Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review
Abstract
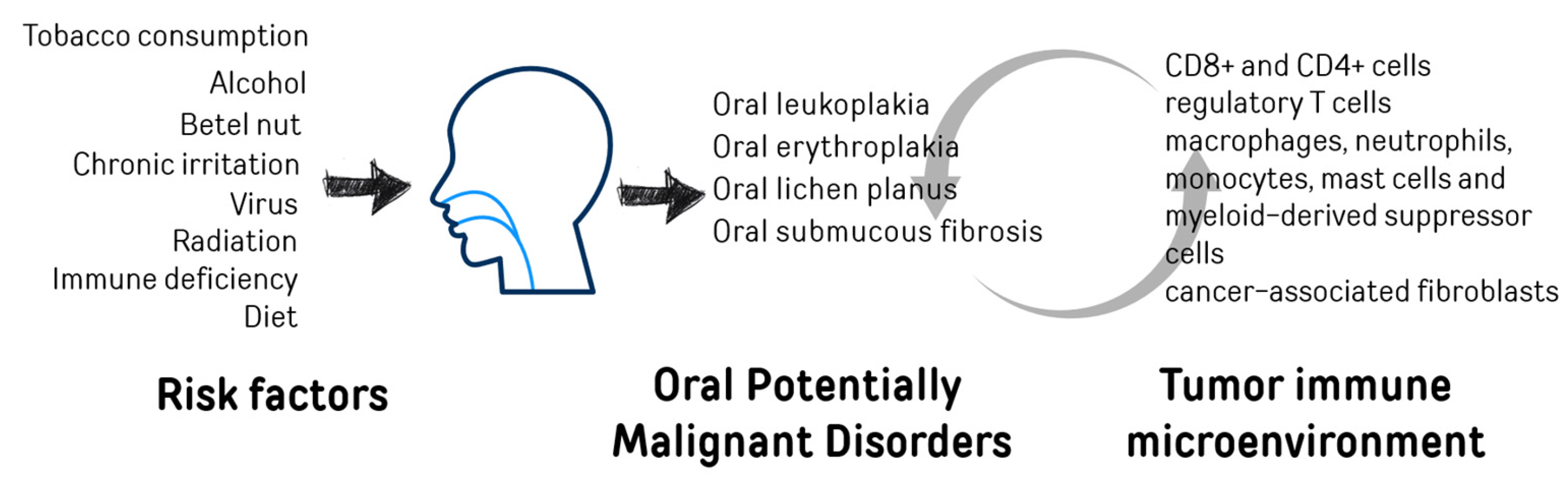
1. Oral Cancer and Oral Potentially Malignant Disorders (OPMDs)
2. Tumor Immune Microenvironment–TIME
3. Tumor Associated Myeloid-Derived Suppressor Cells (MDSCs) and Dendritic Cells (DCs)
4. Tumor-Associated Mast Cells
5. Tumor-Infiltrating Lymphocytes (TILs)
6. Tumor-Associated Macrophages
7. Tumor-Associated Neutrophils (TANs)
8. Cancer Associated Fibroblast (CAF)
9. Enhancing Tumor Treatment Through Immune Modulation and Combination Therapies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4NQO | 4-Nitroquinoline 1-oxide |
| ADU-S100 | STING Pathway Agonist |
| AP2M1 | Adaptor related protein complex 2 mu 1 subunit |
| Arg1 | Arginase 1 |
| BRD4 | Bromodomain Containing Protein 4 |
| CAFs | Cancer-Associated Fibroblasts |
| Cal-27 | Human oral squamous cell carcinoma (OSCC) cell line. |
| CATSPER1 | Cation channel sperm-associated 1 |
| CCL2/CCR2 | Chemokine (C-C Motif) Ligand 2 / Receptor 2 |
| CCL9/CCR1 | Chemokine (C-C Motif) Ligand 9 / Receptor 1 |
| CCR7 | CC Chemokine Receptor 7 |
| CD4+ | Cluster of Differentiation 4 Positive |
| CD45RO+ | Memory T Cell Marker |
| CD57+ | Natural Killer Cell Marker |
| CD8+ | Cluster of Differentiation 8 Positive |
| Ce6 | Chlorin e6 |
| CMTM6 | CKLF-like MARVEL Transmembrane Domain-containing 6. |
| CRT | Chemoradiotherapy |
| CTLs | Cytotoxic T Lymphocytes |
| CXCL14 | C-X-C Motif Chemokine Ligand 14 |
| CXCR1/2/4 | C-X-C Chemokine Receptor 1/2/4 |
| DCs | Dendritic Cells |
| DOX | Doxorubicin |
| EGFR | Epidermal Growth Factor Receptor |
| ErbB2 | Erythroblastic Leukemia Viral Oncogene Homolog 2 |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| ESCC | esophageal squamous cell carcinoma |
| FDA | Food and Drug Administration |
| FISH | Fluorescence In Situ Hybridization. |
| Foxp3 | Forkhead box P3 |
| FP score | Ferroptosis Score |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HFD | high-fat diet |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HSP90B1 | Heat shock protein 90 beta family member 1 |
| HSV-1 | Herpes Simplex Virus Type 1 |
| IFN-β | Interferon beta |
| IFN-γ | Interferon-gamma |
| IL-23/IL-12/IL-10/IL-6/IL-1 | Interleukin-23/12/10/6/1 |
| iNOS | inducible Nitric Oxide Synthase |
| JAK | Janus Kinase |
| JQ1 | BET Bromodomain Inhibitor |
| KIT | KIT proto-oncogene, receptor tyrosine kinase |
| LAT | Linker for activation of T cells |
| LINC00996 | Long intergenic non-protein coding RNA 996 |
| M-MDSCs | monocytes- derived Myeloid-Derived Suppressor Cells |
| M1 | anti-tumor macrophages |
| M2 | pro-tumor macrophages |
| MCP1 | monocyte chemotactic protein-1 |
| MCs | Mast Cells |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MHC I | Major Histocompatibility Complex Class I |
| MHC II | Major Histocompatibility Complex Class II |
| MIP-1α | macrophage inflammatory protein-1alpha |
| MMPs | Matrix Metalloproteinases |
| N1 | anti-tumor neutrophils |
| N2 | pro-tumor neutrophils |
| nab-paclitaxel | Nanoparticle Albumin-Bound Paclitaxel |
| NETs | Neutrophil Extracellular Traps |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| nmPg | Nanomedicine from Porphyromonas gingivalis |
| NOS | oxide synthase |
| NPsR | Nanoparticles loaded with Rose Bengal |
| OLE | Oral Lupus Erythematosus |
| OLP | Oral lichen planus |
| OPMDs | Oral Potentially Malignant Disorders |
| OSCC | Oral Squamous Cell Carcinoma |
| OSF | Oral Submucous Fibrosis |
| PAMPs | Pathogen-Associated Molecular Patterns. |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PD-L2 | Programmed Death-Ligand 2 |
| PDT | Photodynamic Therapy |
| PI3K/AKT | Phosphoinositide 3-Kinase / Protein Kinase B |
| PLIN2 | adipose differentiation-related protein |
| PMN-MDSCs | Polymorphonuclear Myeloid-Derived Suppressor Cells |
| PTT | Photothermal Therapy |
| PVL | Proliferative Verrucous Leukoplakia |
| RAB32 | RAB32, member RAS oncogene family |
| RANTES | Regulated upon Activation, Normal T cell Expressed and Secreted |
| RGD peptide | Arginine-Glycine-Aspartic Acid Peptide |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| S-1 | Oral Fluoropyrimidine Anti-Cancer Drug |
| SCC25 | human oral squamous cell carcinoma (OSCC) cell line |
| SMYD3 | ET and MYND domain containing 3 |
| SOCS1 | Suppressor of cytokine signaling 1 |
| SP | Spirulina platensis |
| STAT3, 6 | Signal Transducer and Activator of Transcription 3, 6 |
| SX-682 | a small-molecule dual inhibitor of the chemokine receptors CXCR1 and CXCR2 |
| TAMs | Tumor-Associated Macrophages |
| TANs | Tumor-Associated Neutrophils |
| TGF-β | Transforming Growth Factor Beta |
| TH2/17 | T helper type 2/17 cells |
| THBS1 | Thrombospondin 1 |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TILs | Tumor-Infiltrating Lymphocytes |
| TIME | Tumor Immune Microenvironment |
| TiO2@Ru@siRNA | Titanium Dioxide-Ruthenium Conjugated Small Interfering RNA |
| TNF-α | Tumor Necrosis Factor Alpha |
| Tregs | Regulatory T Cells |
| TβRII | Transforming Growth Factor Beta Receptor Type II |
| VEGF | Vascular Endothelial Growth Factor |
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Pisani, P.; Parkin, D.M.; GLOBOCAN 2002. Cancer incidence, mortality and prevalence worldwide. In IARC Cancer Base (2002 Estimates); IARC Press: Lyon, France, 2004. [Google Scholar]
- Llewellyn, C.; Johnson, N.; Warnakulasuriya, K. Risk factors for squamous cell carcinoma of the oral cavity in young people—a comprehensive literature review. Oral Oncol. 2001, 37, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.; Mclaughlin, J.; Winn, D.; Austin, D.; Greenberg, R.; Prestonmartin, S.; Bernstein, L.; Schoenberg, J.; Stemhagen, A.; Fraumeni, J. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- Kierce, J.; Shi, Y.; Klieb, H.; Blanas, N.; Xu, W.; Magalhaes, M. Identification of specific clinical risk factors associated with the malignant transformation of oral epithelial dysplasia. Head Neck 2021, 43, 3552–3561. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Food, nutrition and oral cancer. In Food Constituents and Oral Health. Current Status and Future Prospects; Wilson, M., Ed.; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Petridou, E.; Zavras, A.I.; Lefatzis, D.; Dessypris, N.; Laskaris, G.; Dokianakis, G.; Segas, J.; Douglas, C.W.; Diehl, S.R.; Trichopoulos, D. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer 2002, 94, 2981–2988. [Google Scholar] [CrossRef]
- A O’Shaughnessy, J.; Kelloff, G.J.; Gordon, G.B.; Dannenberg, A.J.; Hong, W.K.; Fabian, C.J.; Sigman, C.C.; Bertagnolli, M.M.; Stratton, S.P.; Lam, S.; et al. Treatment and prevention of intraepithelial neoplasia: An important target for accelerated new agent development. Clin. Cancer Res. 2002, 8, 314–346. [Google Scholar]
- Nagao, T.; Ikeda, N.; Warnakulasuriya, S.; Fukano, H.; Yuasa, H.; Yano, M.; Miyazaki, H.; Ito, Y. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol. 2000, 36, 466–470. [Google Scholar] [CrossRef]
- Wang, G.; Pan, C.; Cao, K.; Zhang, J.; Geng, H.; Wu, K.; Wen, J.; Liu, C. Impacts of Cigarette Smoking on the Tumor Immune Microenvironment in Esophageal Squamous Cell Carcinoma. J. Cancer 2022, 13, 413–425. [Google Scholar] [CrossRef]
- Thompson, M.G.; Navarro, F.; Chitsike, L.; Ramirez, L.; Kovacs, E.J.; Watkins, S.K. Alcohol exposure differentially effects anti-tumor immunity in females by altering dendritic cell function. Alcohol 2016, 57, 1–8. [Google Scholar] [CrossRef]
- National Institute of Dental and Craniofacial Research. Oral Cancer 5-Year Survival Rates by Race, Gender, and Stage of Diagnosis. Available online: https://www.nidcr.nih.gov/research/data-statistics/oral-cancer/survival-rates (accessed on 8 July 2021).
- Speight, P.M.; Epstein, J.; Kujan, O.; Lingen, M.W.; Nagao, T.; Ranganathan, K.; Vargas, P. Screening for oral cancer—A perspective from the Global Oral Cancer Forum. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 123, 680–687. [Google Scholar] [CrossRef]
- McCord, C.; Kiss, A.; Magalhaes, M.A.; Leong, I.T.; Jorden, T.; Bradley, G. Oral Squamous Cell Carcinoma Associated with Precursor Lesions. Cancer Prev. Res. 2021, 14, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.M.; Poh, C.F.; Hovan, A.J.; Ng, S.; Rosin, M.P. Evaluation of a suspicious oral mucosal lesion. J. Can. Dent. Assoc. 2008, 74, 275–280. [Google Scholar]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; López, S.P.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Deng, S.; Wang, S.; Shi, X.; Zhou, H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8940. [Google Scholar] [CrossRef]
- Abadeh, A.; Ali, A.A.; Bradley, G.; Magalhaes, M.A. Increase in detection of oral cancer and precursor lesions by dentists Evidence from an oral and maxillofacial pathology service. JADA 2019, 150, 531–539. [Google Scholar] [CrossRef]
- Labarthe, L.; Henriquez, S.; Lambotte, O.; Di Santo, J.P.; Le Grand, R.; Pflumio, F.; Arcangeli, M.-L.; Legrand, N.; Bourgeois, C. Frontline Science: Exhaustion and senescence marker profiles on human T cells in BRGSF-A2 humanized mice resemble those in human samples. J. Leukoc. Biol. 2020, 107, 27–42. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Pierides, C.; Christodoulou, M.-I.; Costeas, P.; Kyriakou, T.-C.; Papageorgis, P. The Role of Tumor-Associated Myeloid Cells in Modulating Cancer Therapy. Front. Oncol. 2020, 10, 899. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Yoshida, S.; Kawai, H.; Eguchi, T.; Sukegawa, S.; Oo, M.W.; Anqi, C.; Takabatake, K.; Nakano, K.; Okamoto, K.; Nagatsuka, H. Tumor Angiogenic Inhibition Triggered Necrosis (TAITN) in Oral Cancer. Cells 2019, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Ali, A.; Magalhaes, M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Sci. Rep. 2020, 10, 9705. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Soares, A.B.; Eymael, D.; Magalhaes, M. Expression of invadopodia markers can identify oral lesions with a high risk of malignant transformation. J. Pathol. Clin. Res. 2021, 7, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef]
- Laliberté, C.; Ng, N.; Eymael, D.; Higgins, K.; Ali, A.; Kiss, A.; Bradley, G.; Magalhaes, M.A.O. Characterization of Oral Squamous Cell Carcinoma Associated Inflammation: A Pilot Study. Front. Oral. Health 2021, 2, 740469. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Pang, X.; Fan, H.-Y.; Tang, Y.-L.; Wang, S.-S.; Cao, M.-X.; Wang, H.-F.; Dai, L.-L.; Wang, K.; Yu, X.-H.; Wu, J.-B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef]
- Kouketsu, A.; Haruka, S.; Kuroda, K.; Hitoshi, M.; Kensuke, Y.; Tsuyoshi, S.; Takahashi, T.; Hiroyuki, K. Myeloid-derived suppressor cells and plasmacytoid dendritic cells are associated with oncogenesis of oral squamous cell carcinoma. J. Oral. Pathol. Med. 2023, 52, 9–19. [Google Scholar] [CrossRef]
- Dar, A.A.; Patil, R.S.; Pradhan, T.N.; Chaukar, D.A.; D’cRuz, A.K.; Chiplunkar, S.V. Myeloid-derived suppressor cells impede T cell functionality and promote Th17 differentiation in oral squamous cell carcinoma. Cancer Immunol. Immunother. 2020, 69, 1071–1086. [Google Scholar] [CrossRef]
- Han, N.; Li, X.; Wang, Y.; Wang, L.; Zhang, C.; Zhang, Z.; Ruan, M.; Zhang, C. Increased tumor-infiltrating plasmacytoid dendritic cells promote cancer cell proliferation and invasion via TNF-α/NF-κB/CXCR-4 pathway in oral squamous cell carcinoma. J. Cancer 2021, 12, 3045–3056. [Google Scholar] [CrossRef]
- Eric, H.; Piersiala, K.; Lagebro, V.; Da Silva, P.F.N.; Petro, M.; Starkhammar, M.; Elliot, A.; Bark, R.; Margolin, G.; Georén, S.K.; et al. High expression of PD-L1 on conventional dendritic cells in tumour-draining lymph nodes is associated with poor prognosis in oral cancer. Cancer Immunol. Immunother. 2024, 73, 165. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xiao, Y.; Yang, Q.-C.; Yang, S.-C.; Yang, L.-L.; Sun, Z.-J. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral Oncol. 2021, 121, 105472. [Google Scholar] [CrossRef] [PubMed]
- A Nguyen, K.; DePledge, L.N.; Bian, L.; Ke, Y.; Samedi, V.; A Berning, A.; Owens, P.; Wang, X.-J.; Young, C.D. Polymorphonuclear myeloid-derived suppressor cells and phosphatidylinositol-3 kinase gamma are critical to tobacco-mimicking oral carcinogenesis in mice. J. Immunother. Cancer 2023, 11, e007110. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431. [Google Scholar] [CrossRef]
- Oo, M.W.; Kawai, H.; Takabatake, K.; Tomida, S.; Eguchi, T.; Ono, K.; Shan, Q.; Ohara, T.; Yoshida, S.; Omori, H.; et al. Resident stroma-secreted chemokine CCL2 governs myeloid-derived suppressor cells in the tumor microenvironment. J. Clin. Investig. 2022, 7, e148960. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, B.; Chen, L.; Lei, W. Mast cell marker gene signature in head and neck squamous cell carcinoma. BMC Cancer 2022, 22, 577. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Reinhardt, L.; Wiechens, B.; Khromov, T.; Schliephake, H.; Brockmeyer, P. Is CCL2 an Important Mediator of Mast Cell–Tumor Cell Interactions in Oral Squamous Cell Carcinoma? Int. J. Mol. Sci. 2023, 24, 3641. [Google Scholar] [CrossRef]
- Jyothsna, M.; Rammanohar, M.; Kumar, K. Histomorphometric Analysis of Angiogenesis using CD31 Immunomarker and Mast Cell Density in Oral Premalignant and Malignant Lesions: A Pilot Study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC37–ZC40. [Google Scholar] [CrossRef]
- Sundararajan, A.; Muthusamy, R.; Siva, K.G.; Harikrishnan, P.; Kumar, S.C.K.; Rathinasamy, S.K. Correlation of Mast Cell and Angiogenesis in Oral Lichen Planus, Dysplasia (Leukoplakia), and Oral Squamous Cell Carcinoma. Rambam Maimonides Med. J. 2021, 12, e0016. [Google Scholar] [CrossRef]
- Parikh, A.; Shin, J.; Faquin, W.; Lin, D.T.; Tirosh, I.; Sunwoo, J.B.; Puram, S.V. Malignant cell-specific CXCL14 promotes tumor lymphocyte infiltration in oral cavity squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e001048. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Wieteska, Ł.; Hinck, C.S.; Yerneni, S.S.; Azambuja, J.H.; Bauer, R.J.; Reichert, T.E.; Hinck, A.P.; Whiteside, T.L. Novel TGFβ Inhibitors Ameliorate Oral Squamous Cell Carcinoma Progression and Improve the Antitumor Immune Response of Anti–PD-L1 Immunotherapy. Mol. Cancer Ther. 2021, 20, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.L.; Korzinkin, M.; Zhavoronkov, A.; Ozerov, I.V.; Walker, M.T.; Higgins, K.; Lingen, M.W.; Izumchenko, E.; Savage, P.A. Effector T cell responses unleashed by regulatory T cell ablation exacerbate oral squamous cell carcinoma. Cell Rep. Med. 2021, 2, 100399. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS Acta Pathol. Microbiol. Et Immunol. Scand. 2018, 126, 732–738. [Google Scholar] [CrossRef]
- Chaves, A.L.F.; Silva, A.G.; Maia, F.M.; Lopes, G.F.M.; De Paulo, L.F.B.; Muniz, L.V.; Dos Santos, H.B.; Soares, J.M.A.; Souza, A.A.; de Oliveira Barbosa, L.A.; et al. Reduced CD8+ T cells infiltration can be associated to a malignant transformation in potentially malignant oral epithelial lesions. Clin. Oral. Investig. 2019, 23, 1913–1919. [Google Scholar] [CrossRef]
- Lahmar, Q.; Keirsse, J.; Laoui, D.; Movahedi, K.; Van Overmeire, E.; Van Ginderachter, J.A. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim. Biophys. Acta 2016, 1865, 23–34. [Google Scholar] [CrossRef]
- Pang, X.; Wang, S.-S.; Zhang, M.; Jiang, J.; Fan, H.-Y.; Wu, J.-S.; Wang, H.-F.; Liang, X.-H.; Tang, Y.-L. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 2021, 70, 1015–1029. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Wang, Y.; Yan, C.; Du, W.-D.; Al-Aroomi, M.A.; Zheng, L.; Lin, S.-F.; Gao, J.-X.; Jiang, S.; Wang, Z.-X.; et al. CC chemokine receptor 7 promotes macrophage recruitment and induces M2-polarization through CC chemokine ligand 19 & 21 in oral squamous cell carcinoma. Discov. Oncol. 2022, 13, 67. [Google Scholar] [CrossRef]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef]
- You, Y.; Tian, Z.; Du, Z.; Wu, K.; Xu, G.; Dai, M.; Wang, Y.; Xiao, M. M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. J. Exp. Clin. Cancer Res. 2022, 41, 10. [Google Scholar] [CrossRef]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Tashiro, K.; Yamashita, Y.; Takahashi, T.; Kumamoto, H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral. Maxillofac. Surg. 2019, 48, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, M.; Koma, Y.; Nishio, M.; Komori, T.; Yokozaki, H. CD163(+) macrophages infiltration correlates with the immunosuppressive cytokine interleukin 10 expression in tongue leukoplakia. Clin. Exp. Dent. Res. 2019, 5, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Battista, R.A.; Pini, G.M.; Finco, A.; Corso, F.; Galli, A.; Arrigoni, G.; Doglioni, C.; Callea, M.; Paccagnella, M.; Porcu, L.; et al. From Tumor Macroenvironment to Tumor Microenvironment: The Prognostic Role of the Immune System in Oral and Lung Squamous Cell Carcinoma. Cancers 2024, 16, 2759. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, X.; Yu, T.; Tang, Q.; Zhou, Z.; Wang, H.; Huang, W.; Huang, T.; Liang, F. Downregulation of VAP-1 in OSCC suppresses tumor growth and metastasis via NF-κB/IL-8 signaling and reduces neutrophil infiltration. J. Oral. Pathol. Med. 2022, 51, 332–341. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Goertzen, C.; Mahdi, H.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047–29063. [Google Scholar] [CrossRef]
- Ska, E.J.O.; Odczyk, B.E.S.; Wawrusiewicz-Kurylonek, N.; Garley, M.; Ratajczak-Wrona, W.; Borys, J.; Towski, A.K. Comparison of B-Cell Activating Factor Expression in Neutrophils in Patients with Potentially Malignant Disorders and Patients with Cancer in the Same Site. Clin. Lab. 2016, 62, 1507–1514. [Google Scholar]
- Moonen, C.G.J.; Hirschfeld, J.; Cheng, L.; Chapple, I.L.C.; Loos, B.G.; Nicu, E.A. Oral Neutrophils Characterized: Chemotactic, Phagocytic, and Neutrophil Extracellular Trap (NET) Formation Properties. Front. Immunol. 2019, 10, 635. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Rubini, C.; Troiano, G.; Lo Muzio, L.; Santarelli, A. Tumour-associated tissue eosinophilia (TATE) in oral squamous cell carcinoma: A comprehensive review. Histol. Histopathol. 2021, 36, 113–122. [Google Scholar] [CrossRef]
- Alkasalias, T.; Moyano-Galceran, L.; Arsenian-Henriksson, M.; Lehti, K. Fibroblasts in the Tumor Microenvironment: Shield or Spear? Int. J. Mol. Sci. 2018, 19, 1532. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Custódio, M.; Biddle, A.; Tavassoli, M. Portrait of a CAF: The Story of Cancer-Associated Fibroblasts in Head and Neck Cancer. Oral Oncol. 2020, 110, 104972. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Kanugula, S.S.; Sudhir, S.; Pereira, M.P.; Jain, S.; Aghi, M.K. The Role of Cancer-Associated Fibroblasts in Tumor Progression. Cancers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Östman, A. PDGF receptors in tumor stroma: Biological effects and associations with prognosis and response to treatment. Adv. Drug Deliv. Rev. 2017, 121, 117–123. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Yorozu, A.; Okazaki, F.; Niinuma, T.; Takasawa, A.; Yamamoto, E.; Kitajima, H.; Kubo, T.; Hatanaka, Y.; Nishiyama, K.; et al. ACLP Activates Cancer-Associated Fibroblasts and Inhibits CD8+ T-Cell Infiltration in Oral Squamous Cell Carcinoma. Cancers 2023, 15, 4303. [Google Scholar] [CrossRef]
- Dourado, M.R.; Guerra, E.N.S.; Salo, T.; Lambert, D.W.; Coletta, R.D. Prognostic Value of the Immunohistochemical Detection of Cancer-Associated Fibroblasts in Oral Cancer: A Systematic Review and Meta-Analysis. J. Oral. Pathol. Med. 2018, 47, 443–453. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-Associated Extracellular Matrix Genes Link Cancer-Associated Fibroblasts to Immune Evasion and Immunotherapy Failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Angadi, P.V.; Patil, P.V.; Kale, A.D.; Hallikerimath, S.; Babji, D. Myofibroblast Presence in Apparently Normal Mucosa Adjacent to Oral Squamous Cell Carcinoma Associated with Chronic Tobacco/Areca Nut Use: Evidence for Field Cancerization. Acta Odontol. Scand. 2014, 72, 502–508. [Google Scholar] [CrossRef]
- Smitha, A.; Rao, K.; Umadevi, H.; Smitha, T.; Sheethal, H.; Vidya, M. Immunohistochemical Study of α-Smooth Muscle Actin Expression in Oral Leukoplakia and Oral Squamous Cell Carcinoma. J. Oral. Maxillofac. Pathol. 2019, 23, 59–64. [Google Scholar] [CrossRef]
- Datar, U.V.; Kale, A.D.; Angadi, P.V.; Hallikerimath, S.; Deepa, M.; Desai, K.M. Role of cancer-associated fibroblasts in oral squamous cell carcinomas, surgical margins, and verrucous carcinomas: An immunohistochemical study. J. Clin. Transl. Res. 2022, 8, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Siddiqui, S.; Faizi, N.; Hassan, J.; Nehal, N.; Siddiqui, A. Role of Stromal Myofibroblasts in the Progression of Oral Lesions from Dysplasia to Invasive Carcinoma. Indian. J. Med. Paediatr. Oncol. 2021, 40, 536–541. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; García-Pedrero, J.M. PD-L1 Expression in Tumor Cells Is an Independent Unfavorable Prognostic Factor in Oral Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Thia, K.; Nguyen, T.; MacDonald, S.; Belobrov, S.; Kranz, S.; Goode, D.; A Trapani, J.; Wiesenfeld, D.; et al. Cancer cell-specific PD-L1 expression is a predictor of poor outcome in patients with locally advanced oral cavity squamous cell carcinoma. J. Immunother. Cancer 2024, 12, e009617. [Google Scholar] [CrossRef] [PubMed]
- Sasaya, T.; Kubo, T.; Murata, K.; Mizue, Y.; Sasaki, K.; Yanagawa, J.; Imagawa, M.; Kato, H.; Tsukahara, T.; Kanaseki, T.; et al. Cisplatin-induced HSF1-HSP90 axis enhances the expression of functional PD-L1 in oral squamous cell carcinoma. Cancer Med. 2023, 12, 4605–4615. [Google Scholar] [CrossRef]
- Liu, L.; Lim, M.A.; Jung, S.-N.; Oh, C.; Won, H.-R.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Chang, J.W.; Koo, B.S. The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine 2021, 92, 153758. [Google Scholar] [CrossRef]
- Boreel, D.F.; Sandker, G.G.W.; Ansems, M.; van den Bijgaart, R.J.E.; Peters, J.P.W.; Span, P.N.; Adema, G.J.; Heskamp, S.; Bussink, J. MHC-I and PD-L1 Expression is Associated with Decreased Tumor Outgrowth and is Radiotherapy-inducible in the Murine Head and Neck Squamous Cell Carcinoma Model MOC1. Mol. Imaging Biol. 2024, 26, 835–846. [Google Scholar] [CrossRef]
- Gu, W.; Kim, M.; Wang, L.; Yang, Z.; Nakajima, T.; Tsushima, Y. Multi-omics Analysis of Ferroptosis Regulation Patterns and Characterization of Tumor Microenvironment in Patients with Oral Squamous Cell Carcinoma. Int. J. Biol. Sci. 2021, 17, 3476–3492. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Li, J.; Xie, X.; Hao, Y.; Zhu, Y.; Zhang, Z.; Fu, J.; Ma, H. Engineered Oxygen Factories Synergize with STING Agonist to Remodel Tumor Microenvironment for Cancer Immunotherapy. Adv. Funct. Mater. 2023, 33, 2300833. [Google Scholar] [CrossRef]
- He, Y.; Dong, Y.; Zhang, X.; Ding, Z.; Song, Y.; Huang, X.; Chen, S.; Wang, Z.; Ni, Y.; Ding, L. Lipid Droplet-Related PLIN2 in CD68+ Tumor-Associated Macrophage of Oral Squamous Cell Carcinoma: Implications for Cancer Prognosis and Immunotherapy. Front. Oncol. 2022, 15, 824235. [Google Scholar] [CrossRef]
- Kondo, Y.; Suzuki, S.; Takahara, T.; Ono, S.; Goto, M.; Miyabe, S.; Sugita, Y.; Ogawa, T.; Ito, H.; Satou, A.; et al. Improving function of cytotoxic T-lymphocytes by transforming growth factor-β inhibitor in oral squamous cell carcinoma. Cancer Sci. 2021, 112, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiu, W.; Li, S.; Wang, S.; Xie, J.; Yang, Q.; Xu, J.; Zhang, J.; Xu, Z.; Sun, Z. A Dual-Responsive STAT3 Inhibitor Nanoprodrug Combined with Oncolytic Virus Elicits Synergistic Antitumor Immune Responses by Igniting Pyroptosis. Adv. Mater. 2023, 35, e2209379. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, G.; Wang, L.; Hu, J.; Ju, Z.; Tao, H.; Li, Q.; Li, J.; Zhang, W.; Sheng, J.; et al. Spatial proteomic profiling elucidates immune determinants of neoadjuvant chemo-immunotherapy in esophageal squamous cell carcinoma. Oncogene 2024, 43, 2751–2767. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Shan, T.; Wang, H.; Mao, L.; Liang, Y.; Cao, M.; Wu, Q.; Li, C.; Wang, Y.; Wang, Y. A Bacterial Nanomedicine Combines Photodynamic-Immunotherapy and Chemotherapy for Enhanced Treatment of Oral Squamous Cell Carcinoma. Small 2023, 19, e2304014. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Wang, W.-J.; Zhang, C.-Y.; Ling, Y.-Y.; Hong, X.-J.; Su, Q.; Li, W.-G.; Mao, Z.-W.; Cheng, B.; Tan, C.-P.; et al. Ru(II)-modified TiO2 nanoparticles for hypoxia-adaptive photo-immunotherapy of oral squamous cell carcinoma. Biomaterials 2022, 289, 121757. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, K.; Huang, S.; Zhang, X.; Zhu, X.; He, Y.; Chen, X.; Tang, Y.; Yuan, L.; Yu, D. Graphdiyne Oxide-Mediated Photodynamic Therapy Boosts Enhancive T-Cell Immune Responses by Increasing Cellular Stiffness. Int. J. Nanomed. 2023, 15, 797–812. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Dai, C.; Wu, J.; Li, Q. Immune-enhanced and tumor-targeted PDT cascade therapy for oral squamous cell carcinoma utilizing a carrier-free BRD4 inhibitor/PDT agent nanocomplex. Chem. Eng. J. 2024, 485, 149446. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Molska, G.R.; Yeo, H.; Esfandiari, N.; Jeong, W.; Huang, M.; Magalhaes, M. Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 6650. https://doi.org/10.3390/ijms26146650
Ali A, Molska GR, Yeo H, Esfandiari N, Jeong W, Huang M, Magalhaes M. Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(14):6650. https://doi.org/10.3390/ijms26146650
Chicago/Turabian StyleAli, Aiman, Graziella Rigueira Molska, Huiling Yeo, Najmeh Esfandiari, Will Jeong, Michelle Huang, and Marco Magalhaes. 2025. "Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review" International Journal of Molecular Sciences 26, no. 14: 6650. https://doi.org/10.3390/ijms26146650
APA StyleAli, A., Molska, G. R., Yeo, H., Esfandiari, N., Jeong, W., Huang, M., & Magalhaes, M. (2025). Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review. International Journal of Molecular Sciences, 26(14), 6650. https://doi.org/10.3390/ijms26146650





