Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine
Abstract
1. Introduction
2. Results
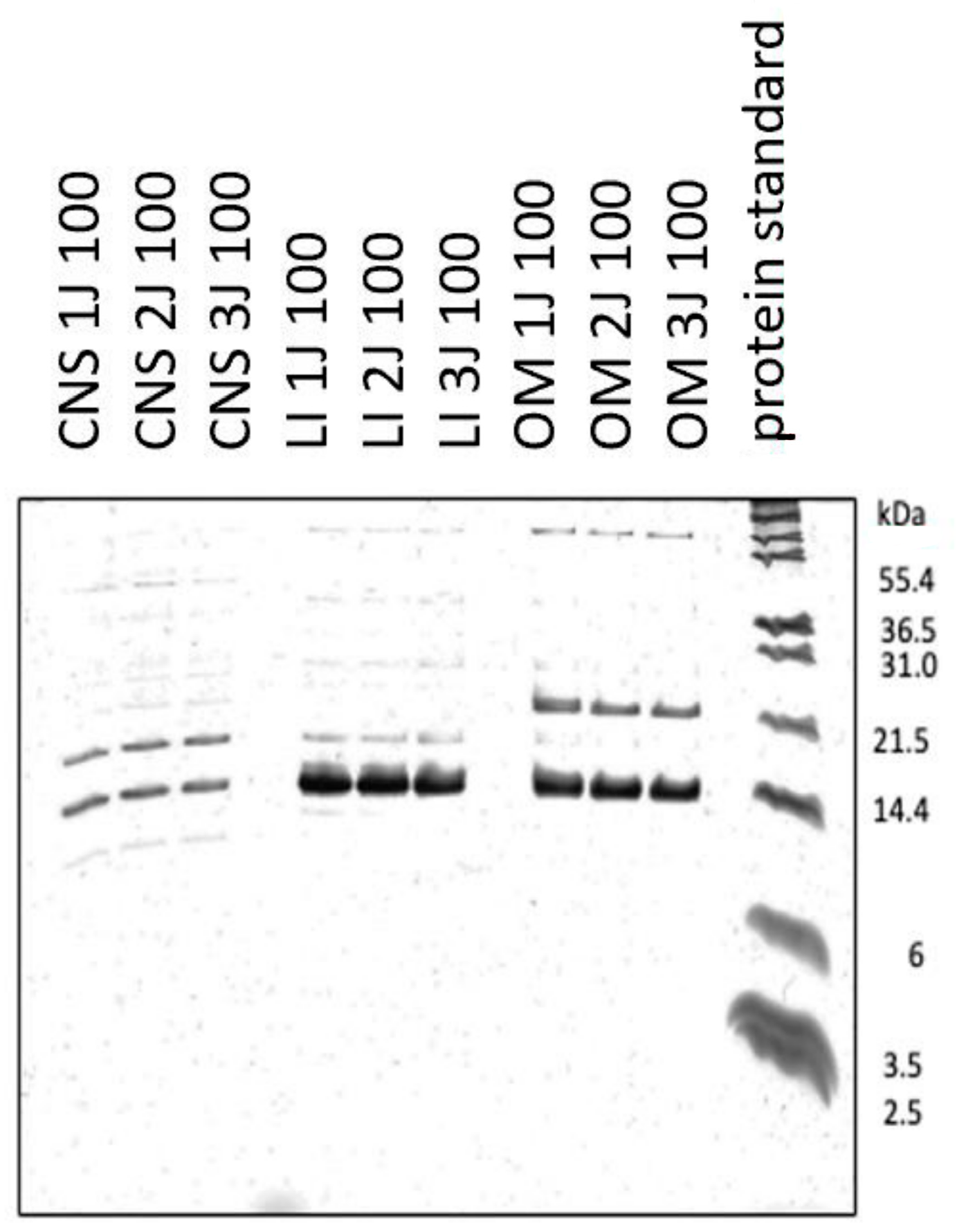
2.1. Characterization of 100 kDa Ultrafiltrates from Post-Natal Rabbit Brain, Liver and OM
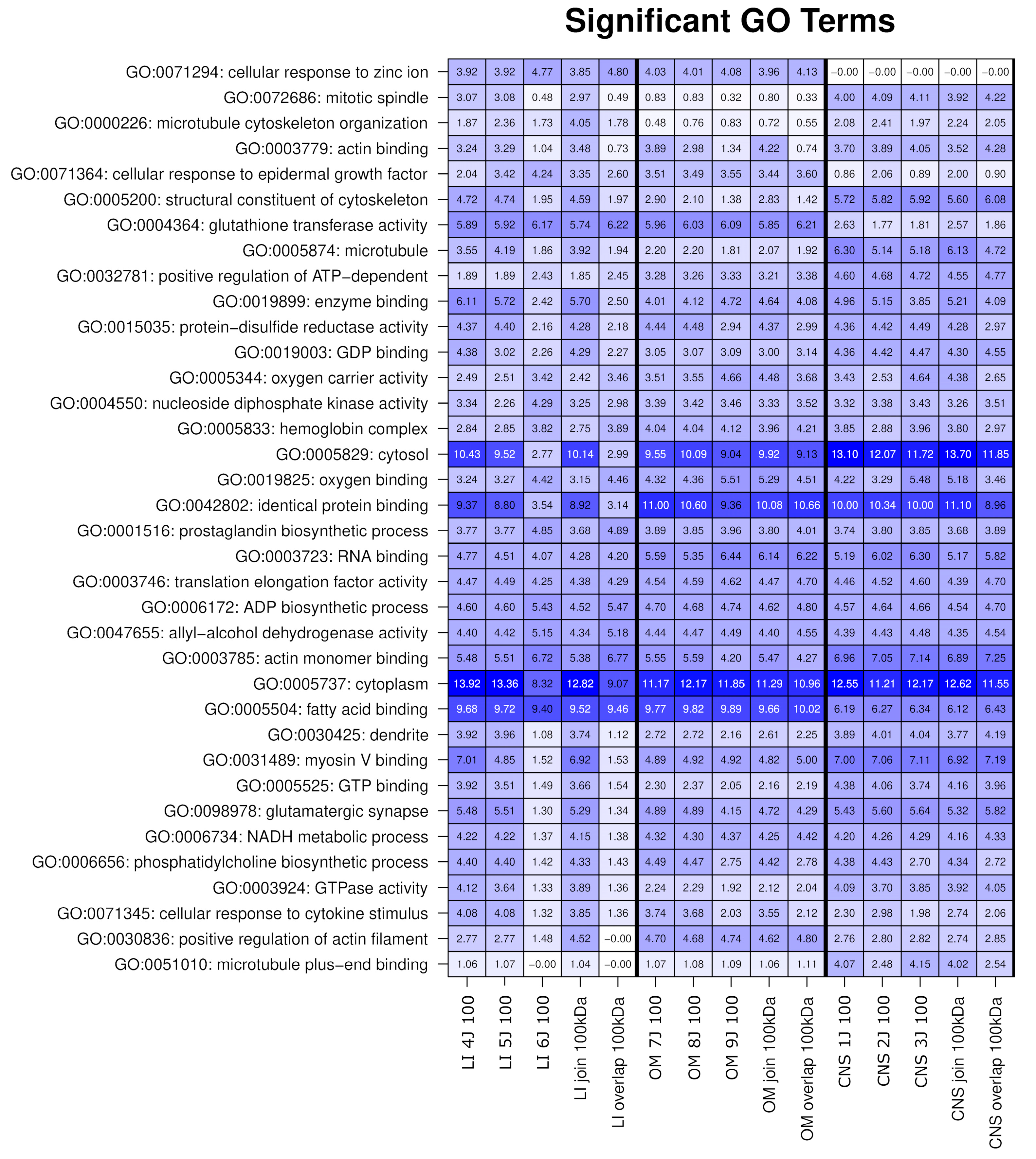
2.2. The OSPs Showed GO Enrichment for Glutathione Transferase Activity, Fatty Acid Binding, Identical Protein Binding, RNA Binding, and KEGG Pathways
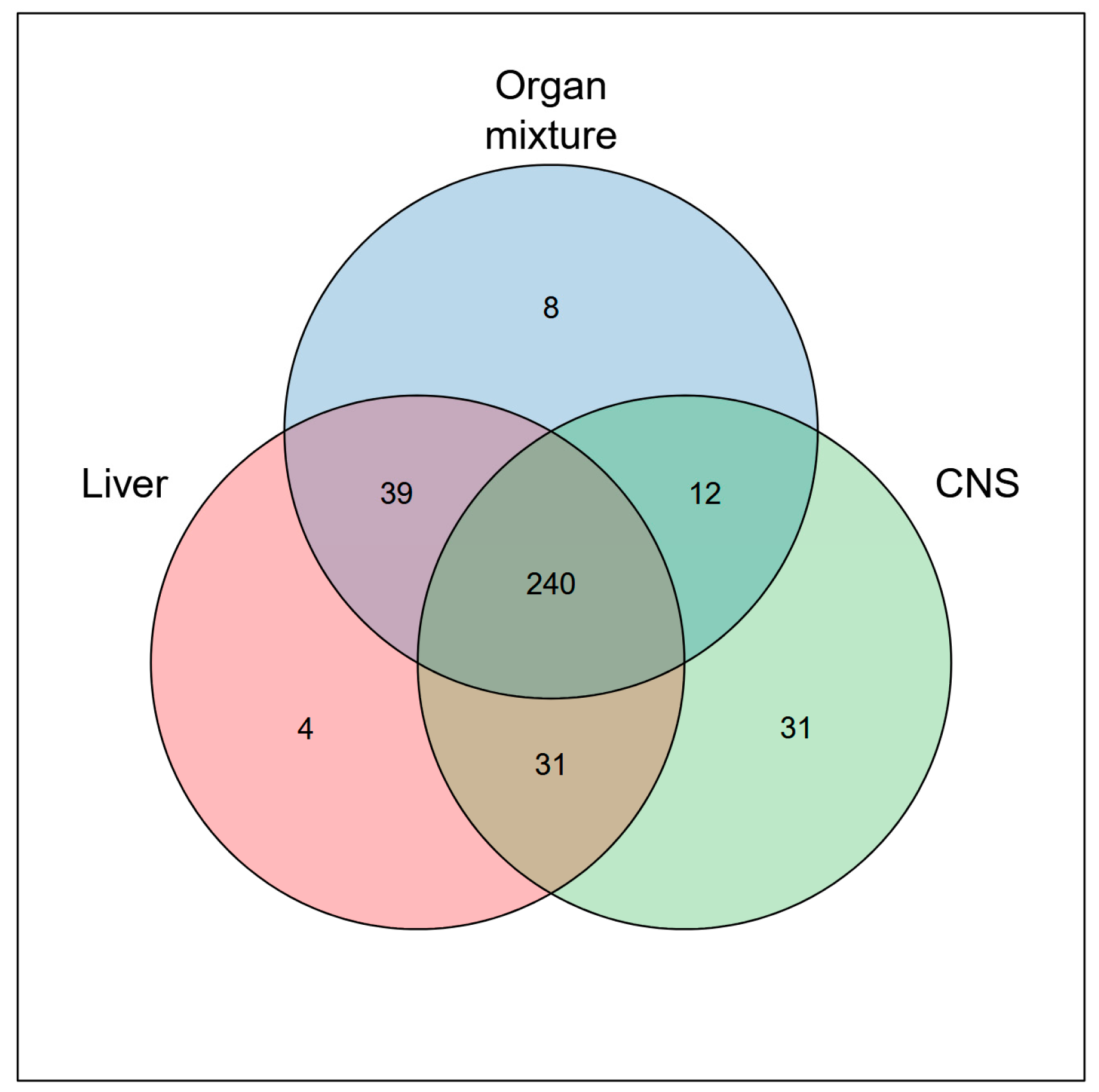
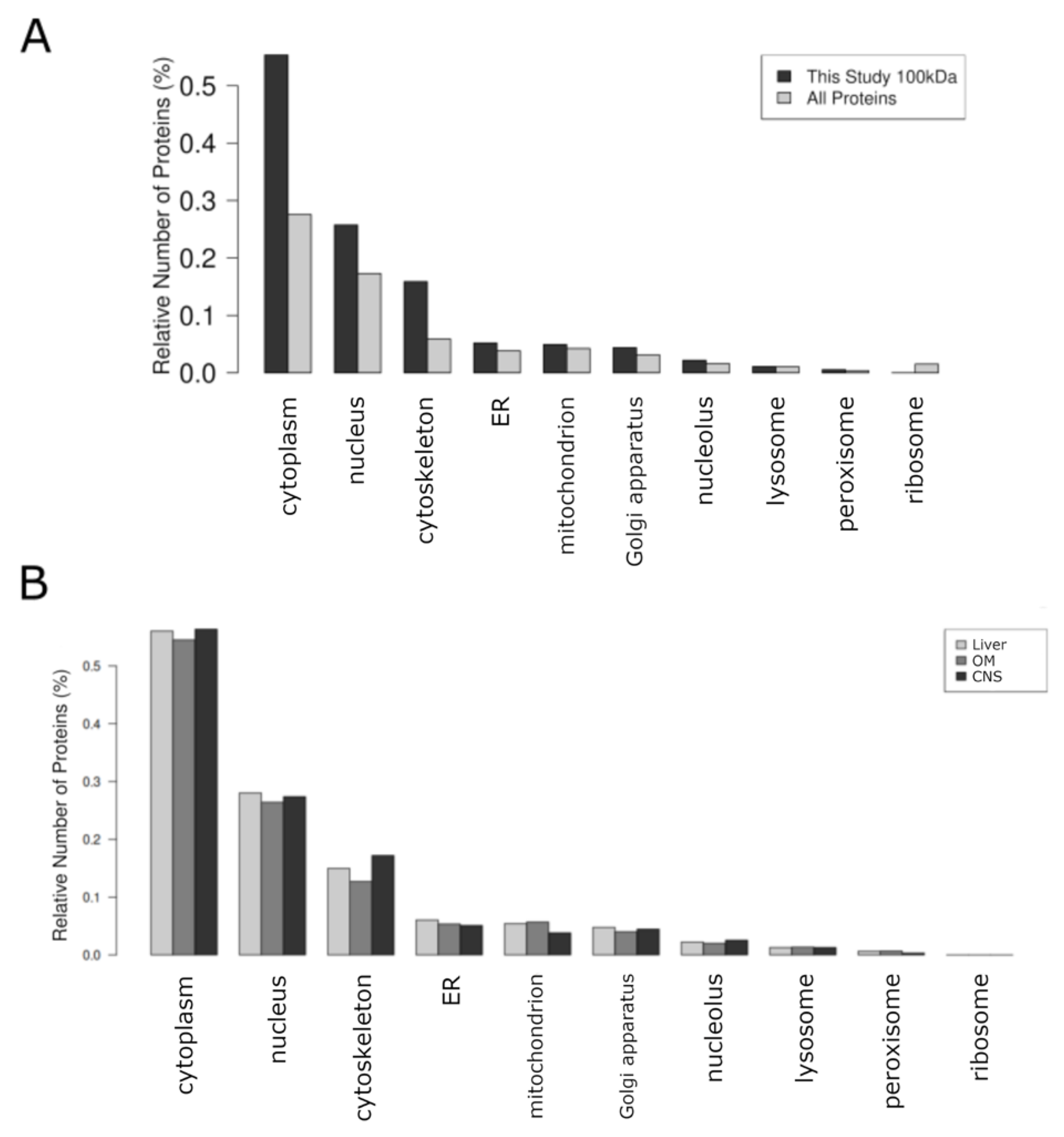
2.3. Integrative Analysis Reveals Distinct Functional Profiles Across Organ-Specific Proteomes
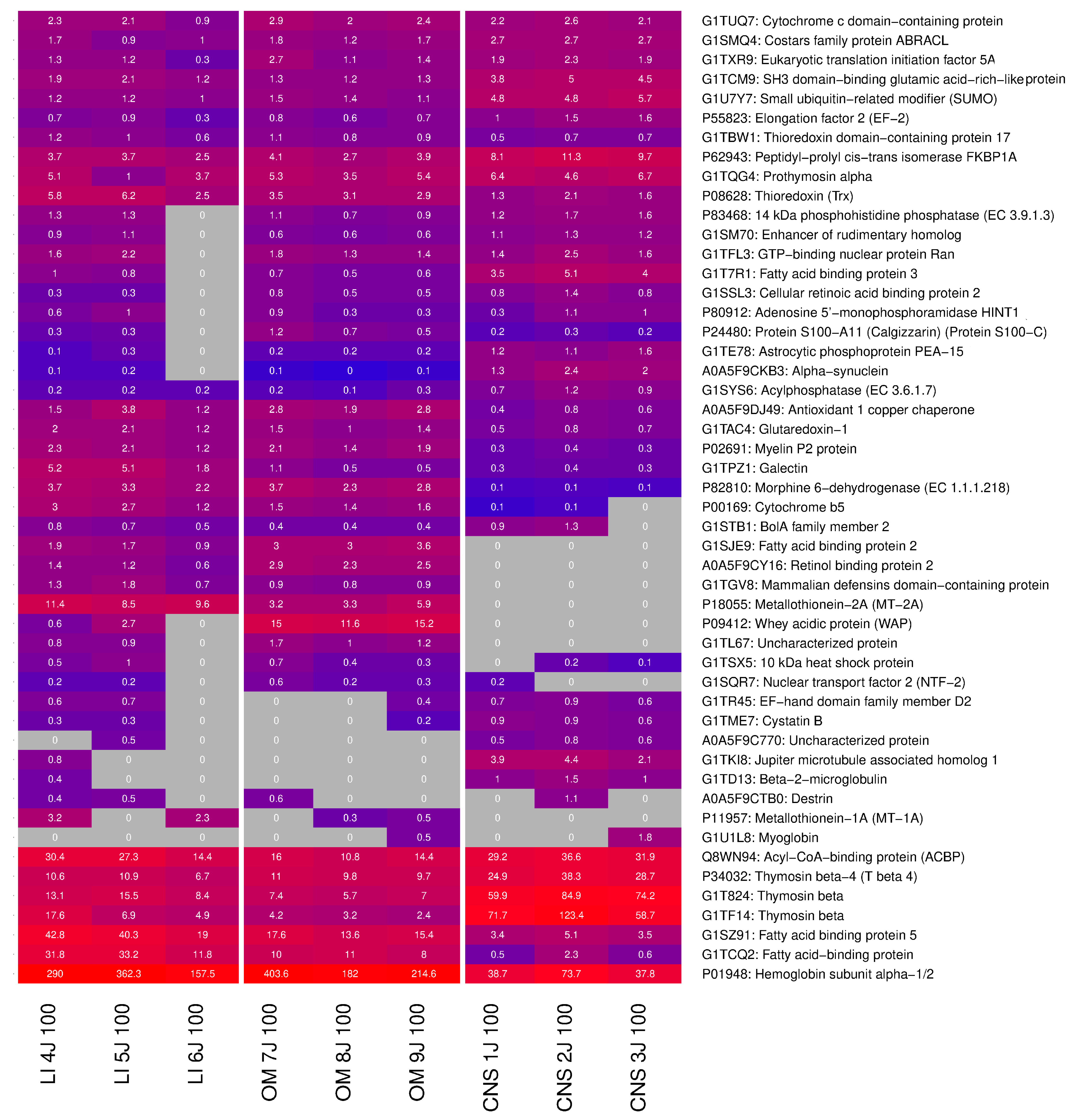
2.4. Identification of Highly Expressed Small Proteins
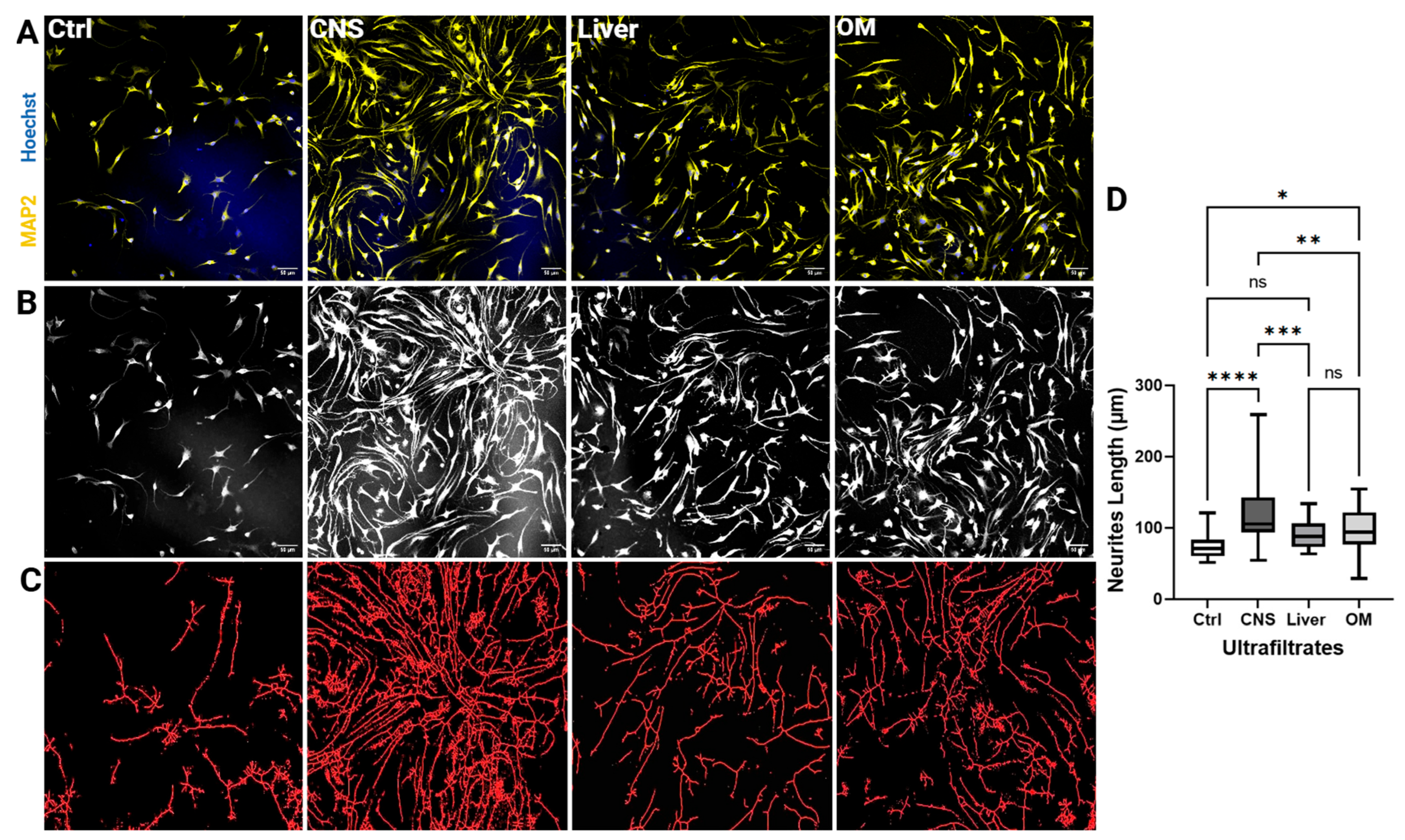
2.5. CNS Extracts of 100 kDa Stimulate Neurite Outgrowth of NSCs
3. Discussion
3.1. Efficacy of CNS Extracts
3.2. The Efficacy of Liver and OM Extracts
3.3. Wider Outcomes for Neuroregenerative Therapy
3.4. Mechanistic vs. Network-Oriented Analysis
3.5. Endogenous Proteolysis as a Functional Network Component
3.6. Integrating Reductionist and Systems Perspectives
3.7. Methodological Advances and Limitations
4. Materials and Methods
4.1. Production of 100 kDa Ultrafiltrates
4.2. Analysis by Tris-Tricine-Polyacrylamide Gel Electrophoresis
4.3. Analysis Using Liquid Proteolysis
4.4. Liquid Chromatography Mass Spectrometry/Mass Spectrometry (LC-MS/MS) Analysis
4.5. Bioinformatics Analysis
4.6. Identification of Proteins/Genes with Uniprot, g:Profiler, and the Human Protein Atlas
4.7. Neural Stem Cell Isolation, Culture, and Characterization by Immunostaining
4.8. Neurite Outgrowth Assay
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinberg, R.; Koch, H.-G. The Largely Unexplored Biology of Small Proteins in Pro- and Eukaryotes. FEBS J. 2021, 288, 7002–7024. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, K.; Bauer, C.; Bendner, G.; Dietrich, H.; Slivka, J.P.; Wink, M.; Wong, M.B.F.; Chan, M.K.S.; Skutella, T. Modulation of Cellular Senescence in HEK293 and HepG2 Cells by Ultrafiltrates UPla and ULu Is Partly Mediated by Modulation of Mitochondrial Homeostasis under Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 6748. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive Peptides of Animal Origin: A Review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Morozov, G.; Khavinson, V.K. Natural and Synthetic Thymic Peptides as Therapeutics for Immune Dysfunction. Int. J. Immunopharmacol. 1997, 19, 501–505. [Google Scholar] [CrossRef]
- Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent Advances in (Therapeutic Protein) Drug Development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Wu, S.; Li, Y.; Guo, L.; Li, Y.; Shah, D.K. Effect of the Size of Protein Therapeutics on Brain Pharmacokinetics Following Systematic Administration. AAPS J. 2022, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, P.B.W.; Gohil, K.; Moon, K.-M.; Foster, L.J.; Williams, F.J. Proteomic Investigation of Neurotrophic Trans-Banglene Reveals Potential Link to Iron Homeostasis. In Molecular Neurobiology; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Kandigian, S.E.; Ethier, E.C.; Kitchen, R.R.; Lam, T.T.; Arnold, S.E.; Carlyle, B.C. Proteomic Characterization of Post-Mortem Human Brain Tissue Following Ultracentrifugation-Based Subcellular Fractionation. Brain Commun. 2022, 4, fcac103. [Google Scholar] [CrossRef]
- Zhang, G.; Bowling, H.; Hom, N.; Kirshenbaum, K.; Klann, E.; Chao, M.V.; Neubert, T.A. In-Depth Quantitative Proteomic Analysis of de Novo Protein Synthesis Induced by Brain-Derived Neurotrophic Factor. J. Proteome Res. 2014, 13, 5707–5714. [Google Scholar] [CrossRef]
- Gentile, J.E.; Carrizales, M.G.; Koleske, A.J. Control of Synapse Structure and Function by Actin and Its Regulators. Cells 2022, 11, 603. [Google Scholar] [CrossRef]
- Cabri, W.; Cantelmi, P.; Corbisiero, D.; Fantoni, T.; Ferrazzano, L.; Martelli, G.; Mattellone, A.; Tolomelli, A. Therapeutic Peptides Targeting PPI in Clinical Development: Overview, Mechanism of Action and Perspectives. Front. Mol. Biosci. 2021, 8, 697586. [Google Scholar] [CrossRef] [PubMed]
- Kolkova, K.; Novitskaya, V.; Pedersen, N.; Berezin, V.; Bock, E. Neural Cell Adhesion Molecule-Stimulated Neurite Outgrowth Depends on Activation of Protein Kinase C and the Ras-Mitogen-Activated Protein Kinase Pathway. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yuan, D.-J.; Li, S.; Liang, X.-S.; Gao, Y.; Lan, X.-Y.; Qin, H.-M.; Ma, Y.-F.; Xu, G.-Y.; Schachner, M.; et al. NCAM Regulates Temporal Specification of Neural Progenitor Cells via Profilin2 during Corticogenesis. J. Cell Biol. 2019, 219, e201902164. [Google Scholar] [CrossRef]
- Strittmatter, S.; Igarashi, M.; Fishman, M. GAP-43 Amino Terminal Peptides Modulate Growth Cone Morphology and Neurite Outgrowth. J. Neurosci. 1994, 14, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fisher, D.A.; Storm, D.R. Intracellular Sorting of Neuromodulin (GAP-43) Mutants Modified in the Membrane Targeting Domain. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 5807–5817. [Google Scholar] [CrossRef]
- Strittmatter, S.M.; Vartanian, T.; Fishman, M.C. GAP-43 as a Plasticity Protein in Neuronal Form and Repair. J. Neurobiol. 1992, 23, 507–520. [Google Scholar] [CrossRef]
- Sébastien, M.; Paquette, A.L.; Prowse, E.N.P.; Hendricks, A.G.; Brouhard, G.J. Doublecortin Restricts Neuronal Branching by Regulating Tubulin Polyglutamylation. Nat. Commun. 2025, 16, 1749. [Google Scholar] [CrossRef]
- Ayanlaja, A.A.; Xiong, Y.; Gao, Y.; Ji, G.; Tang, C.; Abdikani Abdullah, Z.; Gao, D. Distinct Features of Doublecortin as a Marker of Neuronal Migration and Its Implications in Cancer Cell Mobility. Front. Mol. Neurosci. 2017, 10, 199. [Google Scholar] [CrossRef]
- Dema, A.; Charafeddine, R.A.; van Haren, J.; Rahgozar, S.; Viola, G.; Jacobs, K.A.; Kutys, M.L.; Wittmann, T. Doublecortin Reinforces Microtubules to Promote Growth Cone Advance in Soft Environments. Curr. Biol. 2024, 34, 5822–5832.e5. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, X.; Yao, Q.; Li, J.; Ju, G. The Promotive Effects of Thymosin Beta4 on Neuronal Survival and Neurite Outgrowth by Upregulating L1 Expression. Neurochem. Res. 2008, 33, 2269–2280. [Google Scholar] [CrossRef]
- Mollinari, C.; Ricci-Vitiani, L.; Pieri, M.; Lucantoni, C.; Rinaldi, A.M.; Racaniello, M.; De Maria, R.; Zona, C.; Pallini, R.; Merlo, D.; et al. Downregulation of Thymosin Β4 in Neural Progenitor Grafts Promotes Spinal Cord Regeneration. J. Cell Sci. 2009, 122, 4195–4207. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Jia, L.; Lu, X.; Szalad, A.; Zhang, Y.; Zhang, R.; Zhang, Z.G. Therapeutic Benefit of Extended Thymosin Β4 Treatment Is Independent of Blood Glucose Level in Mice with Diabetic Peripheral Neuropathy. J. Diabetes Res. 2015, 2015, 173656. [Google Scholar] [CrossRef]
- Shigyo, M.; Kuboyama, T.; Sawai, Y.; Tada-Umezaki, M.; Tohda, C. Extracellular Vimentin Interacts with Insulin-like Growth Factor 1 Receptor to Promote Axonal Growth. Sci. Rep. 2015, 5, 12055. [Google Scholar] [CrossRef] [PubMed]
- Boyne, L.J.; Fischer, I.; Shea, T.B. Role of Vimentin in Early Stages of Neuritogenesis in Cultured Hippocampal Neurons. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 1996, 14, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Brenes, O.; Giachello, C.N.G.; Corradi, A.M.; Ghirardi, M.; Montarolo, P.G. Synapsin Knockdown Is Associated with Decreased Neurite Outgrowth, Functional Synaptogenesis Impairment, and Fast High-Frequency Neurotransmitter Release. J. Neurosci. Res. 2015, 93, 1492–1506. [Google Scholar] [CrossRef]
- Vasin, A.; Zueva, L.; Torrez, C.; Volfson, D.; Littleton, J.T.; Bykhovskaia, M. Synapsin Regulates Activity-Dependent Outgrowth of Synaptic Boutons at the Drosophila Neuromuscular Junction. J. Neurosci. 2014, 34, 10554–10563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Swann, J.W. Expression of Calretinin in Diverse Neuronal Populations during Development of Rat Hippocampus. Neuroscience 1997, 81, 1137–1154. [Google Scholar] [CrossRef]
- Smith, K.M.; Browne, T.J.; Davis, O.C.; Coyle, A.; Boyle, K.A.; Watanabe, M.; Dickinson, S.A.; Iredale, J.A.; Gradwell, M.A.; Jobling, P.; et al. Calretinin Positive Neurons Form an Excitatory Amplifier Network in the Spinal Cord Dorsal Horn. eLife 2019, 8, e49190. [Google Scholar] [CrossRef]
- Iwasaki, K.; Isaacs, K.R.; Jacobowitz, D.M. Brain-Derived Neurotrophic Factor Stimulates Neurite Outgrowth in a Calretinin-Enriched Neuronal Culture System. Int. J. Dev. Neurosci. 1998, 16, 135–145. [Google Scholar] [CrossRef]
- Kawasaki, A.; Okada, M.; Tamada, A.; Okuda, S.; Nozumi, M.; Ito, Y.; Kobayashi, D.; Yamasaki, T.; Yokoyama, R.; Shibata, T.; et al. Growth Cone Phosphoproteomics Reveals That GAP-43 Phosphorylated by JNK Is a Marker of Axon Growth and Regeneration. iScience 2018, 4, 190–203. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gates, M.A.; Witke, W.; Menzies, A.S.; Wehman, A.M.; Macklis, J.D.; Kwiatkowski, D.; Soriano, P.; Gertler, F.B. Mena Is Required for Neurulation and Commissure Formation. Neuron 1999, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Meiri, K.F.; Saffell, J.L.; Walsh, F.S.; Doherty, P. Neurite Outgrowth Stimulated by Neural Cell Adhesion Molecules Requires Growth-Associated Protein-43 (GAP-43) Function and Is Associated with GAP-43 Phosphorylation in Growth Cones. J. Neurosci. 1998, 18, 10429–10437. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Eckert, A. Ginkgo Biloba Extract Increases Neurite Outgrowth and Activates the Akt/mTOR Pathway. PLoS ONE 2019, 14, e0225761. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Baez, R.; Hernández-Ortega, K.; Martínez-Martínez, E. Insights into the Proteomic Profiling of Extracellular Vesicles for the Identification of Early Biomarkers of Neurodegeneration. Front. Neurol. 2020, 11, 580030. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.-M.; Saijilafu; Zhou, F.-Q. Growing the Growth Cone: Remodeling the Cytoskeleton to Promote Axon Regeneration. Trends Neurosci. 2012, 35, 164–174. [Google Scholar] [CrossRef]
- Dovas, A.; Couchman, J.R. RhoGDI: Multiple Functions in the Regulation of Rho Family GTPase Activities. Biochem. J. 2005, 390, 1–9. [Google Scholar] [CrossRef]
- Auer, M.; Schweigreiter, R.; Hausott, B.; Thongrong, S.; Höltje, M.; Just, I.; Bandtlow, C.; Klimaschewski, L. Rho-Independent Stimulation of Axon Outgrowth and Activation of the ERK and Akt Signaling Pathways by C3 Transferase in Sensory Neurons. Front. Cell. Neurosci. 2012, 6, 43. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, H.; Deng, J.; Liu, J.; Wen, J.; Li, L.; Wang, X.; Pan, M.; Hu, X.; Guo, J. RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and P60-Katanin. Cells 2020, 9, 230. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.A. Neurotrophin Binding to the P75 Receptor Modulates Rho Activity and Axonal Outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef]
- Catlin, J.P.; Tooley, C.E.S. Exploring Potential Developmental Origins of Common Neurodegenerative Disorders. Biochem. Soc. Trans. 2024, 52, 1035–1044. Available online: https://portlandpress.com/biochemsoctrans/article-abstract/52/3/1035/234369/Exploring-potential-developmental-origins-of?redirectedFrom=fulltext (accessed on 18 March 2025). [CrossRef]
- Marrone, L.; Drexler, H.C.A.; Wang, J.; Tripathi, P.; Distler, T.; Heisterkamp, P.; Anderson, E.N.; Kour, S.; Moraiti, A.; Maharana, S.; et al. FUS Pathology in ALS Is Linked to Alterations in Multiple ALS-Associated Proteins and Rescued by Drugs Stimulating Autophagy. Acta Neuropathol. (Berl.) 2019, 138, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Jokwitz, M.; Witke, W.; Pilo Boyl, P. Specificity and Redundancy of Profilin 1 and 2 Function in Brain Development and Neuronal Structure. Cells 2021, 10, 2310. [Google Scholar] [CrossRef]
- Issa, S.; Fayoud, H.; Shaimardanova, A.; Sufianov, A.; Sufianova, G.; Solovyeva, V.; Rizvanov, A. Growth Factors and Their Application in the Therapy of Hereditary Neurodegenerative Diseases. Biomedicines 2024, 12, 1906. [Google Scholar] [CrossRef]
- Thanos, S.; Böhm, M.R.R.; Meyer zu Hörste, M.; Prokosch-Willing, V.; Hennig, M.; Bauer, D.; Heiligenhaus, A. Role of Crystallins in Ocular Neuroprotection and Axonal Regeneration. Prog. Retin. Eye Res. 2014, 42, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Monti, C.; Alberio, T. A Systems Biology-Led Insight into the Role of the Proteome in Neurodegenerative Diseases. Expert Rev. Proteomics 2016, 13, 845–855. [Google Scholar] [CrossRef]
- Alberghina, L.; Höfer, T.; Vanoni, M. Molecular Networks and System-Level Properties. J. Biotechnol. 2009, 144, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Liu, J.; Wu, B.; Yu, M.; Wegner, L.H. Strong Emergence in Biological Systems: Is It Open to Mathematical Reasoning? Acta Biotheor. 2021, 69, 841–856. [Google Scholar] [CrossRef]
- Bhalla, U.S.; Iyengar, R. Emergent Properties of Networks of Biological Signaling Pathways. Science 1999, 283, 381–387. [Google Scholar] [CrossRef]
- Dang, V.; Voigt, B.; Marcotte, E.M. Progress toward a Comprehensive Brain Protein Interactome. Biochem. Soc. Trans. 2025, 53, 303–314. [Google Scholar] [CrossRef]
- Basu, A.; Ash, P.E.; Wolozin, B.; Emili, A. Protein Interaction Network Biology in Neuroscience. Proteomics 2021, 21, 1900311. [Google Scholar] [CrossRef]
- Fang, F.C.; Casadevall, A. Reductionistic and Holistic Science. Infect. Immun. 2011, 79, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.C.; Zamdborg, L.; Ahlf, D.R.; Lee, J.E.; Catherman, A.D.; Durbin, K.R.; Tipton, J.D.; Vellaichamy, A.; Kellie, J.F.; Li, M.; et al. Mapping Intact Protein Isoforms in Discovery Mode Using Top Down Proteomics. Nature 2011, 480, 254–258. [Google Scholar] [CrossRef]
- Kesić, S. Systems Biology, Emergence and Antireductionism. Saudi J. Biol. Sci. 2016, 23, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Vaudel, M.; Burkhart, J.M.; Zahedi, R.P.; Oveland, E.; Berven, F.S.; Sickmann, A.; Martens, L.; Barsnes, H. PeptideShaker Enables Reanalysis of MS-Derived Proteomics Data Sets. Nat. Biotechnol. 2015, 33, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.-L.; Gulbahce, N.; Loscalzo, J. Network Medicine: A Network-Based Approach to Human Disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Padula, M.P.; Berry, I.J.; O′Rourke, M.B.; Raymond, B.B.A.; Santos, J.; Djordjevic, S.P. A Comprehensive Guide for Performing Sample Preparation and Top-Down Protein Analysis. Proteomes 2017, 5, 11. [Google Scholar] [CrossRef]
- Jarome, T.J.; Werner, C.T.; Kwapis, J.L.; Helmstetter, F.J. Activity Dependent Protein Degradation Is Critical for the Formation and Stability of Fear Memory in the Amygdala. PLoS ONE 2011, 6, e24349. [Google Scholar] [CrossRef]
- Boname, J.M.; Bloor, S.; Wandel, M.P.; Nathan, J.A.; Antrobus, R.; Dingwell, K.S.; Thurston, T.L.; Smith, D.L.; Smith, J.C.; Randow, F.; et al. Cleavage by Signal Peptide Peptidase Is Required for the Degradation of Selected Tail-Anchored Proteins. J. Cell Biol. 2014, 205, 847–862. [Google Scholar] [CrossRef]
- Teplyashina, E.A.; Komleva, Y.K.; Lychkovskaya, E.V.; Deikhina, A.S.; Salmina, A.B. Regulation of neurogenesis and cerebral angiogenesis by cell protein proteolysis products. RUDN J. Med. 2021, 25, 114–126. [Google Scholar] [CrossRef]
- Gomez-Cardona, E.; Eskandari-Sedighi, G.; Fahlman, R.; Westaway, D.; Julien, O. Application of N-Terminal Labeling Methods Provide Novel Insights into Endoproteolysis of the Prion Protein in Vivo. ACS Chem. Neurosci. 2024, 15, 134–146. [Google Scholar] [CrossRef]
- Klimaschewski, L. Ubiquitin-Dependent Proteolysis in Neurons. Physiology 2003, 18, 29–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiménez-Huete, A.; Lievens, P.M.J.; Vidal, R.; Piccardo, P.; Ghetti, B.; Tagliavini, F.; Frangione, B.; Prelli, F. Endogenous Proteolytic Cleavage of Normal and Disease-Associated Isoforms of the Human Prion Protein in Neural and Non-Neural Tissues. Am. J. Pathol. 1998, 153, 1561–1572. [Google Scholar] [CrossRef]
- Ahn, A.C.; Tewari, M.; Poon, C.-S.; Phillips, R.S. The Limits of Reductionism in Medicine: Could Systems Biology Offer an Alternative? PLoS Med. 2006, 3, e208. [Google Scholar] [CrossRef]
- Maguire, G. Using a Systems-Based Approach to Overcome Reductionist Strategies in the Development of Diagnostics. Expert Rev. Mol. Diagn. 2013, 13, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, F. The Limits of Reductionism in Biology: What Alternatives? E-LOGOS 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Marcus, K.; Rabilloud, T. How Do the Different Proteomic Strategies Cope with the Complexity of Biological Regulations in a Multi-Omic World? Critical Appraisal and Suggestions for Improvements. Proteomes 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Asad, N. Big Data Meets Proteomics: Leveraging Systems Biology for Mining the Proteome. J. Syst. Biol. Proteome Res. 2023, 4, 146. [Google Scholar] [CrossRef]
- Munro, V.; Kelly, V.; Messner, C.B.; Kustatscher, G. Cellular Control of Protein Levels: A Systems Biology Perspective. Proteomics 2024, 24, e2200220. [Google Scholar] [CrossRef] [PubMed]
- Noben, J.-P.; Dumont, D.; Kwasnikowska, N.; Verhaert, P.; Somers, V.; Hupperts, R.; Stinissen, P.; Robben, J. Lumbar Cerebrospinal Fluid Proteome in Multiple Sclerosis: Characterization by Ultrafiltration, Liquid Chromatography, and Mass Spectrometry. J. Proteome Res. 2006, 5, 1647–1657. [Google Scholar] [CrossRef]
- Kangas, P.; Nyman, T.A.; Metsähonkala, L.; Burns, C.; Tempest, R.; Williams, T.; Karttunen, J.; Jokinen, T.S. Towards Optimised Extracellular Vesicle Proteomics from Cerebrospinal Fluid. Sci. Rep. 2023, 13, 9564. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Nikolov, Z.L. Feasibility of Membrane Ultrafiltration as a Single-Step Clarification and Fractionation of Microalgal Protein Hydrolysates. Front. Bioeng. Biotechnol. 2022, 10, 957268. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.R. One-Dimensional SDS Gel Electrophoresis of Proteins. Curr. Protoc. Toxicol. 2007, 32, A.3F.1–A.3F.38. [Google Scholar] [CrossRef] [PubMed]
- topGO. Available online: http://bioconductor.org/packages/topGO/ (accessed on 27 April 2025).
- PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences|Nucleic Acids Research|Oxford Academic. Available online: https://academic.oup.com/nar/article/50/D1/D543/6415112 (accessed on 18 March 2025).
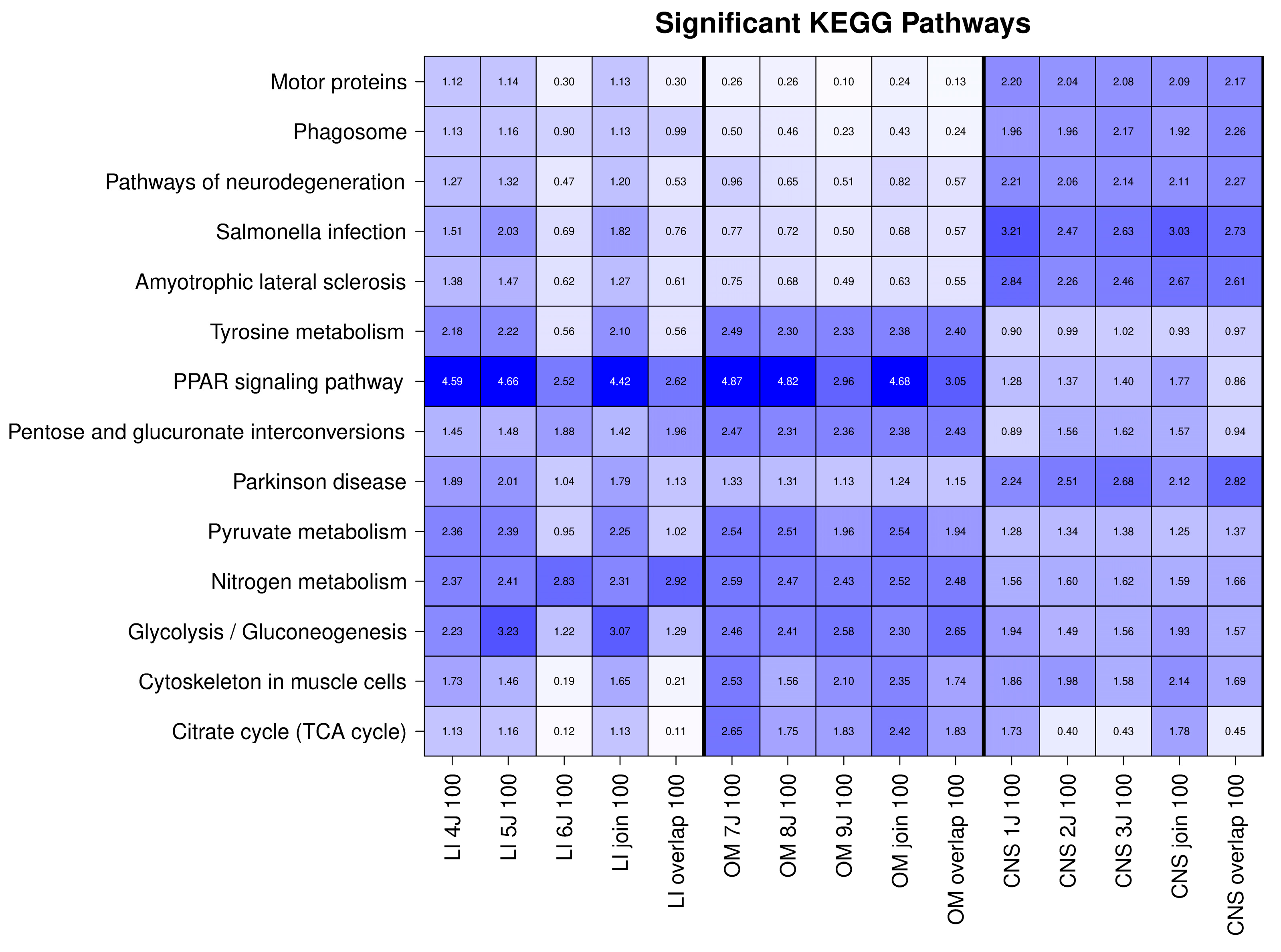
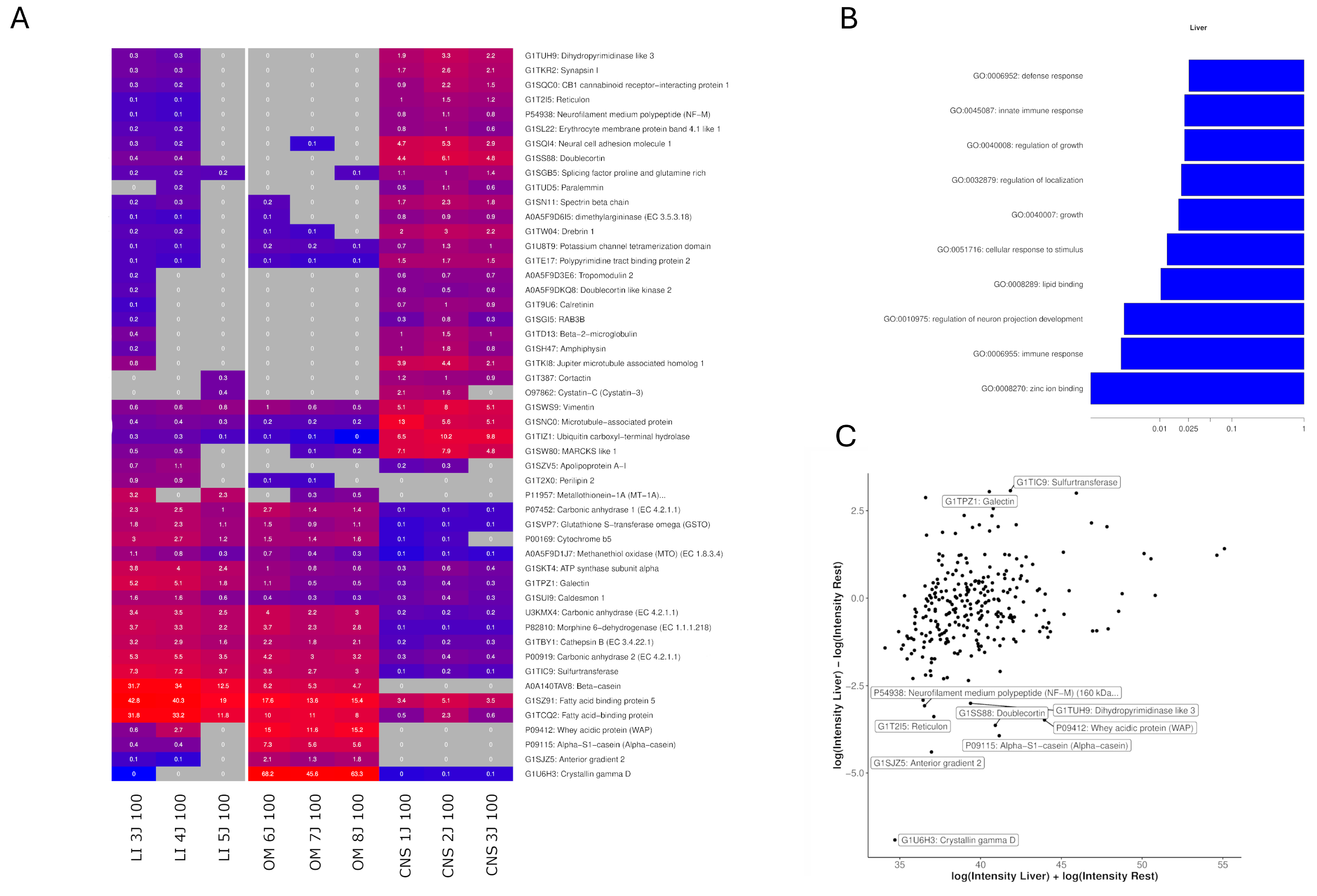
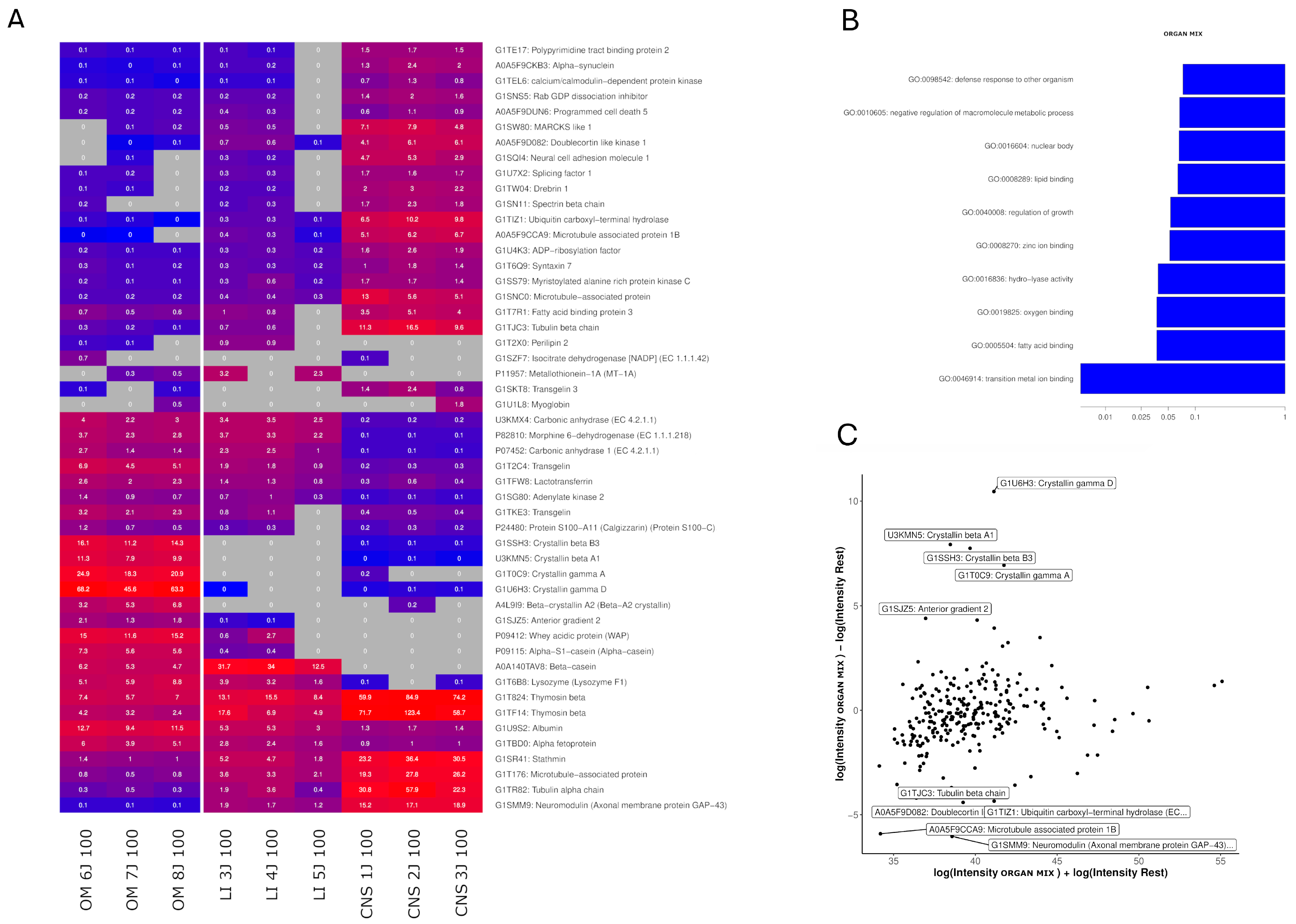
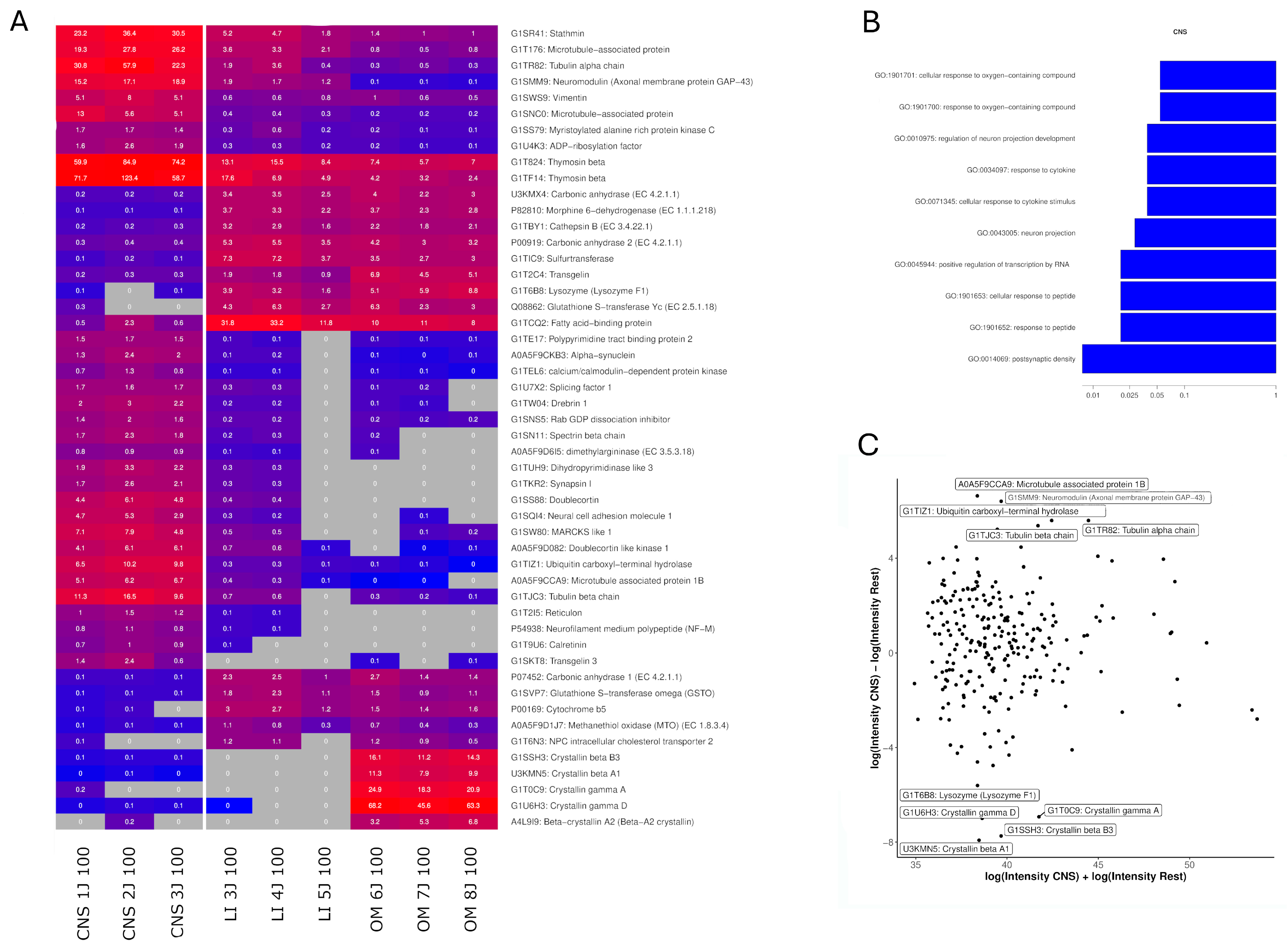
| KEGG Pathway | Proteins |
|---|---|
| Pathways of neurodegeneration | Vesicle-associated membrane protein-associated protein B/C; Tubulin beta-4A chain; ATP synthase subunit beta, mitochondrial; Neurofilament medium polypeptide; Ubiquitin carboxyl-terminal hydrolase isozyme L1; Polyubiquitin-B; microtubule-associated protein; FUS RNA binding protein; Alpha-synuclein; Tubulin alpha chain; Parkinsonism-associated deglycase; Tubulin beta chain; calcium/calmodulin-dependent protein kinase |
| Glycolysis/Glucone-ogenesis | Aldo-keto reductase family 1 member A1; Aldose 1-epimerase; phosphopyruvate hydratase; phosphoenolpyruvate carboxykinase (GTP); Phosphoglycerate mutase; Triosephosphate isomerase (TIM) |
| PPAR signaling pathway | Fatty acid binding protein 7; Fatty acid binding protein 5; Fatty acid binding protein 3; Apolipoprotein A-I; Acyl-CoA-binding protein (ACBP); Hydroxymethylglutaryl-CoA synthase (HMG-CoA synthase); phosphoenolpyruvate carboxykinase (GTP); Fatty acid binding protein 2; Perilipin 2; Sterol carrier protein 2 |
| Identified Proteins | Sources for Neuronal Outgrowth |
|---|---|
| N-CAM | [13,14] |
| Neuromodulin (GAP-43) | [15,16,17] |
| Doublecortin (DCX) | [18,19,20] |
| Thymosin Beta-4 | [21,22,23] |
| Vimentin | [24,25] |
| Synapsin I | [26,27] |
| Calretinin | [28,29,30] |
| KEGG Pathway | Identified Proteins |
|---|---|
| Neurotrophin signaling pathway | 14-3-3 protein epsilon Calcium/calmodulin-dependent protein kinase II alpha Crk-like protein Growth factor receptor-bound protein 2 |
| Axon guidance | Calcium/calmodulin-dependent protein kinase II alpha p21-activated kinase 3 Cofilin-2 Enabled homolog (Mena) |
| MAPK signaling pathway | Crk-like protein Growth factor receptor-bound protein 2 Microtubule-associated protein tau |
| Sample | Protein |
|---|---|
| Liver | Ribosome binding protein 1 |
| Hydroxyacyl-CoA dehydrogenase | |
| Alpha-lactalbumin (Lactose synthase B protein) | |
| HCV F-transactivated protein 1 | |
| Organ Mixture | Zinc finger FYVE-type containing 1 |
| Pepsin F (EC 3.4.23.1) | |
| Gamma-crystallin S (Beta-crystallin S) | |
| Crystallin gamma N | |
| Nebulin | |
| Crystallin beta A4 | |
| Crystallin beta B1 | |
| Lambda-crystallin | |
| CNS/Brain | Myelin expression factor 2 |
| Serine/threonine-protein kinase PAK 3 | |
| protein-tyrosine-phosphatase (EC 3.1.3.48) | |
| Small ArfGAP 1 | |
| Formin-binding protein 1-like | |
| Synaptosome associated protein 91 | |
| Metadherin | |
| SPG11 vesicle trafficking associated | |
| RUN and FYVE domain containing 3 | |
| Dynein cytoplasmic 1 intermediate chain 2 | |
| ADP-ribosylation factor | |
| Ankyrin 2 | |
| Alpha-tubulin N-acetyltransferase 1 (Alpha-TAT1) (TAT) | |
| Microtubule-associated protein RP/EB family member 2 | |
| ENAH actin regulator | |
| RAN binding protein 1 | |
| Peptidyl-prolyl cis-trans isomerase (EC 5.2.1.8) | |
| Amidohydrolase-related domain-containing protein | |
| Eukaryotic translation initiation factor 3 subunit J (eIF3j) | |
| Profilin | |
| Dynein axonemal heavy chain 7 | |
| Microtubule-associated protein RP/EB family member 3 | |
| UBC core domain-containing protein | |
| Protein kinase domain-containing protein | |
| Coronin | |
| VLIG-type G domain-containing protein | |
| Tubulin beta chain | |
| SRC kinase signaling inhibitor 1 | |
| Myopalladin | |
| Pleckstrin homology domain containing A7 | |
| Hydroxymethylglutaryl-CoA synthase (HMG-CoA synthase) |
| Sample | First Independent Production Run | Second Independent Production Run | Third Independent Production Run |
|---|---|---|---|
| CNS | CNS 1J 100 | CNS 2J 100 | CNS 3J 100 |
| Organ Mixture | OM 7J 100 | OM 8J 100 | OM 9J 100 |
| Liver | LI 4J 100 | LI 5J 100 | LI 6J 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slivka, J.P.; Bauer, C.; Halhouli, T.; Younsi, A.; Wong, M.B.F.; Chan, M.K.S.; Skutella, T. Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine. Int. J. Mol. Sci. 2025, 26, 6659. https://doi.org/10.3390/ijms26146659
Slivka JP, Bauer C, Halhouli T, Younsi A, Wong MBF, Chan MKS, Skutella T. Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine. International Journal of Molecular Sciences. 2025; 26(14):6659. https://doi.org/10.3390/ijms26146659
Chicago/Turabian StyleSlivka, Jakub Peter, Chris Bauer, Tasneem Halhouli, Alexander Younsi, Michelle B. F. Wong, Mike K. S. Chan, and Thomas Skutella. 2025. "Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine" International Journal of Molecular Sciences 26, no. 14: 6659. https://doi.org/10.3390/ijms26146659
APA StyleSlivka, J. P., Bauer, C., Halhouli, T., Younsi, A., Wong, M. B. F., Chan, M. K. S., & Skutella, T. (2025). Organ-Specific Small Protein Networks in 100 kDa Ultrafiltrates: Functional Analysis and Implications for Neuroregenerative Medicine. International Journal of Molecular Sciences, 26(14), 6659. https://doi.org/10.3390/ijms26146659






