The Role of Cutibacterium acnes in the Etiopathogenesis of Sarcoidosis: Current Insights and Future Study Directions
Abstract
1. Introduction
2. Cutibacterium acnes: Commensal Bacterium and Opportunistic Pathogen
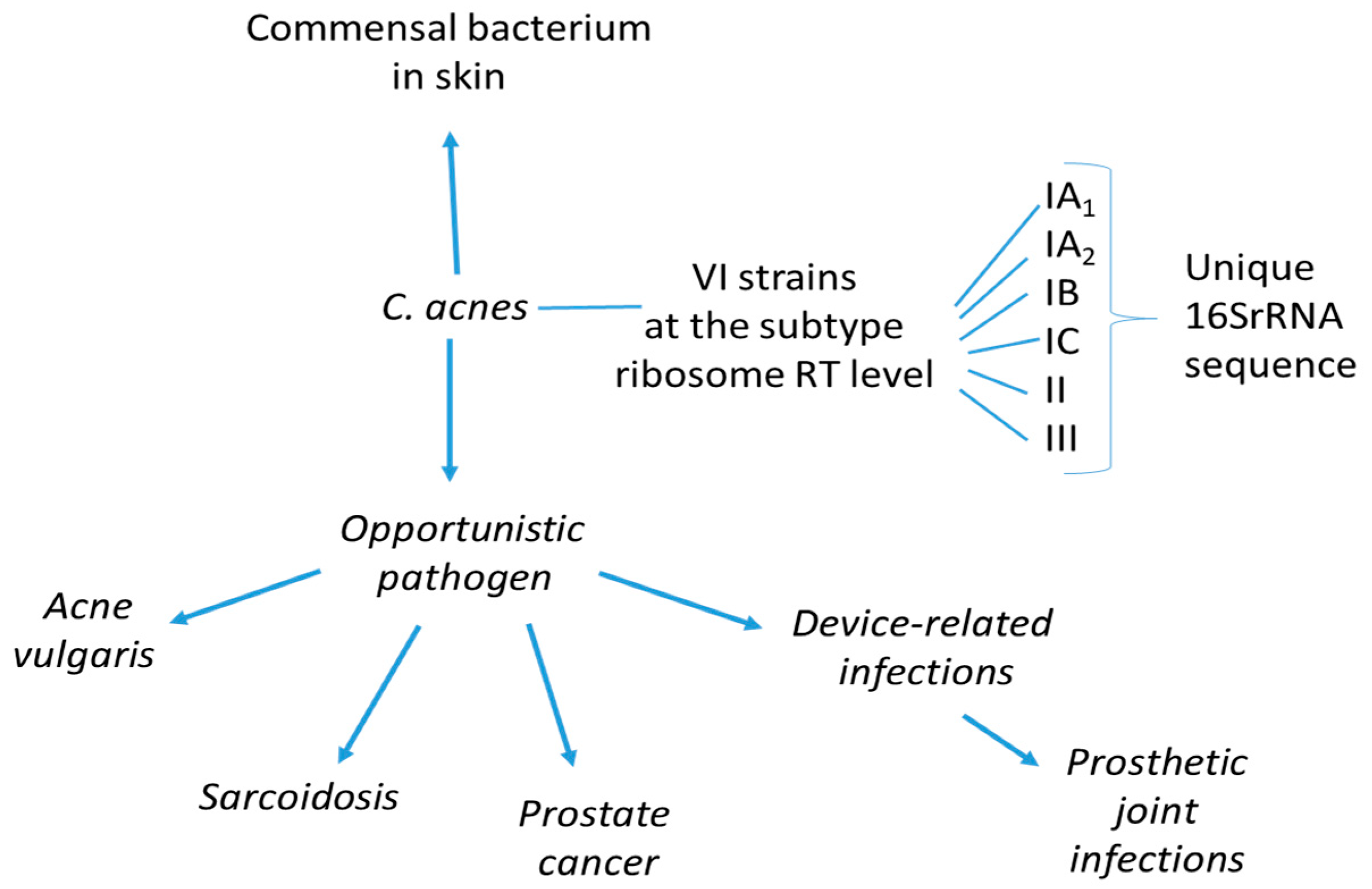
2.1. Molecular Markers of Cutibacterium acnes
2.2. Innate and Acquired Immunity in the Pathogenicity of Cutibacterium acnes
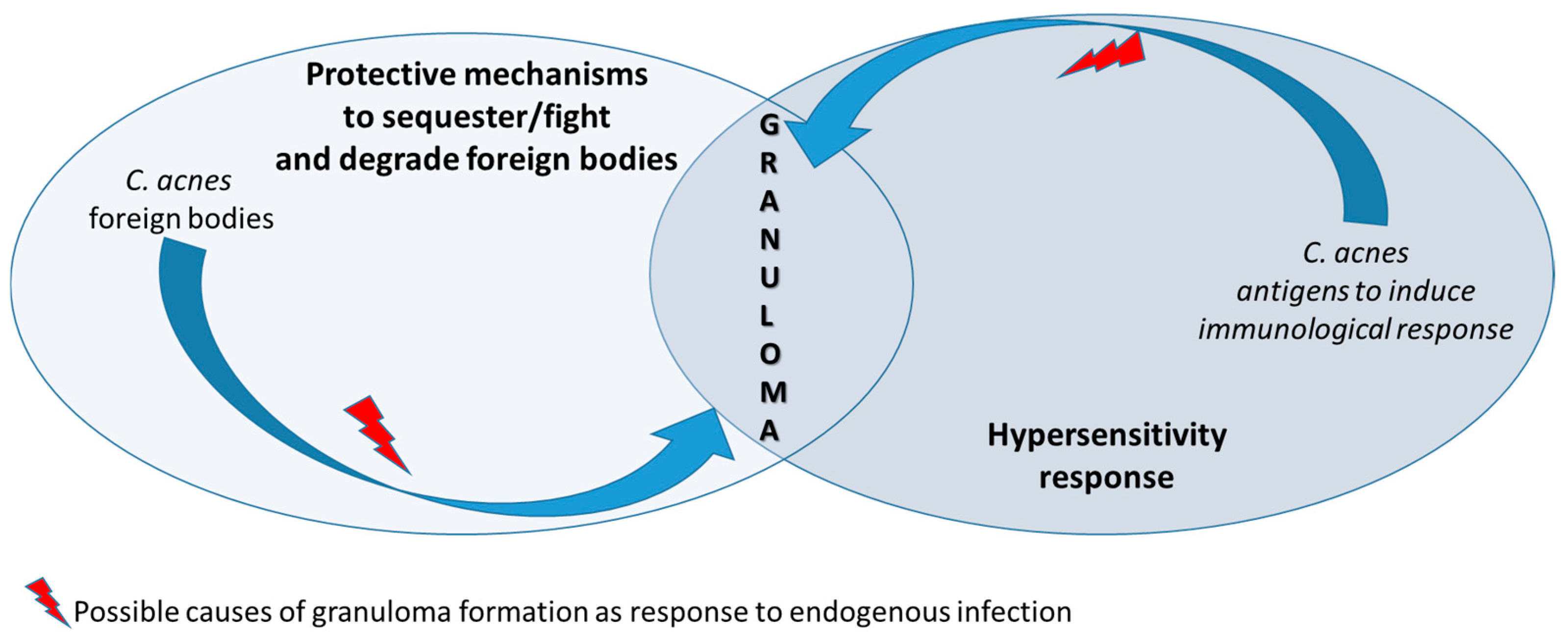
2.3. Association of Cutibacterium acnes with Sarcoidosis
3. Sarcoidosis
3.1. Genetics
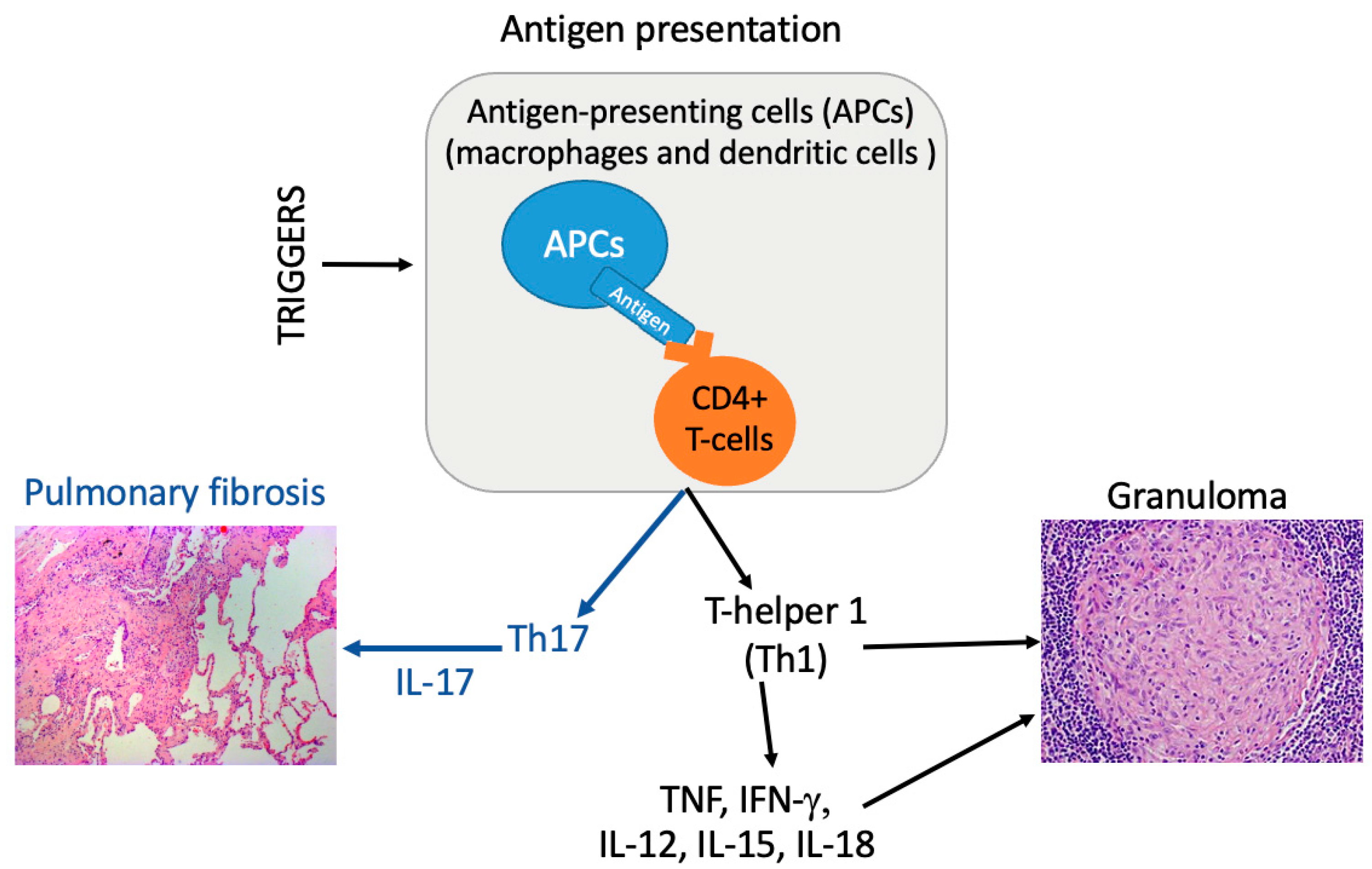
3.2. Immune Pathways
3.3. Etiopathogenesis: What Role for Cutibacterium acnes?
4. Novel Therapeutic Options
5. Cutibacterium acnes, Sarcoidosis, and Malignant Tumors
6. Future Directions for Research and Treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Anti-microbial peptides |
| APC | Antigen-presenting cell |
| PAMPs | Pathogen-associated molecular patterns |
| HA | Hyaluronic acid |
| HYL | Hyaluronate lyase |
| INF-γ | Interferon-gamma |
| KAT | Catalase |
| NLR | NOD-like receptor |
| NLRP3 | NLR pyrin domain-containing 3 |
| PAB | Propionibacterium acnes-specific monoclonal antibody |
| PRRs | Pattern recognition receptors |
| PCa | Prostate cancer |
| PC | Phosphatidylcholine |
| PG | Prostaglandin |
| ROS | Reactive oxygen species |
| RT | Ribotype |
| SM | Sphingomyelin |
| TLR | Toll-like receptors |
| TNF | Tumor necrosis factor |
References
- Brito-Zerón, P.; Pérez-Álvarez, R.; Ramos-Casals, M. Sarcoidosis. Med. Clin. 2022, 159, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Dekio, I.; Sugiura, Y.; Hamada-Tsutsumi, S.; Murakami, Y.; Tamura, H.; Suematsu, M. What Do We See in Spectra?: Assignment of High-Intensity Peaks of Cutibacterium and Staphylococcus Spectra of MALDI-TOF Mass Spectrometry by Interspecies Comparative Proteogenomics. Microorganisms 2021, 9, 1243. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Hall, G.S.; Pratt-Rippin, K.; Meisler, D.M.; Washington, J.A.; Roussel, T.J.; Miller, D. Growth Curve for Propionibacterium acnes. Curr. Eye Res. 1994, 13, 465–466. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; NISC Comparative Sequencing Program; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A wave of regulatory t cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef]
- Borkowski, A.W.; Gallo, R.L. The Coordinated Response of the Physical and Antimicrobial Peptide Barriers of the Skin. J. Investig. Dermatol. 2011, 131, 285–287. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Assa, M.; Mruwat, N.; Sailer, M.; Regmi, S.; Kridin, K. Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions. Microorganisms 2024, 12, 2601. [Google Scholar] [CrossRef]
- McDowell, A.; Valanne, S.; Ramage, G.; Tunney, M.M.; Glenn, J.V.; McLorinan, G.C.; Bhatia, A.; Maisonneuve, J.F.; Lodes, M.; Persing, D.H.; et al. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J. Clin. Microbiol. 2005, 43, 326–334. [Google Scholar] [CrossRef]
- Davidsson, S.; Soderquist, B.; Elgh, F.; Olsson, J.; Andren, O.; Unemo, M.; Mölling, P. Multilocus sequence typing and repetitive-sequence-based PCR (DiversiLab) for molecular epidemiological characterization of Propionibacterium acnes isolates of heterogeneous origin. Anaerobe 2012, 18, 392–399. [Google Scholar] [CrossRef]
- Lomholt, H.B.; Kilian, M. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS ONE 2010, 5, e12277. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Gao, A.; Barnard, E.; Fink, C.; Murray, P.I.; Dowson, C.G.; Nagy, I.; Lambert, P.A.; Patrick, S. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology 2011, 157, 1990–2003. [Google Scholar] [CrossRef]
- Mak, T.N.; Yu, S.H.; De Marzo, A.M.; Bruggemann, H.; Sfanos, K.S. Multilocus sequence typing (MLST) analysis of Propionibacterium acnes isolates from radical prostatectomy specimens. Prostate 2013, 73, 770–777. [Google Scholar] [CrossRef]
- Fassi Fehri, L.; Mak, T.N.; Laube, B.; Brinkmann, V.; Ogilvie, L.A.; Mollenkopf, H.; Lein, M.; Schmidt, T.; Meyer, T.F.; Brüggemann, H. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int. J. Med. Microbiol. 2011, 301, 69–78. [Google Scholar] [CrossRef]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.E.; Corvec, S.; Borens, O.; Trampuz, A. Propionibacterium acnes: An underestimated pathogen in implant-associated infections. Biomed Res. Int. 2013, 2013, 804391. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Molling, P.; Rider, J.R.; Unemo, M.; Karlsson, M.G.; Carlsson, J.; Andersson, S.O.; Elgh, F.; Söderquis, B.; Andrén, O. Frequency and typing of Propionibacterium acnes in prostate tissue obtained from men with and without prostate cancer. Infect. Agents Cancer 2016, 11, 26. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and Acne Vulgaris: A Brief Look at the Latest Updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Renz, N.; Mudrovcic, S.; Perka, C.; Trampuz, A. Orthopedic Implant-Associated Infections Caused by Cutibacterium spp.—A Remaining Diagnostic Challenge. PLoS ONE 2018, 13, e0202639. [Google Scholar] [CrossRef]
- Teramoto, K.; Okubo, T.; Yamada, Y.; Sekiya, S.; Iwamoto, S.; Tanaka, K. Classification of Cutibacterium acnes at phylotype level by MALDI-MS proteotyping. Proc. Jpn. Acad. Ser. B 2019, 95, 612–623. [Google Scholar] [CrossRef]
- Leheste, J.R.; Ruvolo, K.E.; Chrostowski, J.E.; Rivera, K.; Husko, C.; Miceli, A.; Selig, M.K.; Brüggemann, H.; Torres, G.P. acnes-driven disease pathology: Current knowledge and future directions. Front. Cell Infect. Microbiol. 2017, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, A.; Bromke, M.A.; Gamian, A.; Paściak, M. Comprehensive lipidomic analysis of the genus Cutibacterium. mSphere 2024, 9, e0005424. [Google Scholar] [CrossRef] [PubMed]
- Both, A.; Huang, J.; Hentschke, M.; Tobys, D.; Christner, M.; Klatte, T.O.; Seifert, H.; Aepfelbacher, M.; Rohde, H. Genomics of Invasive Cutibacterium acnes Isolates from Deep-Seated Infections. Microbiol. Spectr. 2023, 11, e0474022. [Google Scholar] [CrossRef]
- Petersson, F.; Kilsgård, O.; Shannon, O.; Lood, R. Platelet activation and aggregation by the opportunistic pathogen Cutibacterium (Propionibacterium) acnes. PLoS ONE 2018, 13, e0192051. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Perry, A.L.; Lambert, P.A.; Patrick, S. A new phylogenetic group of Propionibacterium acnes. J. Med. Microbiol. 2008, 57, 218–224. [Google Scholar] [CrossRef]
- Geiger, O.; López-Lara, I.M.; Sohlenkamp, C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 503–513. [Google Scholar] [CrossRef]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of Sphingolipids in Microbial Pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef]
- Nazipi, S.; Stødkilde, K.; Scavenius, C.; Brüggemann, H. The Skin Bacterium Propionibacterium acnes Employs Two Variants of Hyaluronate Lyase with Distinct Properties. Microorganisms 2017, 5, 57. [Google Scholar] [CrossRef]
- McDowell, A.; Nagy, I.; Magyari, M.; Barnard, E.; Patrick, S. The Opportunistic Pathogen Propionibacterium acnes: Insights into Typing, Human Disease, Clonal Diversification and CAMP Factor Evolution. PLoS ONE 2013, 8, e70897. [Google Scholar] [CrossRef] [PubMed]
- Marion, C.; Stewart, J.M.; Tazi, M.F.; Burnaugh, A.M.; Linke, C.M.; Woodiga, S.A.; King, S.J. Streptococcus pneumoniae Can Utilize Multiple Sources of Hyaluronic Acid for Growth. Infect. Immun. 2012, 80, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.N.; Muto, J.; Nizet, V.; Gallo, R.L. Hyaluronan Breakdown Contributes to Immune Defense Against Group A Streptococcus. J. Biol. Chem. 2014, 289, 26914–26921. [Google Scholar] [CrossRef]
- Yorozu, P.; Furukawa, A.; Uchida, K.; Akashi, T.; Kakegawa, T.; Ogawa, T.; Minami, J.; Suzuki, Y.; Awano, N.; Furusawa, H.; et al. Propionibacterium acnes catalase induces increased Th1 immune response in sarcoidosis patients. Respir. Investig. 2015, 53, 161–169. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Castela, M.; Marcelin, A.G.; Calvez, V.; Dupin, N. Characterization of a Cutibacterium acnes Camp Factor 1-Related Peptide as a New TLR-2 Modulator in In Vitro and Ex Vivo Models of Inflammation. Int. J. Mol. Sci. 2022, 23, 5065. [Google Scholar] [CrossRef]
- McCaskill, J.G.; Chason, K.D.; Hua, X.; Neuringer, I.P.; Ghio, A.J.; Funkhouser, W.K.; Tilley, S.L. Pulmonary immune responses to Propionibacterium acnes in C57bl/6 and BALB/c mice. Am. J. Respir. Cell Mol. Biol. 2006, 35, 347–356. [Google Scholar] [CrossRef]
- Spittaels, K.-J.; Van Uytfanghe, K.; Zouboulis, C.C.; Stove, C.; Crabb’e, A.; Coenye, T. Porphyrins produced by acneic Cutibacterium acnes strains activate the inflammasome by inducing K+ leakage. iScience 2021, 24, 102575. [Google Scholar] [CrossRef] [PubMed]
- Chronnell, C.M.; Ghali, L.R.; Ali, R.S.; Quinn, A.G.; Holland, D.B.; Bull, J.J.; Cunliffe, W.J.; McKay, I.A.; Philpott, M.P.; Müller-Röver, S. Human beta defensin-1 and -2 expression in human pilosebaceous units: Upregulation in acne vulgaris lesions. J. Investig. Dermatol. 2001, 117, 1120–1125. [Google Scholar] [CrossRef]
- Kalis, C.; Gumenscheimer, M.; Freudenberg, N.; Tchaptchet, S.; Fejer, G.; Heit, A.; Akira, S.; Galanos, C.; Freudenberg, M.A. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 2005, 174, 4295–4300. [Google Scholar] [CrossRef]
- Grange, P.A.; Chéreau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef]
- Girvan, R.C.; Knight, D.A.; O’loughlin, C.J.; Hayman, C.M.; Hermans, I.F.; Webster, G.A. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine 2011, 29, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.-T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, S.R.; Tan, B.; Li, H.; Ochoa, M.T.; Liu, P.T.; Sharfstein, S.E.; Graeber, T.G.; Sieling, P.A.; Liu, Y.J.; Rea, T.H.; et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005, 11, 653–660. [Google Scholar] [CrossRef]
- Graham, G.M.; Farrar, M.D.; Cruse-Sawyer, J.E.; Holland, K.T.; Ingham, E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br. J. Dermatol. 2004, 150, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Lee, H.G.; Bae, I.-H.; Kim, W.; Park, J.; Lee, T.R.; Cho, E.G. Propionibacterium acnes-derived extracellular vesicles promote acne-like phenotypes in human epidermis. J. Investig. Dermatol. 2018, 138, 1371–1379. [Google Scholar] [CrossRef]
- Thoraval, L.; Varin-Simon, J.; Ohl, X.; Velard, F.; Reffuveille, F.; Tang-Fichaux, M. Cutibacterium acnes and its complex host interaction in prosthetic joint infection: Current insights and future directions. Res. Microbiol. 2025, 17, 104265. [Google Scholar] [CrossRef]
- Abe, C.; Iwai, K.; Mikami, R.; Hosoda, Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1984, 256, 541–547. [Google Scholar] [CrossRef]
- Homma, J.Y.; Abe, C.; Chosa, H.; Ueda, K.; Saegusa, J.; Nakayama, M.; Homma, H.; Washizaki, M.; Okano, H. Bacteriological investigation on biopsy specimens from patients with sarcoidosis. Jpn. J. Exp. Med. 1978, 48, 251–255. [Google Scholar]
- Hiramatsu, J.; Kataoka, M.; Nakata, Y.; Okazaki, K.; Tada, S.; Tanimoto, M.; Eishi, Y. Propionibacterium acnes DNA detected in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis. Vasc. Diffuse. Lung. Dis. 2003, 20, 197–203. [Google Scholar]
- Inoue, Y.; Suga, M. Granulomatous diseases and pathogenic microorganism. Kekkaku 2008, 83, 115–130. (In Japanese) [Google Scholar]
- Isshiki, T.; Homma, S.; Eishi, Y.; Yabe, M.; Koyama, K.; Nishioka, Y.; Yamaguchi, T.; Uchida, K.; Yamamoto, K.; Ohashi, K.; et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms 2021, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Akata, K.; Yamasaki, K.; Nemoto, K.; Ikegami, H.; Kawaguchi, T.; Noguchi, S.; Kawanami, T.; Fukuda, K.; Mukae, H.; Yatera, K. Sarcoidosis Associated with Enlarged Mediastinal Lymph Nodes with the Detection of Streptococcus gordonii and Cutibacterium acnes Using a Clone Library Method. Intern. Med. 2024, 63, 299–304. [Google Scholar] [CrossRef]
- Eishi, Y. Potential Association of Cutibacterium acnes with Sarcoidosis as an Endogenous Hypersensitivity Infection. Microorganisms 2023, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Ryu, J.H.; Matteson, E.L. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 358–375. [Google Scholar] [CrossRef]
- Inaoka, P.T.; Shono, M.; Kamada, M.; Espinoza, J.L. Host-microbe interactions in the pathogenesis and clinical course of sarcoidosis. J. Biomed. Sci. 2019, 26, 45. [Google Scholar] [CrossRef]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef]
- Rybicki, B.A.; Iannuzzi, M.C.; Frederick, M.M.; Thompson, B.W.; Rossman, M.D.; Bresnitz, E.A.; Terrin, M.L.; Moller, D.R.; Barnard, J.; Baughman, R.P.; et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am. J. Respir. Crit. Care Med. 2001, 164, 2085–2091. [Google Scholar] [CrossRef]
- Rossides, M.; Grunewald, J.; Eklund, A. Familial aggregation and heritability of sarcoidosis: A Swedish nested case−control study. Eur. Respir. J. 2018, 52, 1800385. [Google Scholar] [CrossRef]
- Wijnen, P.A.; Voorter, C.E.; Nelemans, P.J.; Verschakelen, J.A.; Bekers, O.; Drent, M. Butyrophilin-like 2 in pulmonary sarcoidosis: A factor for susceptibility and progression? Hum. Immunol. 2011, 72, 342–347. [Google Scholar] [CrossRef]
- Wolin, A.; Lahtela, E.L.; Anttila, V.; Petrek, M.; Grunewald, J.; van Moorsel, C.H.M.; Eklund, A.; Grutters, J.C.; Kolek, V.; Mrazek, F.; et al. SNP Variants in Major Histocompatibility Complex Are Associated with Sarcoidosis Susceptibility—A Joint Analysis in Four European Populations. Front. Immunol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Jamilloux, Y.; Cohen-Aubart, F.; Chapelon-Abric, C.; Maucort-Boulch, D.; Marquet, A.; Pérard, L.; Bouillet, L.; Deroux, A.; Abad, S.; Bielefeld, P.; et al. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin. Arthritis Rheum. 2017, 47, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Nuñez, G. The NOD: A signaling module that regulates apoptosis and host defense against pathogens. Oncogene 2001, 20, 6473–6481. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Moller, D.R.; Rybicki, B.A.; Hamzeh, N.Y.; Montgomery, C.G.; Chen, E.S.; Drake, W.; Fontenot, A.P. Genetic, immunologic, and environmental basis of sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14, S429–S436. [Google Scholar] [CrossRef]
- Eishi, Y.; Suga, M.; Ishige, I.; Kobayashi, D.; Yamada, T.; Takemura, T.; Takizawa, T.; Koike, M.; Kudoh, S.; Costabel, U.; et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J. Clin. Microbiol. 2002, 40, 198–204. [Google Scholar] [CrossRef]
- Tanabe, T.; Ishige, I.; Suzuki, Y.; Aita, Y.; Furukawa, A.; Ishige, Y.; Uchida, K.; Suzuki, T.; Takemura, T.; Ikushima, S.; et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2006, 1762, 794–801. [Google Scholar] [CrossRef]
- Ragusa, F. Sarcoidosis and Th1 chemokines. Clin. Ter. 2015, 166, e72–e76. [Google Scholar]
- Georas, S.N.; Chapman, T.J.; Crouser, E.D. Sarcoidosis and T-helper cells. Th1, Th17, or Th17.1? Am. J. Respir. Crit. Care Med. 2016, 193, 1198–1200. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, L.; Wang, Y.; Aimurola, H.; Zhao, Y.; Li, S.; Xu, Z. The Circulating Treg/Th17 Cell Ratio Is Correlated with Relapse and Treatment Response in Pulmonary Sarcoidosis Patients After Corticosteroid Withdrawal. PLoS ONE 2016, 11, e0148207. [Google Scholar] [CrossRef]
- Song, J.; Zhao, M.; Li, Q.; Lu, L.; Zhou, Y.; Zhang, Y.; Chen, T.; Tang, D.; Zhou, N.; Yin, C.; et al. IL-17A Can Promote Propionibacterium acnes-Induced Sarcoidosis-Like Granulomatosis in Mice. Front. Immunol. 2019, 10, 1923. [Google Scholar] [CrossRef]
- Ishige, I.; Usui, Y.; Takemura, T.; Eishi, Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999, 354, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, Y.R.; Zhang, Y.; Du, S.S.; Baughman, R.P.; Li, H.P. Real-time quantitative reverse transcription—Polymerase chain reaction to detect propionibacterial ribosomal RNA in the lymph nodes of Chinese patients with sarcoidosis. Clin. Exp. Immunol. 2015, 181, 511–517. [Google Scholar] [CrossRef]
- Furusawa, H.; Suzuki, Y.; Miyazaki, Y.; Inase, N.; Eishi, Y. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir. Investig. 2012, 50, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Furukawa, A.; Yoneyama, A.; Furusawa, H.; Kobayashi, D.; Ito, T.; Yamamoto, K.; Sekine, M.; Miura, K.; Akashi, T.; et al. Propionibacterium acnes-Derived Circulating Immune Complexes in Sarcoidosis Patients. Microorganisms 2021, 9, 2194. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Eishi, Y.; Tahara, N.; Asakura, M.; Sakamoto, N.; Nakamura, K.; Takaya, Y.; Nakamura, T.; Yazaki, Y.; Yamaguchi, T.; et al. Japanese antibacterial drug management for cardiac sarcoidosis (J-ACNES): A multicenter, open-label, randomized, controlled study. J. Arrhythmia. 2018, 34, 520–526. [Google Scholar] [CrossRef]
- Deknuydt, F.; Roquilly, A.; Cinotti, R.; Altare, F.; Asehnoune, K. An in vitro model of mycobacterial granuloma to investigate the immune response in brain-injured patients. Crit. Care Med. 2013, 41, 245–254. [Google Scholar] [CrossRef]
- Landgraeber, S.; von Knoch, M.; Löer, F.; Brankamp, J.; Tsokos, M.; Grabellus, F.; Schmid, K.W.; Totsch, M. Association between apoptosis and CD4(+)/CD8(+) T-lymphocyte ratio in aseptic loosening after total hip replacement. Int. J. Biol. Sci. 2009, 5, 182–191. [Google Scholar] [CrossRef]
- Portillo, M.E.; Salvadó, M.; Alier, A.; Sorli, L.; Martínez, S.; Horcajada, J.P.; Puig, L. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin. Orthop. Relat. Res. 2013, 471, 3672–3678. [Google Scholar] [CrossRef]
- Loke, W.S.; Herbert, C.; Thomas, P.S. Sarcoidosis: Immunopathogenesis and Immunological Markers. Int. J. Chronic Dis. 2013, 2013, 928601. [Google Scholar] [CrossRef] [PubMed]
- Kistowska, M.; Meier, B.; Proust, T.; Feldmeyer, L.; Cozzio, A.; Kuendig, T.; Contassot, E.; French, L.E. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J. Investig. Dermatol. 2015, 135, 110–118. [Google Scholar] [CrossRef]
- Aubin, G.G.; Ada Da Silva, G.; Eishi, Y.; Jacqueline, C.; Altare, F.; Corvec, S.; Asehnoune, K. Immune discrepancies during in vitro granuloma formation in response to Cutibacterium (formerly Propionibacterium) acnes infection. Anaerobe 2017, 48, 172–176. [Google Scholar] [CrossRef]
- Yamamoto, K.; Uchida, K.; Furukawa, A.; Tamura, T.; Ishige, Y.; Negi, M.; Kobayashi, D.; Ito, T.; Kakegawa, T.; Hebisawa, A.; et al. Catalase expression of Propionibacterium acnes may contribute to intracellular persistence of the bacterium in sinus macrophages of lymph nodes affected by sarcoidosis. Immunol. Res. 2019, 67, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Sakhamuru, S.; Kambampati, S.; Wasim, S.; Kukkar, V.; Malik, B.H. The Role of Propionibacterium acnes in the Pathogenesis of Sarcoidosis and Ulcerative Colitis: How This Connection May Inspire Novel Management of These Conditions. Cureus 2020, 12, e10812. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, E.; Ando, M.; Fukami, T.; Nureki, S.I.; Eishi, Y.; Kumamoto, T. Minocycline for the treatment of sarcoidosis: Is the mechanism of action immunomodulating or antimicrobial effect? Clin. Rheumatol. 2008, 27, 1195–1197. [Google Scholar] [CrossRef]
- Takemori, N.; Nakamura, M.; Kojima, M.; Eishi, Y. Successful treatment in a case of Propionibacterium acnes-associated sarcoidosis with clarithromycin administration: A case report. J. Med. Case Rep. 2014, 8, 15. [Google Scholar] [CrossRef]
- Bachelez, H.; Senet, P.; Candranel, J.; Kaoukhov, A.; Dubertret, L. The use of tetracyclines for the treatment of sarcoidosis. Arch. Dermatol. 2001, 137, 69–73. [Google Scholar] [CrossRef]
- Sheu, J.; Saavedra, A.P.; Mostaghimi, A. Rapid response of tattoo-associated cutaneous sarcoidosis to minocycline: Case report and review of the literature. Dermatol. Online J. 2014, 20, 13030/qt6dd1m2j9. [Google Scholar] [CrossRef]
- Hirai, Y.; Hamada, Y.; Sasaki, S.; Suzuki, M.; Ito, Y.; Katayama, K.; Uno, H.; Nakada, T.; Kitami, Y.; Uchida, K.; et al. Sarcoidosis and sarcoidal foreign body reaction after permanent eye makeup application: Analysis by immunohistochemistry with commercially available antibodies specific to Cutibacterium acnes and Mycobacteria. J. Cutan. Pathol. 2022, 49, 651–657. [Google Scholar] [CrossRef]
- Nagai, S.; Yokomatsu, T.; Tanizawa, K.; Ikezoe, K.; Handa, T.; Ito, Y.; Ogino, S.; Izumi, T. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern. Med. 2014, 53, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical identification of Propionibacterium acnes in granuloma and inflammatory cells of myocardial tissues obtained from cardiac sarcoidosis patients. PLoS ONE 2017, 12, e0179980. [Google Scholar] [CrossRef]
- Hiraga, Y.; Omichi, M.; Yamada, G. The effect of antibacterial drug for sarcoidosis. In Ministry of Health and Welfare Specific disease Diffuse Lung Disease Research Team Research Report; Ministry of Health, Labour and Welfare: Tokyo, Japan, 1995; pp. 174–175. [Google Scholar]
- Smith, C.S.; Aerts, A.; Saunderson, P.; Kawuma, J.; Kita, E.; Virmond, M. Multidrug therapy for leprosy: A game changer on the path to elimination. Lancet Infect. Dis. 2017, 17, e293–e297. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Chintalapati, S.S.V.V.; Iwata, S.; Miyahara, M.; Miyako, E. Tumor-isolated Cutibacterium acnes as an effective tumor suppressive living drug. Biomed. Pharmacother. 2024, 170, 116041. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Sutton, R.C.; Conley, F.K. Effect of intracerebrally injected Corynebacterium parvum on the development and growth of metastatic brain tumor in mice. Neurosurgery 1989, 25, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Cantrell, J.L.; Lichtenstein, A.K.; Hacker, N.F.; Knox, R.M.; Nieberg, R.K.; Poth, T.; Elashoff, R.M.; Lagasse, L.D.; Zighelboim, J. Immunotherapy with biochemically dissociated fractions of Propionibacterium acnes in a murine ovarian cancer model. Cancer Res. 1984, 44, 1871–1875. [Google Scholar]
- Roszkowski, W.; Ko, H.L.; Szmigielski, S.; Jeljaszewicz, J.; Pulverer, G. The correlation of susceptibility of different Propionibacterium strains to macrophage killing and antitumor activity. Med. Microbiol. Immunol. 1980, 169, 1–8. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tsuchida, T.; Tsuji, Y.; Hamaoka, T. Preventive effect of Propionibacterium acnes on metastasis in mice rendered tolerant to tumor-associated transplantation antigens. GANN Jpn. J. Cancer Res. 1980, 71, 692–698. [Google Scholar]
- Tsuda, K.; Yamanaka, K.; Linan, W.; Miyahara, Y.; Akeda, T.; Nakanishi, T.; Kitagawa, H.; Kakeda, M.; Kurokawa, I.; Shiku, H.; et al. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS ONE 2011, 6, e29020. [Google Scholar] [CrossRef]
- Talib, W.H.; Saleh, S. Propionibacterium acnes Augments Antitumor, Anti-Angiogenesis and Immunomodulatory Effects of Melatonin on Breast Cancer Implanted in Mice. PLoS ONE 2015, 10, e0124384. [Google Scholar] [CrossRef] [PubMed]
- Fanning, M.M.; Kazura, J.W. Lack of biological significance of in vitro Brugia malayi microfilarial cytotoxicity mediated by Propionibacterium acnes (“Corynebacterium parvum”)-and Mycobacterium bovis BCG-activated macrophages. Infect. Immun. 1986, 52, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.M.; Pasciuto, G.; Verrecchia, E.; Sicignano, L.L.; Gerardino, L.; Massaro, M.G.; Urbani, A.; Manna, R. Sarcoidosis and Cancer: The Role of the Granulomatous Reaction as a Double-Edged Sword. J. Clin. Med. 2024, 13, 5232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Li, G.; Jin, G.; Xu, L.; Li, Y.; Wei, P.; Zhang, L. Leprosy: Treatment, prevention, immune response and gene function. Front. Immunol. 2024, 15, 1298749. [Google Scholar] [CrossRef]


| Cutibacterium acnes |
|---|
| Aerotolerant anaerobe |
| Non-spore-forming |
| Gram-positive |
| Rod bacterium, diphtheroid, or coryneform (slightly curved, with a width of 0.4–0.7 µm and a length of 3–5 µm) |
| Cell wall consists of phosphatidylinositol, triacylglycerol, other lipids, and peptidoglycan with L-acid, L-diaminopelic acid, and D-alanine in the peptide chain |
| It expresses the following proteins (for oxidative phosphorylation): NAPDH dehydrogenase/complex I, cytochrome c reductase, cytochrome c oxidase, and FoF1-type ATP synthase |
| Indigenous to skin and mucosal surfaces: it predominantly resides in the pilo-sebaceous follicle of the skin and also normally resides in the oral cavity, conjunctiva, external ear canal, and gut |
| Slow-growing (5-to-7 days with a division time of about five hours) |
| The genus comprises five species (Cutibacterium acnes, Cutibacterium avidum, Cutibacterium granulosum, Cutibacterium namnetense, and Cutibacterium modestum) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Francesco, A.M.; Pasciuto, G.; Verrecchia, E.; Sicignano, L.L.; Gerardino, L.; Rigante, D.; Manna, R. The Role of Cutibacterium acnes in the Etiopathogenesis of Sarcoidosis: Current Insights and Future Study Directions. Int. J. Mol. Sci. 2025, 26, 6652. https://doi.org/10.3390/ijms26146652
Di Francesco AM, Pasciuto G, Verrecchia E, Sicignano LL, Gerardino L, Rigante D, Manna R. The Role of Cutibacterium acnes in the Etiopathogenesis of Sarcoidosis: Current Insights and Future Study Directions. International Journal of Molecular Sciences. 2025; 26(14):6652. https://doi.org/10.3390/ijms26146652
Chicago/Turabian StyleDi Francesco, Angela Maria, Giuliana Pasciuto, Elena Verrecchia, Ludovico Luca Sicignano, Laura Gerardino, Donato Rigante, and Raffaele Manna. 2025. "The Role of Cutibacterium acnes in the Etiopathogenesis of Sarcoidosis: Current Insights and Future Study Directions" International Journal of Molecular Sciences 26, no. 14: 6652. https://doi.org/10.3390/ijms26146652
APA StyleDi Francesco, A. M., Pasciuto, G., Verrecchia, E., Sicignano, L. L., Gerardino, L., Rigante, D., & Manna, R. (2025). The Role of Cutibacterium acnes in the Etiopathogenesis of Sarcoidosis: Current Insights and Future Study Directions. International Journal of Molecular Sciences, 26(14), 6652. https://doi.org/10.3390/ijms26146652







