Smart Polymeric Micelles with Aggregation-Induced Emission and pH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis Route of BT-PGA-TPE-HNPE
2.2. Analysis and Characterization of BT-PGA-TPE-HNPE
2.2.1. 1H NMR Analysis
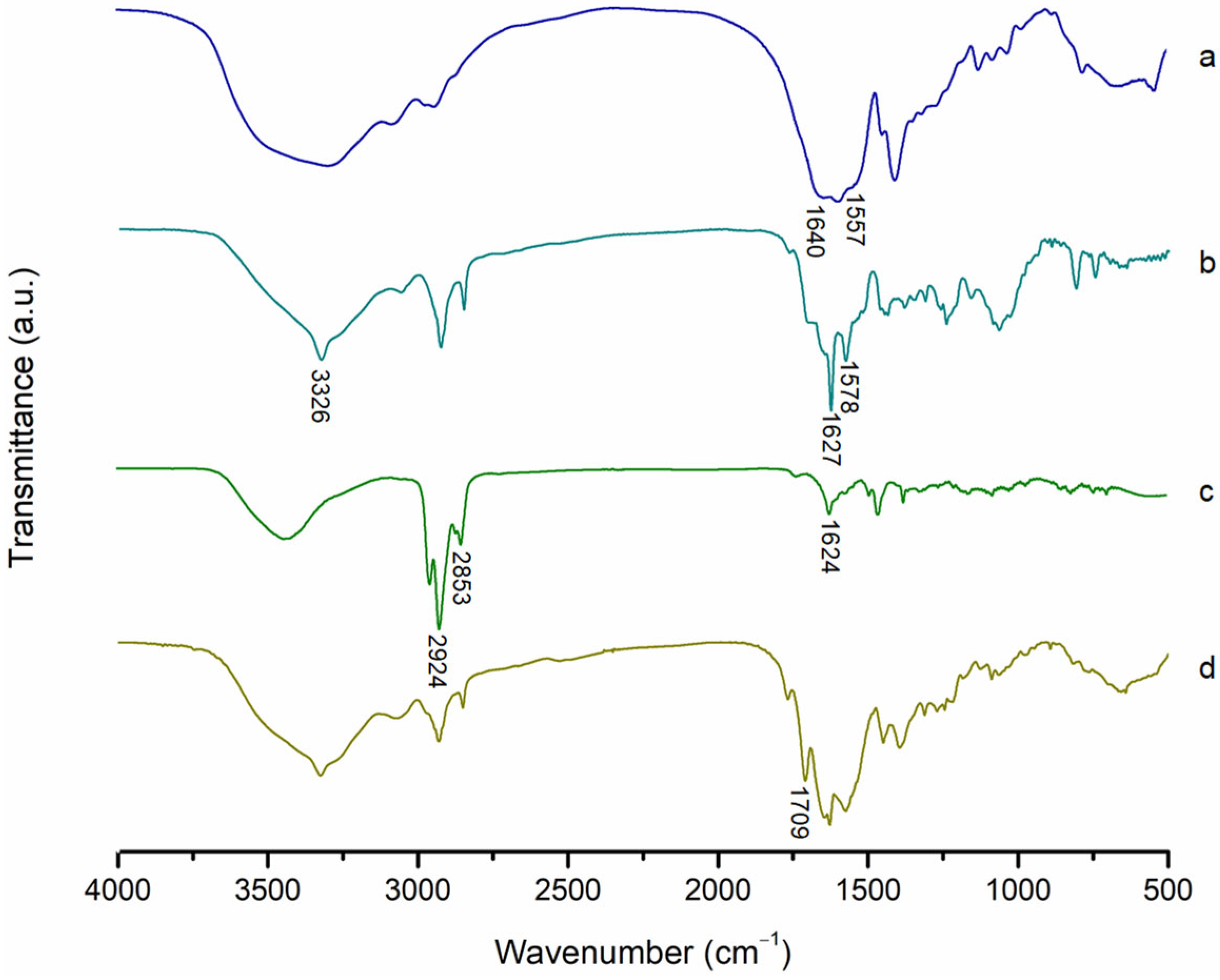
2.2.2. FTIR Analysis
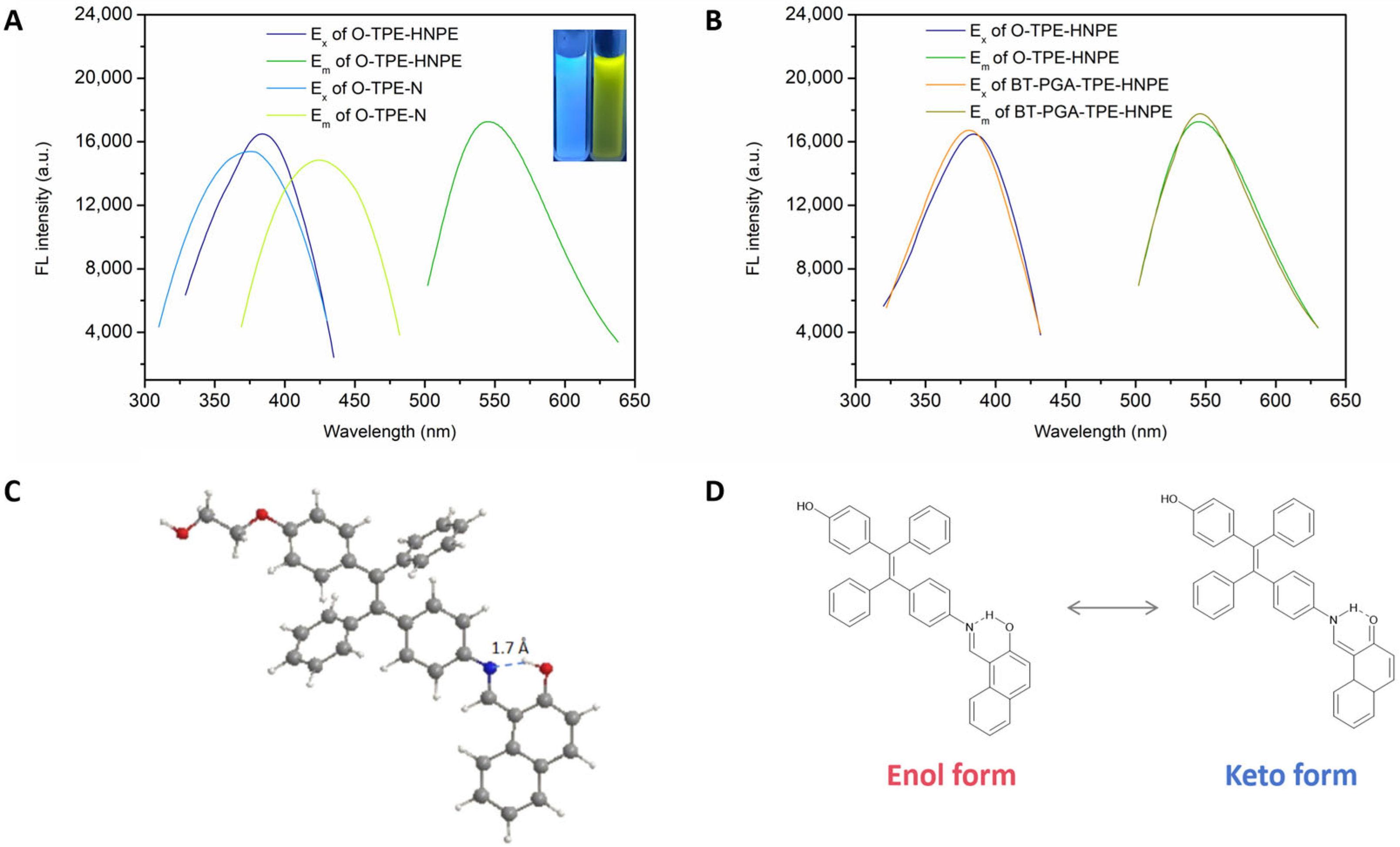
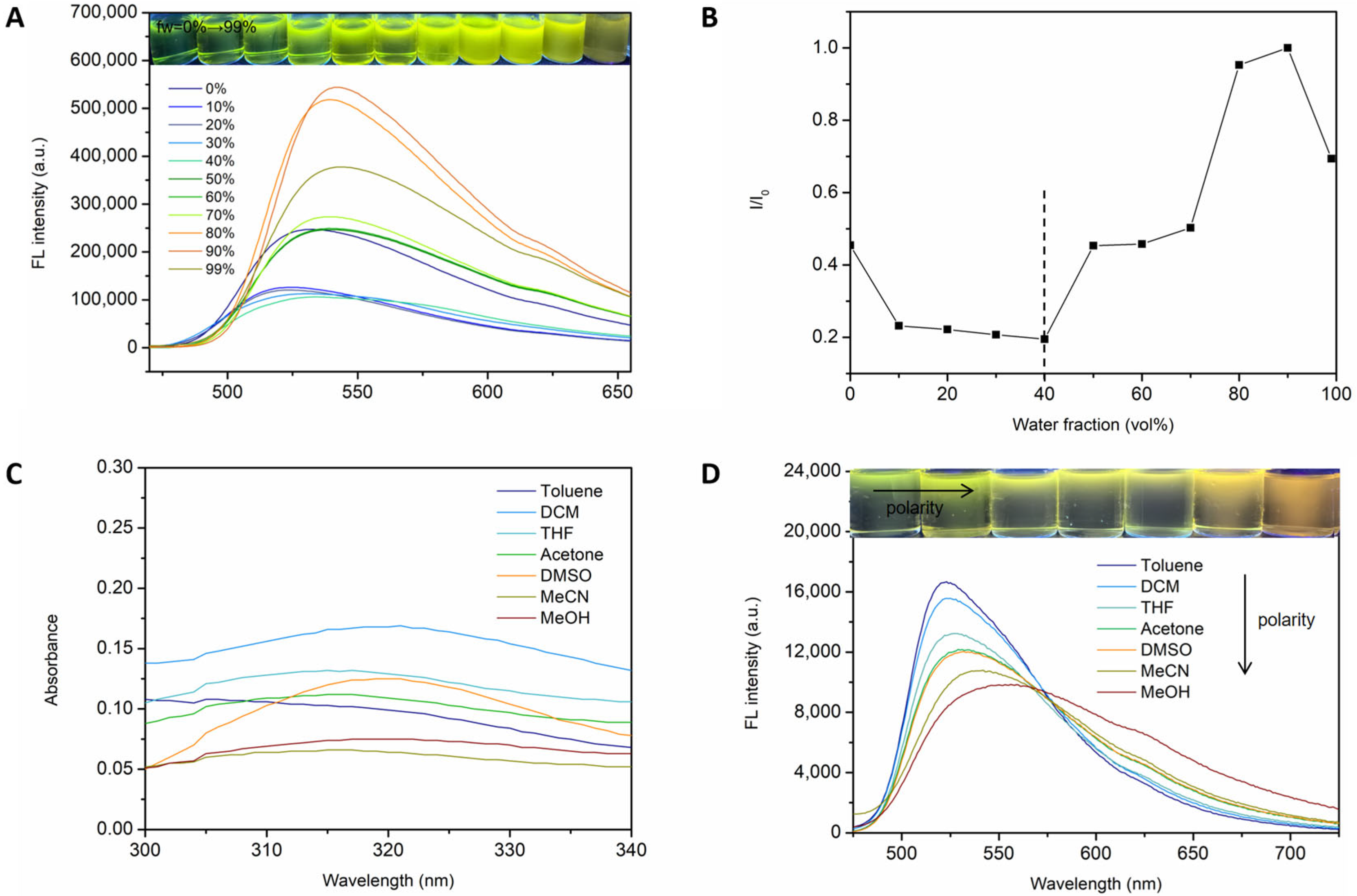
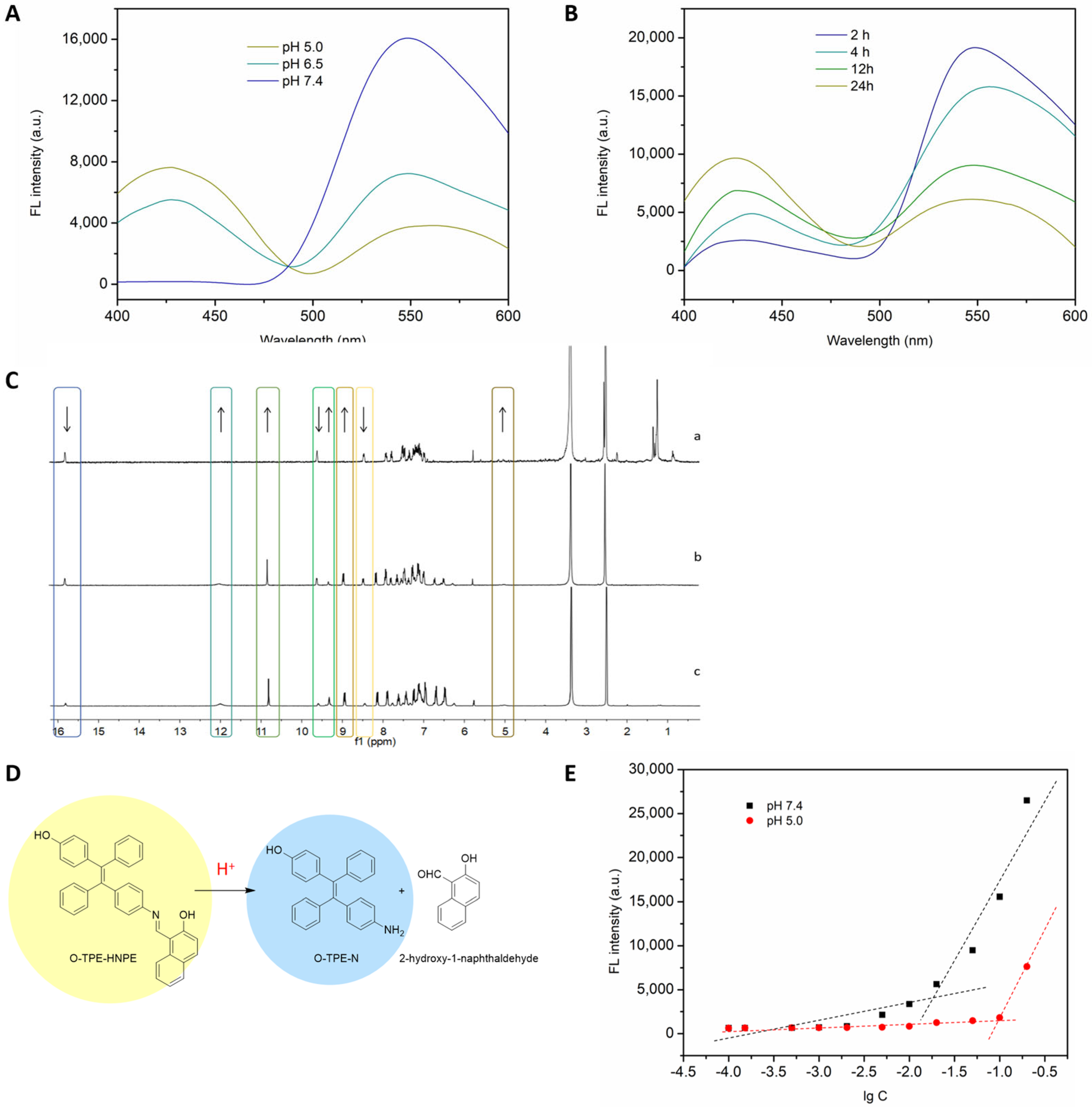
2.2.3. Optical Properties Investigation
2.2.4. CMC Determination
2.3. Characterization of BT-PGA-TPE-HNPE Micelles
2.4. pH-Responsive Drug Release Behavior
2.5. Cellular Imaging and Cellular Uptake Behavior
2.6. In Vitro Cytotoxicity Assay
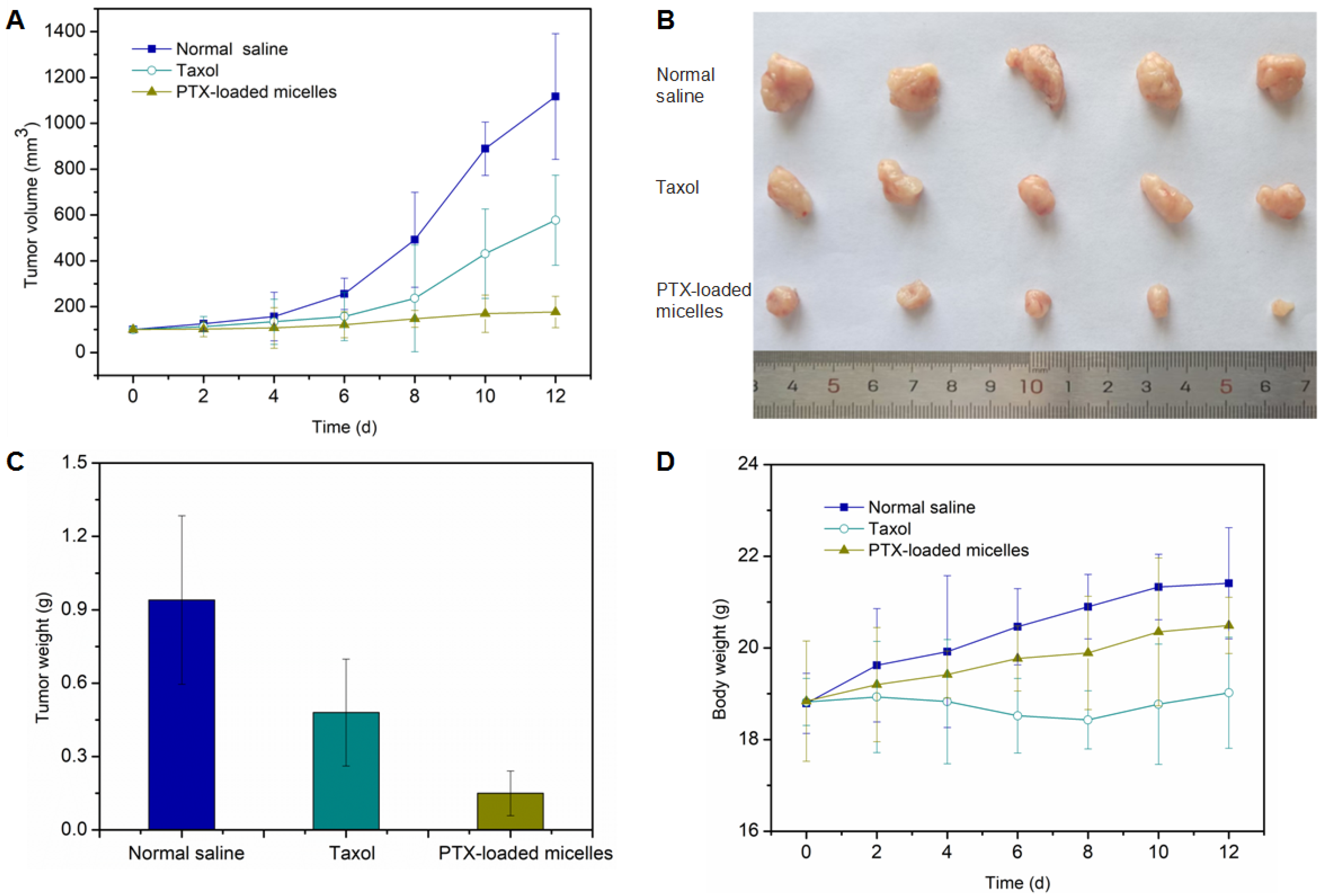
2.7. In Vivo Antitumor Efficacy Assessment
3. Materials and Methods
3.1. Materials
3.2. Cells and Animals
3.3. Synthesis of BT-PGA-TPE-HNPE
3.3.1. Synthesis of O-TPE-HNPE
3.3.2. Synthesis of BT-PGA
3.3.3. Synthesis of BT-PGA-TPE-HNPE
3.4. Characterization of BT-PGA-TPE-HNPE
3.4.1. Confirmation of Polymer Formation
3.4.2. Investigations of AIE Behavior and pH-Sensitive Fluorescence Changes
3.4.3. Determination of Critical Micelle Concentration (CMC)
3.5. Preparation and Characterization of PTX-Loaded BT-PGA-TPE-HNPE Micelles
3.6. In Vitro Drug Release Study
3.7. Cytotoxicity Investigation
3.8. Cellular Imaging Capability and Cellular Uptake Behavior Investigation
3.9. In Vivo Antitumor Activity Study
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Rezaei, S.J.; Sarbaz, L.; Niknejad, H. Folate-decorated redox/pH dual-responsive degradable prodrug micelles for tumor triggered targeted drug delivery. RSC Adv. 2016, 6, 62630–62639. [Google Scholar] [CrossRef]
- He, H.; Zhuang, W.; Ma, B.; Su, X.; Yu, T.; Hu, J.; Chen, L.; Peng, R.; Li, G.; Wang, Y. Oxidation-responsive and aggregation-induced emission polymeric micelles with two-photon excitation for cancer therapy and bioimaging. ACS Biomater. Sci. Eng. 2019, 5, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent progress in nanostructured smart drug delivery systems for cancer therapy: A review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef]
- Hellmers, F.; Ferguson, P.; Koropatnick, J.; Krull, R.; Margaritis, A. Characterization and in vitro cytotoxicity of doxorubicin-loaded γ-polyglutamic acid-chitosan composite nanoparticles. Biochem. Eng. J. 2013, 75, 72–78. [Google Scholar] [CrossRef]
- Manocha, B.; Margaritis, A. Controlled release of doxorubicin from doxorubicin/γ-polyglutamic acid ionic complex. J. Nanomater. 2010, 2010, 780171. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Chen, M.M.; Tang, X.; Li, J.J.; Chen, F.Y.; Jiang, Z.T.; Fu, R.; Li, H.B.; Hu, X.Y.; Geng, W.C.; Guo, D.S. Active targeting tumor therapy using host-guest drug delivery system based on biotin functionalized azocalix[4]arene. J. Control. Release 2024, 368, 691–702. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Gebrie, H.T.; Thankachan, D.; Tsai, H.C.; Lai, J.Y.; Chang, H.M.; Wu, S.Y. Doxorubicin-loaded polymeric biotin-PEG-SeSe-PBLA micelles with surface binding of biotin-mediated cancer cell targeting and redox-responsive drug release for enhanced anticancer efficacy. Colloids Surf. B. Biointerfaces 2024, 241, 114028. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiu, Y.; Zhang, Y.; Wang, Y.; Xu, J.; Yu, W.; Xing, D. Recent advances in biotin-based therapeutic agents for cancer therapy. Nanoscale 2025, 17, 1812–1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, Q.; Li, J.; Jiang, J.; Deng, T.; Yu, C. Biomimetic macrophage membrane-coated gold-quantum dots with tumor microenvironment stimuli-responsive capability for tumor theranostic. Mater. Today Bio 2022, 16, 100359. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.C.; Ting, C.W.; Chiang, W.H. Functionalized polymeric nanogels with pH-sensitive benzoic-imine cross-linkages designed as vehicles for indocyanine green delivery. J. Colloid Interface Sci. 2020, 561, 11–22. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Q.; Xue, H.; Liao, W.; Feng, Y.; Yuan, J.; Tao, L.; Wei, Y. Synthesis of an aggregation-induced emission (AIE) dye with pH-sensitivity based on tetraphenylethylene-pyridine for fluorescent nanoparticles and its applications in bioimaging and in vitro anti-tumor effect. Colloids Surf. B. Biointerfaces 2024, 234, 113750. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.K.; Law, W.C.; Sun, H.; Prasad, P.N.; Cheng, C. A degradable brush polymer–drug conjugate for pH-responsive release of doxorubicin. Polym. Chem. 2015, 6, 953–961. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Zuo, Y.; Wang, M.; Chen, H.; Wang, C.; Cao, Y.; Zeng, Y.; Chen, Y.; Zhang, T.; et al. Dual targeting of FR+CD44 overexpressing tumors by self-assembled nanoparticles quantitatively conjugating folic acid-hyaluronic acid to the gsh-sensitively modified podophyllotoxin. Chem. Eng. J. 2025, 505, 159276. [Google Scholar] [CrossRef]
- Yu, T.; Zhuang, W.; Su, X.; Ma, B.; Hu, J.; He, H.; Li, G.; Wang, Y. Dual-responsive micelles with aggregation-induced emission feature and two-photon aborsption for accurate drug delivery and bioimaging. Bioconjugate Chem. 2019, 30, 2075–2087. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Ren, J.; Qiao, C.; Ma, C.; Han, Y.; Zhang, S.; Xu, Q.; Wu, S. AIEgen-based fluorescent polymer films with tunable janus luminescence and structural color. Chem. Eng. J. 2024, 482, 149025. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Gu, X.; Tang, B.Z. Photoactivatable biomedical materials based on luminogens with aggregation-induced emission (AIE) characteristics. Adv. Healthc. Mater. 2021, 10, 2101177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Li, Z.; Wu, X.; Wang, D.; He, Y.; Cheng, H.; Fan, B.; Zhu, D.; Li, M.; et al. Engineered strategies for lipid droplets-targeted AIEgens based on tetraphenylethene. Molecules 2024, 29, 5904. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lv, Y.; Hou, X.; Yang, X.; Tao, Q.; Li, G. Chitosan based fluorescent probe with AIE property for detection of Fe3+ and bacteria. Int. J. Biol. Macromol. 2024, 279, 135478. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Q.; Zhang, X.; Xue, H.; Liao, W.; Yin, C.; Yuan, J.; Tao, L.; Wei, Y. A series of tetraphenylene-acetonitrile AIE compounds with D-A-D’ structure for drugs delivery systems of paclitaxel: Synthesis, structure-activity relationship and anti-tumors effect. Colloids Surf. B. Biointerfaces 2024, 244, 114136. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational design of a water-soluble NIR AIEgen, and its application in ultrafast wash-free cellular imaging and photodynamic cancer cell ablation. Chem. Sci. 2018, 9, 3685–3693. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, W.; Guo, X.; Li, Y.; Ma, Y.; Chen, L.; Zhang, H.; Wang, T.; Zhang, X.; Wang, Z. Mitochondria-targeted polydopamine nanocomposite with AIE photosensitizer for image-guided photodynamic and photothermal tumor ablation. Small 2019, 15, 1902352. [Google Scholar] [CrossRef]
- Wang, P.; Zou, Y.; Li, Y.; Qin, Z.; Liu, X.; Zhang, H. pH-sensitive self-assembled nanofibers based on electrostatic interaction and schiff base bonding for controlled release of curcumin. Food Hydrocoll. 2022, 131, 107805. [Google Scholar] [CrossRef]
- Porubský, M.; Gurská, S.; Stanková, J.; Hajdúch, M.; Džubák, P.; Hlaváč, J. Aminobodipy conjugates for targeted drug delivery systems and real-time monitoring of drug release. Mol. Pharm. 2021, 18, 2385–2396. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Liang, X.; Wang, X. Dual reduction-sensitive nanomicelles for antitumor drug delivery with low toxicity to normal cells. ACS Appl. Nano Mater. 2024, 7, 19952–19962. [Google Scholar] [CrossRef]
- Singh, A.K.; Kundu, M.; Roy, S.; Roy, B.; Shah, S.S.; Nair, A.V.; Pal, B.; Mondal, M.; Singh, N.D.P. A two-photon responsive naphthyl tagged p-hydroxyphenacyl based drug delivery system: Uncaging of anti-cancer drug in the phototherapeutic window with real-time monitoring. Chem. Commun. 2020, 56, 9986–9989. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Li, R.; Wang, Z.; Chen, N.; Wei, Y.; Ye, Y.; Jiang, H.; Xie, L. The location of benzothiazole on the skeleton of rofecoxib defines an AIEgen and its potential application as multifunctional materials. J. Mol. Struct. 2023, 1279, 134983. [Google Scholar] [CrossRef]
- Ojha, M.; Banerjee, M.; Mandal, M.; Singha, T.; Ray, S.; Datta, P.K.; Mandal, M.; Anoop, A.; Singh, N.D.P. Two-photon-responsive “TICT + AIE” active naphthyridine-BF2 photoremovable protecting group: Application for specific staining and killing of cancer cells. ACS Appl. Mater. Interfaces 2024, 16, 21486–21497. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Pi, M.; Pan, Z.; Song, N.; He, X.; Li, J.; Luo, F.; Tan, H.; Li, Z. Stable, bioresponsive, and macrophage-evading polyurethane micelles containing an anionic tripeptide chain extender. ACS Omega 2019, 4, 16551–16563. [Google Scholar] [CrossRef]
- Singh, A.K.; Mengji, R.; Nair, A.V.; Shah, S.S.; Avijit, J.; Singh, N.D.P. Photoactivable AIEgen-based lipid-droplet-specific drug delivery model for live cell imaging and two-photon light-triggered anticancer drug delivery. ACS Appl. Bio Mater. 2023, 6, 4372–4382. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Z.; Yang, W.; Guo, Y.; Shao, J.; Si, W.; Xu, H.; Wang, W.; Dong, X. H2O2 responsive CO gas release platform with CA IX-targeting for inhibiting tumor growth and metastasis. Chem. Eng. J. 2023, 463, 142371. [Google Scholar] [CrossRef]
- Li, S.; Li, K.; Gong, X.; Yan, P.; Sun, S. pH-induced color changeable AIE-featured polymeric micelles for self-indicating anticancer drug delivery. Sens. Actuators B Chem. 2025, 422, 136637. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, L.; Qi, Z.; Zhang, C.; Wang, C. Preparation of pH-responsive solanesol-based poly (glutamic acid) micellar carrier for doxorubicin delivery. Mater. Today Commun. 2022, 33, 104800. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Zheng, F.; Fang, Y.; Gao, F.; Chen, Q.; Zeng, Q.; Zeng, W. Debut of a novel AIE-based fluorescent probe as tyrosinase tracer to image skin pigmentary disorders. Sens. Actuators B Chem. 2023, 389, 133889. [Google Scholar] [CrossRef]
- Liao, G.; Luo, J.; Cui, T.; Zou, J.; Xu, M.; Ma, Y.; Shi, L.; Jia, J.; Ma, C.; Li, H.; et al. Microwave-assisted one-pot synthesis of carbon dots for highly sensitive and selective detection of selenite. Microchem. J. 2022, 179, 107440. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Sun, X.; Xu, T.; Guo, Z.; Du, X.; Li, S.; Li, X.; Xing, X.; Qiu, D. Visible-light-induced diselenide-crosslinked polymeric micelles for ROS-triggered drug delivery. Molecules 2024, 29, 3970. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Peng, J.; Zhang, Y.; Chen, L.; Luo, F.; Wang, C.; Qian, Z. Tumor neovasculature-targeted APRPG-PEG-PDLLA/MPEG-PDLLA mixed micelle loading combretastatin A-4 for breast cancer therapy. ACS Biomater. Sci. Eng. 2018, 4, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Sahragard, A.; Alavi, Z.; Abolhassanzadeh, Z.; Moein, M.; Mohammadi-Bardbori, A.; Omidi, M.; Zarshenas, M.M. Assessment of the cytotoxic activity of triphala: A semisolid traditional formulation on HepG2 cancer cell line. Biomed. Res. Int. 2021, 2021, 6689568. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Z.; Dai, H.; Lv, X.; Ma, Q.; Yang, D.P.; Shao, J.; Xu, Z.; Dong, X. Boosting O2•− photogeneration via promoting intersystem crossing and electron-donating efficiency of Aza-BODIPYBased nanoplatforms for hypoxic-tumor photodynamic therapy. Small Methods 2023, 7, 2300889. [Google Scholar] [CrossRef]





| Items | BT-PGA-TPE-HNPE | CCBT |
|---|---|---|
| Quantum yield | 26.6% | / |
| Synthesis steps | 5 | 6 |
| CMC | 0.0186 mg/mL | 0.021 mg/mL |
| Drug loading | 15.0% | 10.6% |
| Encapsulation efficiency | 90.1% | 83.4% |
| Cumulative drug release at a pH of 5.0 | 81.9% | <80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zong, Z.; Li, X.; Sun, S. Smart Polymeric Micelles with Aggregation-Induced Emission and pH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 6654. https://doi.org/10.3390/ijms26146654
Liu Z, Zong Z, Li X, Sun S. Smart Polymeric Micelles with Aggregation-Induced Emission and pH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(14):6654. https://doi.org/10.3390/ijms26146654
Chicago/Turabian StyleLiu, Zhenrong, Zhe Zong, Xiaoxin Li, and Shaoping Sun. 2025. "Smart Polymeric Micelles with Aggregation-Induced Emission and pH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy" International Journal of Molecular Sciences 26, no. 14: 6654. https://doi.org/10.3390/ijms26146654
APA StyleLiu, Z., Zong, Z., Li, X., & Sun, S. (2025). Smart Polymeric Micelles with Aggregation-Induced Emission and pH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy. International Journal of Molecular Sciences, 26(14), 6654. https://doi.org/10.3390/ijms26146654







