Bioactive Compounds and In Vitro Health-Promoting Activity of the Fruit Skin and Flesh of Different Haskap Berry (Lonicera caerulea var. kamtschatica Sevast.) Cultivars
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Content of Iridoid and Polyphenolic Compounds
2.3. Sugars and Organic Acids
2.4. Antioxidant Activity
2.5. Antidiabetic Activity
2.6. Anti-Inflammatory Activity
2.7. Cytotoxic Activity Against Cancer Cells
2.8. Antimicrobial Activity
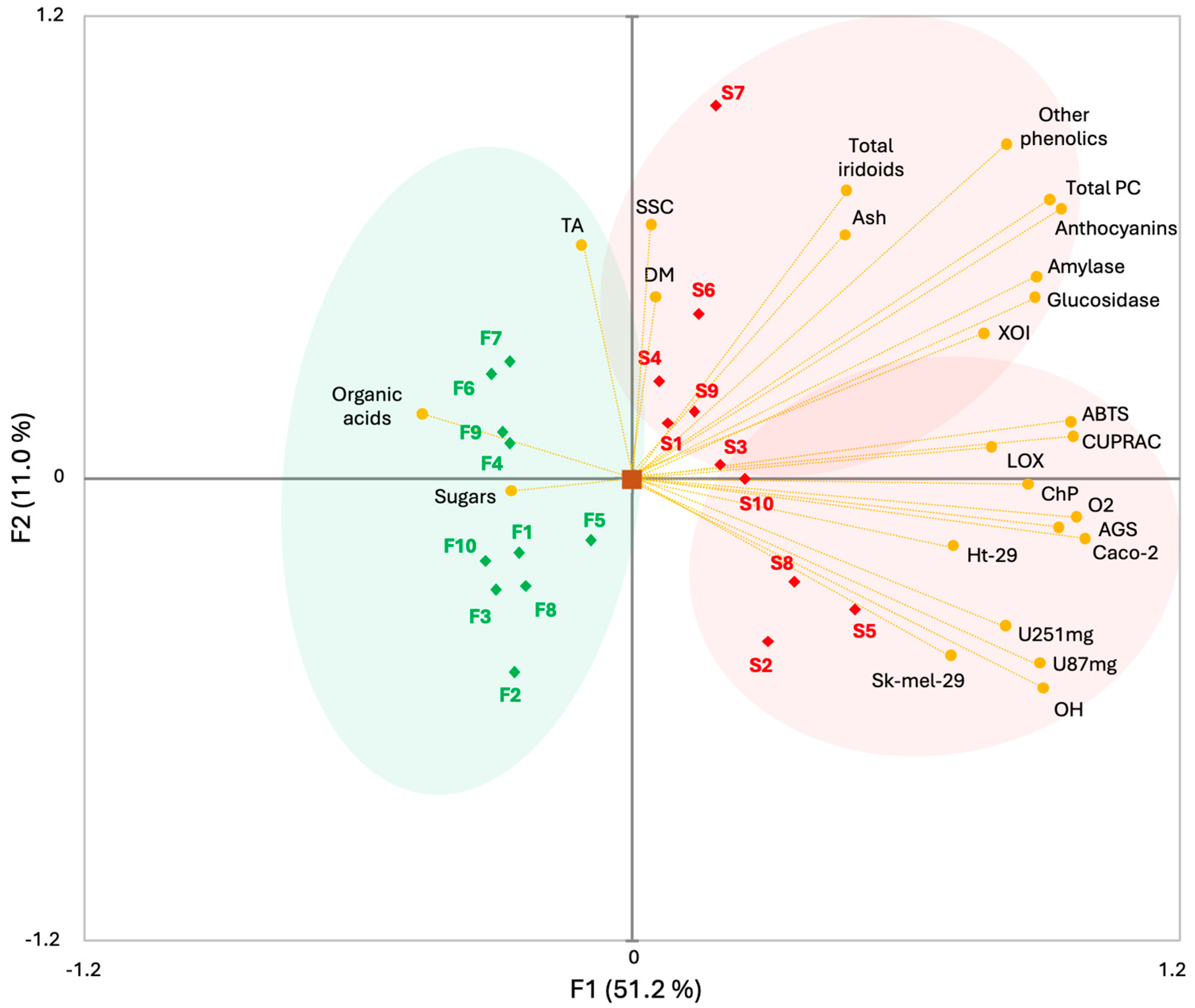
2.9. Principal Component Analysis
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Analysis of Physicochemical Properties
3.4. Analysis of Sugars and Organic Acids by HPLC
3.5. Analysis of Iridoids and Polyphenolic Compounds by UPLC-PDA-MS/MS
3.6. Antioxidant Activity Assay
3.7. Antidiabetic Activity Assay
3.8. Anti-Inflammatory Activity Assay
3.9. Assessment of Cytotoxic Activity Against Cancer Cells
3.10. Assessment of Antimicrobial Activity Against Selected Microorganisms
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochmian, I.D.; Skupien, K.; Grajkowski, J.; Smolik, M.; Ostrowska, K. Chemical Composition and Physical Characteristics of Fruits of Two Cultivars of Blue Honeysuckle (Lonicera caerulea L.) in Relation to Their Degree of Maturity and Harvest Date. Not. Bot. Hort. Agrobot. Cluj. 2012, 40, 155. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Different Extraction Processes Affect the Metabolites in Blue Honeysuckle (Loniceracaerulea L. Subsp. Edulis) Food Products. Turk. J. Agric. 2019, 43, 576–585. [Google Scholar] [CrossRef]
- Dayar, E.; Cebova, M.; Lietava, J.; Panghyova, E.; Pechanova, O. Antioxidant Effect of Lonicera caerulea L. in the Cardiovascular System of Obese Zucker Rats. Antioxidants 2021, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef]
- Ali, S.M.; Ourth, A.; Che, C.-T.; Wang, M.-Y.; Munirathinam, G. Anti-Cancer Evaluation of Various Solvent Extracts of Blue Honeysuckle Berry (Lonicera caerulea L.) against Prostate Cancer Cells. Cancer Res. 2017, 77, 1592. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu-Grama, A.-M.; Mihai, E.; Utoiu, E.; Negreanu-Pirjol, T.; Lupu, C.E.; Artem, V.; Ranca, A.; Negreanu-Pirjol, B.-S. The Chemical Profile, Antioxidant, and Anti-Lipid Droplet Activity of Fluid Extracts from Romanian Cultivars of Haskap Berries, Bitter Cherries, and Red Grape Pomace for the Management of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 16849. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Guo, S.; Fang, H.; Chang, X. Inhibition of Pancreatic α-Amylase by Lonicera caerulea Berry Polyphenols in Vitro and Their Potential as Hyperglycemic Agents. LWT 2020, 126, 109288. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Xiao, Z.; Sun, A.; Zhao, M.; Wang, Y.; Huang, D.; Sui, X.; Huo, J.; Zhang, Y. Polyphenols in Twenty Cultivars of Blue Honeysuckle (Lonicera caerulea L.): Profiling, Antioxidant Capacity, and α-Amylase Inhibitory Activity. Food Chem. 2023, 421, 136148. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, J.; Szczypka, M.; Gorczykowski, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Evaluation of Immunotropic Activity of Iridoid-Anthocyanin Extract of Honeysuckle Berries (Lonicera caerulea L.) in the Course of Experimental Trichinellosis in Mice. Molecules 2022, 27, 1949. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The Potential Health Benefits of Haskap (Lonicera caerulea L.): Role of Cyanidin-3- O -Glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Żurek, N.; Pluta, S.; Seliga, Ł.; Lachowicz-Wiśniewska, S.; Kapusta, I.T. Comparative Evaluation of the Phytochemical Composition of Fruits of Ten Haskap Berry (Lonicera caerulea Var. Kamtschatica Sevast.) Cultivars Grown in Poland. Agriculture 2024, 14, 1734. [Google Scholar] [CrossRef]
- Kucharska, A.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera Caerulea Var. Kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of Dried Powder Preparation Process on Polyphenolic Content and Antioxidant Activity of Blue Honeysuckle Berries (Lonicera caerulea L. Var. Kamtschatica). LWT—Food Sci. Technol. 2016, 67, 214–222. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kucharska, A.Z. Effect of Pre-Treatment of Blue Honeysuckle Berries on Bioactive Iridoid Content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of Modified (MAP) and Controlled Atmosphere (CA) Storage on the Quality and Bioactive Compounds of Blue Honeysuckle Fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue Honeysuckle (Lonicera caerulea Subsp. Edulis (Turcz. Ex Herder) Hultén.) Berries and Changes in Their Ingredients across Different Locations: Changes in Blue Honeysuckle Berry Ingredients across Different Locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Nowicka, P.; Turkiewicz, I.; Golis, T. Characterization in Vitro Potency of Biological Active Fractions of Seeds, Skins and Flesh from Selected Vitis Vinifera L. Cultivars and Interspecific Hybrids. J. Funct. Foods 2019, 56, 353–363. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant Compounds in Skin and Pulp of Fruits Change among Genotypes and Maturity Stages in Highbush Blueberry (Vaccinium corymbosum L.) Grown in Southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, Ł.; Pluta, S. Distribution of Phytochemicals and Antioxidative Potency in Fruit Peel, Flesh, and Seeds of Saskatoon Berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, J.; Mikhailovich, M.S.; Wang, L.; Chen, Y.; Ji, X.; She, H.; Zhang, L.; Zhang, Y.; Huo, J. Comprehensive Structural Analysis of Anthocyanins in Blue Honeysuckle (Lonicera caerulea L.), Bilberry (Vaccinium uliginosum L.), Cranberry (Vaccinium macrocarpon Ait.), and Antioxidant Capacity Comparison. Food Chem. X 2024, 23, 101734. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Blagojević, B.; Agić, D.; Serra, A.T.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in Vitro and in Silico Evaluation of Bioactive Potential of Cornelian Cherry (Cornus mas L.) Extracts Rich in Polyphenols and Iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and Anthocyanins in Cornelian Cherry (Cornus mas L.) Cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Česonienė, L.; Labokas, J.; Jasutienė, I.; Šarkinas, A.; Kaškonienė, V.; Kaškonas, P.; Kazernavičiūtė, R.; Pažereckaitė, A.; Daubaras, R. Bioactive Compounds, Antioxidant, and Antibacterial Properties of Lonicera caerulea Berries: Evaluation of 11 Cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Promising Antioxidant and Antimicrobial Food Colourants from Lonicera caerulea L. Var. Kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of Phytochemical Properties and Content of Bioactive Compounds in Lonicera caerulea L. Var. Kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, J.; Żebrowska, J.; Pabich, M.; Jackowska, I.; Kowalczyk, K.; Dyduch-Siemińska, M. Phytochemical Characterization of Blue Honeysuckle in Relation to the Genotypic Diversity of Lonicera sp. Appl. Sci. 2020, 10, 6545. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, J.W.; Ku, S.-K.; Choi, J.-S.; Lee, H.-J. Anti-Diabetic Effects of Blue Honeyberry on High-Fed-Diet-Induced Type II Diabetic Mouse. Nutr. Res. Pr. 2019, 13, 367. [Google Scholar] [CrossRef]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical Compositions and α-Glucosidase Inhibitory Effects of Anthocyanidins from Blueberry, Blackcurrant and Blue Honeysuckle Fruits. Food Chem. 2019, 299, 125102. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.; Boehm, M.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A. Anti-Inflammatory Activity of Haskap Cultivars Is Polyphenols-Dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef]
- Wu, S.; Yano, S.; Chen, J.; Hisanaga, A.; Sakao, K.; He, X.; He, J.; Hou, D.-X. Polyphenols from Lonicera caerulea L. Berry Inhibit LPS-Induced Inflammation through Dual Modulation of Inflammatory and Antioxidant Mediators. J. Agric. Food Chem. 2017, 65, 5133–5141. [Google Scholar] [CrossRef]
- Cai, M.; Liu, M.; Chen, P.; Liu, H.; Wang, Y.; Yang, D.; Zhao, Z.; Ding, P. Iridoids with Anti-Inflammatory Effect from the Aerial Parts of Morinda Officinalis How. Fitoterapia 2021, 153, 104991. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; He, Y.; Zhang, Q.; Zhu, B.; Shen, Y.; Liu, M.; Zhu, L.; Xin, H.; Qin, L.; et al. Iridoid Glycosides from Morinda Officinalis How. Exert Anti-Inflammatory and Anti-Arthritic Effects through Inactivating MAPK and NF-κB Signaling Pathways. BMC Complement. Med. Ther. 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yang, Y.; Wang, L.; Li, H. Isolation of Three Cyanins from Lonicera caerulea L. Fruits and Its Anticancer Activity. J. Cent. South Univ. 2017, 24, 1573–1581. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-Tumor Properties of Anthocyanins from Lonicera caerulea ‘Beilei’ Fruit on Human Hepatocellular Carcinoma: In Vitro and in Vivo Study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Potocki, L.; Oklejewicz, B.; Kuna, E.; Szpyrka, E.; Duda, M.; Zuczek, J. Application of Green Algal Planktochlorella Nurekis Biomasses to Modulate Growth of Selected Microbial Species. Molecules 2021, 26, 4038. [Google Scholar] [CrossRef]
- PN-EN 12147:2000; Fruit and Vegetable Juices—Determination of Titratable Acidity. Polish Committee for Standardization (PKN): Warsaw, Poland, 2000. (In Polish)
- PN-A-75101-03:1990; Fruit and Vegetable Products. Sample Preparation and Physicochemical Test Methods. Determination of Dry Mass Content by Gravimetric Method. Polish Committee for Standardization (PKN): Warsaw, Poland, 1996. (In Polish)
- PN-90/A-75101/08; Fruit and Vegetable Products Sample Preparation and Physicochemical Test Methods. Determination of Total Ash Content and Alkalinity. Polish Committee for Standardization (PKN): Warsaw, Poland, 1996. (In Polish)
- Żurek, N.; Kapusta, I.; Cebulak, T. Impact of Extraction Conditions on Antioxidant Potential of Extracts of Flowers, Leaves and Fruits of Hawthorn (Crataegus × Macrocarpa L.). Food Sci. Technol. Qual. 2020, 27, 130–141. [Google Scholar] [CrossRef]
- Żurek, N.; Świeca, M.; Pawłowska, A.; Kapusta, I.T. Microencapsulation of Blueberry (Vaccinium myrtillus L.) Extracts via Ionotropic Gelation: In Vitro Assessment of Bioavailability of Phenolic Compounds and Their Activity against Colon Cancer Cells. Appl. Sci. 2024, 14, 7842. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Świeca, M.; Kapusta, I.T. Berries, Leaves, and Flowers of Six Hawthorn Species (Crataegus L.) as a Source of Compounds with Nutraceutical Potential. Molecules 2024, 29, 5786. [Google Scholar] [CrossRef] [PubMed]
- Żurek, N.; Kępska, A.; Kapusta, I. Characterization of Blueberry Fruit Powders as Functional Food Ingredients Rich in Polyphenolic Compounds. J. Cent. Eur. Agric. 2025, 26, 484–494. [Google Scholar] [CrossRef]
- Lachowicz, S.; Świeca, M.; Pejcz, E. Biological Activity, Phytochemical Parameters, and Potential Bioaccessibility of Wheat Bread Enriched with Powder and Microcapsules Made from Saskatoon Berry. Food Chem. 2021, 338, 128026. [Google Scholar] [CrossRef]
- Żurek, N.; Pawłowska, A.; Kapusta, I. Obtaining Preparations with Increased Content of Bioactive Compounds from Eight Types of Berries. J. Berry Res. 2023, 13, 307–323. [Google Scholar] [CrossRef]
| Cultivars and Parts | SSC | TA | DM | Ash |
|---|---|---|---|---|
| °Brix | g/100 g f.m. | g/100 g f.m. | % | |
| Skin | ||||
| Boreal Beauty | 13.51 ± 0.07 e | 3.01 ± 0.02 d | 14.01 ± 0.07 bc | 0.55 ± 0.00 b |
| Boreal Beast | 14.32 ± 0.06 gh | 2.22 ± 0.01 a | 13.82 ± 0.05 b | 0.53 ± 0.00 a |
| Boreal Blizzard | 13.80 ± 0.08 ef | 2.41 ± 0.01 ab | 15.58 ± 0.01 f | 0.57 ± 0.01 b |
| Aurora | 15.71 ± 0.04 ij | 2.89 ± 0.01 cd | 15.41 ± 0.05 ef | 0.51 ± 0.00 a |
| Honeybee | 14.07 ± 0.06 g | 3.11 ± 0.06 e | 17.20 ± 0.05 i | 0.55 ± 0.00 b |
| Vostorg | 12.68 ± 0.05 cd | 3.18 ± 0.03 f | 16.03 ± 0.06 g | 0.60 ± 0.00 b |
| Jugana | 13.71 ± 0.07 e | 3.12 ± 0.04 e | 15.41 ± 0.0.6 ef | 0.61 ± 0.00 b |
| Usłada | 16.04 ± 0.16 j | 2.58 ± 0.07 b | 14.37 ± 0.15 d | 0.63 ± 0.01 b |
| Lawina | 14.47 ± 0.09 h | 2.81 ± 0.05 cd | 14.18 ± 0.05 c | 0.67 ± 0.00 c |
| Sinij Uties | 15.46 ±0.11 i | 2.89 ± 0.02 cd | 15.20 ± 0.06 ef | 0.56 ± 0.00 b |
| Flesh | ||||
| Boreal Beauty | 12.13 ± 0.11 c | 2.84 ± 0.02 cd | 13.54 ± 0.12 a | 0.46 ± 0.00 a |
| Boreal Beast | 10.68 ± 0.09 ab | 2.06 ± 0.01 a | 13.51 ± 0.05 a | 0.50 ± 0.01 a |
| Boreal Blizzard | 10.22 ± 0.10 a | 2.32 ± 0.02 ab | 15.34 ± 0.07 ef | 0.51 ± 0.00 a |
| Aurora | 12.10 ± 0.04 c | 2.78 ± 0.01 cd | 15.22 ± 0.06 ef | 0.49 ± 0.00 a |
| Honeybee | 12.07 ± 0.04 c | 2.94 ± 0.05 cd | 16.84 ± 0.10 h | 0.52 ± 0.00 a |
| Vostorg | 11.23 ± 0.06 b | 3.10 ± 0.05 e | 15.88 ± 0.11 g | 0.58 ± 0.01 b |
| Jugana | 13.01 ± 0.11 d | 2.86 ± 0.02 cd | 15.07 ± 0.07 e | 0.58 ± 0.00 b |
| Usłada | 14.22 ± 0.12 g | 2.53 ± 0.02 b | 14.18 ± 0.08 c | 0.60 ± 0.00 b |
| Lawina | 14.01 ± 0.02 f | 2.63 ± 0.04 b | 14.11 ± 0.11 c | 0.61 ± 0.00 b |
| Sinij Uties | 13.46 ± 0.07 e | 2.69 ± 0.04 c | 14.98 ± 0.14 e | 0.50 ± 0.00 a |
| Mean | ||||
| Skin | 14.33 | 2.81 | 15.12 | 0.58 |
| Flesh | 12.31 | 2.68 | 14.87 | 0.54 |
| Cultivars and Parts | Anthocyanins (mg/g d.m.) | |||||||||||
| (Epi)catechin-Cyanidin 3-O-glucoside | Cyanidin 3-O-sophoroside-5-O-glucoside | Cyanidin 3-O-rutinoside-5-O-glucoside | Cyanidin 3,5-O-diglucoside | Cyanidin 3-O-glucoside | Cyanidin 3-O-rutinoside | Pelargonidin 3-O-glucoside | Peonidin 3-O-glucoside | Peonidin 3-O-rutinoside | Delphinidin 3-O-rhamnoside | Sum Anthocyanins | ||
| Skin | ||||||||||||
| Boreal Beauty | <LLQ | <LLQ | <LLQ | 1.23 ± 0.01 e | 51.48 ± 0.50 f | 4.59 ± 0.41 i | 0.60 ± 0.03 e | 2.25 ± 0.04 h | 0.65 ± 0.04 e | <LLQ | 61.71 ± 3.24 h | |
| Boreal Beast | <LLQ | <LLQ | <LLQ | 4.18 ± 0.30 j | 63.59 ± 0.44 j | 5.03 ± 0.31 j | 0.58 ± 0.03 e | 2.32 ± 0.02 h | 0.59 ± 0.00 d | <LLQ | 77.50 ± 1.01 m | |
| Boreal Blizzard | <LLQ | <LLQ | <LLQ | 2.98 ± 0.20 h | 64.17 ± 0.33 k | 4.19 ± 0.13 h | 0.60 ± 0.00 e | 2.98 ± 0.11 m | 0.60 ± 0.02 d | <LLQ | 75.49 ± 0.74 k | |
| Aurora | <LLQ | <LLQ | <LLQ | 1.39 ± 0.04 f | 56.29 ± 0.74 g | 2.04 ± 0.02 de | 0.65 ± 0.04 f | 2. 52 ± 0.11 j | 0.45 ± 0.03 c | <LLQ | 64.33 ± 2.13 i | |
| Honeybee | <LLQ | <LLQ | <LLQ | 3.44 ± 0.31 i | 82.34 ± 0.30 m | 7.66 ± 0.24 n | 0.80 ± 0.01 g | 2.73 ± 0.11 k | 0.64 ± 0.03 d | <LLQ | 98.50 ± 1.91 n | |
| Vostorg | <LLQ | <LLQ | <LLQ | 1.48 ± 0.03 fg | 64.68 ± 0.93 l | 5.49 ± 0.03 k | 0.47 ± 0.02 d | 2.97 ± 0.23 l | 0.64 ± 0.01 d | <LLQ | 76.44 ± 1.03 l | |
| Jugana | <LLQ | <LLQ | <LLQ | 2.86 ± 0.11 h | 51.58 ± 0.54 f | 2.77 ± 0.04 g | 0.45 ± 0.01 d | 1.69 ± 0.03 f | 0.48 ± 0.00 c | <LLQ | 60.39 ± 1.59 g | |
| Usłada | <LLQ | <LLQ | <LLQ | 1.60 ± 0.01 g | 58.56 ± 0.31 h | 7.72 ± 0.22 n | 0.56 ± 0.00 e | 2.06 ± 0.04 g | 0.70 ± 0.00 e | <LLQ | 71.88 ± 0.73 j | |
| Lawina | <LLQ | <LLQ | <LLQ | 1.46 ± 0.04 fg | 59.38 ± 0.38 i | 5.96 ± 0.13 l | 0.83 ± 0.03 g | 2.38 ± 0.11 hi | 0.66 ± 0.00 e | <LLQ | 71.78 ± 0.77 j | |
| Sinij Uties | <LLQ | <LLQ | <LLQ | 1.63 ± 0.03 g | 63.30 ± 0.83 j | 6.83 ± 0.33 m | 0.93 ± 0.01 h | 2.74 ± 0.22 k | 0.71 ± 0.00 e | <LLQ | 76.61 ± 1.20 l | |
| Flesh | ||||||||||||
| Boreal Beauty | <LLQ | <LLQ | <LLQ | 0.32 ± 0.00 ab | 17.57 ± 0.17 b | 2.44 ± 0.04 f | 0.13 ± 0.00 a | 0.96 ± 0.01 c | 0.22 ± 0.02 b | <LLQ | 22.03 ± 1.08 c | |
| Boreal Beast | <LLQ | <LLQ | <LLQ | 0.61 ± 0.00 c | 16.97 ± 0.05 b | 1.60 ± 0.03 c | 0.14 ± 0.01 a | 0.69 ± 0.03 ab | 0.14 ± 0.00 a | <LLQ | 20.14 ± 0.41 b | |
| Boreal Blizzard | <LLQ | <LLQ | <LLQ | 0.88 ± 0.03 d | 20.41 ± 0.18 de | 1.93 ± 0.03 d | 0.18 ± 0.00 b | 1.32 ± 0.02 e | 0.17 ± 0.00 b | <LLQ | 24.91 ± 1.88 ef | |
| Aurora | <LLQ | <LLQ | <LLQ | 0.37 ± 0.02 b | 20.86 ± 0.21 e | 0.90 ± 0.01 a | 0.24 ± 0.02 b | 1.27 ± 0.03 e | <LLQ | <LLQ | 24.01 ± 0.77 e | |
| Honeybee | <LLQ | <LLQ | <LLQ | 0.80 ± 0.10 d | 16.20 ± 0.11 a | 1.21 ± 0.02 b | <LLQ | 0.64 ± 0.02 a | <LLQ | <LLQ | 19.04 ± 1.07 a | |
| Vostorg | <LLQ | <LLQ | <LLQ | 0.19 ± 0.01 a | 17.25 ± 0.32 b | 1.54 ± 0.03 c | <LLQ | 0.90 ± 0.01 c | <LLQ | <LLQ | 19.66 ± 0.70 ab | |
| Jugana | <LLQ | <LLQ | <LLQ | 0.70 ± 0.01 cd | 21.01 ± 0.42 e | 2.14 ± 0.13 e | 0.17 ± 0.00 b | 0.81 ± 0.02 b | 0.08 ± 0.00 a | <LLQ | 25.08 ± 1.52 f | |
| Usłada | <LLQ | <LLQ | <LLQ | 0.31 ± 0.00 ab | 17.43 ± 0.11 b | 2.73 ± 0.14 g | 0.15 ± 0.00 b | 0.78 ± 0.03 b | 0.20 ± 0.00 b | <LLQ | 21.77 ± 0.32 c | |
| Lawina | <LLQ | <LLQ | <LLQ | 0.40 ± 0.00 b | 19.85 ± 0.18 d | 2.84 ± 0.07 g | 0.29 ± 0.00 c | 1.13 ± 0.01 d | 0.21 ± 0.00 b | <LLQ | 25.10 ± 0.77 f | |
| Sinij Uties | <LLQ | <LLQ | <LLQ | 0.39 ± 0.00 b | 18.95 ± 0.47 c | 2.45 ± 0.10 f | 0.32 ± 0.00 c | 1.04 ± 0.00 c | 0.20 ± 0.00 b | <LLQ | 23.52 ± 0.35 d | |
| Mean | ||||||||||||
| Skin | 0.00 | 0.00 | 0.00 | 2.22 | 61.54 | 5.23 | 0.65 | 2.46 | 0.61 | 0.00 | 73.46 | |
| Flesh | 0.00 | 0.00 | 0.00 | 0.50 | 18.64 | 1.97 | 0.20 | 0.95 | 0.18 | 0.00 | 22.52 | |
| Cultivars and Parts | Other Phenolics (mg/g d.m.) | |||||||||||
| Neochlorogenic Acid | Procyanidin dimer B-type | Chlorogenic Acid | Procyanidin Dimer B-type | Luteolin 7-O-glucoside | Quercetin 3-O-rutinoside-7-O-rhamnoside | Quercetin 3-O-pentoside-glucoside I | Quercetin 3-O-rutinoside | Quercetin 3-O-glucoside | Quercetin 3-O-rhmanoside | |||
| Skin | ||||||||||||
| Boreal Beauty | 0.16 ± 0.00 b | 0.10 ± 0.00 a | 1.75 ± 0.20 d | 0.15 ± 0.00 b | 0.83 ± 0.00 c | 0.13 ± 0.00 a | 0.56 ± 0.01 e | 0.51 ± 0.03 b | 0.30 ± 0.00 b | <LLQ | ||
| Boreal Beast | 0.27 ± 0.01 c | <LLQ | 1.74 ± 0.10 c | 0.16 ± 0.00 b | 1.09 ± 0.03 de | 0.18 ± 0.01 b | 0.15 ± 0.01 b | 1.90 ± 0.14 g | 0.21 ± 0.01 ab | 0.08 ± 0.00 a | ||
| Boreal Blizzard | 0.11 ± 0.00 a | 0.06 ± 0.00 a | 1.93 ± 0.04 d | 0.15 ± 0.01 b | 1.16 ± 0.04 e | 0.09 ± 0.00 a | 0.18 ± 0.01 b | 1.21 ± 0.04 d | 0.19 ± 0.03 ab | <LLQ | ||
| Aurora | 0.11 ± 0.00 a | <LLQ | 1.31 ± 0.03 a | 0.10 ± 0.00 a | 0.94 ± 0.01 c | 0.07 ± 0.00 a | 0.12 ± 0.00 a | 0.56 ± 0.03 c | 0.19 ± 0.01 ab | <LLQ | ||
| Honeybee | 0.27 ± 0.03 c | <LLQ | 4.40 ± 0.41 i | 0.34 ± 0.01 c | 1.75 ± 0.04 f | 0.08 ± 0.00 a | 0.22 ± 0.00 b | 1.53 ± 0.10 e | 0.52 ± 0.00 c | 0.09 ± 0.00 a | ||
| Vostorg | 0.27 ± 0.01 c | <LLQ | 2.16 ± 0.30 f | 0.19 ± 0.00 b | 1.15 ± 0.04 e | 0.14 ± 0.0 a | 0.41 ± 0.00 d | 1.66 ± 0.20 f | 0.26 ± 0.00 b | <LLQ | ||
| Jugana | 0.26 ± 0.02 c | <LLQ | 1.58 ± 0.10 c | 0.13 ± 0.00 a | 0.92 ± 0.02 d | <LLQ | 0.19 ± 0.00 b | 0.28 ± 0.00 a | 0.21 ± 0.00 ab | <LLQ | ||
| Usłada | 0.29 ± 0.01 c | <LLQ | 2.64 ± 0.08 g | 0.22 ± 0.02 b | 1.03 ± 0.04 d | 0.09 ± 0.00 a | 0.23 ± 0.00 b | 1.93 ± 0.04 g | 0.23 ± 0.01 ab | <LLQ | ||
| Lawina | 0.24 ± 0.00 b | <LLQ | 2.69 ± 0.11 gh | 0.21 ± 0.00 b | 1.07 ± 0.04 de | 0.17 ± 0.00 b | 0.25 ± 0.00 c | 1.34 ± 0.00 d | 0.29 ± 0.00 b | 0.07 ± 0.00 a | ||
| Sinij Uties | 0.25 ± 0.01 c | <LLQ | 2.84 ± 0.11 gh | 0.25 ± 0.01 c | 1.19 ± 0.04 e | 0.19 ± 0.01 b | 0.28 ± 0.02 c | 1.47 ± 0.04 e | 0.32 ± 0.03 b | 0.09 ± 0.00 a | ||
| Flesh | ||||||||||||
| Boreal Beauty | 0.13 ± 0.00 a | 0.05 ± 0.00 a | 1.66 ± 0.17 c | 0.06 ± 0.00 a | 0.56 ± 0.04 ab | 0.09 ± 0.00 a | 0.40 ± 0.00 d | 0.38 ± 0.00 b | 0.22 ± 0.00 a | 0.05 ± 0.00 a | ||
| Boreal Beast | 0.15 ± 0.00 b | <LLQ | 1.47 ± 0.11 b | 0.05 ± 0.00 a | 0.56 ± 0.02 ab | 0.06 ± 0.00 a | 0.05 ± 0.00 a | 0.46 ± 0.01 b | 0.08 ± 0.00 a | <LLQ | ||
| Boreal Blizzard | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 1.92 ± 0.15 d | 0.07 ± 0.00 a | 0.79 ± 0.03 c | 0.06 ± 0.00 a | 0.13 ± 0.00 a | 0.72 ± 0.02 c | 0.14 ± 0.00 a | <LLQ | ||
| Aurora | 0.07 ± 0.00 a | 0.05 ± 0.00 a | 1.42 ± 0.06 ab | <LLQ | 0.72 ± 0.03 ab | 0.05 ± 0.00 a | 0.08 ± 0.00 a | 0.37 ± 0.04 b | 0.18 ±0.00 a | <LLQ | ||
| Honeybee | 0.17 ± 0.01 b | 0.06 ± 0.00 a | 3.05 ± 0.04 h | 0.07 ± 0.00 a | 0.81 ± 0.00 c | <LLQ | 0.07 ± 0.00 a | 0.41 ± 0.00 b | 0.16 ± 0.00 a | <LLQ | ||
| Vostorg | 0.13 ± 0.00 a | <LLQ | 1.60 ± 0.03 c | <LLQ | 0.54 ± 0.04 a | <LLQ | 0.11 ± 0.00 a | 0.37 ± 0.03 b | 0.09 ± 0.00 a | <LLQ | ||
| Jugana | 0.17 ± 0.01 b | 0.05 ± 0.00 a | 1.57 ± 0.04 c | 0.06 ± 0.00 a | 0.50 ± 0.00 a | <LLQ | 0.15 ± 0.00 b | 0.23 ± 0.00 a | 0.17 ± 0.00 a | <LLQ | ||
| Usłada | 0.20 ± 0.00 b | <LLQ | 2.28 ± 0.24 f | 0.06 ± 0.00 a | 0.62 ± 0.00 ab | <LLQ | 0.08 ± 0.00 a | 0.49 ± 0.02 b | 0.10 ± 0.00 a | <LLQ | ||
| Lawina | 0.16 ± 0.00 b | <LLQ | 2.27 ± 0.17 f | 0.07 ± 0.00 a | 0.76 ± 0.02 c | 0.08 ± 0.00 a | 0.12 ± 0.00 a | 0.60 ± 0.03 c | 0.16 ± 0.02 a | 0.05 ± 0.00 a | ||
| Sinij Uties | 0.14 ± 0.00 a | <LLQ | 2.14 ± 0.04 e | 0.06 ± 0.00 a | 0.68 ± 0.03 ab | 0.07 ± 0.00 a | 0.10 ± 0.00 a | 0.49 ±0.00 b | 0.14 ± 0.00 a | <LLQ | ||
| Mean | ||||||||||||
| Skin | 0.22 | 0.02 | 2.30 | 0.19 | 1.11 | 0.12 | 0.26 | 1.24 | 0.26 | 0.07 | ||
| Flesh | 0.14 | 0.04 | 1.94 | 0.06 | 0.65 | 0.06 | 0.13 | 0.45 | 0.14 | 0.03 | ||
| Cultivars and Parts | Other phenolics(mg/g d.m.) | |||||||||||
| Kaempferol 3-O-glucoside-7-O-rhamnoside | Kaempferol 3-O-rutinoside | Kaempferol 3-O-glucoside-7-O-glucuronide | 3,4-di-O-caffeoyl-quinic acid | Quercetin 3-O-(6”-acetylo)-glucoside | Kaempferol 3-O-rhamnoside | Kaempferol 3-O-pentoside I | Kaempferol 3-O-pentoside II | Quercetin 3-O-rhamnoside | Sum Other Phenolics | Total Polyphenols Compounds | ||
| Skin | ||||||||||||
| Boreal Beauty | 0.08 ± 0.00 a | <LLQ | <LLQ | 0.17 ± 0.00 b | 0.06 ± 0.00 a | 4.76 ± 0.31 d | <LLQ | <LLQ | <LLQ | 9.72 ± 0.51 f | 71.44 ± 3.70 k | |
| Boreal Beast | 0.08 ± 0.00 a | <LLQ | 0.07 ± 0.00 a | 0.50 ± 0.00 a | 0.11 ± 0.00 a | 6.24 ± 0.24 f | <LLQ | <LLQ | <LLQ | 12.74 ± 0.63 i | 90.18 ± 4.50 o | |
| Boreal Blizzard | <LLQ | <LLQ | 0.11 ± 0.00 a | 0.09 ± 0.00 a | 0.06 ± 0.00 a | 5.41 ± 0.21 e | <LLQ | <LLQ | <LLQ | 10.83 ± 0.81 g | 86.27 ± 2.19 m | |
| Aurora | <LLQ | 0.06 ± 0.00 a | 0.05 ± 0.00 a | 0.11 ± 0.00 a | 0.07 ± 0.00 a | 3.70 ± 0.31 b | <LLQ | <LLQ | <LLQ | 7.50 ± 0.37 b | 71.41 ± 3.69 k | |
| Honeybee | <LLQ | <LLQ | 0.11 ± 0.00 a | 0.15 ± 0.00 b | 0.09 ± 0.00 a | 9.64 ± 0.10 j | <LLQ | <LLQ | <LLQ | 19.48 ± 1.18 m | 118.01 ± 5.01 r | |
| Vostorg | 0.05 ± 0.00 a | <LLQ | 0.11 ± 0.00 a | 0.11 ± 0.00 a | 0.10 ± 0.00 a | 6.69 ± 0.17 g | <LLQ | <LLQ | <LLQ | 13.51 ± 0.77 j | 89.91 ± 1.40 n | |
| Jugana | <LLQ | <LLQ | <LLQ | 0.24 ± 0.02 b | 0.11 ± 0.00 a | 4.04 ± 0.03 c | <LLQ | <LLQ | <LLQ | 8.19 ± 0.46 c | 68.61 ± 2.01 j | |
| Usłada | <LLQ | 0.08 ± 0.01 a | 0.11 ± 0.02 a | 0.08 ± 0.00 a | <LLQ | 7.06 ± 0.30 h | <LLQ | <LLQ | <LLQ | 14.32 ± 0.90 k | 86.09 ± 3.10 m | |
| Lawina | 0.05 ± 0.00 a | 0.06 ± 0.00 a | 0.09 ± 0.00 a | 0.08 ± 0.00 a | 0.06 ± 0.00 a | 6.66 ± 0.18 g | <LLQ | <LLQ | <LLQ | 13.53 ± 0.17 j | 84.80 ± 1.19 l | |
| Sinij Uties | 0.05 ± 0.00 a | 0.07 ± 0.00 a | 0.09 ± 0.00 a | 0.07 ± 0.00 a | 0.08 ± 0.00 a | 7.25 ± 0.21 i | <LLQ | <LLQ | <LLQ | 14.59 ± 0.20 l | 91.50 ± 3.08 p | |
| Flesh | ||||||||||||
| Boreal Beauty | 0.05 ± 0.00 a | <LLQ | <LLQ | 0.40 ± 0.01 c | 0.05 ± 0.00 a | <LLQ | <LLQ | <LLQ | 4.08 ± 0.22 c | 8.34 ± 0.44 c | 29.80 ± 0.71 c | |
| Boreal Beast | <LLQ | <LLQ | <LLQ | 0.58 ± 0.02 e | 0.05 ± 0.00 a | <LLQ | <LLQ | <LLQ | 3.63 ± 0.11 a | 7.41 ± 0.18 a | 27.77 ± 1.39 b | |
| Boreal Blizzard | <LLQ | <LLQ | <LLQ | 0.39 ± 0.00 c | 0.05 ± 0.00 a | <LLQ | <LLQ | <LLQ | 4.41 ± 0.26 d | 8.81 ± 0.73 d | 33.69 ± 2.45 g | |
| Aurora | <LLQ | <LLQ | <LLQ | 0.41 ± 0.04 c | 0.07 ± 0.00 a | <LLQ | <LLQ | <LLQ | 3.66 ± 0.18 b | 7.43 ± 0.22 a | 31.15 ± 1.88 e | |
| Honeybee | <LLQ | <LLQ | <LLQ | 0.98 ± 0.07 g | 0.06 ± 0.00 a | 0.05 ± 0.00 a | <LLQ | <LLQ | 5.88 ± 0.09 g | 11.85 ± 0.66 h | 37.02 ± 0.77 i | |
| Vostorg | <LLQ | <LLQ | <LLQ | 0.72 ± 0.01 f | 0.10 ± 0.00 a | <LLQ | <LLQ | <LLQ | 3.69 ± 0.22 b | 7.47 ± 0.35 b | 27.51 ± 1.67 b | |
| Jugana | 0.05 ± 0.00 a | <LLQ | <LLQ | 0.47 ± 0.03 d | 0.10 ± 0.00 a | <LLQ | <LLQ | 0.05 ± 0.00 a | 3.59 ± 0.04 a | 7.33 ± 0.40 a | 26.31 ± 1.60 a | |
| Usłada | <LLQ | <LLQ | <LLQ | 0.54 ± 0.02 d | <LLQ | <LLQ | <LLQ | <LLQ | 4.45 ± 0.11 de | 9.14 ± 0.48 e | 30.92 ± 0.8 d | |
| Lawina | <LLQ | 0.05 ± 0.00 a | <LLQ | 0.51 ± 0.00 d | 0.05 ± 0.00 a | <LLQ | <LLQ | <LLQ | 4.90 ± 0.24 f | 9.74 ± 0.31 f | 34.81 ± 2.10 h | |
| Sinij Uties | <LLQ | <LLQ | <LLQ | 0.56 ± 0.02 e | 0.06 ± 0.00 a | <LLQ | <LLQ | <LLQ | 4.56 ± 0.18 e | 9.21 ± 0.14 e | 32.77 ± 2.02 f | |
| Mean | ||||||||||||
| Skin | 0.04 | 0.04 | 0.08 | 0.12 | 0.08 | 6.15 | 0.00 | 0.00 | 0.00 | 12.41 | 85.81 | |
| Flesh | 0.00 | 0.00 | 0.00 | 0.55 | 0.06 | 0.00 | 0.00 | 0.00 | 4.29 | 8.65 | 31.18 | |
| Cultivars and Parts | Iridoids (mg/g d.m.) | ||||||
|---|---|---|---|---|---|---|---|
| Loganic Acid | Sweroside Pentoside Isomer I | Loganin | Loganin Pentoside | Sweroside | Sweroside Pentoside Isomer II | Total Iridoids | |
| Skin | |||||||
| Boreal Beauty | 3.66 ± 0.13 f | 1.67 ± 0.12 d | 6.56 ± 0.48 h | 0.99 ± 0.07 d | 1.22 ± 0.09 e | 0.52 ± 0.04 c | 14.62 ± 0.94 k |
| Boreal Beast | 3.13 ± 0.11 e | 1.11 ± 0.04 ab | 7.63 ± 0.28 k | 0.75 ± 0.03 c | 0.71 ± 0.03 d | 0.17 ± 0.01 b | 13.50 ± 0.49 i |
| Boreal Blizzard | 11.99 ± 0.43 k | 2.86 ± 0.10 h | 0.37 ± 0.01 b | <LLQ | 1.43 ± 0.05 f | 0.15 ± 0.01 b | 16.80 ± 0.61 l |
| Aurora | 4.38 ± 0.16 i | 1.46 ± 0.05 c | 5.15 ± 0.19 e | 0.36 ± 0.01 b | 2.32 ± 0.08 g | 0.12 ± 0.00 a | 13.78 ± 0.50 j |
| Honeybee | 3.76 ± 0.26 f | 6.66 ± 0.45 j | 7.47 ± 0.51 k | 0.65 ± 0.04 c | 0.21 ± 0.01 ab | 0.23 ± 0.02 b | 18.98 ± 1.30 n |
| Vostorg | 4.05 ± 0.15 h | 0.98 ± 0.004 a | 4.27 ± 0.15 c | 0.40 ± 0.01 b | 0.66 ± 0.01 d | 0.12 ± 0.00 a | 10.48 ± 0.38 e |
| Jugana | 15.46 ± 0.56 l | 9.08 ± 0.33 l | 0.40 ± 0.01 b | 0.07 ± 0.00 a | 3.13 ± 0.11 h | 0.17 ± 0.01 b | 28.31 ± 1.02 p |
| Usłada | 2.00 ± 0.07 c | 1.92 ± 0.07 e | 4.17 ± 0.15 c | 0.44 ± 0.02 b | 0.21 ± 0.01 ab | 0.14 ± 0.01 a | 8.89 ± 0.32 b |
| Lawina | 2.21 ± 0.08 c | 1.98 ± 0.07 e | 6.69 ± 0.24 hi | 0.74 ± 0.03 c | 0.51 ± 0.02 c | 0.09 ± 0.00 a | 12.22 ± 0.43 h |
| Sinij Uties | 2.52 ± 0.09 d | 1.79 ± 0.03 d | 6.87 ± 0.07 i | 0.72 ± 0.01 c | 0.49 ± 0.01 c | 0.09 ± 0.00 a | 12.47 ± 0.20 h |
| Flesh | |||||||
| Boreal Beauty | 2.14 ± 0.10 c | 1.06 ± 0.05 ab | 5.71 ± 0.28 f | 0.67 ± 0.03 c | 1.17 ± 0.06 e | 0.45 ± 0.02 c | 11.20 ± 0.55 f |
| Boreal Beast | 0.96 ± 0.07 a | 1.02 ± 0.07 a | 7.90 ± 0.56 l | 1.09 ± 0.08 de | 0.57 ± 0.02 c | 0.18 ± 0.01 b | 11.73 ± 0.81 g |
| Boreal Blizzard | 2.97 ± 0.12 e | 2.72 ± 0.09 g | 0.21 ± 0.01 a | 0.52 ± 0.03 b | 0.67 ± 0.01 d | 0.18 ± 0.01 b | 7.27 ± 0.25 a |
| Aurora | 1.61 ± 0.10 b | 1.24 ± 0.04 b | 4.48 ± 0.34 c | 2.08 ± 0.16 g | 0.08 ± 0.02 a | 0.12 ± 0.01 a | 9.63 ± 0.72 d |
| Honeybee | 3.87 ± 0.38 g | 5.82 ± 0.41 i | 8.78 ± 0.37 m | 1.18 ± 0.11 e | 0.14 ± 0.02 a | 0.15 ± 0.01 b | 19.94 ± 1.31 o |
| Vostorg | 1.14 ± 0.04 a | 0.94 ± 0.03 a | 6.43 ± 0.22 g | 1.07 ± 0.04 de | 0.28 ± 0.01 b | 0.14 ± 0.00 a | 10.01 ± 0.34 e |
| Jugana | 5.33 ± 0.25 j | 8.44 ± 0.48 k | 0.46 ± 0.06 b | 3.07 ± 0.20 h | 0.21 ± 0.02 ab | 0.15 ± 0.01 b | 17.66 ± 1.26 m |
| Usłada | 1.16 ± 0.04 a | 2.11 ± 0.07 f | 4.90 ± 0.17 d | 0.89 ± 0.03 d | 0.07 ± 0.00 a | 0.12 ± 0.00 a | 9.25 ± 0.32 c |
| Lawina | 1.79 ± 0.06 b | 1.98 ± 0.07 e | 6.91 ± 0.22 i | 1.26 ± 0.02 f | 0.07 ± 0.00 a | 0.12 ± 0.00 a | 12.14 ± 0.41 h |
| Sinij Uties | 1.24 ± 0.06 a | 1.28 ± 0.06 b | 7.09 ± 0.45 j | 1.32 ± 0.10 f | 0.09 ± 0.00 a | 0.10 ± 0.00 a | 11.12 ± 0.54 f |
| Mean | |||||||
| Skin | 5.32 | 2.95 | 4.96 | 0.51 | 1.09 | 0.18 | 15.01 |
| Flesh | 2.22 | 2.66 | 5.29 | 1.32 | 0.34 | 0.17 | 11.99 |
| Cultivars and Parts | Organic Acids (g/100 g d.m.) | Sugars (g/100 g d.m.) | |||||
|---|---|---|---|---|---|---|---|
| Citric Acid | Malic Acid | Quinic Acid | Total Acids | Glucose | Fructose | Total Sugars | |
| Skin | |||||||
| Boreal Beauty | 0.90 ± 0.07 a | nd | 0.46 ± 0.04 a | 1.37 ± 0.31 b | 0.44 ± 0.02 a | 0.54 ± 0.01 a | 0.98 ± 0.06 a |
| Boreal Beast | 0.35 ± 0.03 a | nd | 0.13 ± 0.01 a | 0.47 ± 0.16 a | 2.20 ± 0.08 c | 2.62 ± 1.00 bcd | 4.82 ± 0.24 d |
| Boreal Blizzard | 0.86 ± 0.07 a | nd | 0.35 ± 0.03 a | 1.21 ± 0.36 b | 2.05 ± 0.07 bc | 2.33 ± 0.11 ab | 4.38 ± 0.17 d |
| Aurora | 0.95 ± 0.06 a | nd | 0.33 ± 0.02 a | 1.28 ± 0.13 b | 2.24 ± 0.08 c | 2.72 ± 0.07 bcd | 5.01 ± 0.28 e |
| Honeybee | 1.06 ± 0.08 a | nd | 0.32 ± 0.03 a | 1.37 ± 0.52 b | 1.62 ± 0.06 bc | 2.32 ± 0.10 b | 3.94 ± 0.40 c |
| Vostorg | 1.25 ± 0.09 a | nd | 0.50 ± 0.02 a | 1.75 ± 0.53 c | 0.99 ± 0.02 ab | 1.19 ± 0.03 a | 2.18 ± 0.12 b |
| Jugana | 1.01 ± 0.105 a | nd | 0.35 ± 0.02 a | 1.35 ± 0.47 b | 2.04 ± 0.12 bc | 2.35 ± 0.07 ab | 4.39 ± 0.18 d |
| Usłada | 0.58 ± 0.00 a | nd | 0.17 ± 0.01 a | 0.75 ± 0.29 a | 3.53 ± 0.12 d | 3.65 ± 0.13 de | 7.18 ± 0.07 f |
| Lawina | 0.59 ± 0.05 a | nd | 0.22 ± 0.01 a | 0.81 ± 0.27 a | 2.28 ± 0.08 c | 2.32 ± 0.08 b | 4.60 ± 0.03 d |
| Sinij Uties | 0.56 ± 0.06 a | nd | 0.43 ± 0.01 a | 0.98 ± 0.09 ab | 3.90 ± 0.14 d | 3.99 ± 0.14 e | 7.89 ± 0.06 g |
| Flesh | |||||||
| Boreal Beauty | 19.51 ± 1.52 h | 2.97 ± 0.23 d | 5.45 ± 0.42 e | 27.93 ± 8.92 i | 19.32 ± 0.68 e | 22.54 ± 0.79 g | 41.86 ± 2.27 i |
| Boreal Beast | 7.63 ± 0.59 b | 1.86 ± 0.14 c | 2.45 ± 0.19 c | 11.94 ± 3.17 d | 18.85 ± 0.66 e | 20.14 ± 0.71 f | 38.99 ± 0.91 h |
| Boreal Blizzard | 9.67 ± 0.75 c | 1.77 ± 0.14 c | 1.47 ± 0.11 b | 12.91 ± 4.65 e | 22.77 ± 0.80 f | 24.66 ± 0.86 h | 47.43 ± 1.34 j |
| Aurora | 10.92 ± 0.82 d | 1.73 ± 0.13 c | 2.30 ± 0.18 c | 14.94 ± 5.15 f | 19.12 ± 0.67 e | 21.60 ± 0.77 g | 40.72 ± 1.75 h |
| Honeybee | 12.14 ± 0.94 e | 1.33 ± 0.10 b | 5.69 ± 0.44 e | 19.16 ± 5.44 h | 18.70 ± 0.65 e | 22.07 ± 0.76 g | 40.81 ± 2.39 h |
| Vostorg | 15.06 ± 1.17 g | 16.08 ± 0.47 f | 17.37 ± 0.98 f | 48.51 ± 1.15 j | 27.67 ± 0.90 i | 29.43 ± 1.03 j | 57.10 ± 1.24 n |
| Jugana | 14.53 ± 1.13 f | 1.03 ± 0.08 a | 2.16± 0.17 c | 17.72 ± 7.49 g | 25.22 ± 0.88 h | 27.45 ± 0.94 i | 52.66 ± 1.58 l |
| Usłada | 9.57 ± 0.74 c | 3.47 ± 0.27 e | 2.41 ± 0.19 c | 15.45 ± 3.86 f | 30.91 ± 1.08 j | 33.82 ± 1.18 k | 64.73 ± 2.05 o |
| Lawina | 9.54 ± 0.73 c | 2.03 ± 0.15 c | 3.35 ± 0.26 d | 14.86 ± 4.03 f | 24.18 ± 0.85 g | 24.64 ± 0.86 h | 48.82 ± 0.33 k |
| Sinij Uties | 8.82 ± 0.72 c | 1.80 ± 0.14 c | 2.61 ± 0.20 c | 13.24 ± 3.84 e | 27.08 ± 1.01 i | 28.47 ± 1.00 ij | 55.56 ± 0.98 m |
| Mean | |||||||
| Skin | 0.81 | 0.00 | 0.32 | 1.13 | 2.13 | 2.40 | 4.53 |
| Flesh | 11.74 | 3.40 | 4.53 | 19.67 | 23.38 | 25.48 | 48.94 |
| Cultivars and Parts | Antioxidant | Antidiabetic | Anti- Inflammatory | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ABTS (mmol TE/100 g) | CUPRAC (mmol TE/100 g) | ChP (μg/mL) | OH˙ (μg/mL) | O2˙− (μg/mL) | α-Amylase (mg/mL) | α-Glucosidase (mg/mL) | LOXI (mg/mL) | XOI (mg/mL) | |
| Skin | |||||||||
| Boreal Beauty | 68.64 ± 1.80 g | 48.03 ± 0.61 k | 428.91 ± 3.01 e | 800.41 ± 12.00 j | 687.34 ± 18.38 g | 11.92 ± 0.18 e | 10.14 ± 1.07 ab | 0.35 ± 0.02 a | 1.93 ± 0.08 de |
| Boreal Beast | 98.01 ± 3.61 l | 54.83 ± 0.09 n | 259.09 ± 4.77 a | 408.11 ± 3.86 c | 423.14 ± 12.61 b | 6.19 ± 0.11 ab | 15.26 ± 1.02 cde | 0.30 ± 0.03 a | 1.83 ± 0.19 cd |
| Boreal Blizzard | 84.42 ± 2.31 k | 48.82 ± 0.11 l | 315.21 ± 5.75 b | 499.50 ± 13.03 e | 516.82 ± 5.51 d | 7.89 ± 0.57 bc | 11.97 ± 0.31 abcd | 0.61 ± 0.04 cd | 2.12 ± 0.15 e |
| Aurora | 74.70 ± 1.77 h | 41.32 ± 0.60 i | 373.51 ± 11.11 d | 576.66 ± 4.85 g | 765.72 ± 7.65 h | 8.49 ± 0.48 cd | 13.16 ± 1.01 bcd | 0.79 ± 0.03 e | 1.53 ± 0.12 b |
| Honeybee | 103.65 ± 2.16 m | 63.91 ± 0.38 o | 240.04 ± 4.42 a | 334.15 ± 5.07 a | 371.70 ± 11.76 a | 4.84 ± 0.33 a | 8.35 ± 0.33 a | 0.46 ± 0.04 b | 1.11 ± 0.05 a |
| Vostorg | 79.94 ± 1.71 i | 49.78 ± 0.65 m | 366.37 ± 7.80 d | 718.86 ± 5.16 i | 533.54 ± 3.77 d | 5.24 ± 0.24 a | 17.80 ± 0.80 e | 0.57 ± 0.05 c | 1.54 ± 0.13 b |
| Jugana | 75.86 ± 1.42 h | 42.23 ± 0.38 j | 381.61 ± 2.84 d | 833.61 ± 4.28 l | 602.42 ± 3.10 e | 6.92 ± 0.40 abc | 11.33 ± 0.56 abc | 0.60 ± 0.02 cd | 1.83 ± 0.15 cd |
| Usłada | 83.54 ± 1.34 jk | 48.77 ± 0.33 l | 346.22 ± 11.11 c | 368.04 ± 3.02 b | 464.76 ± 9.91 c | 4.86 ± 0.44 a | 10.97 ± 0.42 abc | 0.69 ± 0.06 d | 1.73 ± 0.16 bcd |
| Lawina | 83.91 ± 1.45 jk | 49.31 ± 0.21 m | 335.61 ± 9.90 c | 549.51 ± 6.72 f | 652.51 ± 5.02 f | 6.41 ± 0.82 ab | 14.57 ± 1.34 bcde | 0.76 ± 0.05 e | 1.90 ± 0.21 de |
| Sinij Uties | 81.33 ± 2.51 ij | 54.69 ± 0.90 n | 330.22 ± 9.04 bc | 443.43 ± 4.21 d | 454.56 ± 9.62 c | 5.19 ± 0.31 a | 16.04 ± 0.72 de | 0.65 ± 0.05 cd | 1.74 ± 0.07 bcd |
| Flesh | |||||||||
| Boreal Beauty | 39.88 ± 0.82 de | 24.52 ± 0.25 f | 828.11 ± 7.37 j | 1268.05 ± 7.21 o | 1108.23 ± 12.42 k | 21.94 ± 1.08 i | 25.62 ± 2.86 f | 1.36 ± 0.11 h | 2.89 ± 0.12 i |
| Boreal Beast | 36.70 ± 0.74 bc | 21.88 ± 0.47 c | 890.66 ± 6.27 k | 951.12 ± 10.35 m | 764.91 ± 2.86 h | 17.03 ± 1.57 fg | 33.04 ± 2.18 g | 1.20 ± 0.07 g | 2.84 ± 0.12 ghi |
| Boreal Blizzard | 40.83 ± 0.83 e | 23.94 ± 1.02 e | 806.02 ± 5.31 i | 826.07 ± 9.55 kl | 845.42 ± 8.88 j | 15.01 ± 1.23 f | 37.47 ± 3.45 h | 1.14 ± 0.05 g | 3.06 ± 0.22 i |
| Aurora | 42.37± 0.90 e | 24.64 ± 0.85 f | 537.23 ± 15.90 g | 809.22 ± 5.28 jk | 1217.32 ± 3.28 l | 17.0 1± 0.92 fg | 32.42 ± 2.38 g | 1.17 ± 0.05 g | 1.59 ± 0.12 bc |
| Honeybee | 48.72 ± 0.81 f | 30.17 ± 0.32 h | 439.20 ± 10.60 e | 680.21 ± 9.01 h | 608.01 ± 9.32 e | 10.12 ± 1.62 de | 26.10 ± 4.62 f | 0.98 ± 0.04 f | 2.42 ± 0.20 f |
| Vostorg | 34.41 ± 0.72 b | 20.34 ± 0.11 a | 1241.30 ± 26.41 m | 1414.23 ± 11.42 r | 856.82 ± 5.01 j | 15.54 ± 2.30 fg | 43.86 ± 2.04 i | 1.12 ± 0.07 g | 2.61 ± 0.04 fg |
| Jugana | 37.72 ± 0.65 cd | 21.47 ± 0.50 b | 906.63 ± 6.42 k | 993.94 ± 6.30 n | 820.37 ± 10.42 i | 17.29 ± 0.58 gh | 27.93 ± 1.52 f | 1.15 ± 0.09 g | 2.74 ± 0.11 gh |
| Usłada | 29.40 ± 0.36 a | 20.34 ± 0.77 a | 1122.41 ± 24.22 l | 802.77 ± 4.33 j | 860.32 ± 5.60 j | 19.13 ± 1.62 h | 44.48 ± 3.50 i | 1.10 ± 0.02 g | 3.77 ± 0.07 k |
| Lawina | 41.86 ± 0.37 e | 25.35 ± 0.27 g | 730.42 ± 9.40 h | 1361.09 ± 6.60 p | 1627.62 ± 15.72 m | 16.23 ± 2.10 fg | 34.54 ± 2.02 gh | 1.14 ± 0.05 g | 3.50 ± 0.16 j |
| Sinij Uties | 40.52 ± 0.71 e | 23.68 ± 1.01 d | 501.67 ± 13.66 f | 725.31 ± 6.89 i | 743.51 ± 5.42 h | 15.68 ± 1.55 fg | 46.54 ± 1.90 i | 1.14 ± 0.05 g | 3.57 ± 0.15 jk |
| Mean | |||||||||
| Skin | 83.40 | 50.21 | 338.27 | 553.20 | 547.31 | 6.79 | 12.96 | 0.58 | 1.73 |
| Flesh | 39.22 | 23.58 | 799.38 | 983.24 | 945.23 | 16.50 | 35.20 | 1.15 | 2.90 |
| Cultivars and Parts | IC50 value (μg/mL) | |||||
|---|---|---|---|---|---|---|
| Caco-2 | Ht-29 | U251 mg | U87 mg | AGS | SK-Mel-29 | |
| Skin | ||||||
| Boreal Beauty | 256.21 ± 5.41 h | 233.11 ± 3.33 f | 374.11 ± 2.76 b | 425.28 ± 3.15 h | 283.71 ± 6.02 g | 415.35 ± 12.10 b |
| Boreal Beast | 122.22 ± 6.02 b | 218.07 ± 3.11 de | 432.88 ± 5.56 c | 289.56 ± 1.65 b | 182.11 ± 5.45 cd | 494.76 ± 7.02 cd |
| Boreal Blizzard | 175.67 ± 3.66 de | 390.18 ± 6.10 k | 493.45 ± 5.00 d | 364.85 ± 9.81 e | 187.43 ± 1.51 d | 485.37 ± 6.88 c |
| Aurora | 287.55 ± 13.02 i | 308.64 ± 4.41 hi | 630.89 ± 10.21 h | 419.73 ± 3.13 gh | 208.85 ± 8.11 e | 584.61 ± 8.26 g |
| Honeybee | 107.30 ± 4.65 a | 96.04 ± 3.78 a | 351.10 ± 8.00 a | 249.78 ± 5.66 a | 155.03 ± 3.57 a | 333.10 ± 4.65 a |
| Vostorg | 188.10 ± 9.28 f | 176.91 ± 5.47 c | 588.54 ± 9.31 g | 411.91 ± 4.01 g | 240.00 ± 11.88 f | 584.62 ± 8.28 g |
| Jugana | 206.08 ± 4.43 g | 226.28 ± 3.21 ef | 554.41 ± 10.82 f | 390.67 ± 8.33 f | 236.88 ± 5.02 f | 587.11 ± 11.85 g |
| Usłada | 140.91 ± 6.21 c | 131.45 ± 7.07 b | 352.52 ± 8.86 a | 312.65 ± 1.89 c | 164.06 ± 4.16 ab | 426.91 ± 8.58 b |
| Lawina | 165.88 ± 3.51 d | 301.43 ± 4.31 h | 539.12 ± 12.18 f | 310.40 ± 1.71 c | 187.37 ± 2.02 d | 584.62 ± 8.28 g |
| Sinij Uties | 182.56 ± 2.85 ef | 121.31 ± 8.40 b | 478.03 ± 4.77 d | 307.71 ± 8.00 c | 172.77 ± 0.88 bc | 506.04 ± 7.18 de |
| Flesh | ||||||
| Boreal Beauty | 399.30 ± 10.35 l | 336.71 ± 6.01 j | 518.45 ± 3.89 e | 571.54 ± 9.61 l | 387.41 ± 3.41 k | 550.81 ± 9.81 f |
| Boreal Beast | 323.33 ± 2.55 j | 315.10 ± 5.62 i | 624.61 ± 6.02 h | 356.88 ± 7.89 e | 353.11 ± 6.12 i | 714.72 ± 12.65 h |
| Boreal Blizzard | 416.38 ± 3.60 m | 538.33 ± 9.57 m | >750 | 500.91 ± 7.71 k | 383.40 ± 6.52 k | 481.22 ± 8.66 c |
| Aurora | 693.08 ± 6.03 r | 445.72 ± 7.88 l | >750 | 564.90 ± 4.22 l | 371.30 ± 3.58 j | >750 |
| Honeybee | 294.61 ± 3.11 i | 208.22 ± 9.35 d | 515.78 ± 13.01 e | 342.67 ± 3.78 d | 304.73 ± 7.03 h | 701.21 ± 12.51 h |
| Vostorg | 458.24 ± 4.22 o | 257.68 ± 4.57 g | >750 | 603.03 ± 2.54 m | 418.32 ± 4.03 l | >750 |
| Jugana | 488.54 ± 4.22 p | 327.03 ± 5.77 j | >750 | 568.57 ± 8.72 l | 367.28 ± 7.78 j | >750 |
| Usłada | 350.72 ± 12.81 k | 264.41 ± 12.78 g | 513.16 ± 8.04 e | 481.16 ± 3.10 j | 235.09 ± 7.16 f | 522.80 ± 9.27 e |
| Lawina | 393.11 ± 3.40 l | 435.38 ± 7.68 l | >750 | 477.38 ± 7.26 j | 384.00 ± 9.38 k | >750 |
| Sinij Uties | 432.60 ± 3.68 n | 209.88 ± 3.18 d | 739.02 ± 7.37 i | 440.73 ± 0.94 i | 301.72 ± 7.88 h | >750 |
| Mean | ||||||
| Skin | 183.33 | 220.32 | 479.45 | 348.11 | 201.78 | 500.21 |
| Flesh | 425.04 | 333.78 | 717.91 | 491.26 | 350.60 | 672.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurek, N.; Pluta, S.; Świeca, M.; Potocki, L.; Seliga, Ł.; Kapusta, I. Bioactive Compounds and In Vitro Health-Promoting Activity of the Fruit Skin and Flesh of Different Haskap Berry (Lonicera caerulea var. kamtschatica Sevast.) Cultivars. Int. J. Mol. Sci. 2025, 26, 6618. https://doi.org/10.3390/ijms26146618
Żurek N, Pluta S, Świeca M, Potocki L, Seliga Ł, Kapusta I. Bioactive Compounds and In Vitro Health-Promoting Activity of the Fruit Skin and Flesh of Different Haskap Berry (Lonicera caerulea var. kamtschatica Sevast.) Cultivars. International Journal of Molecular Sciences. 2025; 26(14):6618. https://doi.org/10.3390/ijms26146618
Chicago/Turabian StyleŻurek, Natalia, Stanisław Pluta, Michał Świeca, Leszek Potocki, Łukasz Seliga, and Ireneusz Kapusta. 2025. "Bioactive Compounds and In Vitro Health-Promoting Activity of the Fruit Skin and Flesh of Different Haskap Berry (Lonicera caerulea var. kamtschatica Sevast.) Cultivars" International Journal of Molecular Sciences 26, no. 14: 6618. https://doi.org/10.3390/ijms26146618
APA StyleŻurek, N., Pluta, S., Świeca, M., Potocki, L., Seliga, Ł., & Kapusta, I. (2025). Bioactive Compounds and In Vitro Health-Promoting Activity of the Fruit Skin and Flesh of Different Haskap Berry (Lonicera caerulea var. kamtschatica Sevast.) Cultivars. International Journal of Molecular Sciences, 26(14), 6618. https://doi.org/10.3390/ijms26146618








