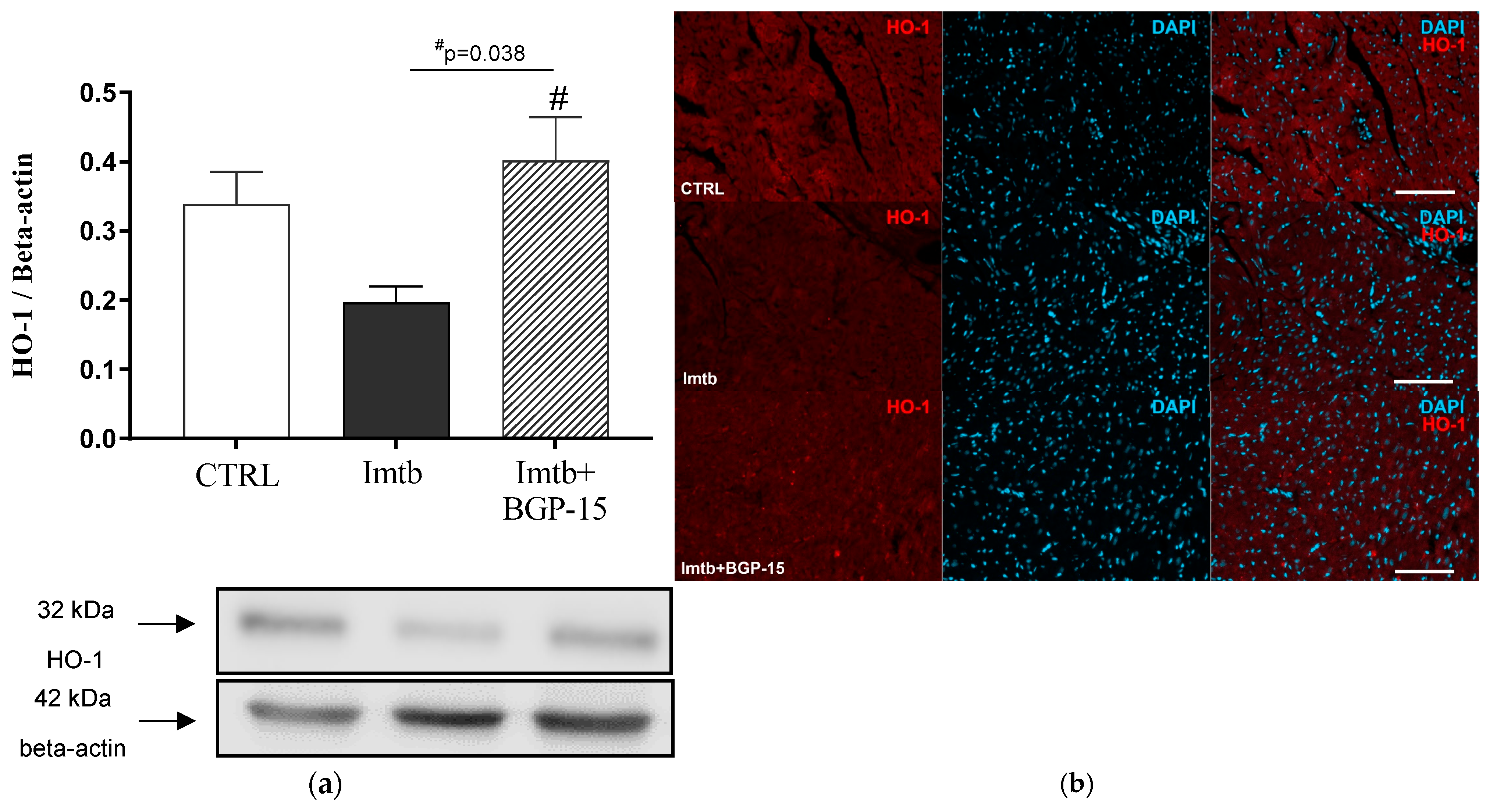
2.4. Effect of Imatinib and BGP-15-Induced Changes on HO-1 Expression in the Heart
2.5. Effect of Imatinib and BGP-15-Induced Changes on Inflammatory Cytokines in the Heart
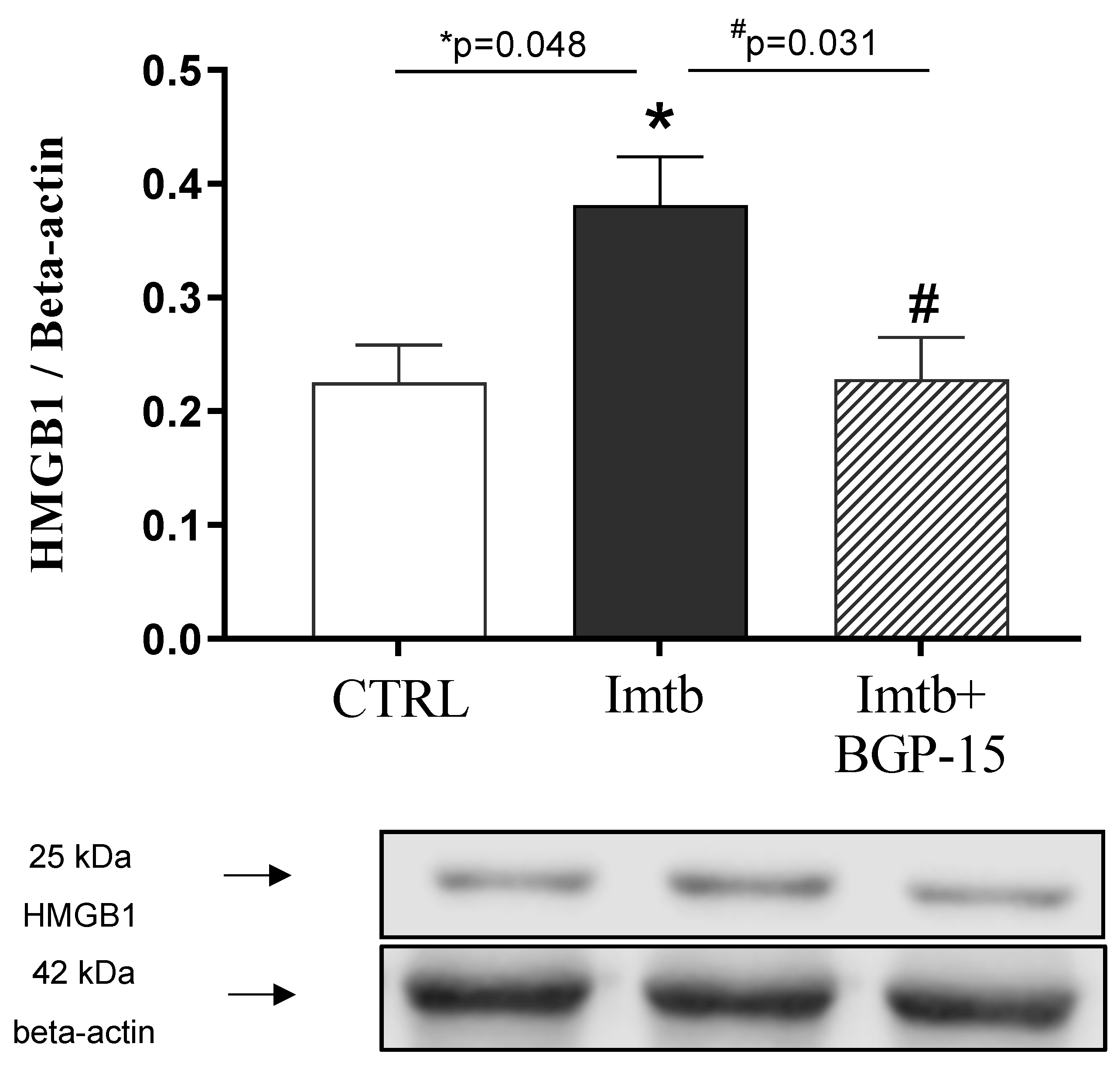
2.6. Effect of Imatinib and BGP-15-Induced Changes on HMGB1 Expression in the Heart
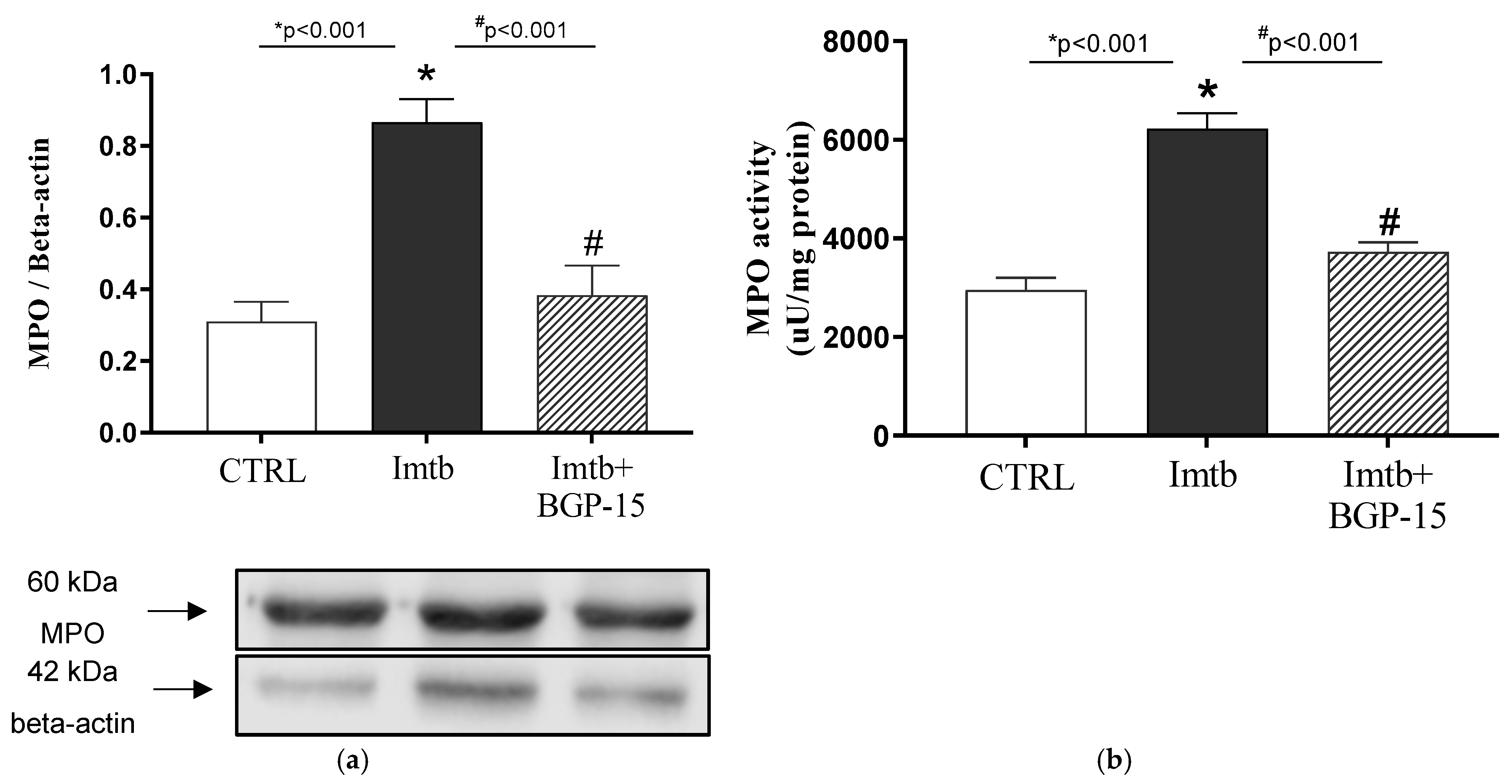
2.7. Effect of Imatinib and BGP-15-Induced Changes on the Expression and Activity of MPO in the Heart
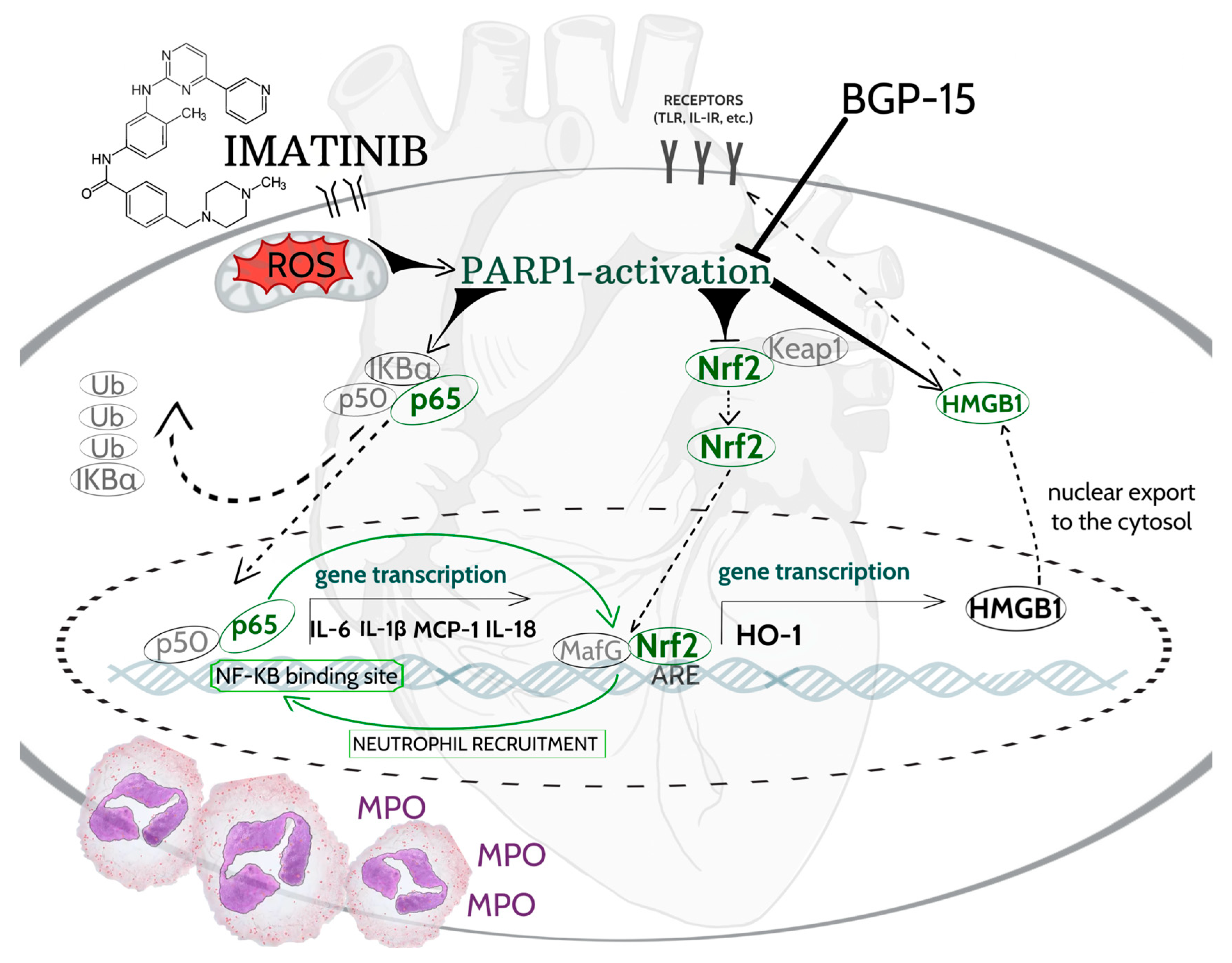
3. Discussion
Limitations
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Measurement of Cardiac PARP1 Concentration
4.3. Western Blotting of NF-κB/p65, Nrf2, HO-1, HMGB1, and MPO
4.4. Determination of Cardiac Inflammatory Cytokines
4.5. Measurement of Cardiac MPO Activity
4.6. Fluorescent Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PARP-1 | poly(ADP-ribose) polymerase-1 |
| Imtb | imatinib |
| NF-κB/p65 | nuclear factor-kappa B/p65 |
| Nrf2 | nuclear transcription factor erythroid-2 related factor |
| HO-1 | heme oxygenase-1 |
| HMGB 1 | high mobility group box 1 |
| MPO | myeloperoxidase |
| IL | interleukin |
References
- World Heath Organization. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 7 July 2025).
- Chianca, M.; Fabiani, I.; Del Franco, A.; Grigoratos, C.; Aimo, A.; Panichella, G.; Giannoni, A.; Castiglione, V.; Gentile, F.; Passino, C.; et al. Management and treatment of cardiotoxicity due to anticancer drugs: 10 questions and answers. Eur. J. Prev. Cardiol. 2022, 29, 2163–2172. [Google Scholar] [CrossRef]
- Nagy, A.; Borzsei, D.; Hoffmann, A.; Torok, S.; Veszelka, M.; Almasi, N.; Varga, C.; Szabo, R. A Comprehensive Overview on Chemotherapy-Induced Cardiotoxicity: Insights into the Underlying Inflammatory and Oxidative Mechanisms. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Li, S.; He, J.; Zhang, X.; Cai, Y.; Liu, J.; Nie, X.; Shi, L. Cardiovascular adverse events in chronic myeloid leukemia patients treated with nilotinib or imatinib: A systematic review, meta-analysis and integrative bioinformatics analysis. Front. Cardiovasc. Med. 2022, 9, 966182. [Google Scholar] [CrossRef]
- Machado, C.B.; da Silva, E.L.; Ferreira, W.A.S.; Pessoa, F.; de Quadros, A.U.; Fantacini, D.M.C.; Furtado, I.P.; Rossetti, R.; Silveira, R.M.; de Lima, S.C.G.; et al. PARP1 Characterization as a Potential Biomarker for BCR::ABL1 p190+ Acute Lymphoblastic Leukemia. Cancers 2023, 15, 5510. [Google Scholar] [CrossRef]
- Moehring, A.; Wohlbold, L.; Aulitzky, W.E.; van der Kuip, H. Role of poly(ADP-ribose) polymerase activity in imatinib mesylate-induced cell death. Cell Death Differ. 2005, 12, 627–636. [Google Scholar] [CrossRef]
- Sarszegi, Z.; Bognar, E.; Gaszner, B.; Konyi, A.; Gallyas, F., Jr.; Sumegi, B.; Berente, Z. BGP-15, a PARP-inhibitor, prevents imatinib-induced cardiotoxicity by activating Akt and suppressing JNK and p38 MAP kinases. Mol. Cell. Biochem. 2012, 365, 129–137. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: The therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 2007, 25, 235–260. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Chen, L.; Yuan, W.; Dong, L.; Zhang, Y.; Wu, H.; Wang, C. PARP-1 mediates LPS-induced HMGB1 release by macrophages through regulation of HMGB1 acetylation. J. Immunol. 2014, 193, 6114–6123. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Peto, A.; Kosa, D.; Feher, P.; Ujhelyi, Z.; Sinka, D.; Vecsernyes, M.; Szilvassy, Z.; Juhasz, B.; Csanadi, Z.; Vigh, L.; et al. Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules 2020, 25, 429. [Google Scholar] [CrossRef]
- Horvath, O.; Ordog, K.; Bruszt, K.; Deres, L.; Gallyas, F.; Sumegi, B.; Toth, K.; Halmosi, R. BGP-15 Protects against Heart Failure by Enhanced Mitochondrial Biogenesis and Decreased Fibrotic Remodelling in Spontaneously Hypertensive Rats. Oxidative Med. Cell. Longev. 2021, 2021, 1250858. [Google Scholar] [CrossRef]
- Sapra, G.; Tham, Y.K.; Cemerlang, N.; Matsumoto, A.; Kiriazis, H.; Bernardo, B.C.; Henstridge, D.C.; Ooi, J.Y.; Pretorius, L.; Boey, E.J.; et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 2014, 5, 5705. [Google Scholar] [CrossRef]
- Crunkhorn, S. Cardiovascular disease: Insulin sensitizer protects the heart. Nat. Rev. Drug Discov. 2015, 14, 93. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Flynn, J.P.; Gerriets, V. Imatinib. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Deininger, M.; Buchdunger, E.; Druker, B.J. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005, 105, 2640–2653. [Google Scholar] [CrossRef]
- Herman, E.H.; Knapton, A.; Rosen, E.; Thompson, K.; Rosenzweig, B.; Estis, J.; Agee, S.; Lu, Q.A.; Todd, J.A.; Lipshultz, S.; et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicol. Pathol. 2011, 39, 1091–1106. [Google Scholar] [CrossRef]
- Kerkela, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Correction to: Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 527. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Sykes, D.; Allen, A.R. Implications of Breast Cancer Chemotherapy-Induced Inflammation on the Gut, Liver, and Central Nervous System. Biomedicines 2021, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Racz, B.; Hanto, K.; Tapodi, A.; Solti, I.; Kalman, N.; Jakus, P.; Kovacs, K.; Debreceni, B.; Gallyas, F., Jr.; Sumegi, B. Regulation of MKP-1 expression and MAPK activation by PARP-1 in oxidative stress: A new mechanism for the cytoplasmic effect of PARP-1 activation. Free Radic. Biol. Med. 2010, 49, 1978–1988. [Google Scholar] [CrossRef]
- Ba, X.; Garg, N.J. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in inflammatory diseases. Am. J. Pathol. 2011, 178, 946–955. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.; Park, S.H.; Lee, H.G.; Park, J.H. A Double-Edged Sword: The Two Faces of PARylation. Int. J. Mol. Sci. 2022, 23, 9826. [Google Scholar] [CrossRef]
- Castri, P.; Lee, Y.J.; Ponzio, T.; Maric, D.; Spatz, M.; Bembry, J.; Hallenbeck, J. Poly(ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-kappaB-dependent signaling. Biochim. Biophys. Acta 2014, 1843, 640–651. [Google Scholar] [CrossRef]
- Luo, J.M.; Lin, H.B.; Weng, Y.Q.; Lin, Y.H.; Lai, L.Y.; Li, J.; Li, F.X.; Xu, S.Y.; Zhang, H.F.; Zhao, W. Inhibition of PARP1 improves cardiac function after myocardial infarction via up-regulated NLRC5. Chem. Biol. Interact. 2024, 395, 111010. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, J.; Lv, X.; Zeng, X.; Ba, X. Novel insights into PARPs in gene expression: Regulation of RNA metabolism. Cell. Mol. Life Sci. 2019, 76, 3283–3299. [Google Scholar] [CrossRef]
- Zerfaoui, M.; Errami, Y.; Naura, A.S.; Suzuki, Y.; Kim, H.; Ju, J.; Liu, T.; Hans, C.P.; Kim, J.G.; Abd Elmageed, Z.Y.; et al. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J. Immunol. 2010, 185, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Seo, Y.E.; Kwon, J.H.; Kim, J.H.; Kim, M.G. Cardioprotective Effects of PARP Inhibitors: A Re-Analysis of a Meta-Analysis and a Real-Word Data Analysis Using the FAERS Database. J. Clin. Med. 2024, 13, 1218. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Arisa, O.; Peer, C.J.; Fojo, A.; Figg, W.D. PARP inhibitors: A review of the pharmacology, pharmacokinetics, and pharmacogenetics. Semin. Oncol. 2024, 51, 19–24. [Google Scholar] [CrossRef]
- Eid, R.A.; Alharbi, S.A.; El-Kott, A.F.; Eleawa, S.M.; Zaki, M.S.A.; El-Sayed, F.; Eldeen, M.A.; Aldera, H.; Al-Shudiefat, A.A.S. Exendin-4 Ameliorates Cardiac Remodeling in Experimentally Induced Myocardial Infarction in Rats by Inhibiting PARP1/NF-kappaB Axis in A SIRT1-Dependent Mechanism. Cardiovasc. Toxicol. 2020, 20, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Literati-Nagy, B.; Peterfai, E.; Kulcsar, E.; Literati-Nagy, Z.; Buday, B.; Tory, K.; Mandl, J.; Sumegi, B.; Fleming, A.; Roth, J.; et al. Beneficial effect of the insulin sensitizer (HSP inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res. Bull. 2010, 83, 340–344. [Google Scholar] [CrossRef]
- Literati-Nagy, B.; Kulcsar, E.; Literati-Nagy, Z.; Buday, B.; Peterfai, E.; Horvath, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: A proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef]
- Kozma, M.; Bombicz, M.; Varga, B.; Priksz, D.; Gesztelyi, R.; Tarjanyi, V.; Kiss, R.; Szekeres, R.; Takacs, B.; Menes, A.; et al. Cardioprotective Role of BGP-15 in Ageing Zucker Diabetic Fatty Rat (ZDF) Model: Extended Mitochondrial Longevity. Pharmaceutics 2022, 14, 226. [Google Scholar] [CrossRef]
- Sumegi, K.; Fekete, K.; Antus, C.; Debreceni, B.; Hocsak, E.; Gallyas, F., Jr.; Sumegi, B.; Szabo, A. BGP-15 Protects against Oxidative Stress- or Lipopolysaccharide-Induced Mitochondrial Destabilization and Reduces Mitochondrial Production of Reactive Oxygen Species. PLoS ONE 2017, 12, e0169372. [Google Scholar] [CrossRef]
- Szabados, E.; Literati-Nagy, P.; Farkas, B.; Sumegi, B. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2000, 59, 937–945. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Li, T.; Liu, C.; Lu, C. Involvement of the crosstalk between Nrf2 and NF-kappaB pathways regulated by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2022, 355, 44. [Google Scholar] [CrossRef] [PubMed]
- Casper, E. The crosstalk between Nrf2 and NF-kappaB pathways in coronary artery disease: Can it be regulated by SIRT6? Life Sci. 2023, 330, 122007. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-kappaB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Bellner, L.; Lebovics, N.B.; Rubinstein, R.; Buchen, Y.D.; Sinatra, E.; Sinatra, G.; Abraham, N.G.; McClung, J.A.; Thompson, E.A. Heme Oxygenase-1 Upregulation: A Novel Approach in the Treatment of Cardiovascular Disease. Antioxid. Redox Signal. 2020, 32, 1045–1060. [Google Scholar] [CrossRef]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. 2021, 139, 111555. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, L.; Ma, J.; Zhang, H.; Xiao, M.; Li, N.; Gong, H.; Yan, M. HMGB1 as an extracellular pro-inflammatory cytokine: Implications for drug-induced organic damage. Cell Biol. Toxicol. 2024, 40, 55. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, Y.; Yan, H.; Gao, Z.; Xu, B.; Yang, B.; He, Q.; Shi, Q.; Luo, P. High-mobility group box 1 protein-mediated necroptosis contributes to dasatinib-induced cardiotoxicity. Toxicol. Lett. 2018, 296, 39–47. [Google Scholar] [CrossRef]
- Tang, P.; Zhou, J.; Liu, H.; Mei, S.; Wang, K.; Ming, H. Depletion of lncRNA MEG3 Ameliorates Imatinib-Induced Injury of Cardiomyocytes via Regulating miR-129-5p/HMGB1 Axis. Anal. Cell. Pathol. 2023, 2023, 1108280. [Google Scholar] [CrossRef]
- Hutchins, E.; Yang, E.H.; Stein-Merlob, A.F. Inflammation in Chemotherapy-Induced Cardiotoxicity. Curr. Cardiol. Rep. 2024, 26, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Tellez, S.A.; Sekheri, M.; Filep, J.G. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants 2022, 11, 2302. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Ja, K.; Chua, J.; Cong, S.; Shim, W.; Hausenloy, D.J. Myeloperoxidase As a Multifaceted Target for Cardiovascular Protection. Antioxid. Redox Signal. 2020, 32, 1135–1149. [Google Scholar] [CrossRef]
- Nettersheim, F.S.; Schluter, J.D.; Kreuzberg, W.; Mehrkens, D.; Grimm, S.; Nemade, H.; Braumann, S.; Hof, A.; Guthoff, H.; Peters, V.; et al. Myeloperoxidase is a critical mediator of anthracycline-induced cardiomyopathy. Basic Res. Cardiol. 2023, 118, 36. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Baccarani, M.; Guilhot, F.; Druker, B.J.; Branford, S.; Kim, D.W.; Pane, F.; Pasquini, R.; Goldberg, S.L.; Kalaycio, M.; et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: Tyrosine kinase inhibitor optimization and selectivity study. J. Clin. Oncol. 2010, 28, 424–430. [Google Scholar] [PubMed]
- Yoo, C.; Ryu, M.H.; Ryoo, B.Y.; Beck, M.Y.; Kang, Y.K. Efficacy, safety, and pharmacokinetics of imatinib dose escalation to 800 mg/day in patients with advanced gastrointestinal stromal tumors. Investig. New Drugs 2013, 31, 1367–1374. [Google Scholar] [CrossRef]
- Szabo, R.; Borzsei, D.; Hoffmann, A.; Lesi, Z.N.; Gesztelyi, R.; Juhasz, B.; Szebeni, G.J.; Osman, J.; Sebestyen, J.; Nagy, A.; et al. Lifestyle-Induced Redox-Sensitive Alterations: Cross-Talk among the RAAS, Antioxidant/Inflammatory Status, and Hypertension. Oxidative Med. Cell. Longev. 2021, 2021, 3080863. [Google Scholar] [CrossRef]
- Ruppert, Z.; Neuperger, P.; Rakoczi, B.; Gemes, N.; Dukay, B.; Hajdu, P.; Peter, M.; Balogh, G.; Tiszlavicz, L.; Vigh, L.; et al. Characterization of obesity-related diseases and inflammation using single cell immunophenotyping in two different diet-induced obesity models. Int. J. Obes. 2024, 48, 1568–1576. [Google Scholar] [CrossRef]
- Barta, B.P.; Onhausz, B.; Egyed-Kolumban, A.; Al Doghmi, A.; Balazs, J.; Szalai, Z.; Ferencz, A.; Hermesz, E.; Bagyanszki, M.; Bodi, N. Intestinal Region-Dependent Impact of NFkappaB-Nrf Crosstalk in Myenteric Neurons and Adjacent Muscle Cells in Type 1 Diabetic Rats. Biomedicines 2024, 12, 2347. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, R.; Börzsei, D.; Nagy, A.; Kiss, V.; Virág, Z.; Kis, G.; Almási, N.; Török, S.; Veszelka, M.; Bagyánszki, M.; et al. Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation. Int. J. Mol. Sci. 2025, 26, 6661. https://doi.org/10.3390/ijms26146661
Szabó R, Börzsei D, Nagy A, Kiss V, Virág Z, Kis G, Almási N, Török S, Veszelka M, Bagyánszki M, et al. Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation. International Journal of Molecular Sciences. 2025; 26(14):6661. https://doi.org/10.3390/ijms26146661
Chicago/Turabian StyleSzabó, Renáta, Denise Börzsei, András Nagy, Viktória Kiss, Zoltán Virág, Gyöngyi Kis, Nikoletta Almási, Szilvia Török, Médea Veszelka, Mária Bagyánszki, and et al. 2025. "Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation" International Journal of Molecular Sciences 26, no. 14: 6661. https://doi.org/10.3390/ijms26146661
APA StyleSzabó, R., Börzsei, D., Nagy, A., Kiss, V., Virág, Z., Kis, G., Almási, N., Török, S., Veszelka, M., Bagyánszki, M., Bódi, N., Barta, B. P., Neuperger, P., Szebeni, G. J., & Varga, C. (2025). Biochemical Insights into the Effects of a Small Molecule Drug Candidate on Imatinib-Induced Cardiac Inflammation. International Journal of Molecular Sciences, 26(14), 6661. https://doi.org/10.3390/ijms26146661









